Transcriptome Analysis Reveals the Important Role of Vitamin B12 in the Response of Natronorubrum daqingense to Salt Stress
Abstract
1. Introduction
2. Results
2.1. Transcriptome Sequencing and Analysis of Differentially Expressed Genes
2.2. Ultraviolet Mutagenesis of Low-Salt Adaptive Mutant LND5
2.3. Construction of ΔcobC and +cobC
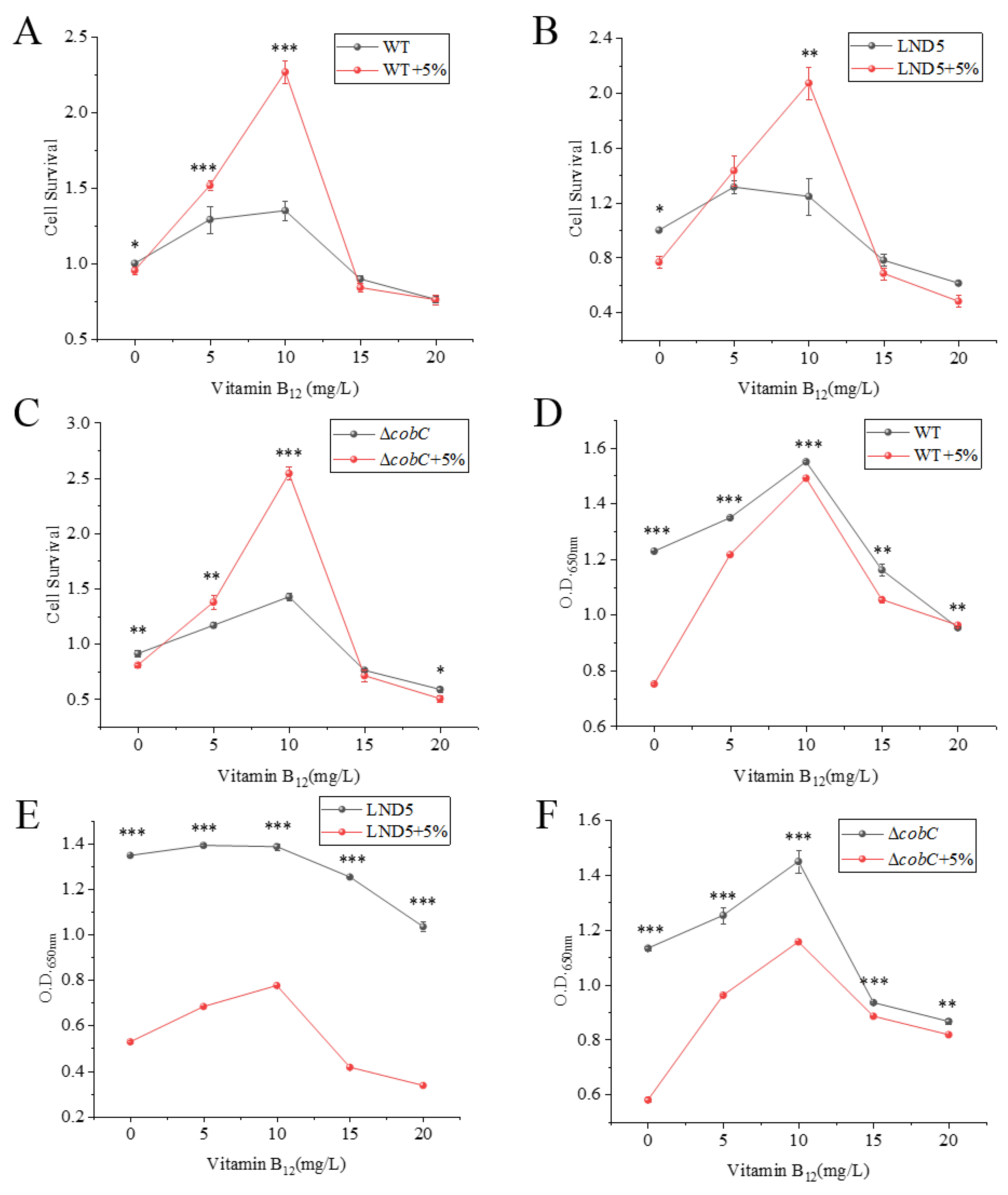
2.4. Effects of Exogenous Vitamin B12 Addition
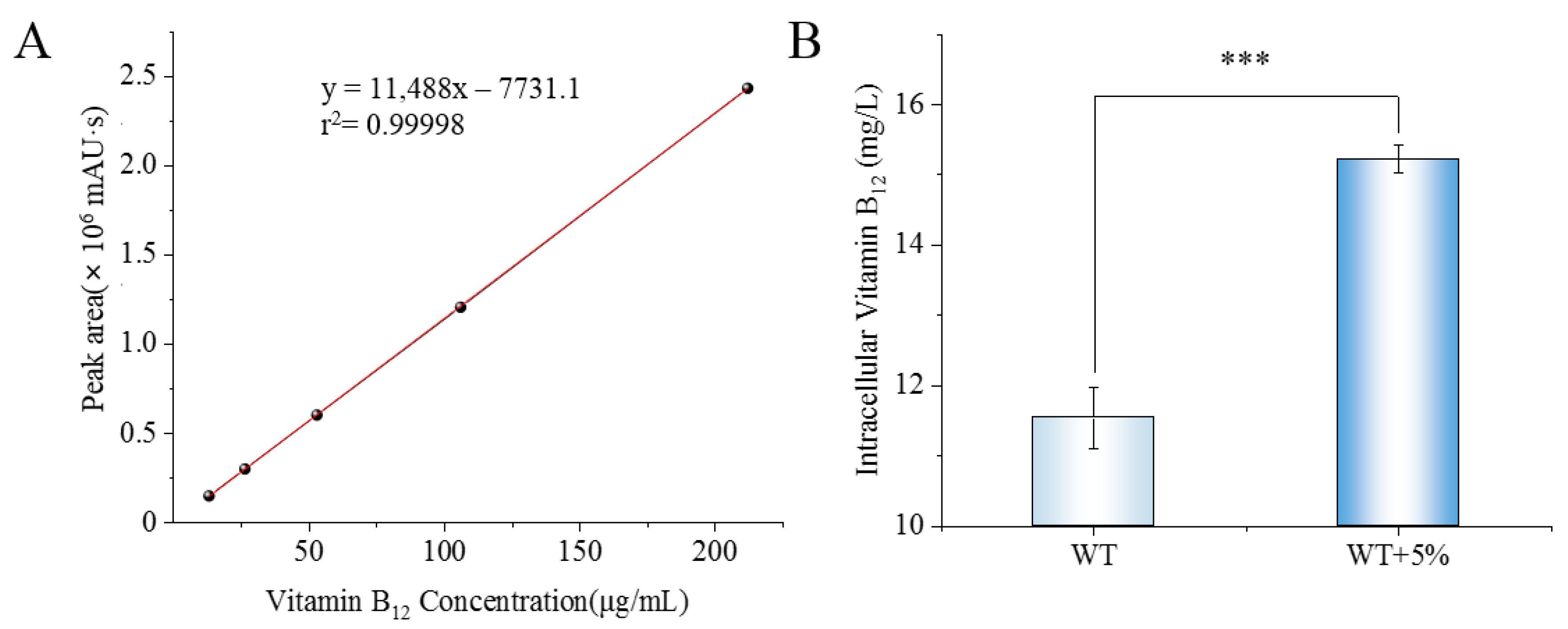
2.5. Detection of Intracellular Vitamin B12 Content
3. Discussion
4. Materials and Methods
4.1. Strains, Plasmids and Growth Conditions
4.2. Transcriptome Sequencing and Analysis
4.3. Ultraviolet Mutagenesis of Low-Salt Adaptive Mutants
4.4. Gene Knockout and Complementation
4.5. Archaeal Protoplast Transformation
4.6. Determination of Intracellular Vitamin B12
4.7. Standard Experimental Procedures and Bioinformatics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, A.D. Microbial water stress. Bacteriol. Rev. 1976, 40, 803–846. [Google Scholar] [CrossRef]
- Brown, A.D.; Simpson, J.R. Water relations of sugar-tolerant yeasts: The role of intracellular polyols. J. Gen. Microbiol. 1972, 72, 589–591. [Google Scholar] [CrossRef]
- Brett, C.L.; Donowitz, M.; Rao, R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. 2005, 288, C223–C239. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Hicks, D.B.; Ito, M. Cation/proton antiporter complements of bacteria: Why so large and diverse? Mol. Microbiol. 2009, 74, 257–260. [Google Scholar] [CrossRef]
- Chong, P.L.; Chang, A.; Yu, A.; Mammedova, A. Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 7616. [Google Scholar] [CrossRef]
- Rao, A.; de Kok, N.A.W.; Driessen, A.J.M. Membrane Adaptations and Cellular Responses of Sulfolobus acidocaldarius to the Allylamine Terbinafine. Int. J. Mol. Sci. 2023, 24, 7328. [Google Scholar] [CrossRef]
- Roberts, M.F. Osmoadaptation and osmoregulation in archaea: Update 2004. Front. Biosci. 2004, 9, 1999–2019. [Google Scholar] [CrossRef]
- da Costa, M.S.; Santos, H.; Galinski, E.A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 1998, 61, 117–153. [Google Scholar] [CrossRef]
- Roberts, M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1, 5. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Roesser, M.; Müller, V. Osmoadaptation in bacteria and archaea: Common principles and differences. Environ. Microbiol. 2001, 3, 743–754. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Guo, X.J.; Li, H.; Huang, Z.Z.; Lin, K.F.; Liu, Y.D. High-throughput screening for a moderately halophilic phenol-degrading strain and its salt tolerance response. Int. J. Mol. Sci. 2015, 16, 11834–11848. [Google Scholar] [CrossRef]
- Cui, S.; Zhou, W.; Tang, X.; Zhang, Q.; Yang, B.; Zhao, J.; Mao, B.; Zhang, H. The Effect of Proline on the Freeze-Drying Survival Rate of Bifidobacterium longum CCFM 1029 and Its Inherent Mechanism. Int. J. Mol. Sci. 2022, 23, 13500. [Google Scholar] [CrossRef]
- Lentzen, G.; Schwarz, T. Extremolytes: Natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 2006, 72, 623–634. [Google Scholar] [CrossRef]
- Cowan, D.A. Cryptic microbial communities in Antarctic deserts. Proc. Natl. Acad. Sci. USA 2009, 106, 19749–19750. [Google Scholar] [CrossRef]
- Casanueva, A.; Tuffin, M.; Cary, C.; Cowan, D.A. Molecular adaptations to psychrophily: The impact of ‘omic’ technologies. Trends Microbiol. 2010, 18, 374–381. [Google Scholar] [CrossRef]
- Klähn, S.; Hagemann, M. Compatible solute biosynthesis in cyanobacteria. Environ. Microbiol. 2011, 13, 551–562. [Google Scholar] [CrossRef]
- Duda, J.; Pedziwilk, Z.; Zodrow, K. Studies on the appearance of vitamin B12 in leguminous plants. Acta Microbiol. Pol. 1952, 6, 233–238. [Google Scholar]
- Calvillo, Á.; Pellicer, T.; Carnicer, M.; Planas, A. Bioprocess Strategies for Vitamin B12 Production by Microbial Fermentation and Its Market Applications. Bioengineering 2022, 9, 365. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.J.; Jahn, D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- Ferry, J.G. CO dehydrogenase. Annu. Rev. Microbiol. 1995, 49, 305–333. [Google Scholar] [CrossRef]
- Weiss, D.S.; Thauer, R.K. Methanogenesis and the unity of biochemistry. Cell 1993, 72, 819–822. [Google Scholar] [CrossRef]
- Frey, P.A. Radical mechanisms of enzymatic catalysis. Annu. Rev. Biochem. 2001, 70, 121–148. [Google Scholar] [CrossRef]
- Roth, J.R.; Lawrence, J.G.; Bobik, T.A. Cobalamin (coenzyme B12): Synthesis and biological significance. Annu. Rev. Microbiol. 1996, 50, 137–181. [Google Scholar] [CrossRef]
- Aureli, A.; Recupero, R.; Mariani, M.; Carlomagno, F.; Bocchini, S.; Nicodemo, M.; Marchili, M.R.; Cianfarani, S.; Cappa, M.; Fintini, D. Low Levels of Serum Total Vitamin B12 Are Associated with Worse Metabolic Phenotype in a Large Population of Children, Adolescents and Young Adults, from Underweight to Severe Obesity. Int. J. Mol. Sci. 2023, 24, 16588. [Google Scholar] [CrossRef]
- Fuschlberger, M.; Putz, P. Vitamin B12 supplementation and health behavior of Austrian vegans: A cross-sectional online survey. Sci. Rep. 2023, 13, 3983. [Google Scholar] [CrossRef]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation-Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, L.; Zhou, X.; Xu, T.; Shen, Q.; Li, T.; Wu, Y. Vitamin B12-Induced Autophagy Alleviates High Glucose-Mediated Apoptosis of Islet β Cells. Int. J. Mol. Sci. 2023, 24, 15217. [Google Scholar] [CrossRef]
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar] [CrossRef]
- Chittaranjan, Y. 91-102: Vitamin B12: An Intergenerational Story. In Global Landscape of Nutrition Challenges in Infants and Children: 93rd Nestlé Nutrition Institute Workshop, Kolkata, March 2019; Nestlé Nutrition Institute Workshop Series; S.Karger AG: Basel, Switzerland, 2020; Volume 93. [Google Scholar] [CrossRef]
- Vásquez, L.; Parra, A.; Quesille-Villalobos, A.M.; Gálvez, G.; Navarrete, P.; Latorre, M.; Toro, M.; González, M.; Reyes-Jara, A. Cobalamin cbiP mutant shows decreased tolerance to low temperature and copper stress in Listeria monocytogenes. Biol. Res. 2022, 55, 9. [Google Scholar] [CrossRef]
- Xie, B.; Bishop, S.; Stessman, D.; Wright, D.; Spalding, M.H.; Halverson, L.J. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013, 7, 1544–1555. [Google Scholar] [CrossRef]
- Keshavarz, H.; Moghadam, R.S.G. Seed priming with cobalamin (vitamin B12) provides significant protection against salinity stress in the common bean. Rhizosphere 2017, 3, 143–149. [Google Scholar] [CrossRef]
- Chi, Y.X.; Yang, L.; Zhao, C.J.; Muhammad, I.; Zhou, X.B.; Hong De Zhu, H.D. Effects of soaking seeds in exogenous vitamins on active oxygen metabolism and seedling growth under low-temperature stress. Saudi J. Biol. Sci. 2021, 28, 3254–3261. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Q.; Liu, Z.H.; Sun, L.; Wei, D.; Zhang, J.Z.; Song, J.Z.; Yuan, H.F. Haloterrigena daqingensis sp. nov., an extremely haloalkaliphilic archaeon isolated from a saline-alkaline soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2267–2271. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Narsing Rao, M.P.; Wei, D.; Sun, L.; Fang, B.Z.; Li, W.Q.; Yu, L.H.; Li, W.J. Complete genome sequencing and comparative genome analysis of the extremely halophilic archea, Haloteriigena daqingensis. Biotechnol. Appl. Biochem. 2022, 69, 1482–1488. [Google Scholar] [CrossRef]
- de la Haba, R.R.; Minegishi, H.; Kamekura, M.; Shimane, Y.; Ventosa, A. Phylogenomics of Haloarchaea: The Controversy of the Genera Natrinema-Haloterrigena. Front. Microbiol. 2021, 12, 740909. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, M.; Song, J. Characterization of Two Na+(K+, Li+)/H+ Antiporters from Natronorubrum daqingense. Int. J. Mol. Sci. 2023, 24, 10786. [Google Scholar] [CrossRef]
- Poolman, B.; Glaasker, E. Regulation of compatible solute accumulation in bacteria. Mol. Microbiol. 1998, 29, 397–407. [Google Scholar] [CrossRef]
- Empadinhas, N.; da Costa, M.S. To be or not to be a compatible solute: Bioversatility of mannosylglycerate and glucosylglycerate. Syst. Appl. Microbiol. 2008, 31, 159–168. [Google Scholar] [CrossRef]
- León, M.J.; Hoffmann, T.; Sánchez-Porro, C.; Heider, J.; Ventosa, A.; Bremer, E. Compatible Solute Synthesis and Import by the Moderate Halophile Spiribacter salinus: Physiology and Genomics. Front. Microbiol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Rodriguez-Valera, F. Betaine is the main compatible solute of halophilic eubacteria. J. Bacteriol. 1984, 160, 478–479. [Google Scholar] [CrossRef]
- Regev, R.; Peri, I.; Gilboa, H.; Avi-Dor, Y. 13C NMR study of the interrelation between synthesis and uptake of compatible solutes in two moderately halophilic eubacteria: Bacterium Ba1 and Vibro costicola. Arch. Biochem. Biophys. 1990, 278, 106–112. [Google Scholar] [CrossRef]
- Welsh, D.T.; Lindsay, Y.E.; Caumette, P.; Herbert, R.A.; Hannan, J. Identification of trehalose and glycine betaine as compatible solutes in the moderately halophilic sulfate reducing bacterium, Desulfovibrio halophilus. FEMS Microbiol. Lett. 1996, 140, 203–207. [Google Scholar] [CrossRef]
- Roth, J.R.; Lawrence, J.G.; Rubenfield, M.; Kieffer-Higgins, S.; Church, G.M. Characterization of the cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J. Bacteriol. 1993, 175, 3303–3316. [Google Scholar] [CrossRef]
- Roessner, C.A.; Scott, A.I. Fine-tuning our knowledge of the anaerobic route to cobalamin (vitamin B12). J. Bacteriol. 2006, 188, 7331–7334. [Google Scholar] [CrossRef][Green Version]
- Antony, C.P.; Doronina, N.V.; Boden, R.; Trotsenko, Y.A.; Shouche, Y.S.; Murrell, J.C. Methylophaga lonarensis sp. nov., a moderately haloalkaliphilic methylotroph isolated from the soda lake sediments of a meteorite impact crater. Int. J. Syst. Evol. Microbiol. 2012, 62, 1613–1618. [Google Scholar] [CrossRef]
- Wang, X.L.; Jiang, C.; Liu, J.H.; Liu, X.P. An efficient genetic knockout system based on linear DNA fragment homologous recombination for halophilic archaea. Hereditas 2015, 37, 388–395. [Google Scholar] [CrossRef]
- Allers, T.; Mevarech, M. Archaeal genetics—The third way. Nat. Rev. Genet. 2005, 6, 58–73. [Google Scholar] [CrossRef]
- Nagata, S.; Wang, Y.; Oshima, A.; Zhang, L.H.; Miyake, H.; Sasaki, H.; Ishida, A. Efficient cyclic system to yield ectoine using Brevibacterium sp. JCM 6894 subjected to osmotic downshock. Biotechnol. 2008, 99, 941–2948. [Google Scholar] [CrossRef][Green Version]
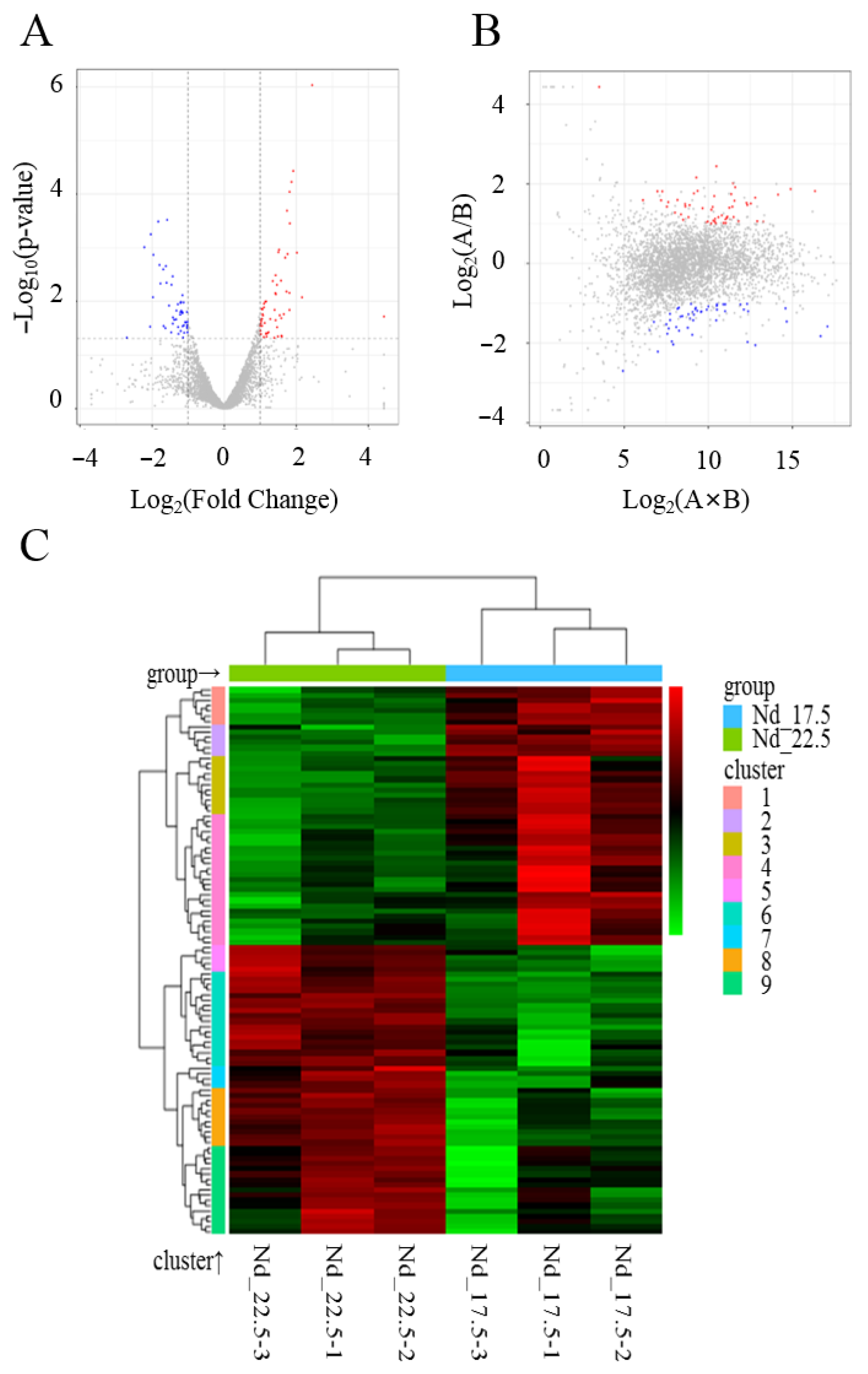
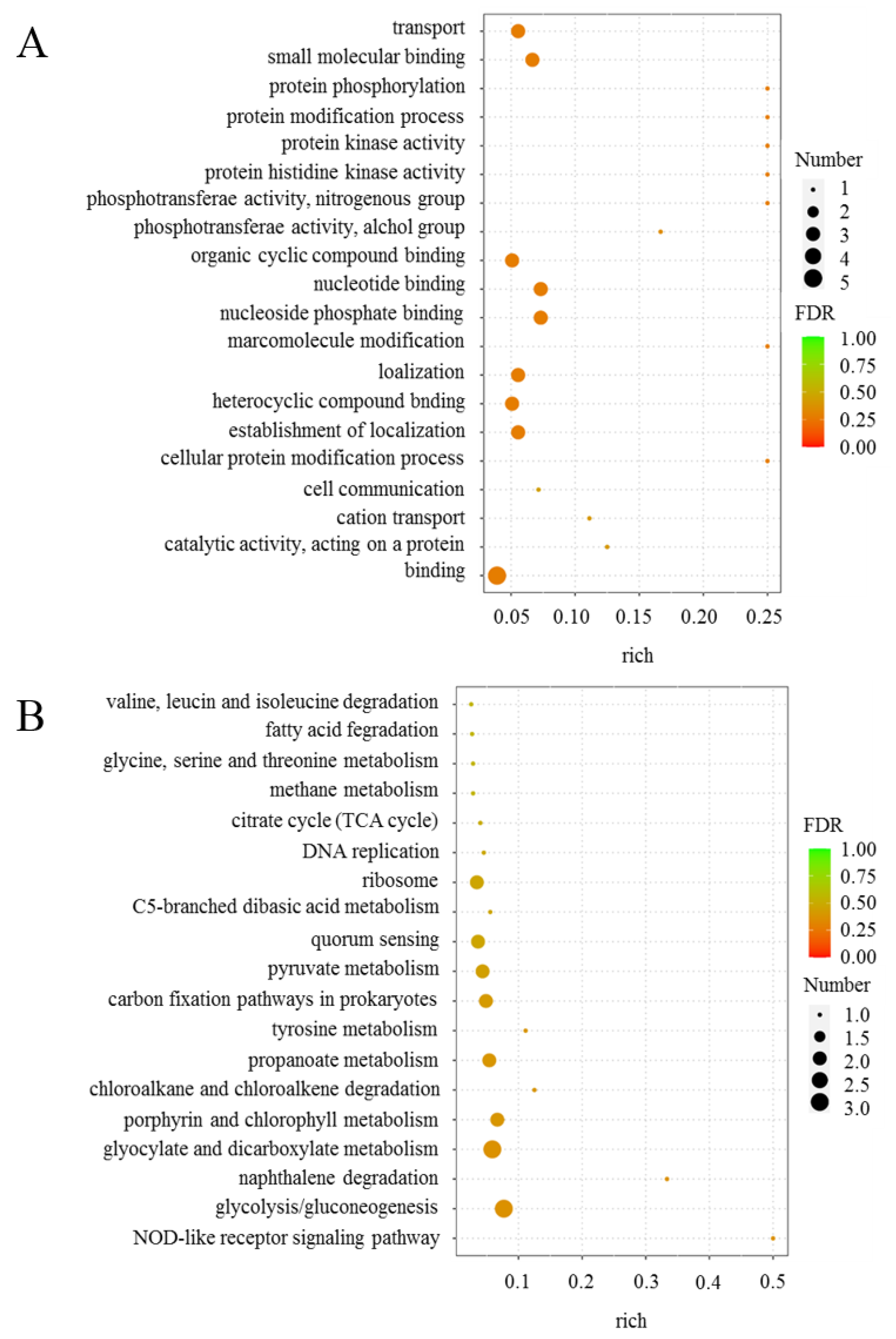
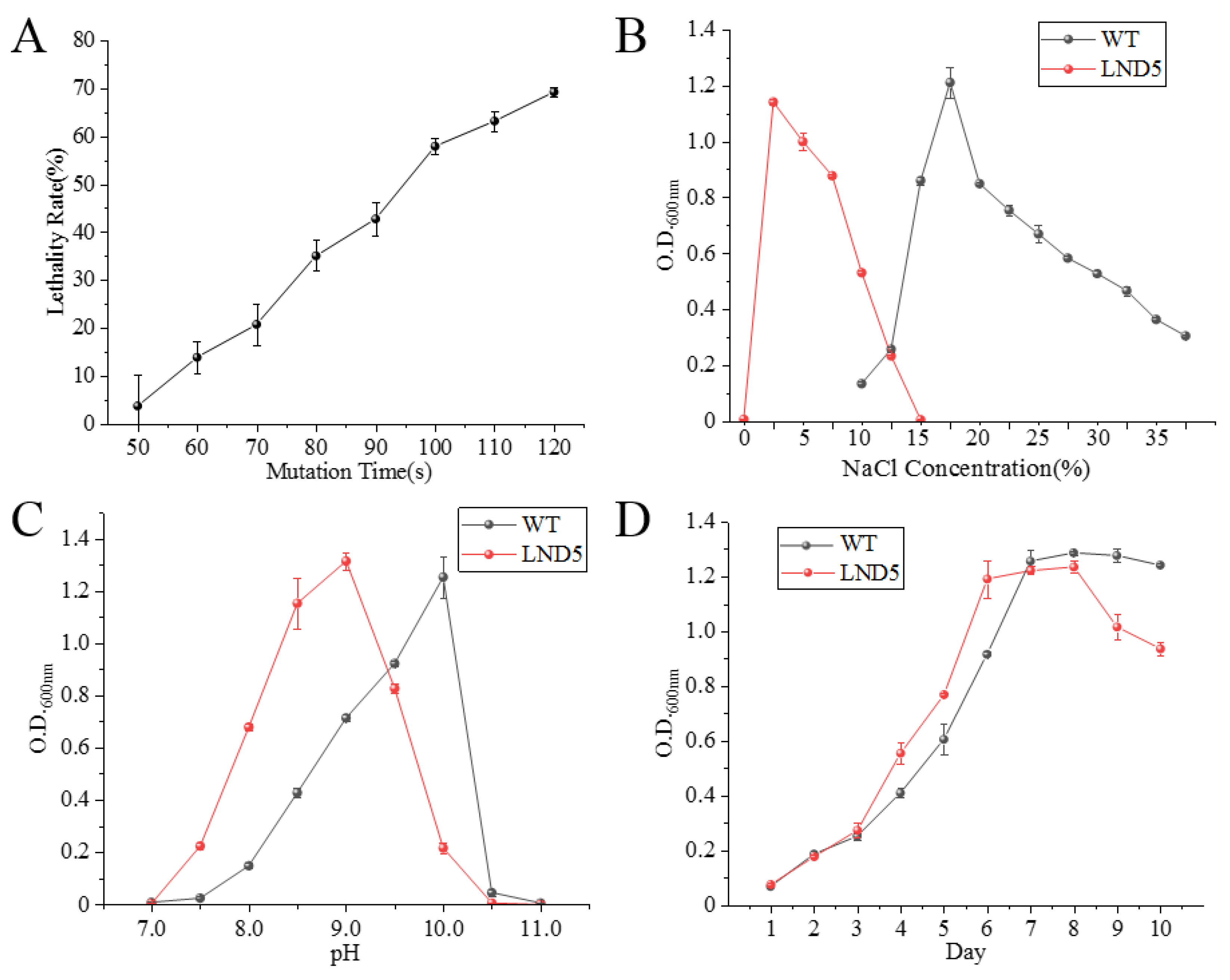
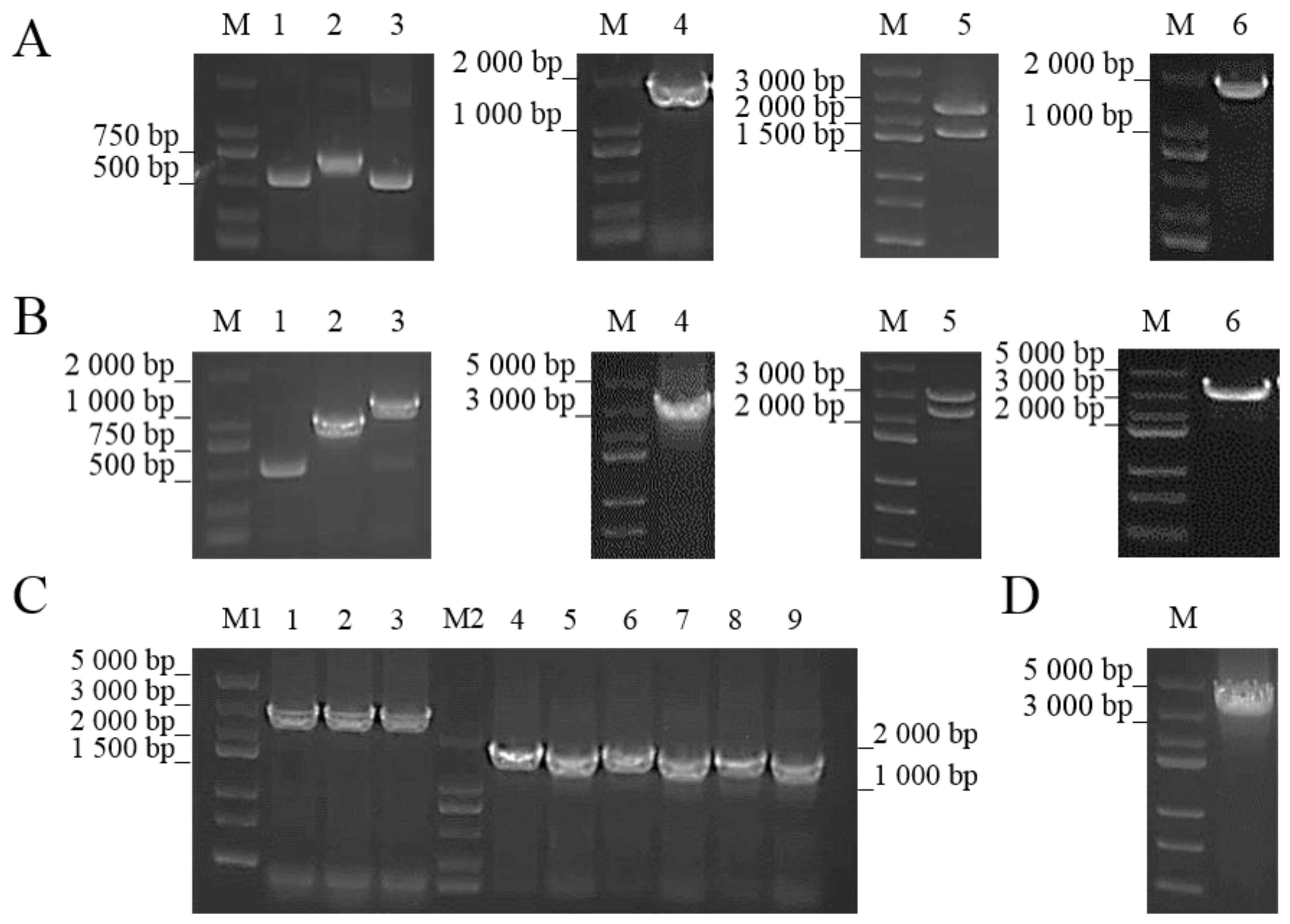

| Gene ID | Name | Fold Change | log2 (Fold Change) | p-Value |
|---|---|---|---|---|
| BB347_RS03400 | cbiE | 2.7626 | 1.4660 | 0.0049 |
| BB347_RS03395 | cobH | 2.3105 | 1.2082 | 0.0392 |
| BB347_RS03460 | cobC | 2.0859 | 1.0607 | 0.0141 |
| Strain or Plasmid | Description | Source or Reference |
|---|---|---|
| Strains | ||
| N. daqingense JX313T | Wild-type strain, an extremely halophilic archaea | Isolated and identified by our lab [31] |
| N. daqingense LND5 | Low-salt adaptive mutant, obtained through UV mutagensis | This study |
| N. daqingense ΔcobC | The cobC gene-knockout strain, vitamin B12 biosynthesis deficiency | This study |
| N. daqingense +cobC | The cobC gene-complementation strain | This study |
| E. coli DH5α | Host strain for cloning | Vazyme Biotech Co., Ltd., Nanjing, China |
| Plasmids | ||
| pUC18 | Cloning vector, AmpR | Comate Biosciences Co., Ltd., Changchun, China |
| E-pUC57 | Conferring erythromycin resistance, AmpR and ErmR | Comate Biosciences Co., Ltd., Changchun, China |
| pSilentI | Conferring hygromycin resistance, AmpR and HygR | MiaoLing Plasmid Platform, Wuhan, China |
| pUC-cobC-ko | Preparation of linear DNA fragments for cobC gene knockout, AmpR and ErmR | This study |
| pUC-cobC-c | Preparation of linear DNA fragments for cobC gene complementation, AmpR and HygR | This study |
| Component | Solution I | Solution II | Solution III | Solution IV | Solution V |
|---|---|---|---|---|---|
| NaCl | 1 M | 1 M | 2.5 M | 2.5 M | 2.5 M |
| KCl | 27 mM | 27 mM | 56 mM | 56 mM | 56 mM |
| Sucrose | 37.5 g | 150 g | 150 g | 150 g | 37.5 g |
| Tris-HCl | 50 mM (pH 8.5) | 50 mM (pH 7.5) | 50 mM (pH 7.5) | 50 mM (pH 7.5) | 50 mM (pH 7.5) |
| MgSO4 | - | - | 150 mM | 134 mM | 134 mM |
| CaCl2 | - | - | 3.75 mM | 3 mM | 3 mM |
| MgCl2 | - | - | - | 150 mM | 150 mM |
| Total | 1 L | 1 L | 1 L | 1 L | 1 L |
| Primers | Description | Amplicon Size (bp) | Sequence (from 5′ to 3′) |
|---|---|---|---|
| 22F | Archaea 16S rDNA [46] | 1500 | ATTCCGGTTGATCCTGC |
| 1540R | AGGAGGTGATCCAGCCGCAG | ||
| M13-47F | Sequencing primers of pUC18 | Depends on the size of inserted fragment | CGCCAGGGTTTTCCCAGTCACGAC |
| M13R | CACACAGGAAACAGCTATGAC | ||
| VF-FP | To clone cobC upstream homologous arm sequence for gene knockout | 500 | TATGACCATGATTACGAATTCACACGCCGGTCGGCATCG |
| VF-RP | TTCTCGTTCATTTGCCCACCTCTTCGACGA | ||
| VE-FP | To clone erythromycin resistance sequence for gene knockout | 735 | GGTGGGCAAATGAACGAGAAAAATATAAAACACAGTCA |
| VE-RP | GTGGTAGTGTATCGAAACCGTTACTTATTAAATAATTTATAGCTATTGAAAAGAGA | ||
| VR-FP | To clone cobC downstream homologous arm sequence for gene knockout | 500 | CGGTTTCGATACACTACCACGA |
| VR-RP | CAGGTCGACTCTAGAGGATCCGCGTCCGGATCGCTCGAG | ||
| VY-FP | To confirm the success of gene knockout or complementation | 2225 (knockout) 3233 (complementation) | GTCGACTTCGACGTGATCC |
| VY-RP | AGCGCGTCGACGTAG | ||
| VBF-RP | To clone cobC upstream homologous arm sequence for gene complementation, with VF-RP | 500 | GGCTTTTTCATTTGCCCACCTCTTCGACGA |
| VH-FP | To clone hygromycin resistance sequence for gene complementation | 1026 | GGTGGGCAAATGAAAAAGCCTGAACTCACCG |
| VH-RP | GTGTCTCGCTCATCTATTCCTTTGCCCTCGGACG | ||
| VBR-FP | To clone cobC and downstream homologous arm sequence for gene complementation, with VR-FP | 1743 | GGAATAGATGAGCGAGACACAGCCCAC |
| cbiE-FP | To detect differentially expressed genes | 135 | TGCTGACCTGTGGCTACAAG |
| cbiE-RP | GTACCTTCCCGACGAACTGG | ||
| cobH-FP | 109 | TGAGACGAGCATGGACATCG | |
| cobH-RP | CAGGTGCTGGAACTCGATGT | ||
| cobC-FP | 139 | TTCGGGAGTTGATCGACGAC | |
| cobC-RP | TCCCCGCAGTTCTCGTAGA | ||
| 16S-FP | As reference gene for RT-qPCR | 162 | GCCGATTAGGTAGACGGTGG |
| 16S-RP | GAGTCCCCTTATCGCACTCG |
| Component | Volume |
|---|---|
| Primer 1 (10 μM) | 0.4 μL |
| Primer 2 (10 μM) | 0.4 μL |
| 2× AceQ qPCR SYBR Green Master Mix | 10 μL |
| 50× ROX Reference Dye 1 | 0.4 μL |
| cDNA | 1 μL |
| ddH2O | up to 20 μL |
| Total | 20 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, Z.; Guan, J.; Song, J. Transcriptome Analysis Reveals the Important Role of Vitamin B12 in the Response of Natronorubrum daqingense to Salt Stress. Int. J. Mol. Sci. 2024, 25, 4168. https://doi.org/10.3390/ijms25084168
Wang Q, Wang Z, Guan J, Song J. Transcriptome Analysis Reveals the Important Role of Vitamin B12 in the Response of Natronorubrum daqingense to Salt Stress. International Journal of Molecular Sciences. 2024; 25(8):4168. https://doi.org/10.3390/ijms25084168
Chicago/Turabian StyleWang, Qi, Zhiwei Wang, Jiaqi Guan, and Jinzhu Song. 2024. "Transcriptome Analysis Reveals the Important Role of Vitamin B12 in the Response of Natronorubrum daqingense to Salt Stress" International Journal of Molecular Sciences 25, no. 8: 4168. https://doi.org/10.3390/ijms25084168
APA StyleWang, Q., Wang, Z., Guan, J., & Song, J. (2024). Transcriptome Analysis Reveals the Important Role of Vitamin B12 in the Response of Natronorubrum daqingense to Salt Stress. International Journal of Molecular Sciences, 25(8), 4168. https://doi.org/10.3390/ijms25084168






