Abstract
The WRKY gene family is crucial for regulating plant growth and development. However, the WRKY gene is rarely studied in naked kernel formation in hull-less Cucurbita pepo L. (HLCP), a natural mutant that lacks the seed coat. In this research, 76 WRKY genes were identified through bioinformatics-based methods in C. pepo, and their phylogenetics, conserved motifs, synteny, collinearity, and temporal expression during seed coat development were analyzed. The results showed that 76 CpWRKYs were identified and categorized into three main groups (I−III), with Group II further divided into five subgroups (IIa−IIe). Moreover, 31 segmental duplication events were identified in 49 CpWRKY genes. A synteny analysis revealed that C. pepo shared more collinear regions with cucumber than with melon. Furthermore, quantitative RT-PCR (qRT-PCR) results indicated the differential expression of CpWRKYs across different varieties, with notable variations in seed coat development between HLCP and CP being attributed to differences in CpWRKY5 expression. To investigate this further, CpWRKY5-overexpression tobacco plants were generated, resulting in increased lignin content and an upregulation of related genes, as confirmed by qRT-PCR. This study offers valuable insights for future functional investigations of CpWRKY genes and presents novel information for understanding the regulation mechanism of lignin synthesis.
1. Introduction
Cucurbita pepo L., commonly known as zucchini, is an annual plant native to Mexico and North America [1]. It is an economically valuable member of the cucurbit fruit family. Because of its carbohydrates, non-cellulosic polysaccharides, phenol, flavonoid contents, and amino acids, as well as its high contents of vitamins (such as vitamin A, E, and C), C. pepo has been recognized as a functional vegetable [2]. In the 1880s, a naturally mutated, hull-less variety of C. pepo (HLCP) was discovered in Austria [3]. Using scanning electron microscopy, Bezold et al. reported that the formation of this HLCP variety occurs due to the absence of lignification and the collapse of the outer four cortices (namely epidermis, subcutaneous tissue, sclerenchyma, and parenchyma) compared with the hulled variety of C. pepo (CP) [4].
Cucurbita pepo seed oil and seed extract have demonstrated remarkable nutritional benefits and medicinal properties, particularly in addressing benign prostatic hyperplasia and inhibiting the proliferation of human papillary thyroid cancer cells [5,6]. CP seeds must be mechanically shelled before eating and processing at a later stage, which increases the production cost. In contrast, HLCP seeds are easy to eat and process, and complete seeds can be obtained without shelling, making them the seeds of choice by the industry [7,8]. According to studies investigating the phenotypic traits and physiological and biochemical parameters associated with HLCP and CP seed coat development, HLCP is formed because of the lack of lignin accumulation [9]. Molecular mechanism studies have shown that CP and HLCP seed coat development is controlled by a pair of nuclear genes present at the same locus. However, some studies have inferred that HLCP seed coat development is controlled by a pair of major genes and some modified genes that control particular quantitative traits [3,10,11,12]. Accordingly, researchers believe that a single gene is involved in seed coat lignification, and the phenotype of HLCP mutants resulted from the combined action of lignin-synthesis-related gene expression and many environmental factors [13,14].
As major modulators of gene expression, transcription factors (TFs) are essential for the development of plant tissues and the signal transmission [15,16]. The WRKY TF family is one of the largest and most well-researched in higher plants [17]. Following the discovery of the first WRKY gene in sweet potato (Ipomoea batatas: SPF1), WRKY genes have since been found in numerous plant species and extensively studied [18], including Dendrobium catenatum (62 DcWRKYs) [19], Cucumis sativus L. (61 CsWRKYs) [17], Citrullus lanatus (63 ClWRKYs) [20], and Taraxacum kok-saghyz Rodin (72 TkWRKYs) [21].
The WRKY DNA-binding domain (DBD) is defined by the N-terminus invariant heptad WRKYGQK amino acid motif and the C-terminus C2H2 or C2HC zinc-binding motif. When WRKY TFs bind to the (T)TGAC(C/T), that is, the W-box cis-element in the target gene promoter, the expression of WRKY genes is induced to achieve cellular homeostasis [22]. WRKY TFs can be categorized into three groups based on the number of WRKY domains and the type of zinc finger motif they possess. Group I WRKY proteins typically contain two WRKY domains, whereas Group II WRKY proteins possess a single WRKY domain and are further classified into five subgroups (IIa–IIe). Both Group I and Group II proteins feature a C2H2 zinc finger motif (C-X4-5-C-X22-23-H-X1-H). Group III proteins have one WRKY domain, followed by a C2HC zinc finger motif (C-X7-C-X23-H-X1-C) [23,24].
WRKY TFs play an important role in regulating different aspects of seed coat formation, including hemicellulose, cellulose, and lignin contents [25], as well as secondary cell wall deposition [26,27]. Recent research using an RNA-Seq method by Xue et al. revealed that WRKY TFs are also involved in the development of seed coats in C. pepo [28]. Among the identified WRKY TFs, seven were significantly upregulated in CP and two were significantly upregulated in HLCP. However, according to the study of Xue et al., WRKY is speculated to play an important role in the seed coat formation [28]. However, The identification of the WRKY gene and its function and mechanism in the development of C. pepo seed coat have not been studied. Therefore, we performed a genome-wide identification of WRKYs in C. pepo, and comprehensively studied their classification, conserved protein domains, chromosomal location, phylogeny, motif composition, structure, and duplication events. Moreover, we investigated the expression profile of WRKY genes between CP and HLCP and explored their potential functions during lignin synthesis and accumulation. These results provide the potential candidate genes for further functional analyses and enrich our understanding of the molecular mechanism underlying lignin synthesis in plants.
2. Results
2.1. Identification and Evaluation of Chromosomal Location, Multiple Sequence Alignment, and Phylogenetic Relationships of CpWRKY Proteins
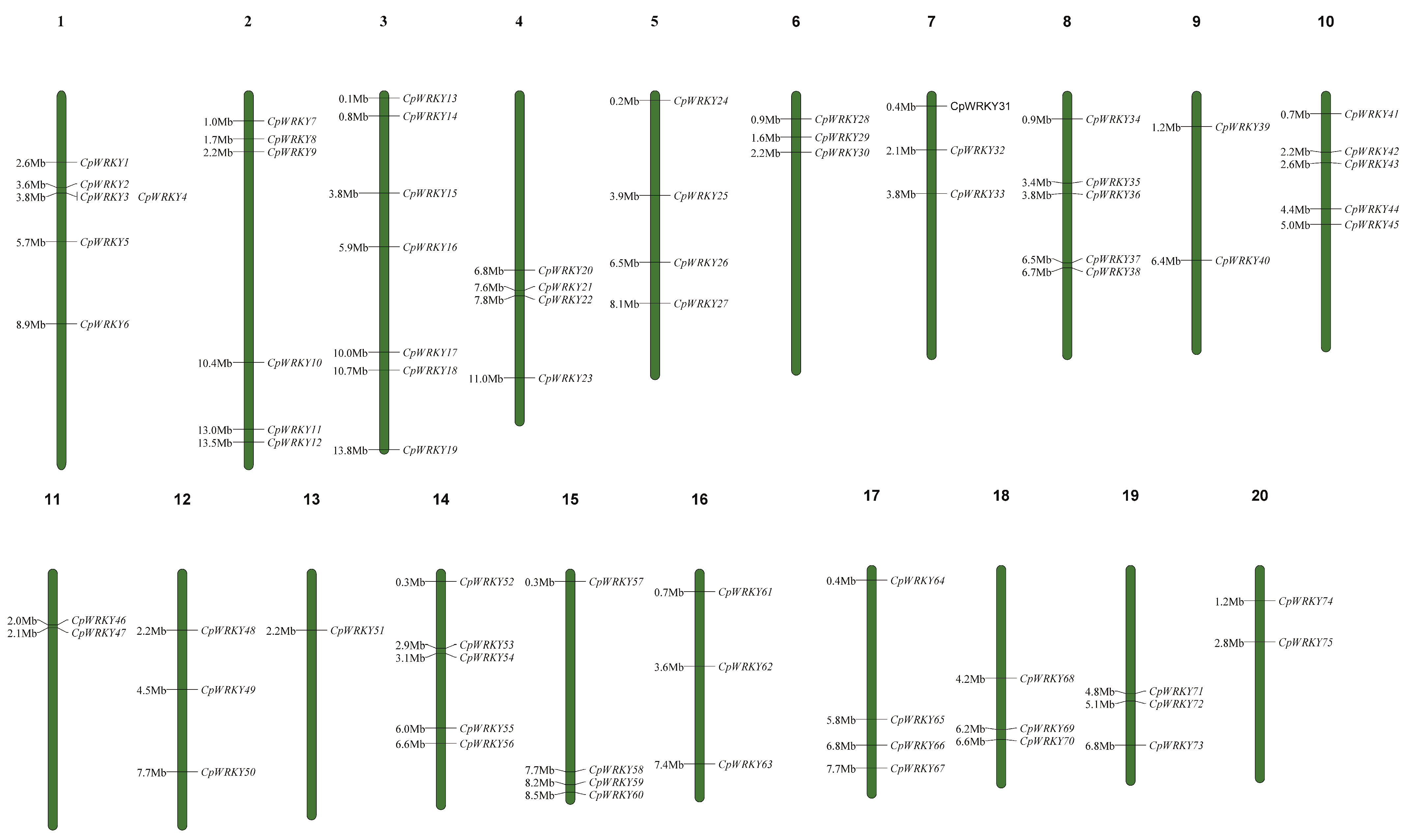
Table S1 presents a total of 76 putative CpWRKY genes. Among these genes, all except CpWRKY76 could be mapped to chromosomes 1–20. These genes were renamed as CpWRKY1–CpWRKY75 according to their order on the chromosomes. (Figure 1). CpWRKY76 could not be definitively assigned to any chromosome in the zucchini genome. Chromosome 3 harbored the highest number of WRKY genes (7), whereas chromosome 13 had the lowest number of CpWRKY genes (1). In addition, six chromosomes (6, 7, 12, 16, 18, and 19) had three CpWRKY genes, while chromosomes 1 and 2 harbored relatively more genes than chromosomes 9, 11, and 20. This indicated that, despite the presence of CpWRKYs on all 20 chromosomes, their distribution across respective chromosomes was uneven.
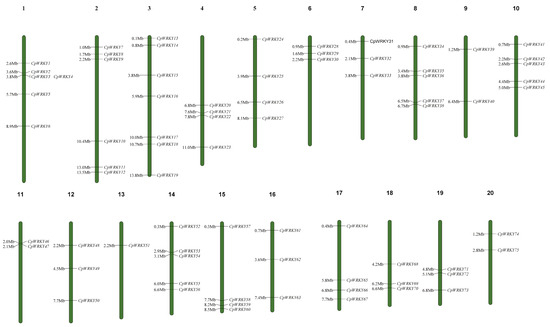
Figure 1.
Distribution of CpWRKY genes on chromosomes. At the top of every chromosome is the number of that chromosome. On the right side of every chromosome are the names of the genes. The distance in mega bases (Mb) between genes or from the gene to the end of the chromosome on the left side of each chromosome.
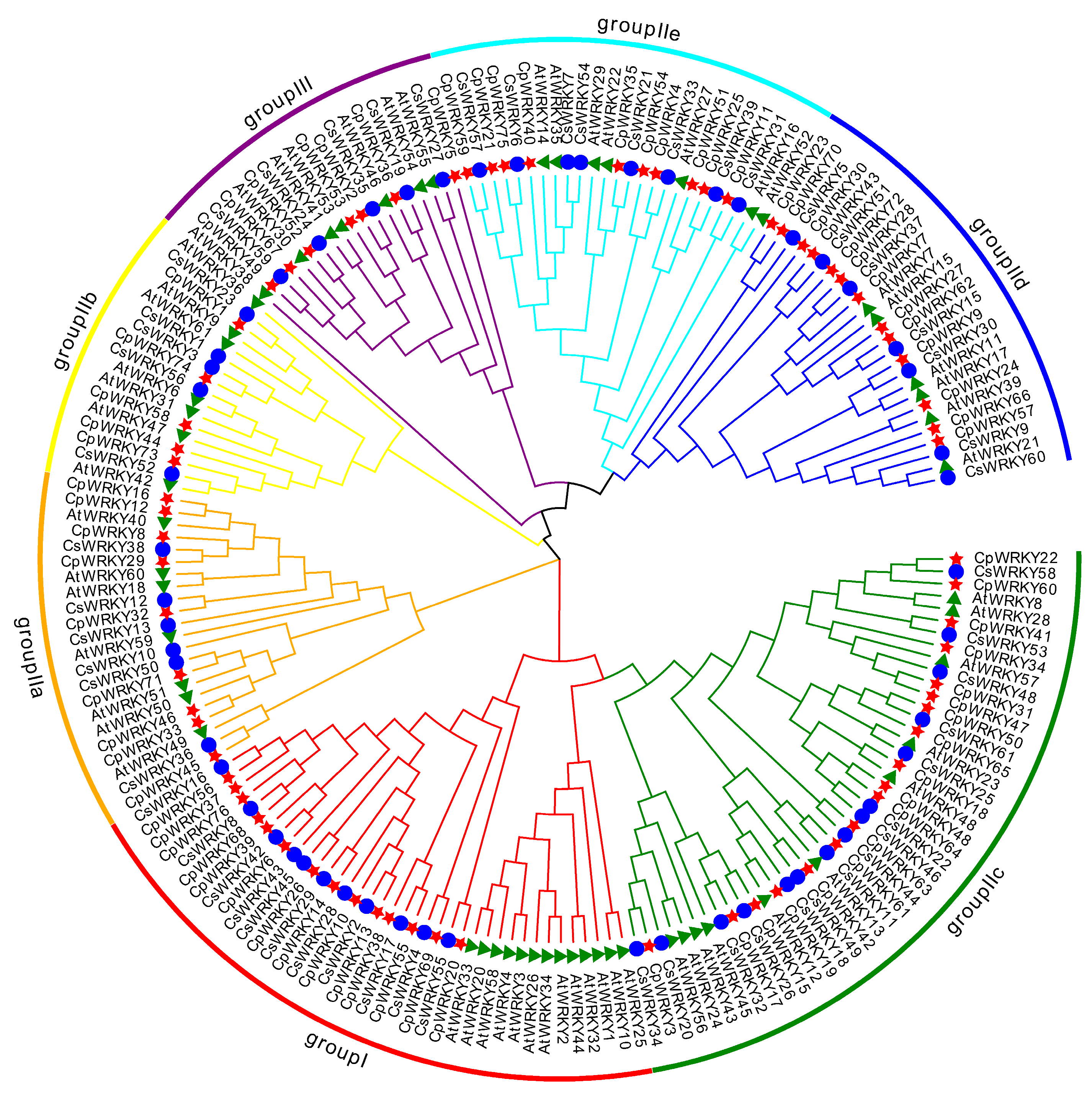
To investigate the evolutionary relationship among WRKY genes in C. pepo, a phylogenetic tree was constructed based on the published WRKY proteins in cucumber and Arabidopsis thaliana following multiple sequence alignment [17]. The phylogenetic tree categorizes these proteins into three groups (Groups I, II, and III) based on the characteristics of the conserved domains (Figure 2). Group I included fourteen members, which contained two conserved WRKY domains, whereas CpWRKY55 protein had lost its C-terminal WRKYGQK-like stretch. Group II included forty-two members, which were further subdivided into five subgroups: IIa (n = 9), IIb (n = 5), IIc (n = 18), IId (n = 13), and IIe (n = 10). In this group, all members possessed a complete WRKYGQK domain except CpWRKY33 and CpWRKY71 protein, which contained a WRKYGKK domain. Moreover, Group III included seven WRKYs that harbored the WRKY domain and the C2HC-type zinc finger (Figure S1).
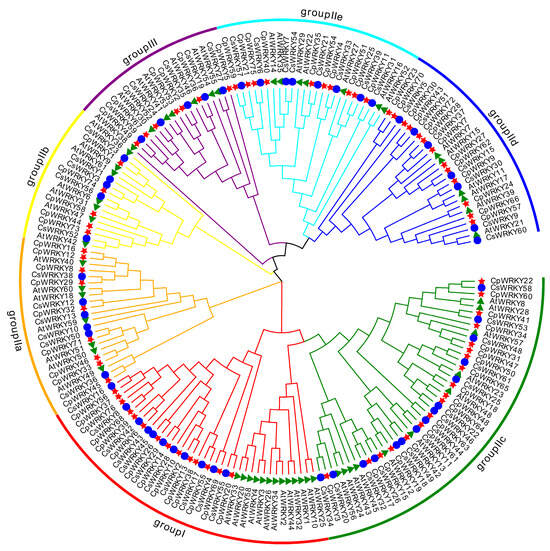
Figure 2.
Phylogenetic analysis and family classification of WRKY domains. Distinct arcs of various colors show diverse groups of the WRKY domain. The WRKY domains from C. pepo, cucumber, and Arabidopsis are denoted by a red star, blue circle, and green triangle, respectively.
2.2. Gene Structure and Conserved Motif Analysis of CpWRKYs
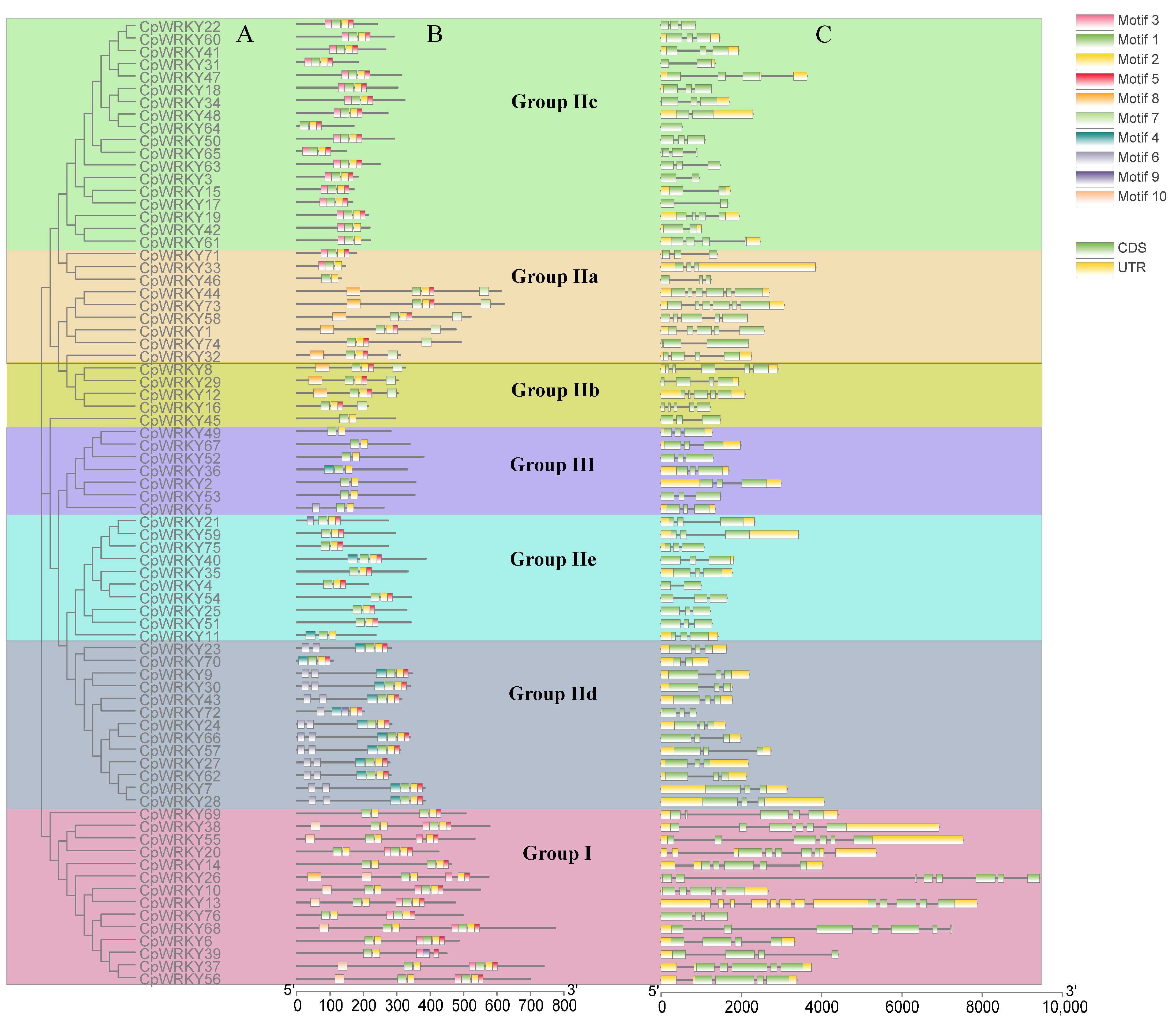
To deepen the comprehension of CpWRKY gene structure through classification, an analysis of exon–intron structures and conserved motifs within these genes was conducted, followed by a phylogenetic clustering (Figure 3A). These classification outcomes aligned with those described in Section 2.1, showing that members within the same subgroup shared comparable gene structure characteristics. A total of 10 conserved motifs were identified and named as motifs 1–10. Details of the 10 putative motifs are presented in Figure S2 (Table S2). Motif 1 was found in most CpWRKY genes. Motifs 1 and 9 contained a WRKYGQK or WRKYGKK sequence. Group III only consisted of motifs 1, 2, 4, and 6. Most CpWRKY proteins within the same group or subgroup possessed similar motifs, and their coding sequences (CDSs) have similar numbers of introns (Figure 3B). The gene structure analysis revealed that each CpWRKY consisted of exons separated by variable numbers of introns. CpWRKY exon–intron structures were analyzed to obtain additional clues about the evolution of CpWRKY family members and their specific features. The introns in CpWRKY genes were variable in size and their number ranged from 0 to 11. Among the 76 CpWRKY genes identified, 8 had 8 exons, 43 had 3 exons, 7 had 4 exons, 11 had 5 exons, and 4 had 6 exons; in addition, CpWRKY64 had 1 exon, CpWRKY68 had 7 exons, and CpWRKY26 had 9 exons (Figure 3C).
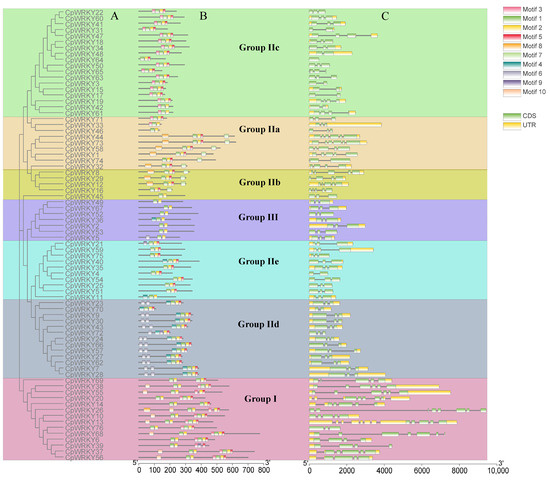
Figure 3.
Phylogenetic grouping, conserved protein patterns, and architecture of CpWRKY genes. (A): The sequence of WRKY domain from CpWRKY protein was used to create a phylogenetic tree. Various categories and subcategories are visualized in distinct hues. (B): Various shaded areas indicate the pattern. (C): Organization of CpWRKY genes. The untranslated 5′- and 3′-segments, exons, and introns are depicted by a green block, yellow block, and black line, respectively.
In terms of the number and position, the distribution patterns of exons and introns were similar within the same group. However, differences in the number of exons were observed within groups. For example, all genes in Groups IId and III had three exons, whereas most members of subfamily IIe exhibited three exons. However, CpWRKY4 in Group IIe had two exons. Additionally, the number of exons in Group I ranged from four to nine. These findings suggest significant structural variations among CpWRKYs, which may correspond to the functional diversification among closely related members.
2.3. Synteny and Collinearity Analyses
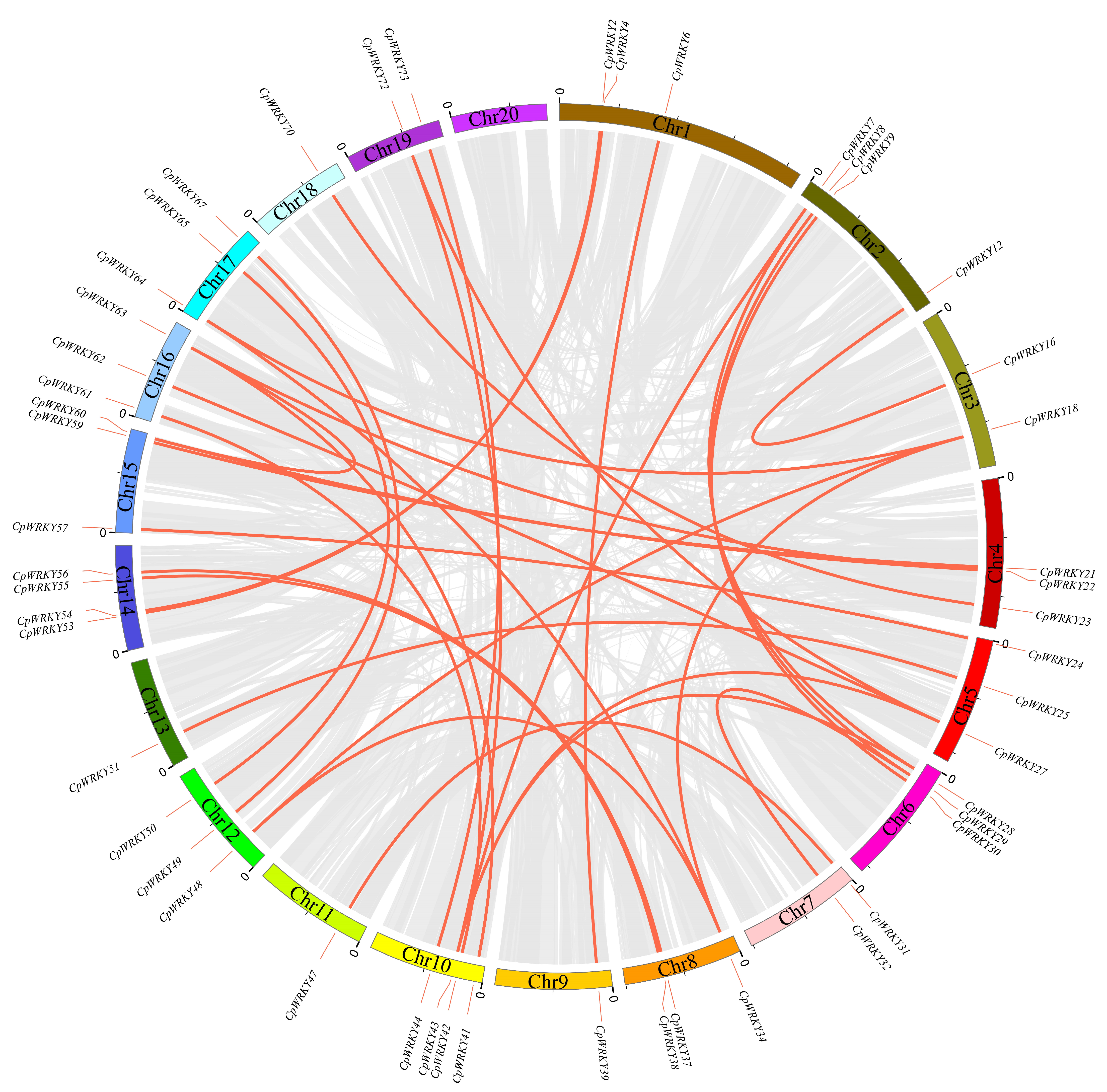
Investigating the segmental duplication events within the WRKY family of C. pepo involved an analysis of the synteny of CpWRKY genes using BLASTP and MCScanX. The results of the synteny analysis revealed an intricate colinear relationship among the members of the WRKY gene family in C. pepo. (Figure 4), suggesting that polyploidization is the main source of WRKY gene family expansion. WRKY members were not only identified on chromosome 20 (Table S3). Thirty-one segmental duplication events involving 49 WRKY genes were observed; however, no tandem duplication events were identified for these genes.
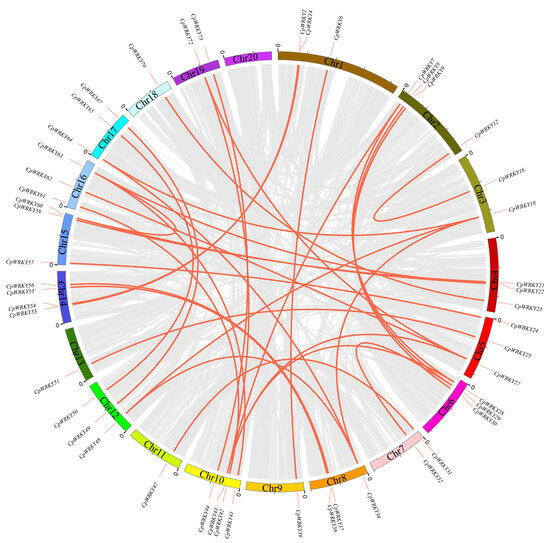
Figure 4.
Chromosomal distribution and synteny blocks of CpWRKY genes. Schematic representation of the chromosomal distribution and interchromosomal relationships of CpWRKY genes. Gray lines indicate all synteny blocks in the C. pepo genome, and red lines indicate segmental duplicated WRKY gene pairs.
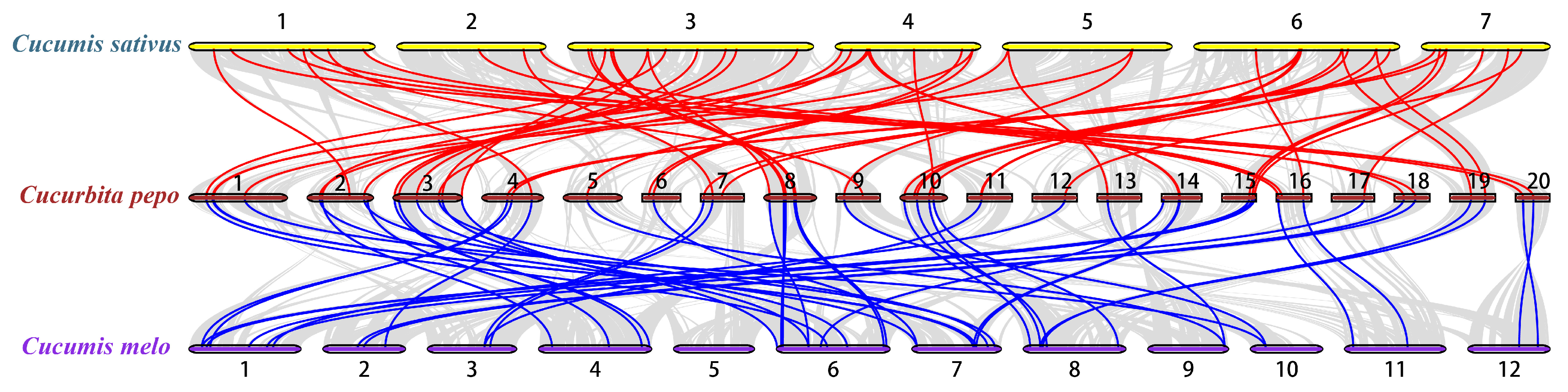
To explore the phylogenetic mechanism of the CpWRKY family, we constructed a homologous map of C. pepo using cucumbers and melons, which belong to the same cucurbit family (Figure 5, Table S4). In total, 54 WRKY collinear gene pairs were identified in C. pepo and cucumber, followed by 49 pairs in C. pepo and melon, indicating that the evolutionary relationship between C. pepo and cucumber is more conservative than that between C. pepo and melon.
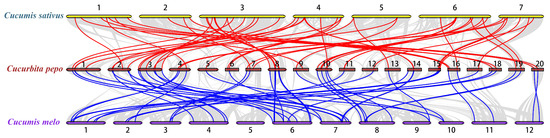
Figure 5.
Collinearity analysis of WRKY gene families was conducted between Cucurbita pepo and representative species. The red line represents the collinearity of the WRKY gene family between C. pepo and Cucumis sativus, while the blue line indicates the collinearity between C. pepo and Cucumis melo. Chromosome numbers are denoted by the numbers on the lines, with other collinearities shown by gray lines.
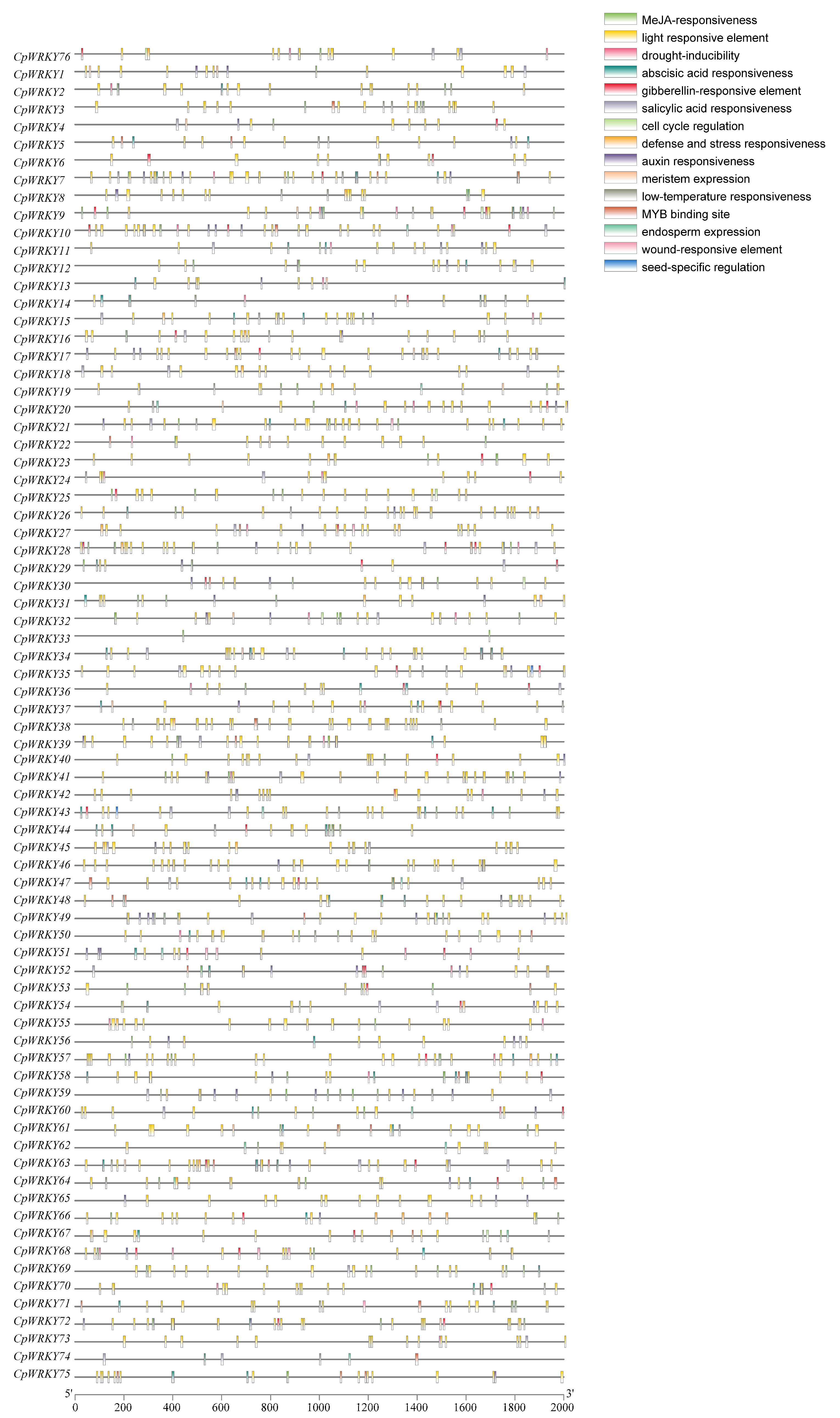
2.4. Analysis of Cis-Acting Elements in the Promoters of CpWRKYs
The cis-acting elements present in the promoters of CpWRKYs were identified and analyzed using PlantCARE. These elements, as shown in Figure 6, primarily pertain to stress responses (such as drought, wound, and low-temperature responsiveness), hormone responses (including abscisic acid, methyl jasmonate, and gibberellin responsiveness), growth and development processes (such as meristem expression, cell cycle regulation, and endosperm expression), and binding sites. Among these, the most frequently observed cis-acting elements were those related to light responsiveness and abscisic acid responsiveness, with 1124 and 233 elements, respectively. Moreover, a significant proportion of these elements were associated with growth and development, indicating the potential pivotal role of CpWRKYs in plant growth and development, as these elements were widespread and abundantly distributed in the promoters of nearly all CpWRKYs.
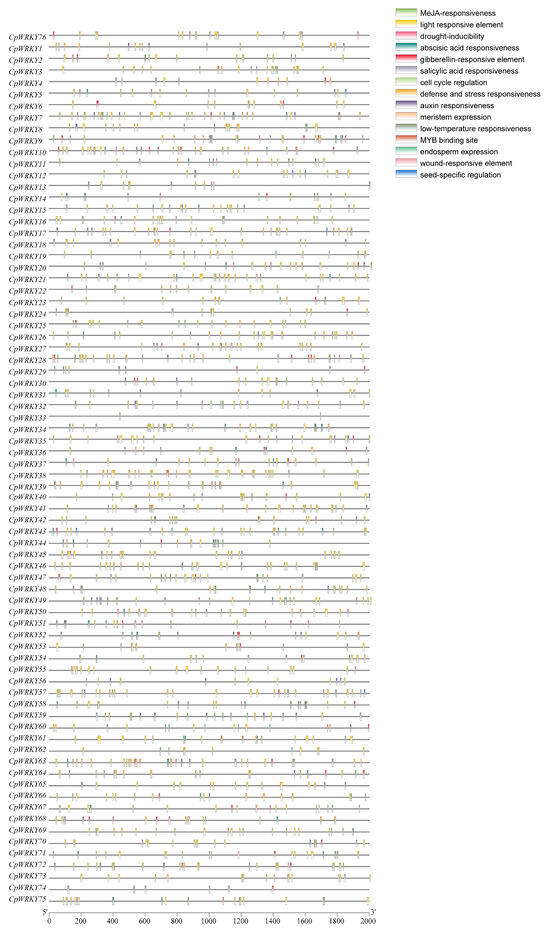
Figure 6.
An analysis of cis-acting elements in CpWRKY promoters was conducted. Diverse colored boxes symbolize various cis-regulatory elements.
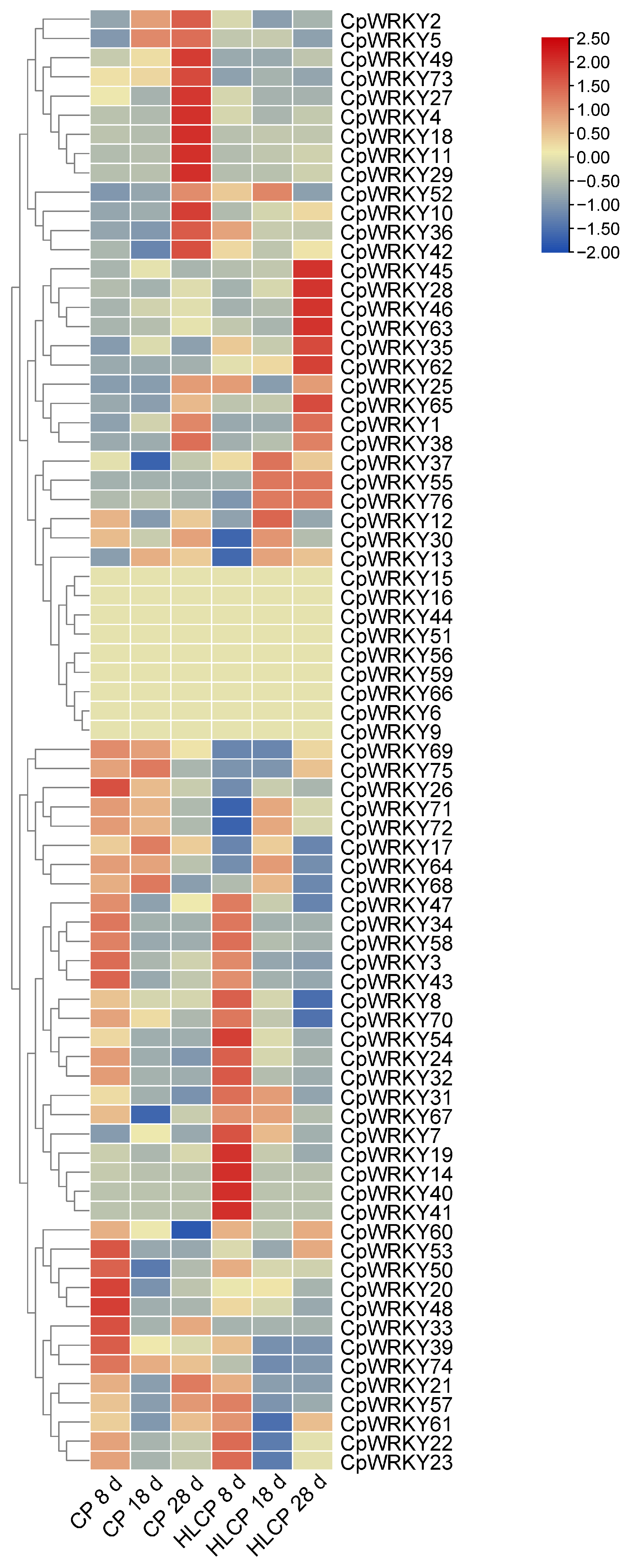
2.5. Expression Analysis of WRKY TFs of Seed Coat Development in C. pepo
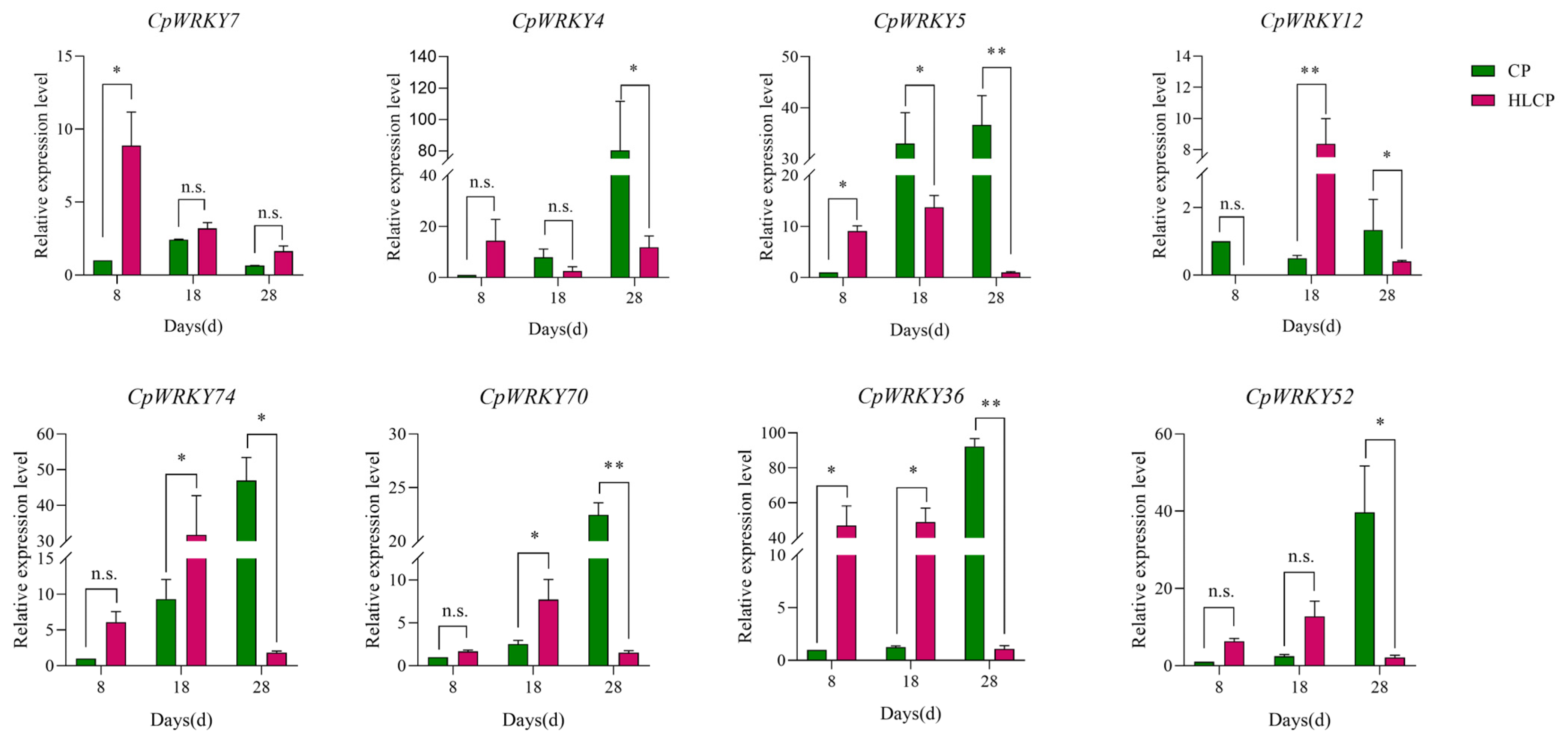
The published RNA-seq data set was used to analyze the relative expression levels of related genes in CpWRKYs during various seed coat development stages of C.pepo [28]. The analysis identified nine genes that were unexpressed in CP and HLCP seed coats at 8d, 18d, and 28d post-pollination. Among these genes, CpWRKY56 and CpWRKY6 were categorized in GroupI, while the remaining seven genes were placed in GroupII (CpWRKY15, CpWRKY16, CpWRKY44, CpWRKY51, CpWRKY59, CpWRKY66, and CpWRKY9). The expression of CpWRKY2 and CpWRKY5 genes showed significant differences in up-regulation between 18d and 28d post-pollination in CP. Furthermore, three genes exhibited significant variation in expression between 18d and 28d post-pollination in HLCP. There was a distinct contrast in the expression levels of 25 CpWRKY genes in CP after 28 days post-pollination, whereas 16 CpWRKY genes showed varied expression in HLCP (Figure 7 and Table S5). From Table S5 FPKM values, eight highly expressed genes were selected for qRT-PCR analysis. The expression of these genes varied between CP and HLCP across different seed coat development stages. Specifically, 8 days post-pollination, seven genes showed strong expression in HLCP: CpWRKY4, CpWRKY5, CpWRKY7, CpWRKY36, CpWRKY52, CpWRKY70, and CpWRKY74. Notably, CpWRKY7 and CpWRKY36 exhibited significantly higher expression in HLCP than in CP (p < 0.05). CpWRKY4 and CpWRKY5 displayed high expression in CP after 18 days post-pollination, with CpWRKY5 showing notably higher expression in CP compared to HLCP. By 28 days post-pollination, the expression levels of CpWRKY5 were significantly increased in CP compared to HLCP (p < 0.01; Figure 8). However, since seed coat lignification primarily occurs between 18 and 28 days after pollination, it is hypothesized that the gene CpWRKY5 plays a crucial role in lignin synthesis in the seed coat [28].
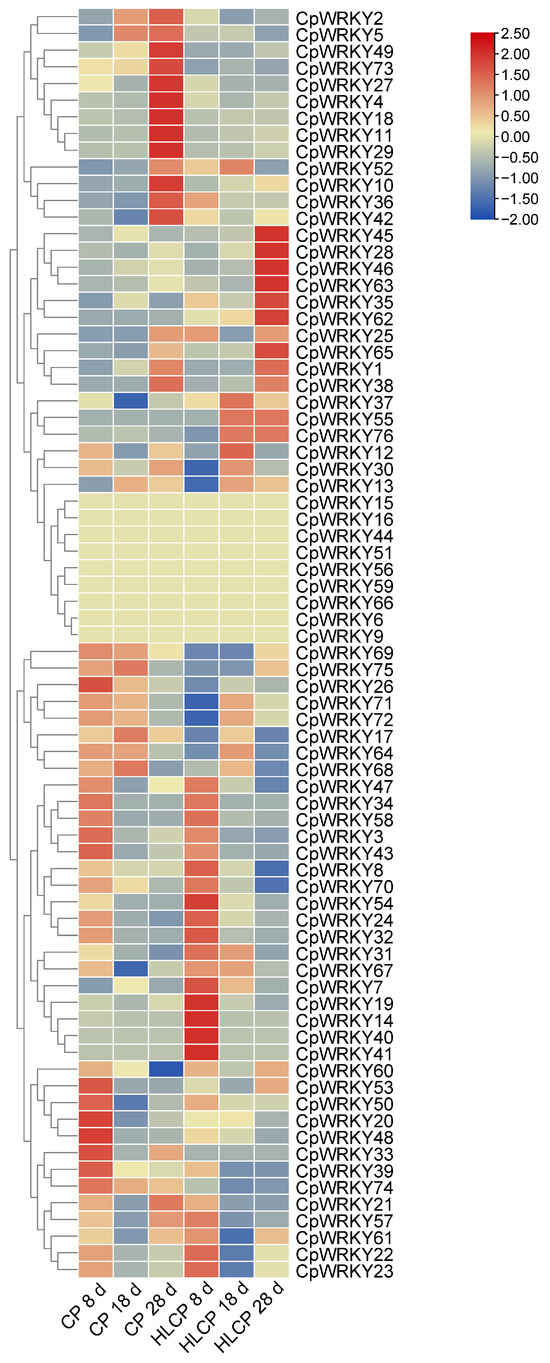
Figure 7.
Cluster analysis of the WRKY gene expression profiles during seed coat development after pollination at different time points in C. pepo. CP: Hulled Cucurbita pepo; HLCP: hull-less Cucurbita pepo.
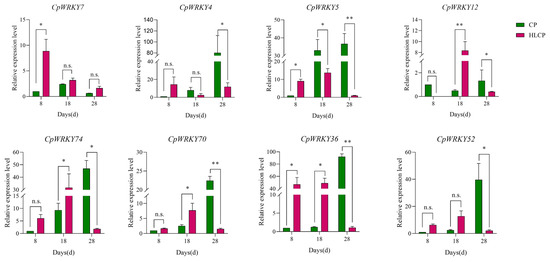
Figure 8.
Expression pattern of CpWRKY genes during C. pepo seed coat development. CP: Hulled C. pepo; HLCP: hull-less C. pepo. n.s. represents p-value > 0.05. * represents significant (p-value < 0.05); ** represents highly significant difference (p-value < 0.01).
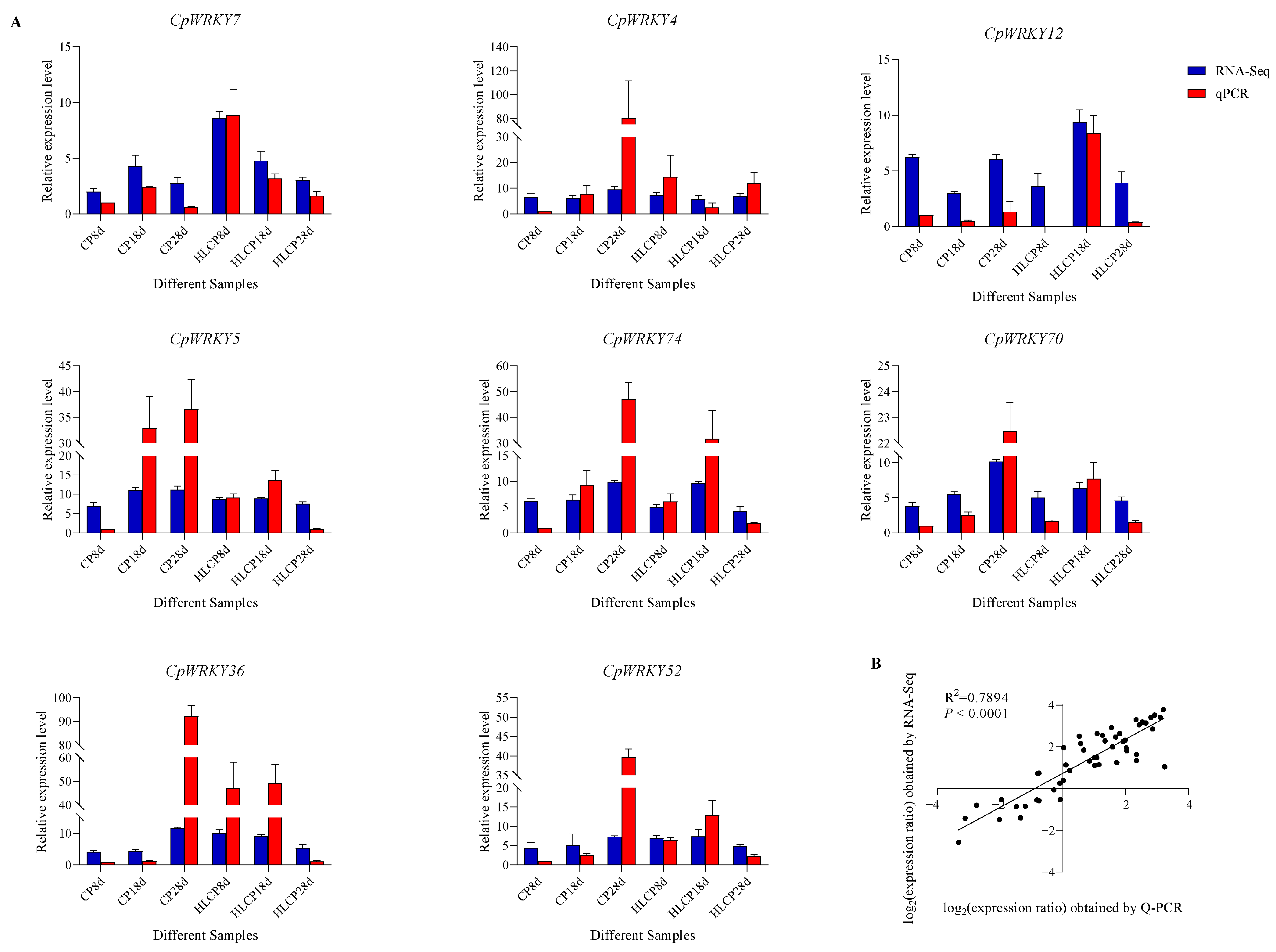
To verify the consistency between RNA-Seq data and RT-qPCR results, log2FPKM was used to represent the expression level of genes in RNA-Seq. Figure 9 illustrates that the relative expression levels obtained through RNA-Seq analysis align with those obtained through qRT-PCR analysis (Figure 9A), with a correlation coefficient of 0.7894 (p < 0.0001; Figure 9B).

Figure 9.
Comparison of RNA-Seq expression levels and qRT-PCR expression levels. (A) The expression levels of eight CpWRKY genes were measured using qRT-PCR and RNA-Seq FPKM analysis. The blue bar represents the relative expression level of RNA-Seq, shown as the log2 FPKM value. The relative gene expression of genes analyzed using qRT-PCR is represented by means of an orange histogram. (B) Correlation analysis of log2 value between RNA-Seq and qRT-PCR values.
2.6. CpWRKY5 Positively Regulates Lignin Synthesis in Transgenic Tobacco
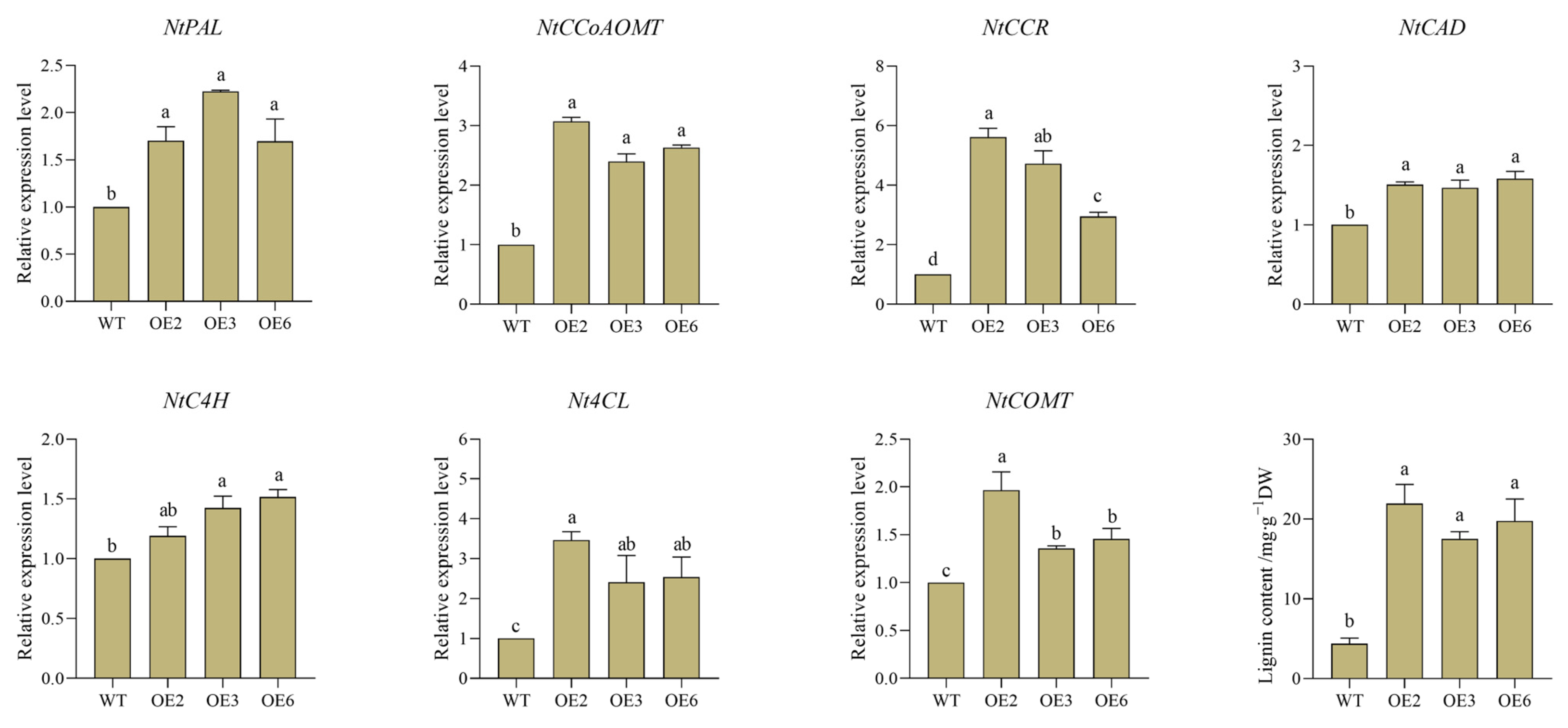
The expression level of the CpWRKY5 gene was higher in CP, with a consistent coding sequence region in both CP and HLCP (Figure S3). To clone the 786 bp CpWRKY5 gene’s coding sequence region, cDNA from CP was used as a template, resulting in the successful construction and transformation of the pCAMBIA2300-CpWRKY5-GFP overexpression vector into tobacco (Figure S4). Three representative overexpression lines (CpWRKY5-OE2, CpWRKY5-OE3, and CpWRKY5-OE6) were selected for experiments to determine the lignin content and the expression of related genes (Figure 10, Table S6). Phenylalanine ammonialyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumarate-CoA ligase (4CL) were closely related to the synthesis of total lignin content. The RT-qPCR results showed that the expression levels of PAL, C4H, and 4CL were 1.2~3.5 times higher in the transgenic lines OE2, OE3, and OE6 compared to the WT (p < 0.05). Cinnamyl alcohol dehydrogenase (CAD) and caffeoyl-CoA O-methyltransferase (CCoA-OMT) are crucial catalytic enzymes in the final stages of lignin biosynthesis. The expression levels of these enzymes were observed to notably increase by 15~30% in the transgenic lines (p < 0.05). Furthermore, lignin content in transgenic lines was measured, and it was discovered that, following CpWRKY5 overexpression, lignin concentration in overexpressing tobacco lines was considerably higher than that in WT (p < 0.05). These results indicated that CpWRKY5 overexpression enhanced the expression of lignin synthesis genes and the accumulation of lignin content in tobacco, and CpWRKY5 served as a positive regulator of lignin synthesis in the plant.
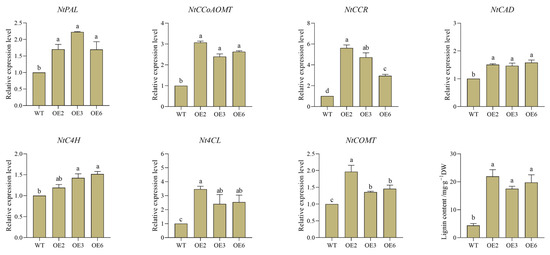
Figure 10.
CpWRKY5 overexpression in transgenic tobacco enhanced the lignin content and the expression of related genes. Lowercase letters indicate significant differences among different strains (p-value < 0.05).
3. Discussion
WRKY TFs represent one of the largest TF families in plants, with multifaceted roles in plant growth and development [18]. The advancement of transgenic technology and genomics [22] has enabled the identification of the WRKY TF family in soybean [29], maize [30], cotton [31], cucumber [17], watermelon [20], and other species, which is of considerable significance when explaining the action mechanism of WRKY TFs in plants. Based on the characteristics of WRKY conserved motifs and zinc finger structures, Eulgem et al. classified WRKY genes in A. thaliana into groups I, II, and III. Using the same classification model [18], Chen et al. performed a cluster analysis of the cucumber WRKY gene family. In the present study, 76 CpWRKY family members were identified based on the C. pepo genome sequences [17]. The number of WRKY genes in C. pepo is higher than those in the same cucurbit plants, such as cucumber, watermelon, and melon [32,33].
Rinerson et al. proposed four major WRKY TF lineages in flowering plants, namely Group I + IIc, Group IIa + IIb, Group IId + IIe, and Group III, which accurately reflect the WRKY family evolution [34]. Based on the characteristics of the WRKY conserved domain and zinc finger structure, along with the phylogenetic relationship of WRKYs with Arabidopsis and cucumber genes, as well as the gene structure, amino acid sequence, and conserved domain, a total of 76 CpWRKY proteins were found to exhibit similarities with typical WRKY family proteins in other species. Within the CpWRKY family, only members of Groups IIe and IId (or Groups I and IIc) in the CpWRKY family were divided into two sub-branches, each involving the same branch. Group IIb, Group III, and Group IId + IIe were merged into the same clade. Group IIa is an independent branch and is believed to have directly evolved from a single-domain algae WRKY gene, independent of other Group I-derived lineages [34].
Domain loss appears to be common in monocots such as rice and maize, and the loss of the WRKY domain is among the divergent forces of WRKY gene family expansion [35,36,37]. In C. pepo, the C-terminal of Group I CpWKRY55 lacked a conserved heptapeptide sequence, and the CpWRKY39 C-terminal demonstrated the loss of the zinc finger structure. The heptapeptide motif WRKYGQK (or WRKYGKK) and the zinc finger motif are believed to be necessary for the high-affinity binding of WRKY TFs with the homologous cis-acting W-box element (TTGACC/T) [18]. Therefore, the heptapeptide motif mutation and the loss of the zinc finger motif may affect the normal CpWRKY–target gene interaction. The binding characteristics and functions of these two CpWRKY proteins need to be further explored. Whole-genome duplications, segmental duplications, and tandem duplications are crucial for gene family expansion [30]. Among 49 CpWRKY genes, 31 segmental duplication events were observed, but no tandem duplication events were noted. Therefore, the main driving force for WRKY gene expansion in C. pepo may be fragment replication.
Lignin, the major component of plant cell walls, is regarded as a crucial factor affecting seed coat formation. Its formation and accumulation are regulated by WRKY genes [38,39]. The WRKY family is also involved in seed coat development and lignin synthesis in Arabidopsis and pomegranate [40,41]. Based on transcriptome sequencing, Xue et al. reported that WRKY TFs were involved in seed coat formation in C. pepo [28]. Liping Zhang et al. found that transgenic Arabidopsis plants that overexpress CcWRKY25 have an increased lignin content, resulting in larger plants with stronger stems [42]. It is important to note that lignin synthesis is a complex process, influenced by multiple genes and transcription factors [43]. Feng Wen et al. found that the heterologous overexpression of Akebia trifoliata WRKY12 in tobacco resulted in suppressed lignin synthesis in key enzyme genes [44]. CpWRKY5 overexpression resulted in the simultaneous upregulation of multiple genes encoding lignin synthesis, leading to increased lignin accumulation. Our preliminary findings suggest that CpWRKY5 may play a role in regulating lignin synthesis; however, further investigation is required to elucidate the molecular mechanisms involved.
4. Materials and Methods
4.1. Plant Materials
Two seed varieties, namely 04LAg-26-2 (hulled C. Pepo, CP) and 04LAg-26-28 (hull-less C. Pepo, HLCP), were provided by Wuwei Golden Apple Co., Ltd. (Wuwei, China), After sowing 100% of the germinated CP and HLCP seeds in nutrient soil and vermiculite in a 2:1 volume ratio, the pots were placed in a growth chamber with a 12 h photoperiod, relative humidity of 50%, and a temperature of 25 °C [45]. Following strict self-pollination, CP and HLCP fruits were collected at 8, 18, and 28 days after pollination. The seeds of these fruits were dissected with a blade on an aseptic operation table. Finally, the seed coats were collected and frozen in liquid nitrogen and refrigerated at −80 °C [28].
The SR tobacco seeds were rinsed with sterile water, disinfected with 75% ethanol and sodium hypochlorite solution for 1 and 15 min, respectively, rinsed with sterile water 3–5 times, and dried with sterile filter paper. The dried seeds were inoculated on MS medium (pH 5.8). After 4 weeks, sterile tender leaves of the plant were collected. Their edges and main veins were removed and cut into 0.5 × 0.5 cm small pieces, which were then used as explants.
4.2. Identification and Characterization of Putative WRKY Genes in C. pepo
Genome and annotation files of C. pepo were downloaded from The CuGenDB (http://cucurbitgenomics.org/ftp/genome/Cucurbita_pepo, accessed on 20 June 2022) [46]. The hidden Markov model files (PF03106) of WRKY TFs downloaded from the pfam database (http://pfam.xfam.org/, accessed on 21 June 2022) were used to search all WRKY TFs. The hmmer3.0 software was used to search for C. pepo protein sequences (E ≤ 1.2 × 10−28), and the obtained results were deduplicated using SMART (http://smart.embl-heidelberg.de/, accessed on 21 June 2022) and NCBI-CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi/, accessed on 22 June 2022) databases for further identification and screening. Finally, the CpWRKY family protein sequences were obtained [19]. The basic physicochemical properties of the obtained CpWRKY protein sequences were analyzed using ProtParam in the online tool Ex PASy (https://www.expasy.org/, accessed on 27 June 2022). The subcellular localization of these proteins was predicted and analyzed using the online software Plant-mPLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/plant/, accessed on 27 June 2022).
4.3. Sequence Alignment, Phylogenetic Tree, Classification, Gene Structure, and Conserved Motif Analyse
The CpWRKY gene family protein sequences obtained from C. pepo were aligned using ClustalW in software MEGA 7.0 and visually edited using DNAMAN to analyze the conserved WRKY core domain (60 amino acids). Using the neighbor-joining (NJ) method with 1000 bootstrap replications in MEGA 7.0 software, a phylogenetic tree of WRKY gene family members from C. pepo, Arabidopsis thaliana (At), and Cucumis sativus (Cs) was constructed [17].
The exon–intron structure of the CpWRKY gene family was analyzed using the Gene Structure Display Server (GSDS 2.0) (http://gsds.gao-lab.org/index.php/, accessed on 28 June 2022). The conserved motifs of CpWRKY gene family protein sequences were analyzed using the MEME (https://meme-suite.org/meme/, accessed on 28 June 2022) online tool. The parameter settings were as follows: number of motifs: 10; motif minimum width: 6; and motif maximum width: 100. Finally, the phylogenetic tree, gene structure, and conserved sequence were merged and visualized using TBtools v2.07 software.
4.4. Chromosomal Location, Gene Duplication, Collinearity Analyses, and Cis-Acting Elements in the Promoters of the CpWRKY Family Genes
Information about the specific location of 76 CpWRKYs on C. pepo chromosomes was obtained from the C. pepo genome database and visualized using Map Chart v1.0 software. Gene duplication events were analyzed using MCScanX v1.1 software. The diagram clarifying the collinearity of CpWRKYs with cucumber WRKY (CsWRKYs) and melon WRKY (CmWRKYs) was constructed using Circos tool [17,32]. The 2000 bp gene sequence upstream of the initiation codon (ATG) of CpWRKYs was identified as the gene promoter sequence in the C. pepo genome. Cis-acting elements in the promoter region were analyzed using the online PlantCARE tool and then visualized with TBtools [47].
4.5. Gene Expression Analysis
The Illumina RNA-seq data set (SRR15439210 to SRR15439227) was acquired from NCBI to investigate the expression patterns of CpWRKY genes in CP and HLCP at 8, 18, and 28 days post-pollination. Following data normalization using logarithm 2 (TPM + 1), TBtools software was utilized to generate a heatmap.
To examine changes in CpWRKY expression during seed development, RNA was extracted from the seed coat by using the Plant RNA Kit (Omega Bio-Tek, Guangzhou, China) according to the manufacturer’s instructions. RNA integrity and concentration were detected through 1% agarose gel electrophoresis and by using a microspectrophotometer, respectively. The first-strand cDNA was synthesized using the Revert Aid First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Beijing, China). Specific primers for the selected eight CpWRKY genes were designed using Premier 5 software (Table S7). qRT-PCR was performed using the SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biology, Changsha, China) in a QuanStudio 5 Real-Time PCR System (Thermo Fisher Scientific, Beijing, China). CpAct was used as an internal control gene [48]. For each reaction, three independent biological and technical replicates were used. The relative expression level of each gene was calculated using the 2−ΔΔCt method [49]. SPSS 21 software was used for statistical analysis.
4.6. Construction of Transgenic Nicotiana tabacum Overexpressing CpWRKY5
The recombinant CpWRKY5 overexpression vector was generated by cutting the pCAMBIA2300-GFP vector with the restriction endonuclease Sac I/Xba I. By using CP intermediate cDNA as a template, the full-length CDS sequence of CpWRKY5 was amplified through PCR. The sequence was recovered and cloned into the 35S-promoter-driven pCAMBIA2300-GFP vector. The recombinant plasmids were introduced into the Agrobacterium tumefaciens strain GV3101 using the heat shock method. To induce bud formation, the generated explants were co-cultured with the plasmid-containing A. tumefaciens GV3101 in MS + 6-BA medium for 3 days and transferred into the bud induction and differentiation medium (MS + 6-BA 1 mg/L + timentin 300 mg/L + Kan 100 mg/L, pH 5.8). When the resistant buds grew to 2 cm, they were transferred into the rooting medium (1/2MS + timentin 300 mg/L + NAA 0.1 mg/L + Kan 100 mg/L, pH 5.8) to induce root formation. After the roots were formed, the plants were transferred to pots containing nutrient soil and vermiculite (1:1) and grown at 25 °C with high humidity (>50%) under a 16 h light/8 h dark photoperiod. After the T1 generation grew out of the leaves, genomic DNA was extracted from the collected seeds using the TransDirect Plant Tissue PCR Kit (TransGen Biotech, Beijing, China). The positive plants and the gene expression were detected through PCR and qRT-PCR, respectively. NtHSC70-1 was used as an internal control (Table S7) [50].
4.7. Determination of Lignin Content and Related Gene Expression
Primers of lignin-biosynthesis-related genes expressed in tobacco leaves during maturation (Table S7), as specified by Song et al., were used [51]. Lignin content was measured following the protocol outlined by Bruce, using a Solarbio lignin content detection kit (Solarbio, Beijing, China), with some adaptations [52]. To determine the expression of lignin synthesis-related genes in wild-type and transgenic tobacco leaves grown for approximately 4 weeks, qRT-PCR was performed using the SYBR Green Premix Pro Taq HS qPCR Kit (Precision Biology, Changsha, China) in the Quan Studio 5 Real-Time PCR System (Thermo Fisher Scientific, Inc., USA). The gene NtHSC70-1 was used as an internal reference [50]. The experiments were carried out in triplicate, and the mean standard deviation was calculated. The relative expression level of each gene was calculated using the 2−ΔΔCt method [49]. Statistical analysis was conducted using SPSS 21 software, utilizing a one-way ANOVA followed by a Tukey–Kramer post hoc test (p ≤ 0.05) to determine statistically significant differences. GraphPad Prism 9.0 software was employed for generating graphs.
5. Conclusions
In this study, 76 CpWRKYs were isolated and identified from the whole C. pepo genome. Subsequently, we systematically and comprehensively analyzed CpWRKYs, including their structure, phylogeny, conserved domains, chromosomal location, and duplication events, through bioinformatics analyses. The functions of the selected eight CpWRKYs were verified based on the transcriptome data. The construction of CpWRKY5-overexpressing tobacco verified the role of CpWRKY5 as a positive regulator of lignin content and the expression of related genes. These results add to our knowledge of the CpWRKY gene family structure and the gene’s evolution in C. pepo, providing valuable insights into the mechanism underlying lignin synthesis in the seed coat in C. pepo. To build a framework for the molecular process of lignin synthesis, we will subsequently overexpress the candidate CpWRKY5 in C. pepo and methodically investigate the regulation mechanism of CpWRKY5 on lignin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25084177/s1.
Author Contributions
Conceptualization, F.T. and B.X.; methodology, J.C. and X.L.; data curation, J.C. and F.T.; writing—original draft preparation, J.C.; writing—review and editing, F.T. and Y.X.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31760577).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its Supplementary Materials, published online. All genome sequences and genome annotation files of Cucurbita pepo, Cucumis sativus, and Cucumis melo were obtained from The CuGenDB (http://cucurbitgenomics.org/ftp/genome/Cucurbita_pepo, accessed on 20 June 2022), and the published WRKY sequences of A. thaliana were acquired from the e TAIR database (http://www.arabidopsis.org/, accessed on 20 June 2022). The Cucurbita pepo seed coat transcriptome sequencing data in this study were obtained from a previous report (https://www.ncbi.nlm.nih.gov/sra/?term=SRR15439210/, accessed on 20 June 2022).
Acknowledgments
We thank Wuwei Golden Apple Co., Ltd., Wuwei City, Gansu Province, China for providing the two test varieties of C. pepo. We also want to thank F.T. and Y.X. for their help in the bioinformatic analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Decker, D.S. Origin(s), evolution, and systematic of Cucurbita pepo (Cucurbitaceae). Econ. Bot. 1988, 42, 4–15. [Google Scholar] [CrossRef]
- Cvetkovic, D.; Stanojevic, L.; Zvezdanovic, J.; Stanojevic, J.; Savic, D.; Karabegovic, I.; Danilovic, B. Pumpkin fruit (Cucurbita pepo L.) as a source of phytochemicals useful in food and pharmaceutical industries. J. Food Meas. Charact. 2021, 15, 4596–4607. [Google Scholar] [CrossRef]
- Teppner, H. Cucurbita pepo (Cucurbitaceae)—History, seed coat types, thin coated seeds and their genetics. Phyton 2000, 40, 1–42. [Google Scholar]
- Bezold, T.N.; Mathews, D.; Loy, J.B.; Minocha, S.C. Molecular analysis of the hull-less seed trait in pumpkin: Expression profles of genes related to seed coat development11. Seed Sci. Res. 2005, 3, 205–217. [Google Scholar] [CrossRef]
- Makni, M.; Fetoui, H.; Gargouri, N.K.; Garouri, E.M.; Zeghal, N. Antidiabetic effect of flax and pumpkin seed mixture powder: Effect on hyperlipidemia and antioxidant status in alloxan diabetic rats. J. Diabetes Complicat. 2011, 25, 339–345. [Google Scholar] [CrossRef]
- Bahadori, M.H.; Azari, Z.; Zaminy, A.; Dabirian, S.; Mehrdad, S.M.; Kondori, B.J. Anti-proliferative and apoptotic effects of hull-less pumpkin extract on human papillary thyroid carcinoma cell line. Anat. Cell Biol. 2021, 54, 104–114. [Google Scholar] [CrossRef]
- Lelley, T.; Loy, B.L.; Murkovic, M. Hull-Less oil seed pumpkin. In Oil Crops, Handbook of Plant Breeding; Vollmann, J., Rajcan, I., Eds.; Springer: New York, NY, USA, 2009; pp. 469–492. [Google Scholar]
- Meru, G.; Fu, Y.Q.; Shrestha, S.; Michael, V.N.; Dorval, M.; Mainviel, R. Genomic Position and Markers Associated with the Hull-Less Seed Trait in Pumpkin. Plants 2022, 11, 1238. [Google Scholar] [CrossRef]
- Loy, J. Seed development in Cucurbita pepo: An overview with emphasis on hull-less seeded genotypes of pumpkin. Rep.-Cucurbit Genet. Coop. 2000, 23, 89–95. [Google Scholar]
- Zhou, X. A study on the breeding of naked kernel pumpkin and its genetic behavior. Acta Hortic. Sin. 1987, 2, 115–118. [Google Scholar]
- Zhang, Z. Studies on the heredity of some characters in seed shell-less of squash. Acta Agric. Boreali-Occident. Sin. 2004, 3, 93–96. [Google Scholar]
- Li, Z.; Qu, S.; Cui, C. Development of AFLP and SCAR markers linked to hull-less n gene in pumpkin (Cucurbita pepo L.). J. Northeast Agric. Univ. 2011, 42, 67–71. [Google Scholar]
- Murovec, J.; Draslar, K.; Bohanec, B. Detailed analysis of Cucurbita pepo seed coat types and structures with scanning electron microscopy. Botany 2012, 90, 1161–1169. [Google Scholar] [CrossRef]
- Chahal, G.K. A Single-Gene Mutation Changed the Architecture of Pumpkin Seed: A Review. J. Plant Growth Regul. 2022, 41, 113–118. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Chi, Y.; Yang, Y.; Zhou, Y.; Zhou, J.; Fan, B.; Yu, J.Q.; Chen, Z. Protein-protein interactions in the regulation of WRKY transcription factors. Mol. Plant 2013, 6, 287–300. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Han, J.; Lu, W.; Ren, Z. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Zhang, Z.; Quan, S.; Niu, J.; Guo, C.; Kang, C.; Liu, J.; Yuan, X. Genome-Wide Identification, Classification, Expression and Duplication Analysis of bZIP Family Genes in Juglans regia L. Int. J. Mol. Sci. 2022, 23, 5961. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e0191308. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, J.; Li, H.; Wei, F.; Zhang, Y.; Jiang, H.; Peng, X. Identification of the WRKY Gene Family and Characterization of Stress-Responsive Genes in Taraxacum kok-saghyz Rodin. Int. J. Mol. Sci. 2022, 23, 10270. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Tomo, Y.; et al. Solution structure of an Arabidopsis WRKY DNA binding domain. Plant Cell 2005, 17, 944–956. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, W.; Zhao, L.; Yu, Y.; Chen, S.; Tao, L.; Cheng, L.; Kang, Q.; Song, X.; Wu, J.; et al. Genome-wide identification and expression analysis of the WRKY transcription factor family in flax (Linum usitatissimum L.). BMC Genom. 2021, 22, 375. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kolevski, B.; Smyth, D.R. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 2022, 14, 1359–1375. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; Shadle, G.; Shen, H.; Barros-Rios, J.; Fresquet, C.S.; Wang, H.; Dixon, R.A. Combining enhanced biomass density with reduced lignin level for improved forage quality. Plant Biotechnol. J. 2016, 14, 895–904. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, Z.; Tao, F.; Zhou, J.; Xu, B. Transcriptomic Analysis Reveal the Molecular Mechanisms of Seed Coat Development in Cucurbita pepo L. Front. Plant Sci. 2022, 13, 772685. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Chi, Y.; Fan, B.; Chen, Z. Characterization of Soybean WRKY Gene Family and Identification of Soybean WRKY Genes that Promote Resistance to Soybean Cyst Nematode. Sci. Rep. 2017, 7, 17804. [Google Scholar] [CrossRef]
- Hu, W.; Ren, Q.; Chen, Y.; Xu, G.; Qian, Y. Genome-wide identification and analysis of WRKY gene family in maize provide insights into regulatory network in response to abiotic stresses. BMC Plant Biol. 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Guo, Q.; Xu, P.; Gong, Y.; Shu, H.; Yang, Y.; Ni, W.; Zhang, X.; Shen, X. Transcriptome-wide identification of salt-responsive members of the WRKY gene family in Gossypium aridum. PLoS ONE 2015, 10, e0126148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Wei, B.Q. Identification of WRKY gene family and their expression analysis under low-temperature stress in melon (Cucumis melo). Chin. J. Agric. Biotechol. 2020, 28, 1761–1775. [Google Scholar]
- Ferriol, M.; Picó, B.; Nuez, F. Genetic diversity of a germplasm collection of Cucurbita pepo using SRAP and AFLP markers. TAG. Theoretical and applied genetics. Theor. Appl. Genet. 2003, 107, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Rinerson, C.I.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Rushton, P.J. The evolution of WRKY transcription factors. BMC Plant Biol. 2015, 15, 66. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Hao, Z.; Lu, Y.; Xue, G.; Cao, Z.; Qu, H.; Cheng, T.; Shi, J.; Chen, J. Gibberellin Oxidase Gene Family in L. chinense: Genome-Wide Identification and Gene Expression Analysis. Int. J. Mol. Sci. 2021, 22, 7167. [Google Scholar] [CrossRef]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef]
- Cao, P.; Liu, X.; Guo, J.; Chen, Y.; Li, S.; Wang, C.; Min, Y. Genome-Wide Analysis of Dynamin Gene Family in cassava (Manihot esculenta Crantz) and Transcriptional Regulation of Family Members ARC5 in Hormonal Treatments. Int. J. Mol. Sci. 2019, 20, 5094. [Google Scholar] [CrossRef]
- Diao, W.P.; Snyder, J.C.; Wang, S.B.; Liu, J.B.; Pan, B.G.; Guo, G.J.; Wei, G. Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Capsicum annuum L. Front. Plant Sci. 2016, 7, 211. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Qu, F.; Ma, G.; Tian, Y.; Wen, L. Cloning and expression analysis of key genes for lignin synthesis in Cucurbita pepo L. Braz. J. Bot. 2022, 45, 909–916. [Google Scholar]
- Wang, H.; Avci, U.; Nakashima, J.; Hahn, M.G.; Chen, F.; Dixon, R.A. Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proc. Natl. Acad. Sci. USA 2010, 107, 22338–22343. [Google Scholar] [CrossRef]
- Xue, H.; Cao, S.; Li, H.; Zhang, J.; Niu, J.; Chen, L.; Zhang, F.; Zhao, D. De novo transcriptome assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.). PLoS ONE 2017, 12, e0178809. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Zhang, W.; Shu, H.; Sun, P.; Huang, C.; Deng, Q.; Wang, Z.; Cheng, S. Genome-Wide Identification of WRKY Gene Family and Functional Characterization of CcWRKY25 in Capsicum chinense. Int. J. Mol. Sci. 2023, 24, 11389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guan, Y.; Zhang, Z.; Song, A.; Chen, S.; Jiang, J.; Chen, F. CmMYB8 encodes an R2R3 MYB transcription factor which represses lignin and flavonoid synthesis in chrysanthemum. Plant Physiol. Biochem. 2020, 149, 217–224. [Google Scholar] [CrossRef]
- Wen, F.; Wu, X.; Zhang, L.; Xiao, J.; Li, T.; Jia, M. Molecular Cloning and Characterization of WRKY12, A Pathogen Induced WRKY Transcription Factor from Akebia trifoliata. Genes 2023, 14, 1015. [Google Scholar] [CrossRef]
- Wang, S.S.; Chen, K.; Zhang, J.Y.; Wang, J.Q.; Li, H.S.; Yang, X.Y.; Shi, Q.H. Genome-wide characterization of MATE family members in Cucumis melo L. and their expression profiles in response to abiotic and biotic stress. Hortic. Plant J. 2022, 8, 474–488. [Google Scholar] [CrossRef]
- Montero-Pau, J.; Blanca, J.; Bombarely, A.; Ziarsolo, P.; Esteras, C.; Martí-Gómez, C.; Ferriol, M.; Gómez, P.; Jamilena, M.; Mueller, L.; et al. De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol. J. 2018, 16, 1161–1171. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Fang, H.; Wen, Y.; Wang, Y.; Zhang, J.; Peng, C.; Long, J. Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Neolamarckia cadamba. Int. J. Mol. Sci. 2023, 24, 7537. [Google Scholar] [CrossRef]
- Obrero, A.; Die, J.V.; Román, B.; Gómez, P.; Nadal, S.; González-Verdejo, C.I. Selection of reference genes for gene expression studies in zucchini (Cucurbita pepo) using qPCR. J. Agric. Food Chem. 2011, 59, 5402–5411. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.F.; Lu, P.; Meng, L.J.; Cao, P.J. Identification and validation of tobacco reference genes for qRT-PCR based on microarray data. Tobacco Sci. Technol. 2015, 48, 1–6. [Google Scholar]
- Song, Z.; Wang, D.; Gao, Y.; Li, C.; Jiang, H.; Zhu, X.; Zhang, H. Changes of lignin biosynthesis in tobacco leaves during maturation. Funct. Plant Biol. 2021, 48, 624–633. [Google Scholar] [CrossRef]
- Bruce, R.J.; West, C.A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).