Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors
Abstract
1. Introduction
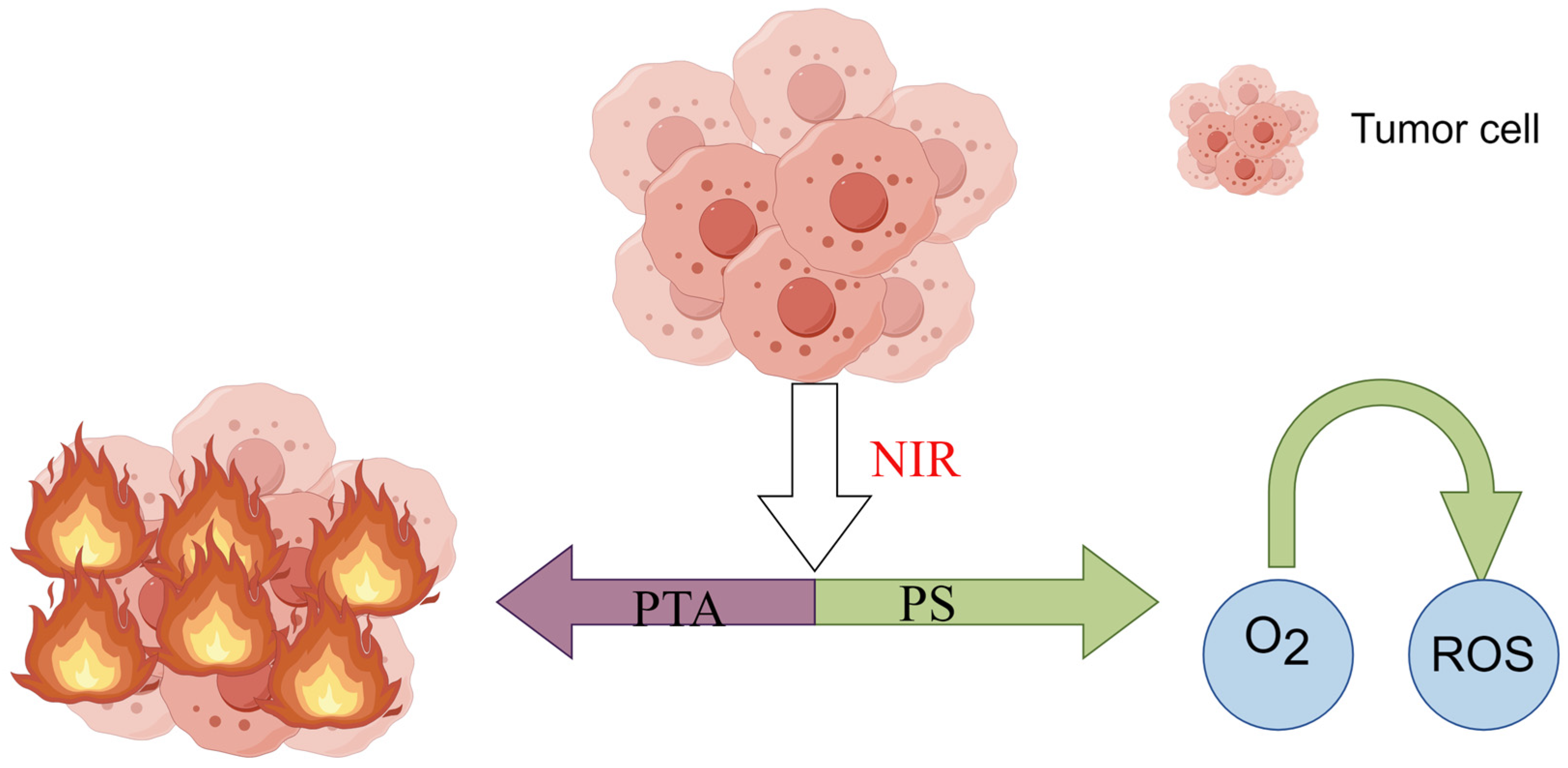
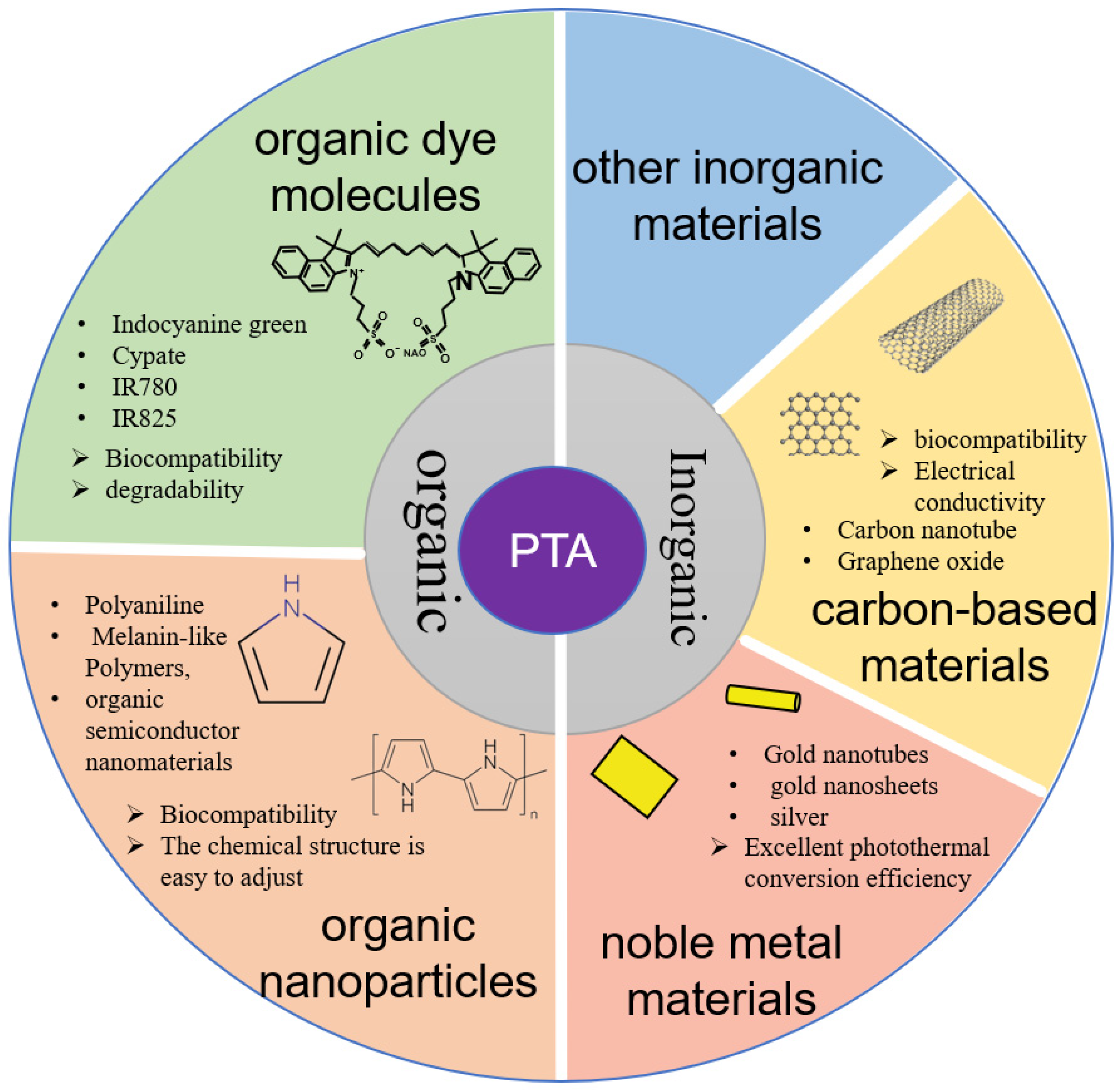
2. Mechanisms and Characteristics of PTT
2.1. Mechanisms of PTT
2.2. Characteristics of PTT
3. Nanocarrier Particles—Target Antitumor Treatment with PTT
3.1. Passive Targeting
3.2. Active Targeting
| Author | Material | PTA | Carrier | Target |
|---|---|---|---|---|
| Xiangtian Deng et al. [53] | IrO2@ZIF-8/BSA-FA (Ce6) | IrO2 and Ce6 | Zeolitic imidazolate framework-8 (metal-organic framework, MOF) | BSA-FA |
| Xue Li et al. [54] | FA-Fe2O3@PDA-miRNA | Fe2O3@PDA | Fe2O3 | FA |
| Hongzhi Hu et al. [55] | AIBI@H-mMnO2-TPP@PDA-RGD(AHTPR) | MnO2 | Hollow mesoporous MnO2 | TPP |
| Wei-Nan Zeng et al. [56] | TPP-PPG@ICG | ICG | Polyethylenimine-modified PEGylated nanographene oxide sheets | TPP |
| Peng Lin et al. [57] | T-ND | Cy7(heptamethine cyanine) | TCF(2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran) | OTP |
| Ying Yuan et al. [58] | SPN-PT | Semiconducting polymer (PCPDTBT) | PCPDTBT | Peptide PT (the original peptide that has undergone PEGylation) |
| Tian, J. et al. [59] | HGNs-PEG-CD271 | HGNs | HGNs | CD271 |
| Xiong, S. et al. [60] | GTN-CD133@ICG@HA | ICG | GTNs | HA, CD133 |
| Jingwei Zhang et al. [61] | CM/SLN/ICG | ICG | SLNs (silica nanoparticles) | CMs (cell membranes) |
| Yanlong Xu et al. [62] | BPQDs-DOX@OPM | Black phosphorus quantum dots (BPQDs) | Black phosphorus quantum dots (BPQDs) | OPM (surface-encapsulated platelet-osteosarcoma hybrid membrane) |
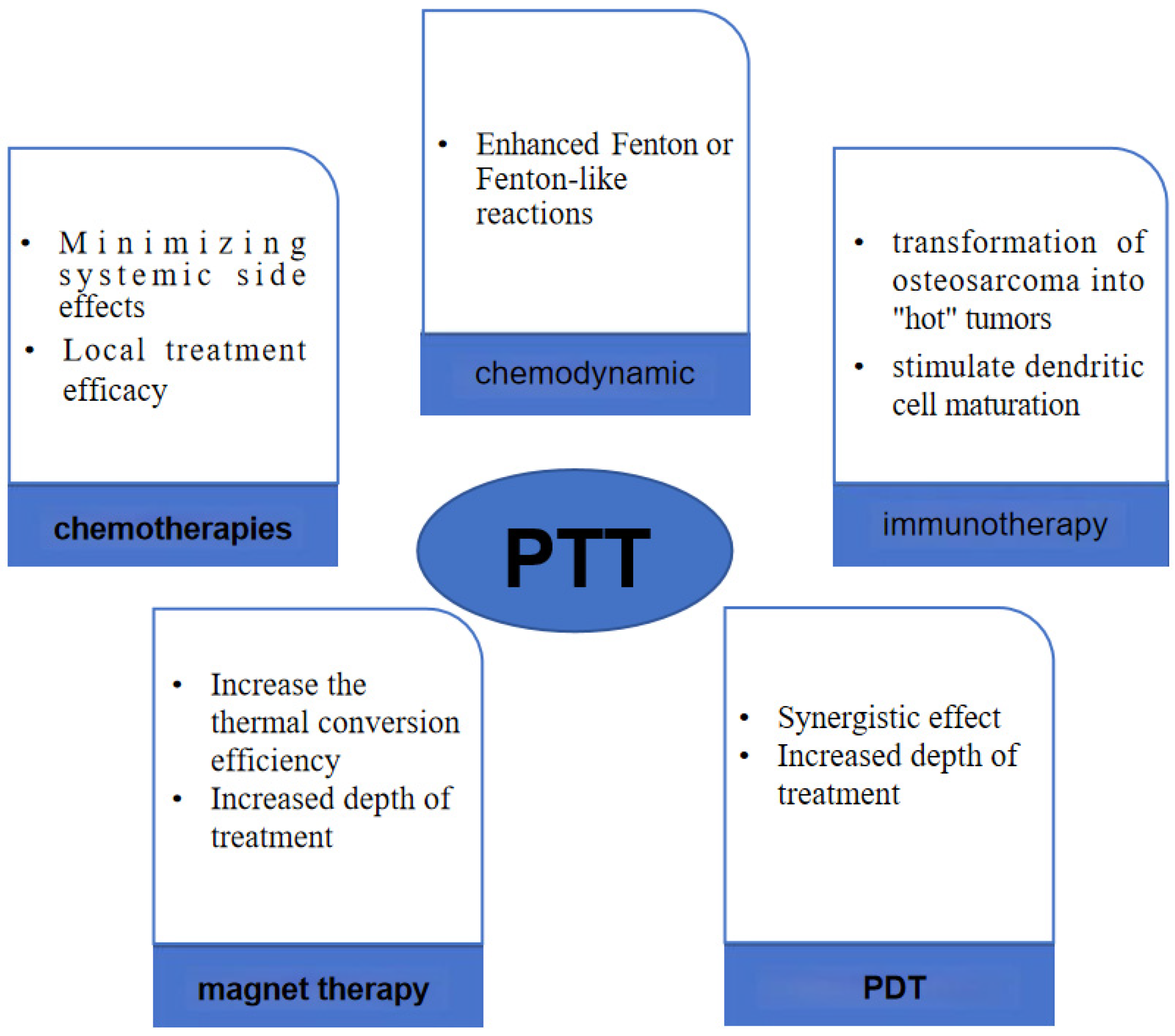
4. PTT Combined Multiple Therapies
4.1. PTT Combined with Chemotherapies
4.2. PTT Combined with Immunotherapy: PTT—Immunomodulation
4.3. PTT Combined with Chemodynamic Therapy—Photothermal-Chemodynamic
4.4. PTT Combined with Magnetic Hyperthermia—Magnetic Photothermal Therapy (MPHT)
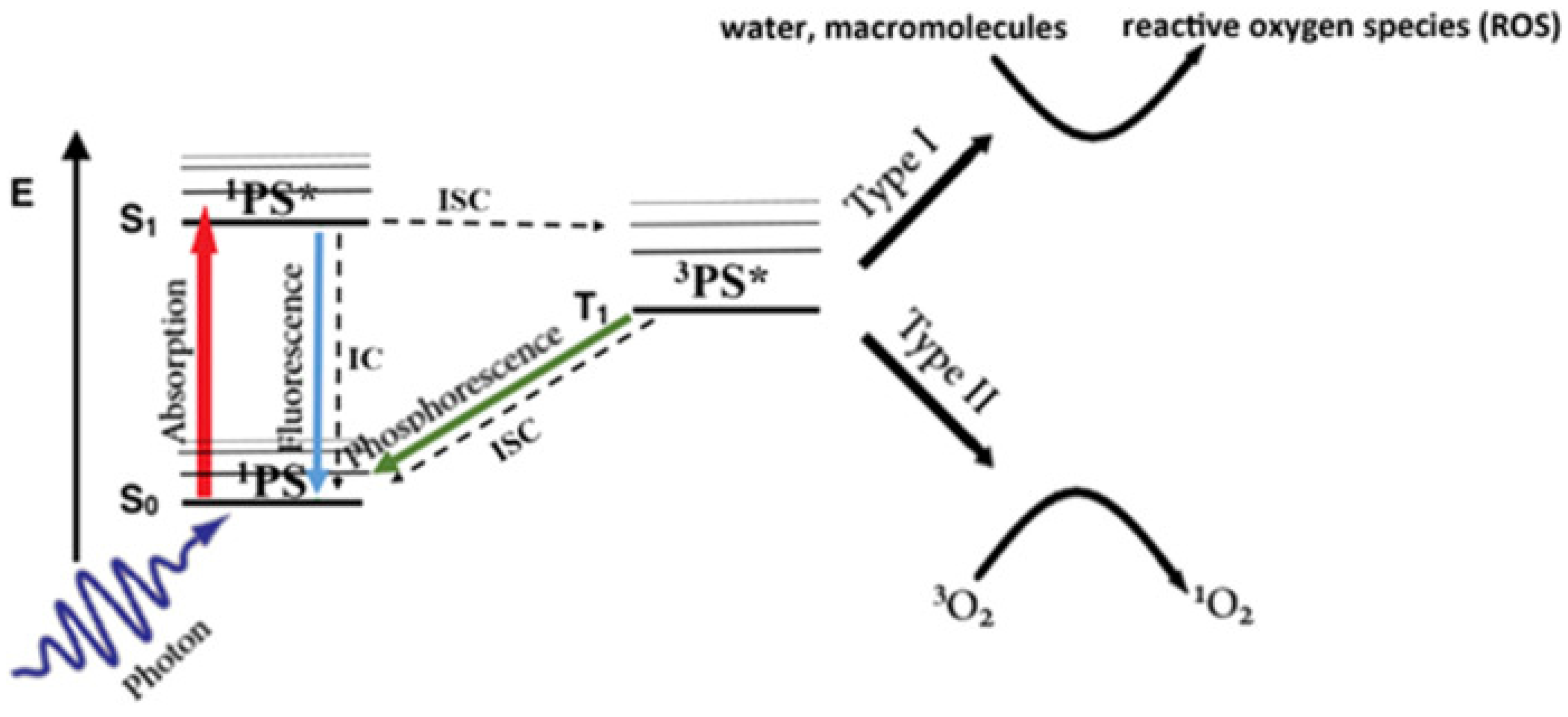
4.5. PTT Combined with PDT
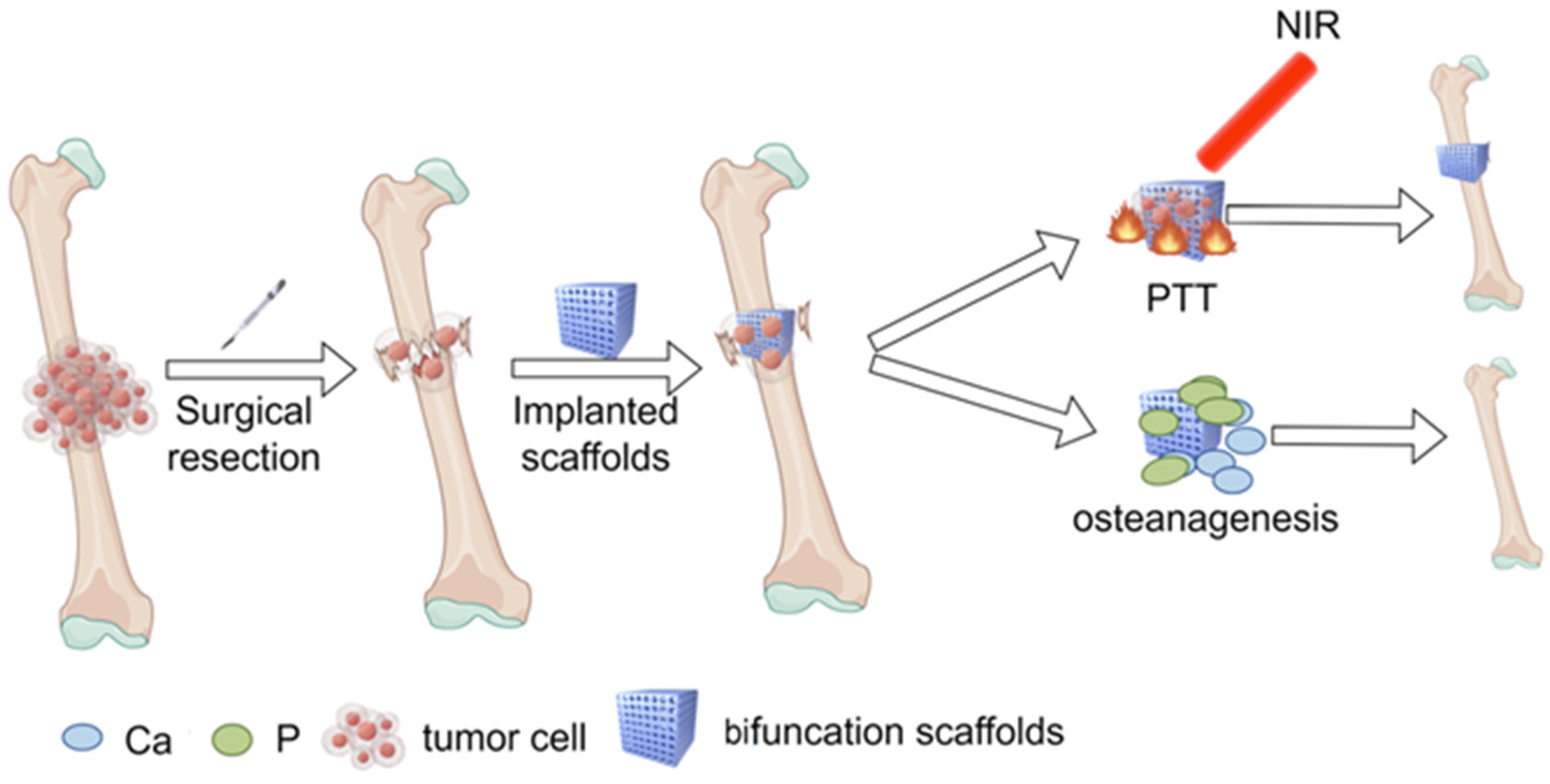
5. Novel Calcium Phosphate Scaffolds—Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration
5.1. Tricalcium Phosphate (TCP)
5.2. Nano-Hydroxyapatite (n-HA)
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Liao, J.; Han, R.; Wu, Y.; Qian, Z. Review of a new bone tumor therapy strategy based on bifunctional biomaterials. Bone Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Meltzer, P.S.; Longo, D.L.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Mosebach, J.; Thierjung, H.; Schlemmer, H.P.; Delorme, S. Multiple Myeloma Guidelines and Their Recent Updates: Implications for Imaging. RoFo Fortschritte Auf Dem Geb. Der Rontgenstrahlen Und Der Nukl. 2019, 191, 998–1009. [Google Scholar] [CrossRef]
- Faisham, W.I.; Mat Saad, A.Z.; Alsaigh, L.N.; Nor Azman, M.Z.; Kamarul Imran, M.; Biswal, B.M.; Bhavaraju, V.M.; Salzihan, M.S.; Hasnan, J.; Ezane, A.M.; et al. Prognostic factors and survival rate of osteosarcoma: A single-institution study. Asia-Pac. J. Clin. Oncol. 2017, 13, e104–e110. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, X.; Hu, Y.; Zhang, Y.; Wang, Z.; Wu, S.; Shen, J.; Ye, Z.; Tu, C.; Zhang, Y.; et al. Clinical Guideline for Microwave Ablation of Bone Tumors in Extremities. Orthop. Surg. 2020, 12, 1036–1044. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhang, T.; Xu, L.; Ke, J.; Ma, L.; Lan, G.; Yao, Z.; Ouyang, L.; Huang, H.; et al. Prevention and control strategies of common post-operative complications of microwave ablation in situ in treatment of bone tumors. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2012, 26, 1473–1476. [Google Scholar]
- Sun, J.; Xing, F.; Braun, J.; Traub, F.; Rommens, P.M.; Xiang, Z.; Ritz, U. Progress of Phototherapy Applications in the Treatment of Bone Cancer. Int. J. Mol. Sci. 2021, 22, 11354. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Geng, C.; Shen, H.; Zhang, Q.; Miao, Y.; Wu, J.; Ouyang, R.; Zhou, S. Two Hawks with One Arrow: A Review on Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration. Nanomaterials 2023, 13, 551. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Lindner, U.; Lawrentschuk, N.; Weersink, R.A.; Davidson, S.R.; Raz, O.; Hlasny, E.; Langer, D.L.; Gertner, M.R.; Van der Kwast, T.; Haider, M.A.; et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: Validation of focal therapy and imaging accuracy. Eur. Urol. 2010, 57, 1111–1114. [Google Scholar] [CrossRef]
- Gough-Palmer, A.L.; Gedroyc, W.M. Laser ablation of hepatocellular carcinoma—A review. World J. Gastroenterol. 2008, 14, 7170–7174. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Fahey, J.M.; Korytowski, W.; Girotti, A.W. Upstream signaling events leading to elevated production of pro-survival nitric oxide in photodynamically-challenged glioblastoma cells. Free Radic. Biol. Med. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CAA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sinica. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Wang, Y.; Li, J. Recent Advances in Engineering Nanomedicines for Second Near-Infrared Photothermal-Combinational Immunotherapy. Nanomaterials 2022, 12, 1656. [Google Scholar] [CrossRef]
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnol. 2022, 20, 141. [Google Scholar] [CrossRef]
- Dinakaran, D.; Wilson, B.C. The use of nanomaterials in advancing photodynamic therapy (PDT) for deep-seated tumors and synergy with radiotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1250804. [Google Scholar] [CrossRef]
- Hunt, C.R.; Pandita, R.K.; Laszlo, A.; Higashikubo, R.; Agarwal, M.; Kitamura, T.; Gupta, A.; Rief, N.; Horikoshi, N.; Baskaran, R.; et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007, 67, 3010–3017. [Google Scholar] [CrossRef]
- Werfel, T.A.; Cook, R.S. Efferocytosis in the tumor microenvironment. Semin. Immunopathol. 2018, 40, 545–554. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, L.; Xiao, P.; Sun, X.; Zhou, L.; Liu, X.; Wang, J.; Wang, G.; Cao, H.; Zhang, P.; et al. Walking Dead Tumor Cells for Targeted Drug Delivery Against Lung Metastasis of Triple-Negative Breast Cancer. Adv. Mater. 2022, 34, e2205462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Ji, W.; Zhou, S.; Qiu, L.; Li, L.; Qian, Z.; Liu, X.; Zhang, H.; Cao, X. Upconverting and persistent luminescent nanocarriers for accurately imaging-guided photothermal therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 191–198. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 degrees C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Kaub, L.; Schmitz, C. Comparison of the Penetration Depth of 905 nm and 1064 nm Laser Light in Surface Layers of Biological Tissue Ex Vivo. Biomedicines 2023, 11, 1355. [Google Scholar] [CrossRef]
- Su, Y.; Yu, B.; Wang, S.; Cong, H.; Shen, Y. NIR-II bioimaging of small organic molecule. Biomaterials 2021, 271, 120717. [Google Scholar] [CrossRef]
- Azari, F.; Zhang, K.; Kennedy, G.T.; Chang, A.; Nadeem, B.; Delikatny, E.J.; Singhal, S. Precision Surgery Guided by Intraoperative Molecular Imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1620–1627. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Yap, P.K.; Xin Lim, G.L.; Mehta, M.; Chan, Y.; Ng, S.W.; Kapoor, D.N.; Negi, P.; Anand, K.; Singh, S.K.; et al. Perspectives and advancements in the design of nanomaterials for targeted cancer theranostics. Chem.-Biol. Interact. 2020, 329, 109221. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G.; Zhao, Y. Precise design of nanomedicines: Perspectives for cancer treatment. Natl. Sci. Rev. 2019, 6, 1107–1110. [Google Scholar] [CrossRef]
- Chu, S.H.; Feng, D.F.; Ma, Y.B.; Li, Z.Q. Hydroxyapatite nanoparticles inhibit the growth of human glioma cells in vitro and in vivo. Int. J. Nanomed. 2012, 7, 3659–3666. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Ravi Kiran, A.; Kusuma Kumari, G.; Krishnamurthy, P.T.; Khaydarov, R.R. Tumor microenvironment and nanotherapeutics: Intruding the tumor fort. Biomater. Sci. 2021, 9, 7667–7704. [Google Scholar] [CrossRef]
- Khawar, I.A.; Kim, J.H.; Kuh, H.J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control. Release Off. J. Control. Release Soc. 2015, 201, 78–89. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release Off. J. Control. Release Soc. 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sinica. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Xu, L.; Yang, P.; He, F.; Yang, D.; An, G.; Ansari, M.B. Redox-responsive UCNPs-DPA conjugated NGO-PEG-BPEI-DOX for imaging-guided PTT and chemotherapy for cancer treatment. Dalton Trans. 2018, 47, 3921–3930. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Tan, X.; Pang, X.; You, Q.; Sun, Q.; Tan, F.; Li, N. Photosensitizer loaded PEG-MoS2-Au hybrids for CT/NIRF imaging-guided stepwise photothermal and photodynamic therapy. J. Mater. Chem. B 2017, 5, 2286–2296. [Google Scholar] [CrossRef]
- Kang, X.; Sun, T.; Zhang, L.; Zhou, C.; Xu, Z.; Du, M.; Xiao, S.; Liu, Y.; Gong, M.; Zhang, D. Synergistic Theranostics of Magnetic Resonance Imaging and Photothermal Therapy of Breast Cancer Based on the Janus Nanostructures Fe3O4-Au(shell)-PEG. Int. J. Nanomed. 2021, 16, 6383–6394. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, Y.; Zhang, Y.; Jia, C.; Wei, B.; Hu, T.; Tang, R.; Li, C. Glucose-Targeted Hydroxyapatite/Indocyanine Green Hybrid Nanoparticles for Collaborative Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37665–37679. [Google Scholar] [CrossRef]
- Xu, W.; Lou, Y.; Chen, W.; Kang, Y. Folic acid decorated metal-organic frameworks loaded with doxorubicin for tumor-targeted chemotherapy of osteosarcoma. Biomed. Technik. Biomed. Eng. 2020, 65, 229–236. [Google Scholar] [CrossRef]
- Carron, P.M.; Crowley, A.; O’Shea, D.; McCann, M.; Howe, O.; Hunt, M.; Devereux, M. Targeting the Folate Receptor: Improving Efficacy in Inorganic Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 2675–2708. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, R.; Song, Q.; Zhang, Y.; Zhao, H.; Hu, H.; Zhang, Z.; Liu, W.; Lin, W.; Wang, G. Synthesis of dual-stimuli responsive metal organic framework-coated iridium oxide nanocomposite functionalized with tumor targeting albumin-folate for synergistic photodynamic/photothermal cancer therapy. Drug Deliv. 2022, 29, 3142–3154. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Gao, Q.; Li, N.; Dong, S.; Gao, Y.; Wang, Z.; Zhang, B.; He, X. MiRNA-520a-3p combined with folic acid conjugated Fe2O3@PDA multifunctional nanoagents for MR imagine and antitumor gene-photothermal therapy. Nanotechnology 2023, 34, 375101. [Google Scholar] [CrossRef]
- Hu, H.; Deng, X.; Song, Q.; Yang, W.; Zhang, Y.; Liu, W.; Wang, S.; Liang, Z.; Xing, X.; Zhu, J.; et al. Mitochondria-targeted accumulation of oxygen-irrelevant free radicals for enhanced synergistic low-temperature photothermal and thermodynamic therapy. J. Nanobiotechnol. 2021, 19, 390. [Google Scholar] [CrossRef]
- Zeng, W.N.; Yu, Q.P.; Wang, D.; Liu, J.L.; Yang, Q.J.; Zhou, Z.K.; Zeng, Y.P. Mitochondria-targeting graphene oxide nanocomposites for fluorescence imaging-guided synergistic phototherapy of drug-resistant osteosarcoma. J. Nanobiotechnol. 2021, 19, 79. [Google Scholar] [CrossRef]
- Lin, P.; Xue, Y.; Mu, X.; Shao, Y.; Lu, Q.; Jin, X.; Yinwang, E.; Zhang, Z.; Zhou, H.; Teng, W.; et al. Tumor Customized 2D Supramolecular Nanodiscs for Ultralong Tumor Retention and Precise Photothermal Therapy of Highly Heterogeneous Cancers. Small 2022, 18, e2200179. [Google Scholar] [CrossRef]
- Yuan, Y.; Diao, S.; Ni, X.; Zhang, D.; Yi, W.; Jian, C.; Hu, X.; Li, D.; Yu, A.; Zhou, W.; et al. Peptide-based semiconducting polymer nanoparticles for osteosarcoma-targeted NIR-II fluorescence/NIR-I photoacoustic dual-model imaging and photothermal/photodynamic therapies. J. Nanobiotechnol. 2022, 20, 44. [Google Scholar] [CrossRef]
- Tian, J.; Gu, Y.; Li, Y.; Liu, T. CD271 antibody-functionalized HGNs for targeted photothermal therapy of osteosarcoma stem cells. Nanotechnology 2020, 31, 305707. [Google Scholar] [CrossRef]
- Xiong, S.; Xiong, G.; Li, Z.; Jiang, Q.; Yin, J.; Yin, T.; Zheng, H. Gold nanoparticle-based nanoprobes with enhanced tumor targeting and photothermal/photodynamic response for therapy of osteosarcoma. Nanotechnology 2021, 32, 155102. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, Y.; Ni, W.; Xiao, H.; Zhang, J. Cancer cell membrane coated silica nanoparticles loaded with ICG for tumour specific photothermal therapy of osteosarcoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2298–2305. [Google Scholar] [CrossRef]
- Xu, Y.; Du, L.; Han, B.; Wang, Y.; Fei, J.; Xia, K.; Zhai, Y.; Yu, Z. Black phosphorus quantum dots camouflaged with platelet-osteosarcoma hybrid membrane and doxorubicin for combined therapy of osteosarcoma. J. Nanobiotechnol. 2023, 21, 243. [Google Scholar] [CrossRef]
- Ma, Z.; Qin, H.; Chen, H.; Yang, H.; Xu, J.; Yang, S.; Hu, J.; Xing, D. Phage display-derived oligopeptide-functionalized probes for in vivo specific photoacoustic imaging of osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 111–121. [Google Scholar] [CrossRef]
- Yang, N.; Ding, Y.; Zhang, Y.; Wang, B.; Zhao, X.; Cheng, K.; Huang, Y.; Taleb, M.; Zhao, J.; Dong, W.F.; et al. Surface Functionalization of Polymeric Nanoparticles with Umbilical Cord-Derived Mesenchymal Stem Cell Membrane for Tumor-Targeted Therapy. ACS Appl. Mater. Interfaces 2018, 10, 22963–22973. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, C.; Zhang, H.; Shi, H.; Mao, F.; Qian, H.; Xu, W.; Wang, D.; Pan, J.; Fang, X.; et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci. Adv. 2022, 8, eabj8207. [Google Scholar] [CrossRef]
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sinica. B 2020, 10, 1534–1548. [Google Scholar] [CrossRef]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhao, G.; Zhang, Y.; Zhan, F.; Chen, Z.; He, T.; Cao, Y.; Hao, L.; Wang, Z.; et al. Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J. Nanobiotechnol. 2022, 20, 83. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; Hong, X.; Cheng, Y.; Tian, Y.; Zhao, S.; Liu, W.; Tang, Y.; Zhao, R.; Song, L.; et al. Adjuvant Photothermal Therapy Inhibits Local Recurrences after Breast-Conserving Surgery with Little Skin Damage. ACS Nano 2018, 12, 662–670. [Google Scholar] [CrossRef]
- Qu, R.; He, D.; Wu, M.; Li, H.; Liu, S.; Jiang, J.; Wang, X.; Li, R.; Wang, S.; Jiang, X.; et al. Afterglow/Photothermal Bifunctional Polymeric Nanoparticles for Precise Postbreast-Conserving Surgery Adjuvant Therapy and Early Recurrence Theranostic. Nano Lett. 2023, 23, 4216–4225. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Xue, C.C.; Li, M.H.; Zhao, Y.; Zhou, J.; Hu, Y.; Cai, K.Y.; Zhao, Y.; Yu, S.H.; Luo, Z. Tumor microenvironment-activatable Fe-doxorubicin preloaded amorphous CaCO3 nanoformulation triggers ferroptosis in target tumor cells. Sci. Adv. 2020, 6, eaax1346. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Song, S.; Qin, Y.; He, Y.; Huang, Q.; Fan, C.; Chen, H.Y. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem. Soc. Rev. 2010, 39, 4234–4243. [Google Scholar] [CrossRef]
- Shi, Y.; Lammers, T. Combining Nanomedicine and Immunotherapy. Acc. Chem. Res. 2019, 52, 1543–1554. [Google Scholar] [CrossRef]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Khorsandi, K.; Gul, A. Curcumin effect on cancer cells’ multidrug resistance: An update. Phytother. Res. PTR 2020, 34, 2534–2556. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar]
- Termini, D.; Den Hartogh, D.J.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Zhang, C.; Yang, T.; Deng, Z.; Song, Y.; Huang, L.; Li, F.; Li, Q.; Lin, S.; et al. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed. Pharmacother. 2021, 136, 111202. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Wang, Y.; He, A.; Hu, H.; Zhang, J.; Han, M.; Huang, Y. Curcumin inhibits the proliferation and invasion of MG-63 cells through inactivation of the p-JAK2/p-STAT3 pathway. OncoTargets Ther. 2019, 12, 2011–2021. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, Y.; Liu, J.S.; Long, S.Z.; Liu, H.L.; Zhang, J.; Zhang, T. The role of miR-21/RECK in the inhibition of osteosarcoma by curcumin. Mol. Cell. Probes 2020, 51, 101534. [Google Scholar] [CrossRef]
- Sun, X.; Meng, Z.; Yu, Q.; Wang, X.; Zhao, Z. Engineering PDA-coated CM-CS nanoparticles for photothermo-chemotherapy of osteosarcoma and bone regeneration. Biochem. Eng. J. 2021, 175, 108138. [Google Scholar] [CrossRef]
- Han, R.; Min, Y.; Li, G.; Chen, S.; Xie, M.; Zhao, Z. Supercritical CO2-assisted fabrication of CM-PDA/SF/nHA nanofibrous scaffolds for bone regeneration and chemo-photothermal therapy against osteosarcoma. Biomater. Sci. 2023, 11, 5218–5231. [Google Scholar] [CrossRef]
- Tan, B.; Wu, Y.; Wu, Y.; Shi, K.; Han, R.; Li, Y.; Qian, Z.; Liao, J. Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-co-Thermal Therapy and Bone Reconstruction. ACS Appl. Mater. Interfaces 2021, 13, 31542–31553. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Wang, Q.; Tong, R.; Lin, H.; Qu, F. Near-infrared light-mediated LA-UCNPs@SiO2-C/HA@mSiO2-DOX@NB nanocomposite for chemotherapy/PDT/PTT and imaging. Dalton Trans. 2017, 46, 14293–14300. [Google Scholar] [CrossRef]
- Conniot, J.; Silva, J.M.; Fernandes, J.G.; Silva, L.C.; Gaspar, R.; Brocchini, S.; Florindo, H.F.; Barata, T.S. Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014, 2, 105. [Google Scholar] [CrossRef]
- Mocellin, S.; Nitti, D. Therapeutics targeting tumor immune escape: Towards the development of new generation anticancer vaccines. Med. Res. Rev. 2008, 28, 413–444. [Google Scholar] [CrossRef]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef]
- Töpfer, K.; Kempe, S.; Müller, N.; Schmitz, M.; Bachmann, M.; Cartellieri, M.; Schackert, G.; Temme, A. Tumor evasion from T cell surveillance. J. Biomed. Biotechnol. 2011, 2011, 918471. [Google Scholar] [CrossRef]
- Xu, Z.; Ramishetti, S.; Tseng, Y.C.; Guo, S.; Wang, Y.; Huang, L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 259–265. [Google Scholar] [CrossRef]
- DeFrancesco, L. CAR-T cell therapy seeks strategies to harness cytokine storm. Nat. Biotechnol. 2014, 32, 604. [Google Scholar] [CrossRef]
- Song, H.; Yang, P.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics 2019, 9, 2299–2314. [Google Scholar] [CrossRef]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2022, 21, 495–508. [Google Scholar] [CrossRef]
- Pockley, A.G. Heat shock proteins as regulators of the immune response. Lancet 2003, 362, 469–476. [Google Scholar] [CrossRef]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol./Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Adkins, I.; Sadilkova, L.; Hradilova, N.; Tomala, J.; Kovar, M.; Spisek, R. Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. Oncoimmunology 2017, 6, e1311433. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Li, X.; Zhao, Y.; Li, M.; Jiang, W.; Tang, X.; Dou, J.; Lu, L.; Wang, F.; et al. Near-Infrared II Phototherapy Induces Deep Tissue Immunogenic Cell Death and Potentiates Cancer Immunotherapy. ACS Nano 2019, 13, 11967–11980. [Google Scholar] [CrossRef]
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy. Theranostics 2019, 9, 126–151. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Z.; Chen, H.; Zeng, X.; Mei, L. An optimal portfolio of photothermal combined immunotherapy. Cell Rep. Phys. Sci. 2022, 3, 100898. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, X.; Ren, T.; Huang, Y.; Zhang, H.; Yu, Y.; Chen, C.; Wang, W.; Niu, J.; Lou, J.; et al. The role of tumor-associated macrophages in osteosarcoma progression—Therapeutic implications. Cell Oncol. 2021, 44, 525–539. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Cao, J.; Dong, R.; Jiang, L.; Gong, Y.; Yuan, M.; You, J.; Meng, W.; Chen, Z.; Zhang, N.; Weng, Q.; et al. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol. Res. 2019, 7, 292–305. [Google Scholar] [CrossRef]
- Deng, X.; Liang, H.; Yang, W.; Shao, Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J. Photochem. Photobiol. BBiol. 2020, 208, 111913. [Google Scholar] [CrossRef]
- Luo, G.; Xu, Z.; Zhong, H.; Shao, H.; Liao, H.; Liu, N.; Jiang, X.; Zhang, Y.; Ji, X. Biodegradable photothermal thermosensitive hydrogels treat osteosarcoma by reprogramming macrophages. Biomater. Sci. 2023, 11, 2818–2827. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Guo, M.; Du, S.; Han, N. Recent strategies for nano-based PTT combined with immunotherapy: From a biomaterial point of view. Theranostics 2021, 11, 7546–7569. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Du, Y.; Zhang, Y.; Wang, X.; Ding, Y.; Yang, X.; Meng, F.; Tu, J.; Luo, L.; et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019, 10, 4871. [Google Scholar] [CrossRef]
- Lagerweij, T.; Pérez-Lanzón, M.; Baglio, S.R. A Preclinical Mouse Model of Osteosarcoma to Define the Extracellular Vesicle-mediated Communication Between Tumor and Mesenchymal Stem Cells. J. Vis. Exp. JoVE 2018, 135, e56932. [Google Scholar] [CrossRef]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the immune microenvironment of osteosarcoma: The outlook for osteosarcoma treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef]
- He, G.; Shuai, Y.; Hai, Y.; Yang, T.; Pan, X.; Liu, Y.; Meng, X.; Yang, H.; Yang, M.; Mao, C. Integration of gold nanodendrites and immune checkpoint blockers to achieve highly efficient photothermal immunotherapy for eradicating primary and distant metastatic osteosarcoma. Mater. Today Nano 2022, 20, 100268. [Google Scholar] [CrossRef]
- Crunkhorn, S. Strengthening the sting of immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 669. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Liu, K.; Liao, Y.; Zhou, Z.; Zhang, L.; Jiang, Y.; Lu, H.; Xu, T.; Yang, D.; Gao, Q.; Li, Z.; et al. Photothermal-triggered immunogenic nanotherapeutics for optimizing osteosarcoma therapy by synergizing innate and adaptive immunity. Biomaterials 2022, 282, 121383. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.X.; Wan, S.S.; Zhang, X.Z. Nanocatalyst-Mediated Chemodynamic Tumor Therapy. Adv. Healthc. Mater. 2022, 11, e2101971. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, Z.; Chen, M.; Chao, Y.; Liu, Z.; Feng, L.; Hao, Y.; Dong, Z.L.; Chen, M.C.; Chao, Y.; et al. Near-infrared light and glucose dual-responsive cascading hydroxyl radical generation for in situ gelation and effective breast cancer treatment. Biomaterials 2020, 228, 119568. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, e2104223. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, F.; Yao, C.; Xu, X.; Akakuru, O.U.; Chen, T.; Yang, F.; Wu, A. Rational design of nanomedicine for photothermal-chemodynamic bimodal cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1682. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Niu, D.; Li, Y.; Liu, X.; Li, C.; Si, W.; Cao, J.; Song, Y.; Wen, G.; et al. Ultrasensitive Chemodynamic Therapy: Bimetallic Peroxide Triggers High pH-Activated, Synergistic Effect/H2O2 Self-Supply-Mediated Cascade Fenton Chemistry. Adv. Healthc. Mater. 2021, 10, e2002126. [Google Scholar] [CrossRef]
- Guan, G.; Wang, X.; Li, B.; Zhang, W.; Cui, Z.; Lu, X.; Zou, R.; Hu, J. “Transformed” Fe3S4 tetragonal nanosheets: A high-efficiency and body-clearable agent for magnetic resonance imaging guided photothermal and chemodynamic synergistic therapy. Nanoscale 2018, 10, 17902–17911. [Google Scholar] [CrossRef]
- Zhu, B.; An, D.; Bi, Z.; Liu, W.; Shan, W.; Li, Y.; Nie, G.; Xie, N.; Al-Hartomy, O.A.; Al-Ghamdi, A.; et al. Two-Dimensional Nitrogen-Doped Ti3C2 Promoted Catalysis Performance of Silver Nanozyme for Ultrasensitive Detection of Hydrogen Peroxide. ChemElectroChem 2022, 9, e202200050. [Google Scholar] [CrossRef]
- Xue, Y.; Niu, W.; Wang, M.; Chen, M.; Guo, Y.; Lei, B. Engineering a Biodegradable Multifunctional Antibacterial Bioactive Nanosystem for Enhancing Tumor Photothermo-Chemotherapy and Bone Regeneration. ACS Nano 2020, 14, 442–453. [Google Scholar] [CrossRef]
- Chen, Q.; Shan, X.; Shi, S.; Jiang, C.; Li, T.; Wei, S.; Zhang, X.; Sun, G.; Liu, J. Tumor microenvironment-responsive polydopamine-based core/shell nanoplatform for synergetic theranostics. J. Mater. Chem. B 2020, 8, 4056–4066. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, C.; Guo, X.; Li, G.; Yang, X.; Yu, J.; Zhong, J.; Xie, Y.; Zheng, L.; Zhao, J. RhRu Alloy-Anchored MXene Nanozyme for Synergistic Osteosarcoma Therapy. Small 2023, 19, e2205511. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Balan, V.; Verestiuc, L. Advanced 3D Magnetic Scaffolds for Tumor-Related Bone Defects. Int. J. Mol. Sci. 2022, 23, 16190. [Google Scholar] [CrossRef]
- Tavares, F.; Soares, P.I.P.; Silva, J.C.; Borges, J.P. Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/pSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 1128. [Google Scholar] [CrossRef]
- Teran, F.J.; Casado, C.; Mikuszeit, N.; Salas, G.; Bollero, A.; Morales, M.P.; Camarero, J.; Miranda, R. Accurate determination of the specific absorption rate in superparamagnetic nanoparticles under non-adiabatic conditions. Appl. Phys. Lett. 2012, 101, 062413. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Liu, X.L.; Herng, T.S.; Peng, E.; Ding, J. GO-Functionalized Large Magnetic Iron Oxide Nanoparticles with Enhanced Colloidal Stability and Hyperthermia Performance. ACS Appl. Mater. Interfaces 2019, 11, 22703–22713. [Google Scholar] [CrossRef]
- Shivanna, A.T.; Dash, B.S.; Chen, J.P. Functionalized Magnetic Nanoparticles for Alternating Magnetic Field- or Near Infrared Light-Induced Cancer Therapies. Micromachines 2022, 13, 1279. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, H.; Xu, Y.; Cao, Y.; Zheng, Y.; Liang, B. Engineering a triple-functional magnetic gel driving mutually-synergistic mild hyperthermia-starvation therapy for osteosarcoma treatment and augmented bone regeneration. J. Nanobiotechnol. 2023, 21, 201. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; Dong, C.; Guo, W.; Xu, Z.; Chen, Y.; Xiang, H.; Zhang, R. Engineering Electronic Band Structure of Binary Thermoelectric Nanocatalysts for Augmented Pyrocatalytic Tumor Nanotherapy. Adv. Mater. 2022, 34, e2106773. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Ren, W.; Wu, A. Therapeutic applications of iron oxide based nanoparticles in cancer: Basic concepts and recent advances. Biomater. Sci. 2018, 6, 708–725. [Google Scholar] [CrossRef]
- Khodaei, A.; Jahanmard, F.; Madaah Hosseini, H.R.; Bagheri, R.; Dabbagh, A.; Weinans, H.; Amin Yavari, S. Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioact. Mater. 2022, 11, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, J.; Li, C.; He, Z.; Wang, X.; Pan, Y.; Zhao, L. Injectable Magnetic Hydrogel Filler for Synergistic Bone Tumor Hyperthermia Chemotherapy. ACS Appl. Bio Mater. 2024, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.M.; Courteau, A.; Bellaye, P.S.; Kohli, E.; Oudot, A.; Doulain, P.E.; Petitot, C.; Walker, P.M.; Decréau, R.; Collin, B. Superparamagnetic Iron Oxide Nanoparticles for Immunotherapy of Cancers through Macrophages and Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Shestovskaya, M.V.; Luss, A.L.; Bezborodova, O.A.; Makarov, V.V.; Keskinov, A.A. Iron Oxide Nanoparticles in Cancer Treatment: Cell Responses and the Potency to Improve Radiosensitivity. Pharmaceutics 2023, 15, 2406. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Rinaldi-Montes, N.; Alonso, J.; Amghouz, Z.; Garaio, E.; García, J.A.; Gorria, P.; Blanco, J.A.; Phan, M.H.; Srikanth, H. Boosted Hyperthermia Therapy by Combined AC Magnetic and Photothermal Exposures in Ag/Fe3O4 Nanoflowers. ACS Appl. Mater. Interfaces 2016, 8, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, A.; Eroglu, Z.; Gurcan, C.; Gazzi, A.; Ekim, O.; Sundu, B.; Gokce, C.; Ceylan, A.; Giro, L.; Unal, M.A.; et al. Synergized photothermal therapy and magnetic field induced hyperthermia via bismuthene for lung cancer combinatorial treatment. Mater. Today. Bio 2023, 23, 100825. [Google Scholar] [CrossRef]
- Martínez-Banderas, A.I.; Aires, A.; Quintanilla, M.; Holguín-Lerma, J.A.; Lozano-Pedraza, C.; Teran, F.J.; Moreno, J.A.; Perez, J.E.; Ooi, B.S.; Ravasi, T.; et al. Iron-Based Core-Shell Nanowires for Combinatorial Drug Delivery and Photothermal and Magnetic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43976–43988. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Yang, F.; Ke, Q.F.; Xie, X.T.; Guo, Y.P. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Gao, Y.; Wang, P.; He, G.; Blum, N.; Lin, J.; Liu, Q.; Wang, X.; Huang, P. Six Birds with One Stone: Versatile Nanoporphyrin for Single-Laser-Triggered Synergistic Phototheranostics and Robust Immune Activation. Adv. Mater. 2022, 34, e2209394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Q.; Yang, N.; Shi, Y.; Ge, W.; Wang, W.; Huang, W.; Song, X.; Dong, X.J.A.F.M. Phase-change materials based nanoparticles for controlled hypoxia modulation and enhanced phototherapy. Adv. Funct. Mater. 2019, 29, 1906805. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Gao, X.; Li, P.; Zhang, W.; Ma, Y.; Wang, H.; Tang, B. Enhanced Photodynamic Therapy by Reduced Levels of Intracellular Glutathione Obtained By Employing a Nano-MOF with Cu(II) as the Active Center. Angew. Chem. 2018, 57, 4891–4896. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jiang, S.; Ding, M.; Sun, S.; Ma, Y.; Younis, M.R.; He, G.; Wang, J.; Lin, J.; Cao, Z.; et al. Ultrasmall Rhodium Nanozyme with RONS Scavenging and Photothermal Activities for Anti-Inflammation and Antitumor Theranostics of Colon Diseases. Nano Lett. 2020, 20, 3079–3089. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Xu, B.; Zhao, Y.; Zhang, T.; Wang, Y.; Wang, D.; Liu, J.; Song, S.; Zhang, H. A Bimetallic Nanozyme with Cascade Effect for Synergistic Therapy of Cancer. ChemMedChem 2022, 17, e202100663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Liu, J.; Zhang, Y. Recent Progress of Rare-Earth Doped Upconversion Nanoparticles: Synthesis, Optimization, and Applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Chu, Z.; Tian, T.; Tao, Z.; Yang, J.; Chen, B.; Chen, H.; Wang, W.; Yin, P.; Xia, X.; Wang, H.; et al. Upconversion nanoparticles@AgBiS2 core-shell nanoparticles with cancer-cell-specific cytotoxicity for combined photothermal and photodynamic therapy of cancers. Bioact. Mater. 2022, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, L.; Jia, W.; Zhang, C.; Ren, W.; Liu, C.; Tang, Y. Composite Nanomaterials of Conjugated Polymers and Upconversion Nanoparticles for NIR-Triggered Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2023. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Aktuglu, K.; Erol, K.; Vahabi, A. Ilizarov bone transport and treatment of critical-sized tibial bone defects: A narrative review. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2019, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Jäger, M.; Mayer, C.; Sowislok, A. Functionalization of Synthetic Bone Substitutes. Int. J. Mol. Sci. 2021, 22, 4412. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Lu, Y.; Meng, L.; Wu, J.; Gong, T.; Zou, J.; Bosiakov, S.; Cheng, L. A Critical Review on the Design, Manufacturing and Assessment of the Bone Scaffold for Large Bone Defects. Front. Bioeng. Biotechnol. 2021, 9, 753715. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef] [PubMed]
- Suleman, A.; Kondiah, P.P.D.; Mabrouk, M.; Choonara, Y.E. The Application of 3D-Printing and Nanotechnology for the Targeted Treatment of Osteosarcoma. Front. Mater. 2021, 8, 668834. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Chanlalit, C.; Shukla, D.R.; Fitzsimmons, J.S.; An, K.N.; O’Driscoll, S.W. Stress shielding around radial head prostheses. J. Hand Surg. 2012, 37, 2118–2125. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate materials in restorative dentistry: A review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Chen, L.; Zhou, Y.; Dang, W.; Chang, J.; Wu, C. Ultrathin Cu-TCPP MOF nanosheets: A new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics 2018, 8, 4086–4096. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jiang, C.; Zhai, D.; Luo, Y.; Chen, Y.; Lv, F.; Yi, Z.; Deng, Y.; Wang, J.; Chang, J.; et al. A Bifunctional Biomaterial with Photothermal Effect for Tumor Therapy and Bone Regeneration. Adv. Funct. Mater. 2016, 26, 1197–1208. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Y.N.; Wan, J.; Cui, R.; Yu, X.; Zhao, G.; Lin, K. A novel multifunctional carbon aerogel-coated platform for osteosarcoma therapy and enhanced bone regeneration. J. Mater. Chem. B 2020, 8, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xia, Y.; Zhuang, P.; Liu, W.; Mu, C.; Liu, Z.; Wang, J.; Chen, L.; Dai, H.; Luo, Z. FePSe3-Nanosheets-Integrated Cryogenic-3D-Printed Multifunctional Calcium Phosphate Scaffolds for Synergistic Therapy of Osteosarcoma. Small 2023, 19, e2303636. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Chen, D.-H. Preparation of LaB6 nanoparticles as a novel and effective near-infrared photothermal conversion material. Chem. Eng. J. 2012, 180, 337–342. [Google Scholar] [CrossRef]
- Yin, C.; Jia, X.; Miron, R.J.; Long, Q.; Xu, H.; Wei, Y.; Wu, M.; Zhang, Y.; Li, Z. Setd7 and its contribution to Boron-induced bone regeneration in Boron-mesoporous bioactive glass scaffolds. Acta Biomater. 2018, 73, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Ma, B.; Huan, Z.; Lin, R.; Wang, X.; Li, T.; Wu, J.; Ma, N.; Zhu, H.; Chang, J.; et al. LaB6 surface chemistry-reinforced scaffolds for treating bone tumors and bone defects. Appl. Mater. Today 2019, 16, 42–55. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.Y.; Lin, L.B.; Zhao, C.; Hu, Z.M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local delivery of insulin/IGF-1 for bone regeneration: Carriers, strategies, and effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, H.; Yang, M.; Wang, L.; Gan, K. Three-dimensional-printed MPBI@β-TCP scaffold promotes bone regeneration and impedes osteosarcoma under near-infrared laser irradiation. FASEB J. 2023, 37, e22924. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ma, Z.; Chen, Q.; Li, W.; Liu, X.; Ma, X.; Mao, Y.; Yang, H.; Ma, H.; Wang, J. Bifunctional, Copper-Doped, Mesoporous Silica Nanosphere-Modified, Bioceramic Scaffolds for Bone Tumor Therapy. Front. Chem. 2020, 8, 610232. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Yi, K.; Ju, E.; Jin, Y.; Xu, Y.; Wang, H.; Chen, W.C.; Wang, K.; Wang, Y.; Tao, Y.; et al. 3D Printed Bioceramic Scaffolds as a Universal Therapeutic Platform for Synergistic Therapy of Osteosarcoma. ACS Appl. Mater. Interfaces 2021, 13, 18488–18499. [Google Scholar] [CrossRef] [PubMed]
- Monroe, E.A.; Votava, W.; Bass, D.B.; McMullen, J. New calcium phosphate ceramic material for bone and tooth implants. J. Dent. Res. 1971, 50, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.G.; Porter, A.E.; Skepper, J.N. Hydroxyapatite nano and microparticles: Correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009, 30, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qiang, L.; Liu, Y.; Fan, M.; Si, X.; Zheng, P. Biomaterial-assisted tumor therapy: A brief review of hydroxyapatite nanoparticles and its composites used in bone tumors therapy. Front. Bioeng. Biotechnol. 2023, 11, 1167474. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, E.; Wilson, P.; Sheela, K.; Ramya, R. Role of triton X-100 and hydrothermal treatment on the morphological features of nanoporous hydroxyapatite nanorods. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, Y.; Song, J.; Huang, D.; An, M.; Chen, W.; Han, P.; Yao, X.; Zhang, X. A multifunctional nano-hydroxyapatite/MXene scaffold for the photothermal/dynamic treatment of bone tumours and simultaneous tissue regeneration. J. Colloid. Interface Sci. 2023, 652, 1673–1684. [Google Scholar] [CrossRef]
- Zhu, C.; He, M.; Sun, D.; Huang, Y.; Huang, L.; Du, M.; Wang, J.; Wang, J.; Li, Z.; Hu, B.; et al. 3D-Printed Multifunctional Polyetheretherketone Bone Scaffold for Multimodal Treatment of Osteosarcoma and Osteomyelitis. ACS Appl. Mater. Interfaces 2021, 13, 47327–47340. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, M.; Li, L.; Wei, S.; Hu, X.; Wang, X.; Shan, G.; Zhang, Y.; Xia, H.; Yin, Q. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 225–233. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Toskas, G.; Cherif, C.; Hund, R.D.; Laourine, E.; Mahltig, B.; Fahmi, A.; Heinemann, C.; Hanke, T. Chitosan(PEO)/silica hybrid nanofibers as a potential biomaterial for bone regeneration. Carbohydr. Polym. 2013, 94, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zou, Q.; Zou, W.; Xie, Z.; Li, Z.; Zhao, X.; Du, C. Bifunctional scaffolds of hydroxyapatite/poly(dopamine)/carboxymethyl chitosan with osteogenesis and anti-osteosarcoma effect. Biomater. Sci. 2021, 9, 3319–3333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Li, M.; Lin, Z.; Wang, L.; Zhang, Y.; Yin, Q.; Xia, H.; Han, G. Zero-Dimensional Carbon Dots Enhance Bone Regeneration, Osteosarcoma Ablation, and Clinical Bacterial Eradication. Bioconjugate Chem. 2018, 29, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.S.; Kim, D.S.; Suhito, I.R.; Choo, S.S.; Kim, S.J.; Song, I.; Kim, T.H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.P.; Zhou, T.; Ye, E.; Liu, S.; Koh, L.D.; Low, M.; Loh, X.J.; Win, K.Y.; Zhang, L.; Han, M.Y. Effective Targeted Photothermal Ablation of Multidrug Resistant Bacteria and Their Biofilms with NIR-Absorbing Gold Nanocrosses. Adv. Healthc. Mater. 2016, 5, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; You, L.; Lan, L.; Lee, H.J.; Chaudhry, S.T.; Li, R.; Cheng, J.X.; Mei, J. Semiconducting Polymer Nanoparticles for Centimeters-Deep Photoacoustic Imaging in the Second Near-Infrared Window. Adv. Mater. 2017, 29, 1703403. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Yang, Y.; Yang, Y.; Zhang, K.; Guo, L.; Ge, H.; Chen, X.; Liu, J.; Feng, H. Molecular Engineering of an Organic NIR-II Fluorophore with Aggregation-Induced Emission Characteristics for In Vivo Imaging. Small 2019, 15, e1805549. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Xu, C.; Pu, K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat. Commun. 2021, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, H.; Wang, Z.; Younis, M.R.; Liu, C.; Wu, C.; Luo, Y.; Huang, P. 3D Printed Wesselsite Nanosheets Functionalized Scaffold Facilitates NIR-II Photothermal Therapy and Vascularized Bone Regeneration. Adv. Sci. 2021, 8, e2100894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Y.; Shi, J.; Yao, X.; Chen, W.; Wei, X.; Zhang, X.; Chu, P.K. Near-infrared light II—Assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials 2021, 269, 120634. [Google Scholar] [CrossRef] [PubMed]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol. Sci. Off. J. Soc. Toxicol. 2006, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Gong, T.; Wang, Y.; Li, Z.; Lu, M.; Luo, Y.; Min, L.; Tu, C.; Zhang, X.; Zeng, Q.; et al. Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors. Int. J. Mol. Sci. 2024, 25, 4139. https://doi.org/10.3390/ijms25084139
Xie M, Gong T, Wang Y, Li Z, Lu M, Luo Y, Min L, Tu C, Zhang X, Zeng Q, et al. Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors. International Journal of Molecular Sciences. 2024; 25(8):4139. https://doi.org/10.3390/ijms25084139
Chicago/Turabian StyleXie, Mengzhang, Taojun Gong, Yitian Wang, Zhuangzhuang Li, Minxun Lu, Yi Luo, Li Min, Chongqi Tu, Xingdong Zhang, Qin Zeng, and et al. 2024. "Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors" International Journal of Molecular Sciences 25, no. 8: 4139. https://doi.org/10.3390/ijms25084139
APA StyleXie, M., Gong, T., Wang, Y., Li, Z., Lu, M., Luo, Y., Min, L., Tu, C., Zhang, X., Zeng, Q., & Zhou, Y. (2024). Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors. International Journal of Molecular Sciences, 25(8), 4139. https://doi.org/10.3390/ijms25084139






