Iron Supplementation of Pregnant Sows to Prevent Iron Deficiency Anemia in Piglets: A Procedure of Questionable Effectiveness
Abstract
1. Introduction
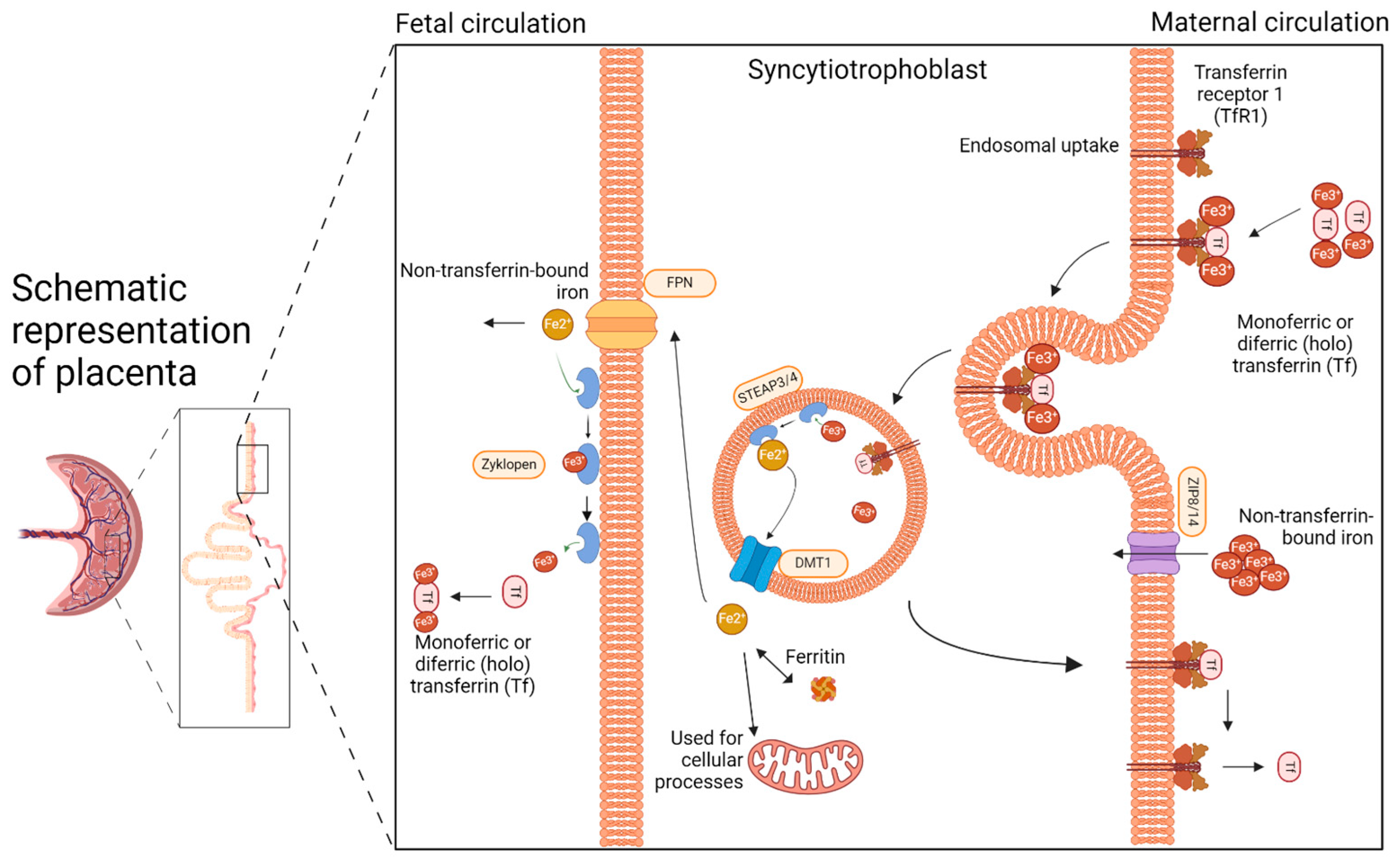
2. The Placenta: The Unappreciated Organ of Iron Metabolism
3. Supplementation of Pregnant Sows with Iron
3.1. Oral vs. Parenteral Iron Supplementation
3.1.1. Oral Iron Supplementation
3.1.2. Parenteral Iron Supplementation
3.2. Doses and Formulations of Supplemental Iron
3.3. The Iron Levels of Pregnant Sows and Their Impact on Piglets
3.4. The Impact of Iron Supplementation in Pregnant Sows on the Iron Content of Milk
| Author (Year) | Formulation | Dose of Fe | Length of Supplementation | Breed of Pigs | Effect on Piglets |
|---|---|---|---|---|---|
| McGowan (1924) [4] | Fe₂O₃ | 40 g per day | Last weeks of pregnancy | n.d. | HGB— RBC—  Liver Fe—n.d. Serum Fe—n.d. |
| Hart et al. (1930) [38] | Fe2(SO4)3 Fe₂O₃ | 10–250 mg per day | During pregnancy | Poland China, Duroc Jersey, and Chester White | HGB— RBC—  Liver Fe—  Serum Fe—n.d. |
| Pilgrim and Qureshi (1952) [59] | iron dextran | 100 mg per day | During pregnancy | n.d. | HGB— RBC—n.d. Liver Fe—n.d. Serum Fe—n.d. |
| Rydberg et al. (1959) [45] | iron dextran solution | 1 g in a single dose | 14 days before parturition | n.d. | HGB— RBC—  Liver Fe—n.d. Serum Fe—n.d. |
| Pond et al. (1961) [44] | iron dextran | 1 g or 5 g in a single dose | From day 100 of pregnancy | Berkshire and Yorkshire | HGB—no change RBC—  Liver Fe—n.d. Serum Fe—n.d. |
| Spruill et al. (1971) [48] | FeSO4 | 200 mg per day | 94 days before prepartum | Hampshire and Yorkshire | HGB— RBC—n.d. Liver Fe—n.d. Serum Fe—no change |
| Lillie and Frobish (1978) [49] | FeSO4 | 30 or 60 mg per day | During pregnancy | Duroc | HGB—no change RBC—n.d. Liver Fe—n.d. Serum Fe—  |
| Ducsay et al. (1984) [47] | iron dextran | 5.5 g (1.1 g per injection) | From day 40 to 60 of pregnancy | n.d. | HGB— RBC—  Liver Fe—  Serum Fe—n.d. |
| O’Connor et al. (1989) [69] | FeSO4x7H2O | 200 mg per day | During pregnancy | n.d. | HGB— RBC—n.d. Liver Fe—n.d. Serum Fe—n.d. |
| Egeli et al. (1998) [75] | amino acid chelated iron or glutamic chelated iron | 300 mg per day 650 mg per day | Last 3 weeks of pregnancy | Norwegian Landrace | HGB— RBC—  Liver Fe—n.d. Serum Fe—no change |
| Peters and Mahan (2008) [15] | chelated to soy proteinor FeSO4 | 20 mg per day | During pregnancy | Yorkshire Landrace | HGB— RBC—n.d. Liver Fe—n.d. Serum Fe—n.d. |
| Wang (2014) [52] | organic iron complex or FeSO4 | 650 mg per day | From day 84 of gestation | n.d. | HGB— RBC—  Liver Fe—n.d. Serum Fe—  |
| Zhao et al. (2015) [76] | chelated iron (bacterial iron) | n.d. | From day 84 of gestation | Yorkshire Landrace | HGB—no change RBC—n.d. Liver Fe—n.d. Serum Fe—no change |
| Jahan et al. (2017) [77] | lactoferrin | 1 g per day | During pregnancy | Large White, Landrace, Duroc | HGB—n.d. RBC—n.d. Liver Fe—n.d. Serum Fe—n.d. |
| Buffler et al. (2017) [71] | Fe(II) SO4 7H2O | 688 mg per day, on average | During pregnancy | German Landrace | HGB— RBC—n.d. Liver Fe—  Serum Fe—no change |
| Li et al. (2018) [72] | Fe-Gly | 150 mg 240 mg 330 mg 420 mg per day, on average | From day 86 of gestation | Landrace x Large White sows | HGB— RBC—  Liver Fe—  Serum Fe—  |
| FeSO4xH2O | HGB— RBC—  Liver Fe—n.d. Serum Fe —  | ||||
| Wan et al. (2018) [70] | ferrous N-carbamylglycinate chelate or FeSO4 | 447 mg per day 457 mg per day | From day 86 of gestation | Landrace x Large Yorkshire | HGB—n.d. RBC—n.d. Liver Fe—  Serum Fe—n.d. |
| Barros et al. (2019) [78] | Yes Minerals Iron® | 1.3 g per day | From day 84 of gestation | Topigs Norsvin® | HGB—n.d. RBC—n.d. Liver Fe—n.d. Serum Fe—n.d. |
| Mazgaj et al. (2020) [57] | sucrosomial ferric pyrophosphate | 60 mg per day | From day 80 of gestation | 990 line | HGB—no change RBC—no change Liver Fe—no change Serum Fe—no change |
| Zhang et al. (2022) [54] | lactoferrin and Fe-Gly | 400 mg 700 mg 1 g per day | From day 80 of gestation | no info | HGB—n.d. RBC—n.d. Liver Fe—no change Serum Fe—  |
| Xing et al. (2023) [55] | lactoferrin or heme iron or Fe-Gly | 700 mg per day 280 mg per day 1.5 g per day | From day 33 of gestation | Landrace x Yorkshire | HGB—n.d. RBC—n.d. Liver Fe—  Serum Fe—  |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Venn, J.A.J.; McCance, R.A.; Widdowson, E.M. Iron Metabolism in Piglet Anaemia. J. Comp. Pathol. Ther. 1947, 57, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Drabek, J. Iron deficiency in suckling piglets: Etiology, clinical aspects and diagnosis. Folia Vet 2005, 49, 104–111. [Google Scholar]
- Kim, J.C.; Wilcock, P.; Bedford, M.R. Iron status of piglets and impact of phytase superdosing on iron physiology: A review. Anim. Feed Sci. Technol. 2018, 235, 8–14. [Google Scholar] [CrossRef]
- Boussingault, J.B. Du fer contenu dans le sang et dans les aliments. Comptes Rendus Acad. Sci. Paris 1872, 74, 1353–1359. [Google Scholar]
- McGowan, J.P.; Crichton, A. Iron Deficiency in Pigs. Biochem. J. 1924, 18, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Friendship, R.; Seip, V.; Amezcua, R. A comparison of 4 iron supplementation protocols to protect suckling piglets from anemia. Can. Vet. J. 2021, 62, 55–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lipiński, P.; Styś, A.; Starzyński, R.R. Molecular insights into the regulation of iron metabolism during the prenatal and early postnatal periods. Cell. Mol. Life Sci. 2013, 70, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Egeli, A.K.; Framstad, T.; Morberg, H. Clinical Biochemistry, Haematology and Body Weight in Piglets. Acta Vet. Scand. 1998, 39, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Vaňhara, J.; Berlinská, J. Parenteral iron administration in suckling piglets—A review. Acta Vet. Brno 2017, 86, 249–261. [Google Scholar] [CrossRef]
- Starzyński, R.R.; Laarakkers, C.M.M.M.; Tjalsma, H.; Swinkels, D.W.; Pieszka, M.; Styś, A.; Mickiewicz, M.; Lipiński, P. Iron Supplementation in Suckling Piglets: How to Correct Iron Deficiency Anemia without Affecting Plasma Hepcidin Levels. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Ginzburg, Y.Z. Hepcidin-Ferroportin Axis in Health and Disease. In Vitamins and Hormones; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 110, pp. 17–45. ISBN 9780128178423. [Google Scholar]
- Ueberschär, S. Sudden death in suckling piglets following administration of iron-dextran-preparation. Dtsch. Tierarztl. Wochenschr. 1966, 73, 145–150. [Google Scholar] [PubMed]
- Lipiński, P.; Starzyński, R.R.; Canonne-Hergaux, F.; Tudek, B.; Oliński, R.; Kowalczyk, P.; Dziaman, T.; Thibaudeau, O.; Gralak, M.A.; Smuda, E.; et al. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 2010, 177, 1233–1243. [Google Scholar] [CrossRef]
- Brady, P.S.; Ku, P.K.; Ullrey, D.E.; Miller, E.R. Evaluation of an Amino Acid-Iron Chelate Hematinic for the Baby Pig. J. Anim. Sci. 1978, 47, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C.; Newton, E.A. Effect of initial breeding weight on macro- and micromineral composition over a three-parity period using a high-producing sow genotype. J. Anim. Sci. 1995, 73, 151. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Mahan, D.C. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 2008, 86, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, Z.; Mazgaj, R.; Starzyński, R.R.; Wang, X.; Opiela, J.; Smorąg, Z.; Gajda, B.; Nicpoń, J.; Lenartowicz, M.; Ogłuszka, M.; et al. Impact of litter size on the hematological and iron status of gilts, sows and new-born piglets: A comparative study of domestic pigs and wild boars. BMC Vet. Res. 2024, 20, 64. [Google Scholar] [CrossRef]
- Cao, C.; Fleming, M.D. The placenta: The forgotten essential organ of iron transport. Nutr. Rev. 2016, 74, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Mazgaj, R.; Lipiński, P.; Edison, E.S.; Bednarz, A.; Staroń, R.; Haberkiewicz, O.; Lenartowicz, M.; Smuda, E.; Jończy, A.; Starzyński, R.R. Marginally reduced maternal hepatic and splenic ferroportin under severe nutritional iron deficiency in pregnancy maintains systemic iron supply. Am. J. Hematol. 2021, 96, 659–670. [Google Scholar] [CrossRef]
- Macdonald, A.A.; Bosma, A.A. Notes on placentation in the Suina. Placenta 1985, 6, 83–91. [Google Scholar] [CrossRef]
- McPherson, R.L.; Ji, F.; Wu, G.; Blanton, J.R.; Kim, S.W. Growth and compositional changes of fetal tissues in pigs. J. Anim. Sci. 2004, 82, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Pajor, E.A.; Fraser, D.; Kramer, D.L. Consumption of solid food by suckling pigs: Individual variation and relation to weight gain. Appl. Anim. Behav. Sci. 1991, 32, 139–155. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.D.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and Iron Homeostasis during Pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Fleming, M.D. Localization and Kinetics of the Transferrin-Dependent Iron Transport Machinery in the Mouse Placenta. Curr. Dev. Nutr. 2021, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Fisher, A.L.; Chua, K.J.; Ruchala, P.; Ganz, T.; Nemeth, E. Maternal hepcidin determines embryo iron homeostasis in mice. Blood 2020, 136, 2206–2216. [Google Scholar] [CrossRef]
- Gambling, L.; Danzeisen, R.; Gair, S.; Lea, R.G.; Charania, Z.; Solanky, N.; Joory, K.D.; Kaila, S.; Srai, S.; McArdle, H.J. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem. J. 2001, 356, 883–889. [Google Scholar] [CrossRef]
- Bastin, J.; Drakesmith, H.; Rees, M.; Sargent, I.; Townsend, A. Localisation of proteins of iron metabolism in the human placenta and liver. Br. J. Haematol. 2006, 134, 532–543. [Google Scholar] [CrossRef]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; DiRenzo, C.; Robine, S.; Andrews, N.C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Investig. 2005, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T.; Shimoda, S.; Ohashi, W.; Bin, B.H.; Koseki, H.; Hirano, T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE 2011, 6, e18059. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Peralta, M.; He, L.; Jorge-Nebert, L.F.; Wang, B.; Miller, M.L.; Eppert, B.L.; Afton, S.; Nebert, D.W. ZIP8 Zinc Transporter: Indispensable Role for Both Multiple-Organ Organogenesis and Hematopoiesis In Utero. PLoS ONE 2012, 7, e36055. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Attieh, Z.K.; Syed, B.A.; Kuo, Y.; Stevens, V.; Fuqua, B.K.; Andersen, H.S.; Naylor, C.E.; Evans, R.W.; Gambling, L.; et al. Identification of Zyklopen, a New Member of the Vertebrate Multicopper Ferroxidase Family, and Characterization in Rodents and Human Cells. J. Nutr. 2010, 140, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.L.; Wilkins, S.J.; McKeating, D.R.; Perkins, A.V.; Whibley, P.E.; Cuffe, J.S.M.; Simmons, D.G.; Fuqua, B.K.; Vulpe, C.D.; Wallace, D.F.; et al. The Placental Ferroxidase Zyklopen Is Not Essential for Iron Transport to the Fetus in Mice. J. Nutr. 2021, 151, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.B.; Elvehjem, C.A.; Steenbock, H.; Kemmerer, A.R.; Bohstedt, G.; Fargo, J.M. A Study of the Anemia of Young Pigs and Its Prevention. J. Nutr. 1930, 2, 277–294. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Chaney, C.H.; Barnhart, C.E. The effect of iron supplementation of sow rations on the prevention of baby pig anemia. Am. J. Vet. Res. 1964, 25, 420–423. [Google Scholar] [CrossRef]
- Abbas, M.; Hayirli, Z.; Drakesmith, H.; Andrews, S.C.; Lewis, M.C. Effects of iron deficiency and iron supplementation at the host-microbiota interface: Could a piglet model unravel complexities of the underlying mechanisms? Front. Nutr. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Ng, S.-W.; Norwitz, S.G.; Norwitz, E.R. The Impact of Iron Overload and Ferroptosis on Reproductive Disorders in Humans: Implications for Preeclampsia. Int. J. Mol. Sci. 2019, 20, 3283. [Google Scholar] [CrossRef] [PubMed]
- Pond, W.G.; Lowrey, R.S.; Maner, J.H.; Loosli, J.K. Parenteral Iron Administration to Sows during Gestation or Lactation. J. Anim. Sci. 1961, 20, 747–750. [Google Scholar] [CrossRef]
- Rydberg, M.E.; Self, H.L.; Kowalczyk, T.; Grummer, R.H. The Effect of Pre-Partum Intramuscular Iron Treatment of Dams on Litter Hemoglobin Levels. J. Anim. Sci. 1959, 18, 415–419. [Google Scholar] [CrossRef]
- Bhattarai, S.; Framstad, T.; Nielsen, J.P. Iron treatment of pregnant sows in a Danish herd without iron deficiency anemia did not improve sow and piglet hematology or stillbirth rate. Acta Vet. Scand. 2019, 61, 60. [Google Scholar] [CrossRef] [PubMed]
- Ducsay, C.A.; Buhi, W.C.; Bazer, F.W.; Roberts, R.M.; Combs, G.E. Role of Uteroferrin in Placental Iron Transport: Effect of Maternal Iron Treatment on Fetal Iron and Uteroferrin Content and Neonatal Hemoglobin. J. Anim. Sci. 1984, 59, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Spruill, D.G.; Hays, V.W.; Cromwell, G.L. Effects of Dietary Protein and Iron on Reproduction and Iron-Related Blood Constituents in Swine. J. Anim. Sci. 1971, 33, 376–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lillie, R.J.; Frobish, L.T. Effect of Copper and Iron Supplement on Performance and Hematology of Confined Sows and their Progeny through Four Reproductive Cycles. J. Anim. Sci. 1978, 46, 678–685. [Google Scholar] [CrossRef]
- Egeli, A.K.; Framstad, T.; Gronningen, D. The Effect of Peroral Administration of Amino Acid-Chelated Iron to Pregnant Sows in Preventing Sow and Piglet Anaemia. Acta Vet. Scand. 1998, 39, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kroe, D.; Kinney, T.D.; Kaufman, N.; Klavins, J.V. The influence of amino acids on iron absorption. Blood 1963, 21, 546–552. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Che, L.; Lin, Y.; Fang, Z.; Xu, S.; Wu, D. Influence of organic iron complex on sow reproductive performance and iron status of nursing pigs. Livest. Sci. 2014, 160, 89–96. [Google Scholar] [CrossRef]
- Jahan, M.; Francis, N.; Wang, B. Milk lactoferrin concentration of primiparous and multiparous sows during lactation. J. Dairy Sci. 2020, 103, 7521–7530. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, D.; Tang, W.; Dong, Z.; Zhang, Y.; Wang, S.; Yin, Y.; Wan, D. Correlations of gestational hemoglobin level, placental trace elements content, and reproductive performances in pregnant sows. J. Anim. Sci. 2022, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhang, C.; Ji, P.; Yang, J.; Li, Q.; Pan, H.; An, Q. Effects of Different Iron Supplements on Reproductive Performance and Antioxidant Capacity of Pregnant Sows as Well as Iron Content and Antioxidant Gene Expression in Newborn Piglets. Animals 2023, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Mazgaj, R.; Szudzik, M.; Lipiński, P.; Jończy, A.; Smuda, E.; Kamyczek, M.; Cieślak, B.; Swinkels, D.; Lenartowicz, M.; Starzyński, R.R. Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets. Animals 2020, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial® iron absorption studied by in vitro and ex-vivo models. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, A.F.; Qureshi, M.A. The effect of iron supplementation of pregnant swine on the iron status and growth of their offspring. J. Nutr. 1952, 47, 37–44. [Google Scholar]
- Rajagopal, A.; Rao, A.U.; Amigo, J.; Tian, M.; Upadhyay, S.K.; Hall, C.; Uhm, S.; Mathew, M.K.; Fleming, M.D.; Paw, B.H.; et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 2008, 453, 1127–1131. [Google Scholar] [CrossRef]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef]
- Qiu, A.; Jansen, M.; Sakaris, A.; Min, S.H.; Chattopadhyay, S.; Tsai, E.; Sandoval, C.; Zhao, R.; Akabas, M.H.; Goldman, I.D. Identification of an Intestinal Folate Transporter and the Molecular Basis for Hereditary Folate Malabsorption. Cell 2006, 127, 917–928. [Google Scholar] [CrossRef]
- Young, M.F.; Griffin, I.; Pressman, E.; Mcintyre, A.W.; Cooper, E.; Mcnanley, T.; Harris, Z.L.; Westerman, M.; O’Brien, K.O. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J. Nutr. 2012, 142, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.R.; Cox, J.S.G.; Fitzmaurice, C.; Moss, G.F. The iron dextran complex. Nature 1965, 208, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Geisser, P.; Baer, M.; Schaub, E. Structure/histotoxicity relationship of parenteral iron preparations. Arzneimittelforschung 1992, 42, 1439–1452. [Google Scholar] [PubMed]
- Thorén-Tolling, K.; Jönsson, L. Cellular distribution of orally and intramuscularly administered iron dextran in newborn piglets. Can. J. Comp. Med. Rev. Can. Med. Comp. 1977, 41, 318–325. [Google Scholar]
- NRC. Nutrient Requirements of Swine: 10th Revised Edition; National Academies Press: Washington, DC, USA, 1998; ISBN 0309549884. [Google Scholar]
- Zhao, P.; Upadhaya, S.D.; Li, J.; Kim, I. Comparison effects of dietary iron dextran and bacterial-iron supplementation on growth performance, fecal microbial flora, and blood profiles in sows and their litters. Anim. Sci. J. 2015, 86, 937–942. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.L.; Picciano, M.F.; Roos, M.A.; Easter, R.A. Iron and Folate Utilization in Reproducing Swine and Their Progeny. J. Nutr. 1989, 119, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Zhang, Y.M.; Wu, X.; Lin, X.; Shu, X.G.; Zhou, X.H.; Du, H.T.; Xing, W.G.; Liu, H.N.; Li, L.; et al. Maternal dietary supplementation with ferrous N-carbamylglycinate chelate affects sow reproductive performance and iron status of neonatal piglets. Animal 2018, 12, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Buffler, M.; Becker, C.; Windisch, W.M. Effects of different iron supply to pregnant sows (Sus scrofa domestica L.) on reproductive performance as well as iron status of new-born piglets. Arch. Anim. Nutr. 2017, 71, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Dong, D.; Jiang, S.; Yang, Z.; Wang, Y. Effect of different sources and levels of iron in the diet of sows on iron status in neonatal pigs. Anim. Nutr. 2018, 4, 197–202. [Google Scholar] [CrossRef]
- Nicolas, G.; Bennoun, M.; Porteu, A.; Mativet, S.; Beaumont, C.; Grandchamp, B.; Sirito, M.; Sawadogo, M.; Kahn, A.; Vaulont, S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 2002, 99, 4596–4601. [Google Scholar] [CrossRef]
- Matte, J.J.; Audet, I. Maternal perinatal transfer of vitamins and trace elements to piglets. Animal 2020, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Egeli, A.K.; Framstad, T. Effect of an Oral Starter Dose of Iron on Haematology and Weight Gain in Piglets Having Voluntary Access to Glutamic Acid-chelated Iron Solution. Acta Vet. Scand. 1998, 39, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Liu, S.-Y.; Wang, H.-J.; Zhang, T.-W.; Yu, P.; Duan, X.-L.; Zhao, S.-E.; Chang, Y.-Z. Effects of Pregnancy and Lactation on Iron Metabolism in Rats. Biomed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Kracht, S.; Ho, Y.; Haque, Z.; Bhattachatyya, B.N.; Wynn, P.C.; Wang, B. Dietary lactoferrin supplementation to gilts during gestation and lactation improves pig production and immunity. PLoS ONE 2017, 12, e0185817. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.A.; Pascoal, L.A.F.; Watanabe, P.H.; Martins, T.D.D.; Andrade, T.S.; Ribeiro, J.E.S. Dietary iron chelate for sows and effects on iron supplementation in piglets. An. Acad. Bras. Cienc. 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Ganz, T.; Nemeth, E. Iron Homeostasis during Pregnancy: Maternal, Placental, and Fetal Regulatory Mechanisms. Annu. Rev. Nutr. 2023, 43, 279–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazgaj, R.; Lipiński, P.; Starzyński, R.R. Iron Supplementation of Pregnant Sows to Prevent Iron Deficiency Anemia in Piglets: A Procedure of Questionable Effectiveness. Int. J. Mol. Sci. 2024, 25, 4106. https://doi.org/10.3390/ijms25074106
Mazgaj R, Lipiński P, Starzyński RR. Iron Supplementation of Pregnant Sows to Prevent Iron Deficiency Anemia in Piglets: A Procedure of Questionable Effectiveness. International Journal of Molecular Sciences. 2024; 25(7):4106. https://doi.org/10.3390/ijms25074106
Chicago/Turabian StyleMazgaj, Rafał, Paweł Lipiński, and Rafał R. Starzyński. 2024. "Iron Supplementation of Pregnant Sows to Prevent Iron Deficiency Anemia in Piglets: A Procedure of Questionable Effectiveness" International Journal of Molecular Sciences 25, no. 7: 4106. https://doi.org/10.3390/ijms25074106
APA StyleMazgaj, R., Lipiński, P., & Starzyński, R. R. (2024). Iron Supplementation of Pregnant Sows to Prevent Iron Deficiency Anemia in Piglets: A Procedure of Questionable Effectiveness. International Journal of Molecular Sciences, 25(7), 4106. https://doi.org/10.3390/ijms25074106






