Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi
Abstract
1. Introduction
2. Results
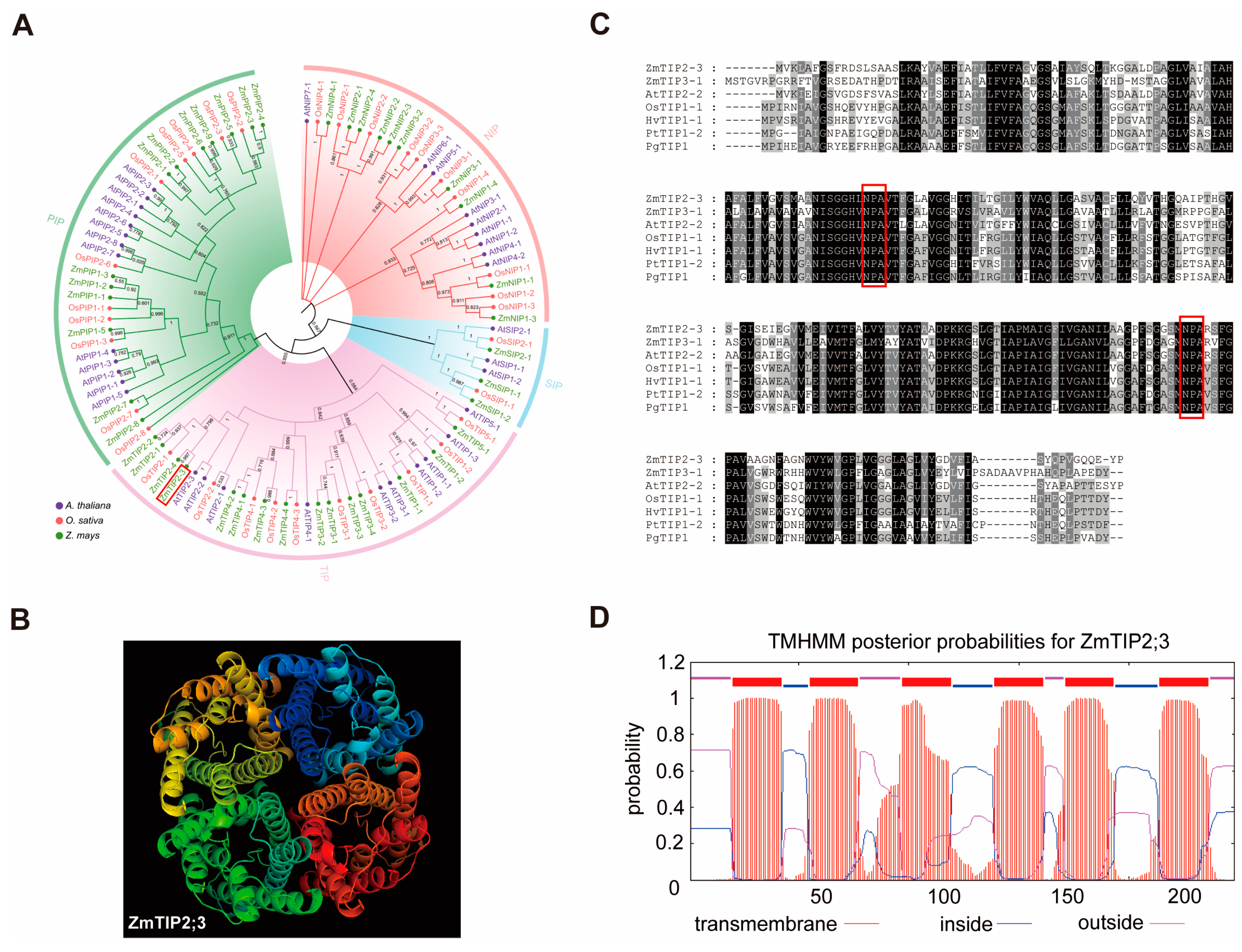
2.1. Bioinformatics Analysis of the ZmTIP2;3 Gene
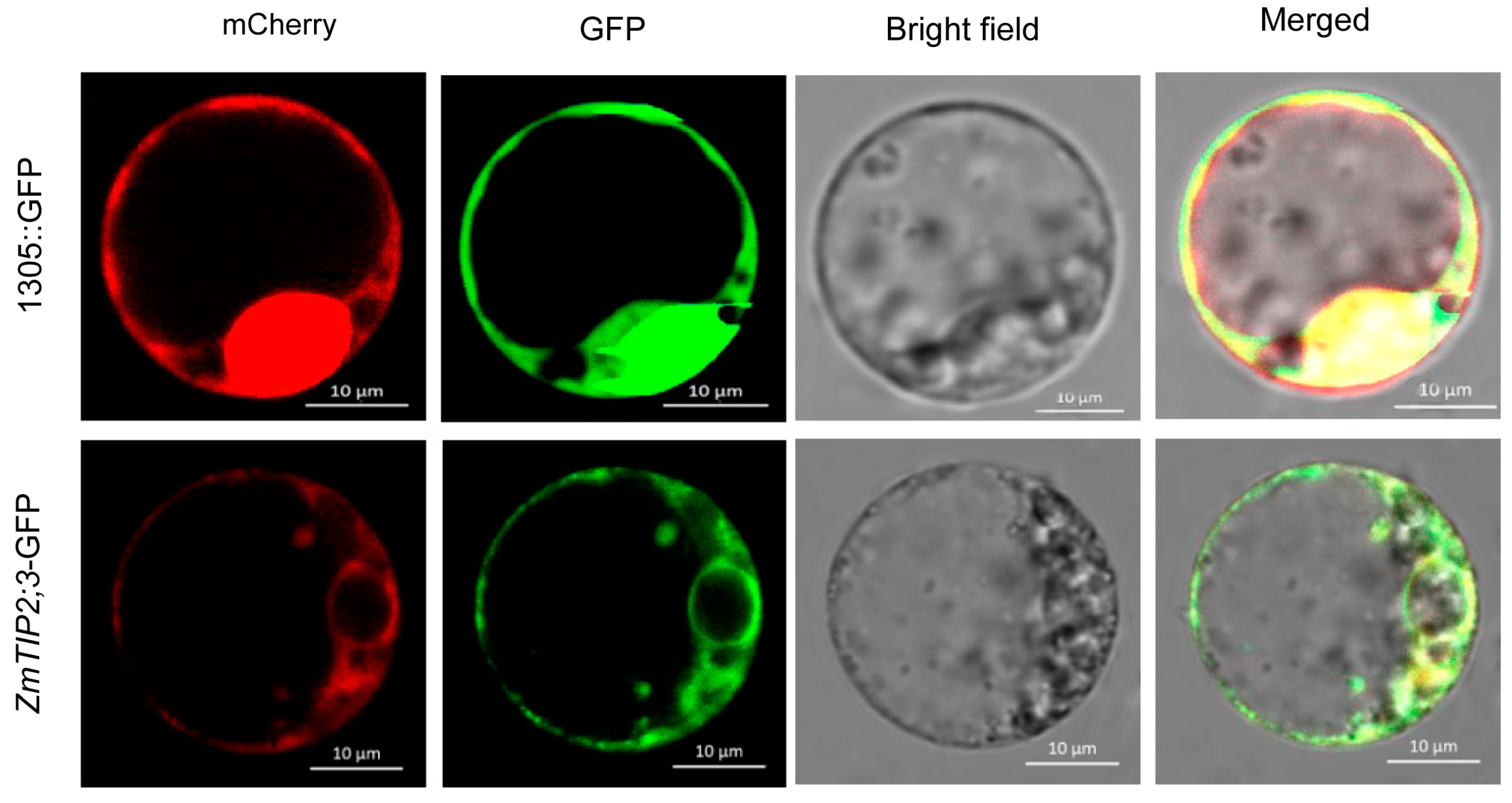
2.2. Subcellular Localization of ZmTIP2;3
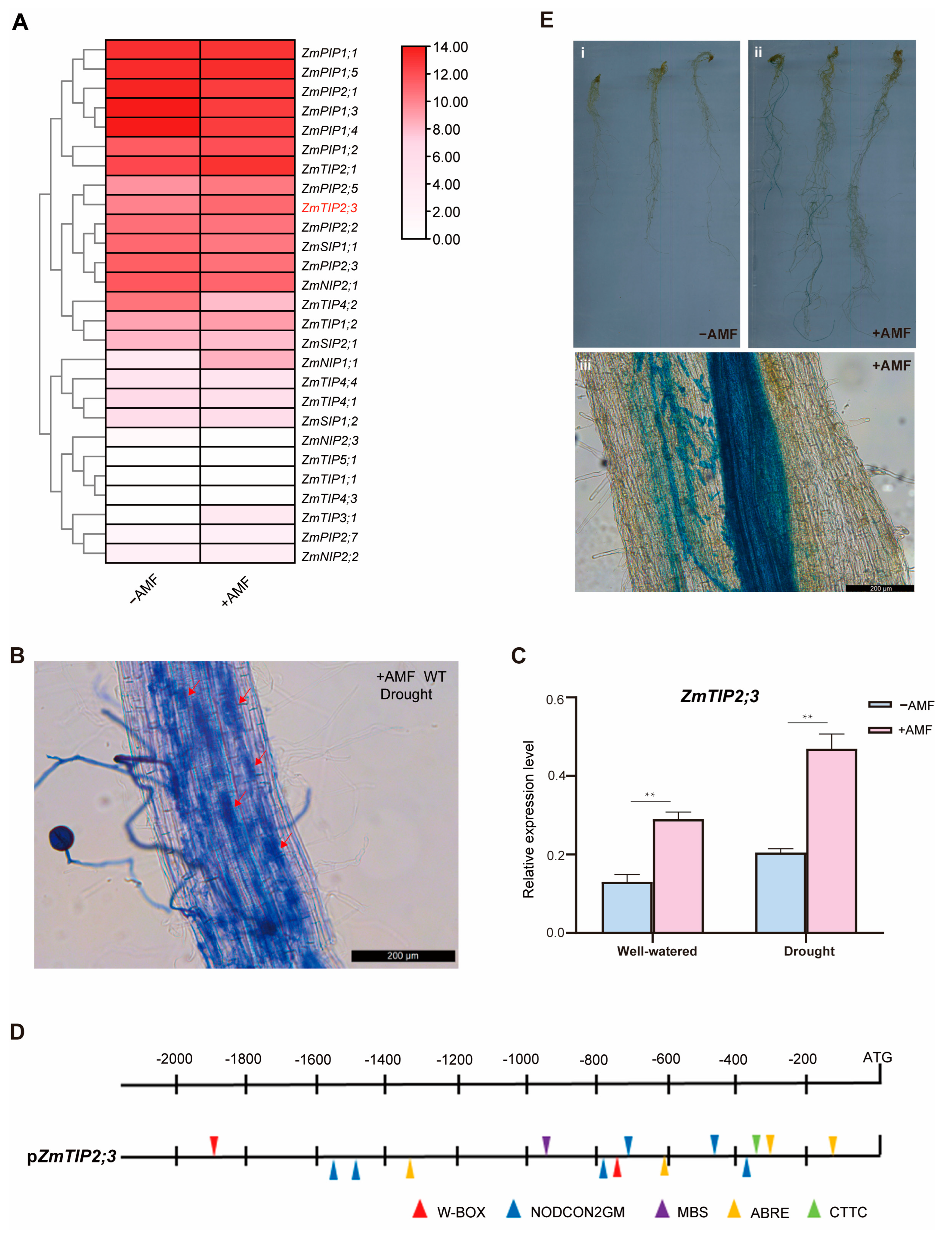
2.3. Mycorrhizal-Inducible Expression of the ZmTIP2;3 Gene
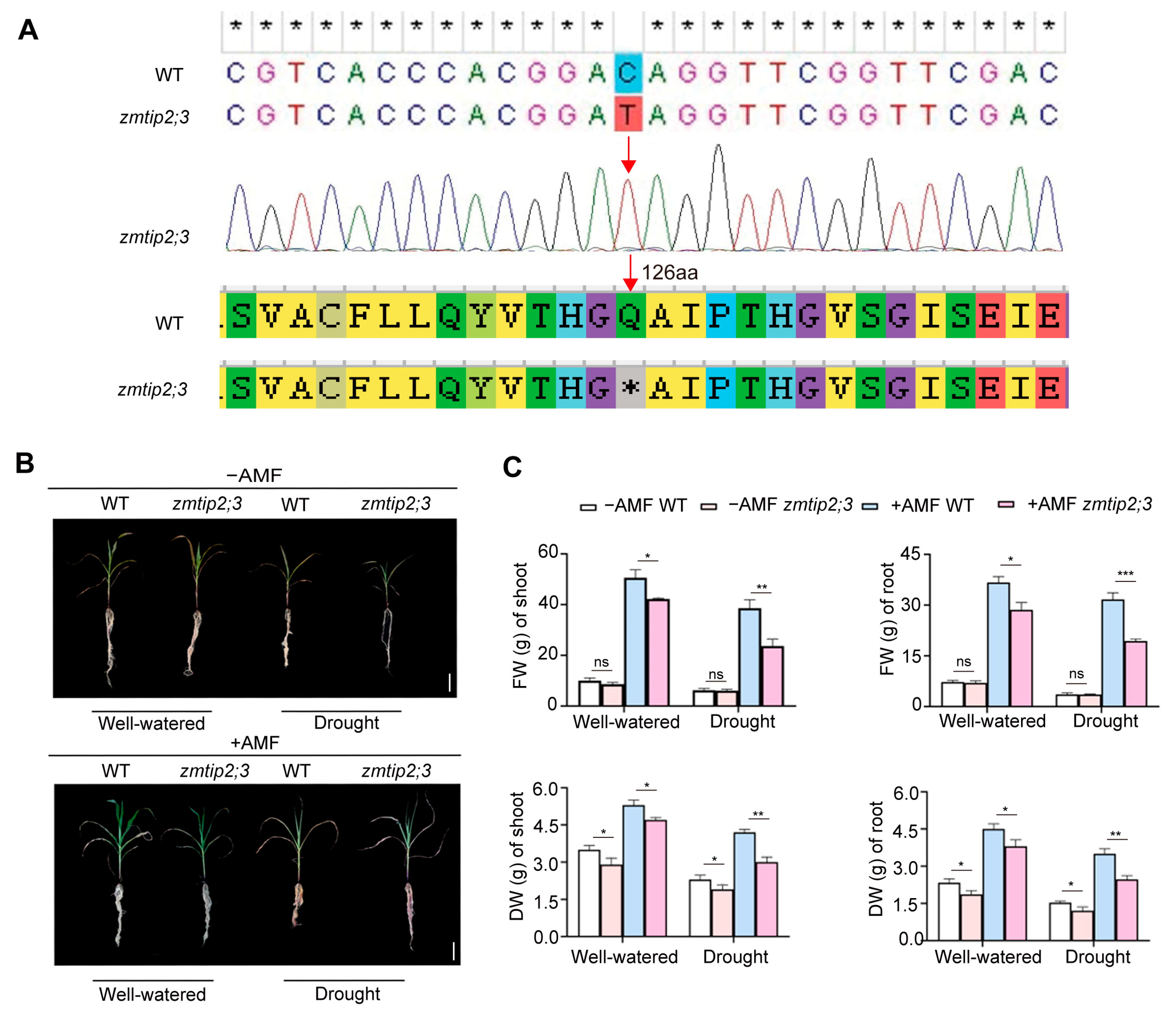
2.4. ZmTIP2;3 Promoted Drought Resistance during AM Fungi Symbiosis in Maize
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growing Environment Used in the Experiment
4.1.1. Plant Materials
4.1.2. Plant Growth and AMF Inoculation
4.2. RNA Isolation and qRT-PCR
4.3. Bioinformatics Analysis
4.4. Vector Construction
4.5. Subcellular Localization in Maize Protoplasts
4.6. Induced Transformation of Hairy Roots of L. japonicus
4.7. Detection of Mycorrhizal Infection Rate
4.8. Determination of Physiological and Biochemical Indicators
4.9. Determination of Relative Water Content of Leaves
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadam, N.N.; Tamilselvan, A.; Lawas, L.M.F.; Quinones, C.; Bahuguna, R.N.; Thomson, M.J. Genetic Control of Plasticity in Root Morphology and Anatomy of Rice in Response to Water Deficit. Plant Physiol. 2017, 174, 2302–2315. [Google Scholar] [CrossRef]
- Mouradi, M.; Farissi, M.; Bouizgaren, A.; Makoudi, B.; Kabbadj, A.; Very, A.-A.; Sentenac, H.; Qaddourya, A.; Ghoulam, C. Effects of water deficit on growth, nodulation and physiological and biochemical processes in Medicago sativa-rhizobia symbiotic association. Arid Land Res. Manag. 2016, 30, 193–208. [Google Scholar] [CrossRef]
- Kim, W.; Iizumi, T.; Nishimori, M. Global Patterns of Crop Production Losses Associated with Droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.T.; Lei, Q.; Feng, C.; Zheng, X.; Zhou, F.; Li, L.; Liu, X.; Wang, Z.; Kong, J. Ectopically expressing MdPIP1;3, an aquaporin gene, increased fruit size and enhanced drought tolerance of transgenic tomatoes. BMC Plant Biol. 2017, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly Regulated Channels Controlling Plant Water Relations~1. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef]
- Verdoucq, L.; Rodrigues, O.; Martinière, A.; Luu, D.T.; Maurel, C. Plant aquaporins on the move: Reversible phosphorylation, lateral motion and cycling. Curr. Opin. Plant Biol. 2014, 22, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef]
- Martre, P.; Morillon, R.; Barrieu, F.; North, G.B.; Nobel, P.S.; Chrispeels, M.J. Plasma Membrane Aquaporins Play a Significant Role during Recovery from Water Deficit. Plant Physiol. 2002, 130, 2101–2110. [Google Scholar] [CrossRef]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef]
- Mahdieh, M.; Mostajeran, A.; Horie, T.; Katsuhara, M. Drought Stress Alters Water Relations and Expression of PIP-Type Aquaporin Genes in Nicotiana tabacum Plants. Plant Cell Physiol. 2008, 49, 801–813. [Google Scholar] [CrossRef]
- Wang, X.; Gao, F.; Bing, J.; Sun, W.; Feng, X.; Ma, X.; Zhou, Y.; Zhang, G. Overexpression of the Jojoba Aquaporin Gene, ScPIP1, Enhances Drought and Salt Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Su, H.; Wang, X.; Ren, Z.; Zhang, K.; Feng, T.; Zhang, M.; Li, Z.; Li, L.; Zhuang, J.; et al. Coronatine promotes maize water uptake by directly binding to the aquaporin ZmPIP2;5 and enhancing its activity. J. Integr. Plant Biol. 2023, 65, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Volpe, V.; Zanini, S.; Vallino, M.; Abbà, S.; Bonfante, P. A Rice GRAS Gene Has an Impact on the Success of Arbuscular Mycorrhizal Colonization. Am. J. Plant Sci. 2015, 6, 1905–1915. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Li, Y. Effects of Mycorrhizal Symbiosis on Growth Behavior and Carbohydrate Metabolism of Trifoliate Orange Under Different Substrate P Levels. J. Plant Growth Regul. 2015, 34, 499–508. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Smith, M.G.N. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Paszkowski, U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 2017, 39, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Xu, G. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 202000926. [Google Scholar] [CrossRef]
- Maurel, C.; Plassard, C. Aquaporins: For more than water at the plant-fungus interface? New Phytol. 2011, 190, 815–817. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant-Microbe Interact. MPMI 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef]
- Uehlein, N.; Fileschi, K.; Eckert, M.; Bienert, G.P.; Bertl, A.; Kaldenhoff, R. Arbuscular mycorrhizal symbiosis and plant aquaporin expression. Phytochemistry 2007, 68, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.R.; Maistriaux, L.C.; Chaumont, F. Toward understanding of the high number of plant aquaporin isoforms and multiple regulation mechanisms. Plant Sci. Int. J. Exp. Plant Biol. 2017, 264, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Pagliarani, C. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Abrahám, E.; Rigó, G.; Székely, G.; Nagy, R.; Koncz, C.; Szabados, L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003, 51, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [PubMed]
- Augé, R. Water relation, drought and VA mycorrhizal symbiosis. Mycorrhiza. Mycorrhiza 2001, 11, 3–42. [Google Scholar]
- Su, Y.; Liu, Z.; Sun, J.; Wu, C.; Li, Y.; Zhang, C.; Zhao, L. Genome-Wide Identification of Maize Aquaporin and Functional Analysis During Seed Germination and Seedling Establishment. Front. Plant Sci. 2022, 13, 831916. [Google Scholar] [CrossRef]
- Quigley, F.; Rosenberg, J.M.; Shachar-Hill, Y.; Bohnert, H.J. From genome to function: The Arabidopsis aquaporins. Genome Biol. 2002, 3, 1. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Reddy, P.S.; Rao, T.; Sharma, K.K.; Vadez, V. Genome-wide identification and characterization of the aquaporin gene family in Sorghum bicolor (L.). Plant Gene 2015, 1, 18–28. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Ali, Z.; Wang, C.B.; Xu, L.; Yi, J.X.; Xu, Z.L.; Liu, X.Q.; He, X.L.; Huang, Y.H.; Khan, I.A.; et al. Genome-wide sequence characterization and expression analysis of major intrinsic proteins in soybean (Glycine max L.). PLoS ONE 2013, 8, e56312. [Google Scholar] [CrossRef] [PubMed]
- Zézé, A.; Brou, Y.C.; Meddich, A.; Marty, F. Molecular identification of MIP genes expressed in the roots of an arbuscular mycorrhizal Trifolium alexandrium L. under water stress. Afr. J. Agric. Res. 2008, 3, 78–83. [Google Scholar]
- Li, T.; Hu, Y.-J.; Hao, Z.-P.; Li, H.; Wang, Y.-S.; Chen, B.-D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Nijjer, S.; Rogers, W.E.; Siemann, E. The effect of mycorrhizal inoculum on the growth of five native tree species and the invasive Chinese Tallow tree (Sapium sebiferum). Plant Cell Physiol. 2004, 56, 357–368. [Google Scholar]
- Özgönen, H.; Akgül, D.S.; Erkiliç, A.O. The effects of arbuscular mycorrhizal fungi on yield and stem rot caused by Sclerotium rolfsii Sacc. in peanut. Afr. J. Agric. Res. 2010, 5, 128–132. [Google Scholar]
- Khalvati, M.A.; Hu, Y.; Mozafar, A.; Schmidhalter, U. Quantification of Water Uptake by Arbuscular Mycorrhizal Hyphae and its Significance for Leaf Growth, Water Relations, and Gas Exchange of Barley Subjected to Drought Stress. Plant Biol. 2005, 7, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.-N.; Wu, Q.-S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Modulation of Aquaporin Genes by the Arbuscular Mycorrhizal Symbiosis in Relation to Osmotic Stress Tolerance. In Symbioses and Stress: Joint Ventures in Biology; Seckbach, J., Grube, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 357–374. [Google Scholar]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouahmane, L.; Duponnois, R.; Boumezzough, A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. Comptes Rendus Biol. 2016, 339, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Rejali, F.; Soleimani, M. Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci. Hortic. 2020, 261, 108923. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Zou, Y.-N.; Wu, Q.-S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef]
- He, F.; Sheng, M. Effects of Rhizophagus irregularis on Photosynthesis and Antioxidative Enzymatic System in Robinia pseudoacacia L. under Drought Stress. Front. Plant Sci. 2017, 2017, 224124. [Google Scholar] [CrossRef] [PubMed]
- Luu, D.T.; Maurel, C. Aquaporins in a challenging environment: Molecular gears for adjusting plant water status. Blackwell Sci. Ltd. 2005, 28, 85–96. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, X.; Zeevaart, J.A. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The Enigmatic LEA Proteins and Other Hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Xin, X.; Liping, S.; Jiaowen, P.; Xiangpei, K.; Maoying, Z.; Dequan, L. ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol. 2013, 6, 944–959. [Google Scholar]
- Yu, J.; Lai, Y.; Wu, X.; Wu, G.; Guo, C. Overexpression of OsEm1 encoding a group I LEA protein confers enhanced drought tolerance in rice. Biochem. Biophys. Res. Commun. 2016, 478, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Planchet, E.; Verdu, I.; Delahaie, J.; Cukier, C.; Girard, C.; Morère-Le Paven, M.C.; Limami, A.M. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicago truncatula. J. Exp. Bot. 2014, 65, 2161–2170. [Google Scholar] [CrossRef]
- Langeroodi, A.R.S.; Osipitan, O.A.; Radicetti, E.; Mancinelli, R. To what extent arbuscular mycorrhiza can protect chicory (Cichorium intybus L.) against drought stress. Sci. Hortic. 2020, 263, 109109. [Google Scholar] [CrossRef]
- Ren, A.; Zhu, Y.; Chen, Y.; Ren, H.; Xiong, Y. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Ren, W.; Yang, Q.; Chai, Z.; Chen, R.; Wang, L.; Zhao, J.; Lang, Z.; Wang, H.; et al. Gene-Indexed Mutations in Maize. Mol. Plant 2018, 11, 496–504. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef]
- Liao, D.; Chen, X.; Chen, A.; Wang, H.; Liu, J.; Liu, J.; Gu, M.; Sun, S.; Xu, G. The characterization of six auxin-induced tomato GH3 genes uncovers a member, SlGH3.4, strongly responsive to arbuscular mycorrhizal symbiosis. Plant Cell Physiol. 2015, 56, 674–687. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization by One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Estimation of Vesicular Arbuscular Mycorrhizal Infection Levels. Research for Methods Having a Functional Significance, Physiological and genetical aspects of mycorrhizae—Aspects physiologiques et genetiques des mycorhizes. In Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France, 1–5 July 1985. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Ni, Y.; Xie, K.; Li, Y.; Wu, W.; Shan, H.; Cheng, B.; Li, X. Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi. Int. J. Mol. Sci. 2024, 25, 4205. https://doi.org/10.3390/ijms25084205
Wang D, Ni Y, Xie K, Li Y, Wu W, Shan H, Cheng B, Li X. Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi. International Journal of Molecular Sciences. 2024; 25(8):4205. https://doi.org/10.3390/ijms25084205
Chicago/Turabian StyleWang, Deyin, Ying Ni, Kailing Xie, Yuanhao Li, Wenxiang Wu, Hanchen Shan, Beijiu Cheng, and Xiaoyu Li. 2024. "Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi" International Journal of Molecular Sciences 25, no. 8: 4205. https://doi.org/10.3390/ijms25084205
APA StyleWang, D., Ni, Y., Xie, K., Li, Y., Wu, W., Shan, H., Cheng, B., & Li, X. (2024). Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi. International Journal of Molecular Sciences, 25(8), 4205. https://doi.org/10.3390/ijms25084205





