Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases
Abstract
:1. Introduction
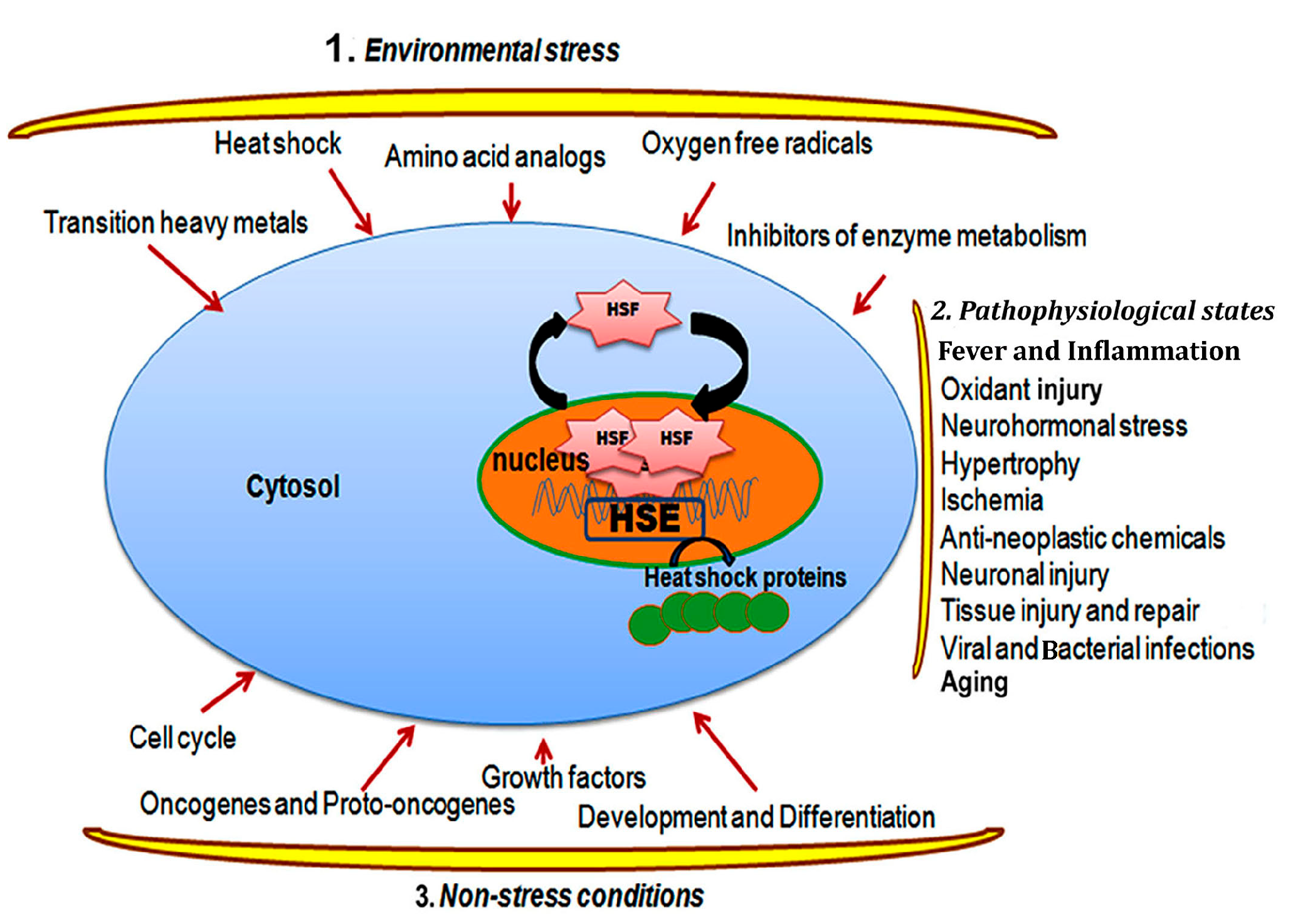
2. Stress Response and Heat Shock Factors (HSFs)
2.1. Heat Shock Response and Regulation
2.2. Heat Shock Transcription Factors and the Regulation of Heat Shock Response
| HSFs | Organism, and Homology | Oligomeric State, and Localization | Activators | Characterization | References |
|---|---|---|---|---|---|
| HSF1 | Humans, mice, and chickens, 92% homology | Monomer (70 kDa), trimer (178 kDa), cyto-nuclear | Heat, metals, amino acid analogs | Constitutive and inducible, phosphorylation and developmental | [2,34] |
| HSF2 | Humans, mice, and chickens, 92% homology | Dimer (127 kDa), trimer (202 kDa), cyto-nuclear | Hemin, embryogenesis | Activated during early blastocyst stage, limb buds, neuronal cells, and spermatogenesis | [2,20,35] |
| HSF3 | Chickens and birds | Dimer, cytosol and nuclear | Heat, metals | Interact with cMyb and G1/S transition in cell cycle | [2,10,36] |
| HSF4 (a/b) | Humans | Trimer, constitutive, nuclear | Development | Active during lens development | [2,37,38] |
2.3. Functional Significance of Heat Shock Response in Thermotolerance and Environmental Adaptation
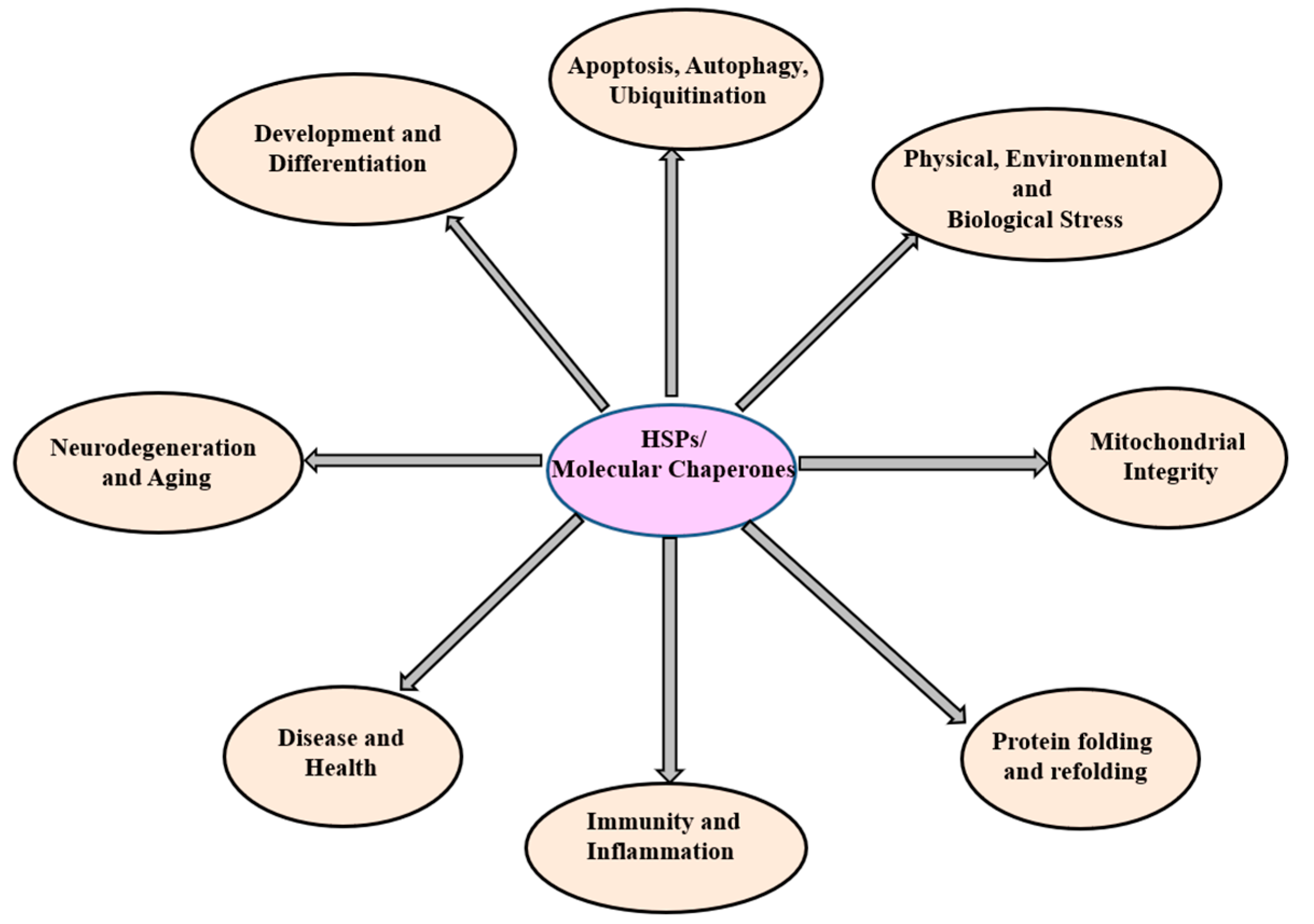
3. The Heat Shock Proteins (Molecular Chaperones)
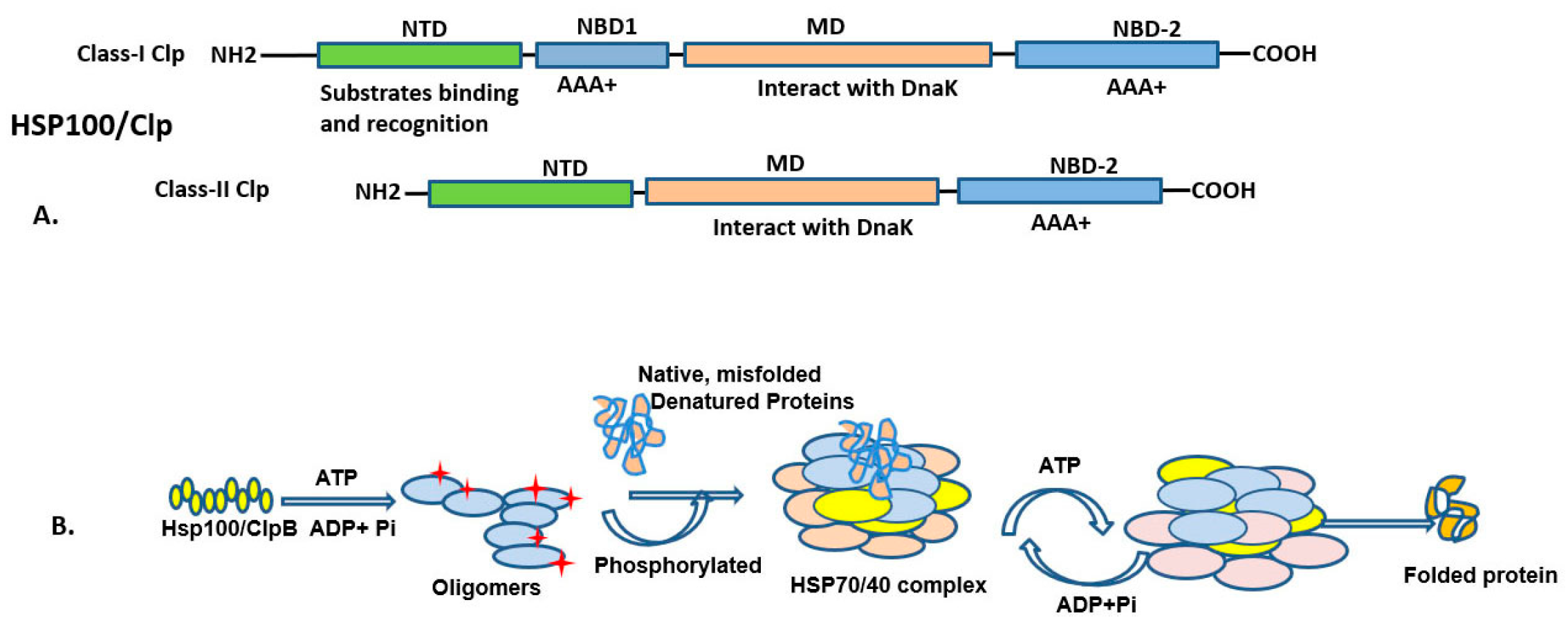
3.1. HSP100 (HSPH; Clp Family) and Functions
3.2. HSP90 (HSPC) and Functions
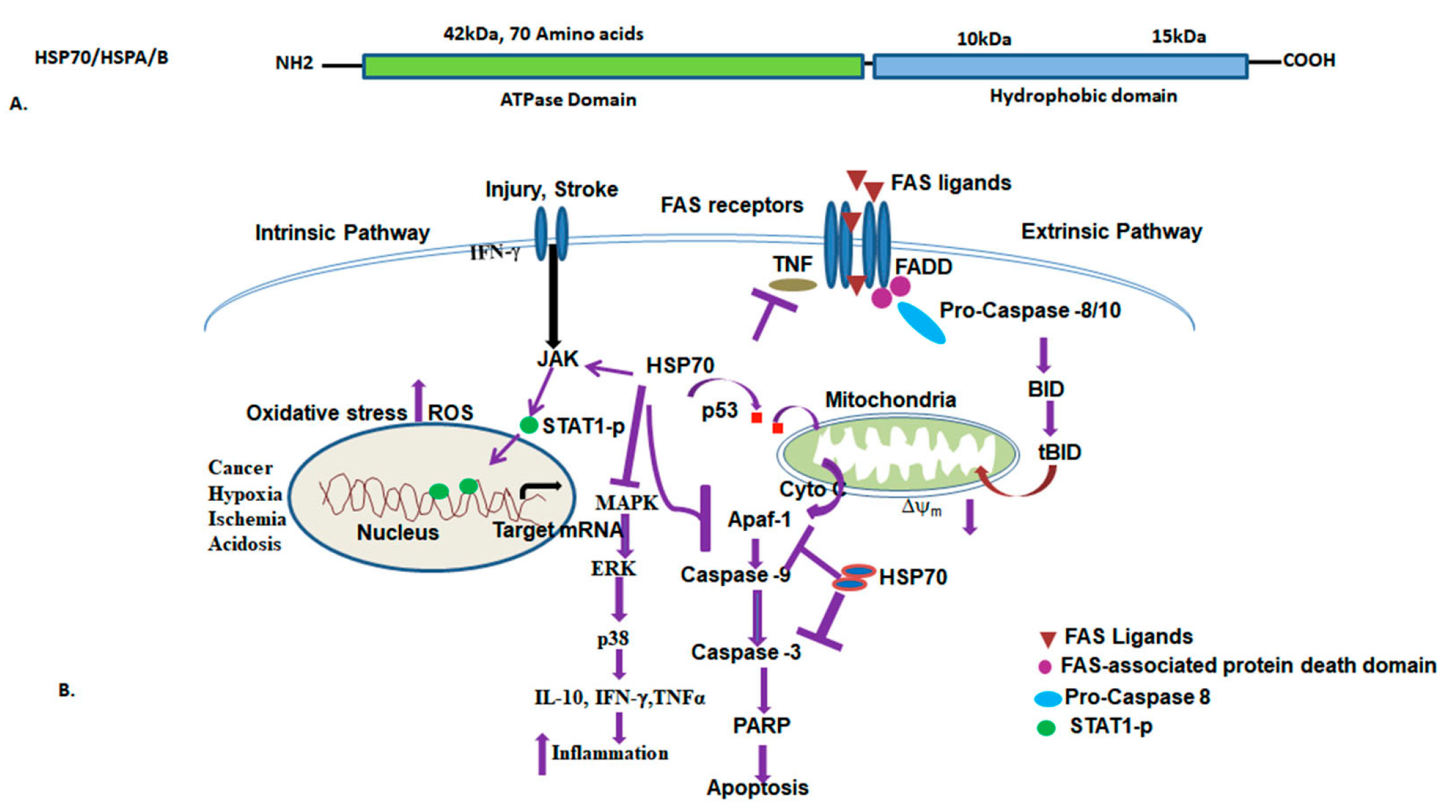
3.3. HSP70 (HSPA/B) and Functions
3.4. HSP60 (HSPD/E) GroEL/ES and Functions
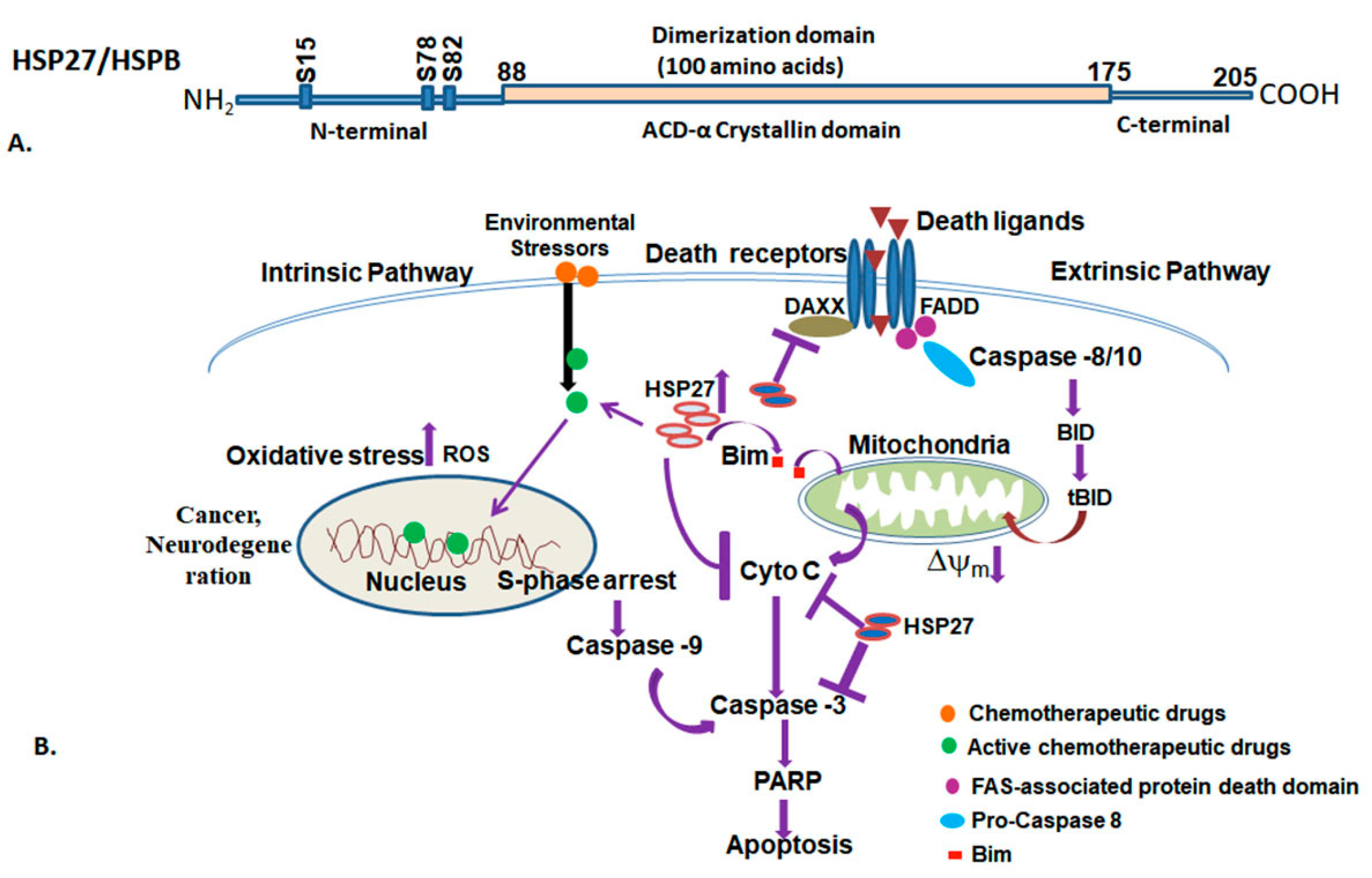
3.5. Small Heat Shock Proteins (HSPB)
3.6. Ubiquitin
| Diseases | Stress Proteins | Role of Stress Proteins | References |
|---|---|---|---|
| Cancers | HSP90 | Affecting client protein interaction | [107,108,127,128] |
| HSP70 | Promoting cell survival | [150,155,156,171,255] | |
| HSP60, | Interact with cytochrome-C and DAXX pro- and anti-apoptotic role | [220] | |
| sHSPs | Anchorage-independent growth, increase invasiveness | [256,257,258] | |
| Ubiquitin | Regulating ER stress, PERK-mediated UPS | [273,283] | |
| Neurodegeneration, dementia, Alzheimer’s disease, Parkinson’s disease | HSP100 | Binding with HSP70 and HSP40 and preventing aggregates | [105,107,108] |
| HSP90 | Cdc37 complex disruption, | [135,136] | |
| HSP70 | Mitochondrial integrity, oxidative stress | [170] | |
| HSP60 | Pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, binds to Aβ oligomers | [205,213] | |
| HSP27 | αβ-crystallin, α- synuclein | [263,264] | |
| Ubiquitin | Parkin, a ubiquitin E3 ligase, misfolded α-synuclein | [138,287] | |
| Auto-immune disease | HSP60 | HSP60 peptides, elicit cytotoxic T cell responses | [195,196,197,198] |
| HSP27 | Cellular stress, 1L-1β in LPS-treated monocytes | [216,240,241] | |
| Infectious diseases | HSP70 | Viral replication | [158,159,160,161] |
| HSP60 | Cell surface expression IL8 | [202] | |
| Inflammation Rheumatoid arthritis | HSP60 | Cytokine signaling processes and release | [206,207,208] |
| Cardiovascular disease | HSP70 | Insulin resistance and anti-inflammatory effect | [170] |
| HSP60 | TLR2 and TLR4 functions | [193] | |
| HSP27 | Desmin-linked | [253] | |
| Metabolic diseases Diabetes | HSP70 | Increases sensitivity to insulin | [165,166,167,171] |
| HSP60 | Modulates the TLRs and IGF-I receptor level, PI3-K/Akt activation | [196,197,198,199,200] | |
| Ubiquitin | IRE1, USP14-mediated regulation | [274,275] |
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schumann, W. Thermosensors in eubacteria: Role and evolution. J. Biosci. 2007, 32, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I.; Santoro, M.G. Stress–inducible responses and heat shock proteins: New pharmacologic targets for cytoprotection. Nat. Biotechnol. 1998, 16, 833–838. [Google Scholar] [CrossRef]
- Tissiéres, A.; Mitchell, H.K.; Tracy, U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J. Mol. Biol. 1974, 84, 389–398. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, S.L.; Meselson, M. Translation in vitro of Drosophila heat-shock messages. J. Mol. Biol. 1977, 117, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S. Regulation of protein synthesis during heat shock. Nature 1981, 293, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Molecular chaperones: Opening and closing the anfinsen cage. Curr. Biol. 1994, 4, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, T.; Heller, J.; Goldenberg, S.; Arad, Z. The heat shock response in congeneric land snails (Sphincterochila) from different habitats. Cell Stress Chaperones 2012, 17, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Östling, P.; Björk, J.K.; Roos-Mattjus, P.; Mezger, V.; Sistonen, L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J. Biol. Chem. 2007, 282, 7077–7086. [Google Scholar] [CrossRef] [PubMed]
- Kanei-Ishii, C.; Tanikawa, J.; Nakai, A.; Morimoto, R.I.; Ishii, S. Activation of heat shock transcription factor 3 by c-Myb in the absence of cellular stress. Science 1997, 277, 246–248. [Google Scholar] [CrossRef]
- Craig, E.A.; Gambill, B.D.; Nelson, R.J. Heat shock proteins: Molecular chaperones of protein biogenesis. Microbiol. Rev. 1993, 57, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Sõti, C.; Nagy, E.; Giricz, Z.; Vígh, L.; Csermely, P.; Ferdinandy, P. Heat shock proteins as emerging therapeutic targets. Br. J. Pharmacol. 2005, 146, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Sorger, P.K. Heat shock factor and the heat shock response. Cell 1991, 65, 363–366. [Google Scholar] [CrossRef]
- Clos, J.; Westwood, J.T.; Becker, P.B.; Wilson, S.; Lambert, K.; Wu, C. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell 1990, 63, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, T.J.; Gallo, G.J.; Sheldon, L.; Tempst, P.; Kingston, R.E. Isolation of a cDNA for HSF2: Evidence for two heat shock factor genes in humans. Proc. Natl. Acad. Sci. USA 1991, 88, 6911–6915. [Google Scholar] [CrossRef] [PubMed]
- Satyal, S.H.; Morimoto, R.I. Biochemical events in the activation and attenuation of the heat shock transcriptional response. J. Biosci. 1998, 23, 303–311. [Google Scholar] [CrossRef]
- Sarge, K.; Zimarino, V.; Holm, K.; Wu, C.; Morimoto, R. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 1991, 5, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Rabindran, S.K.; Giorgi, G.; Clos, J.; Wu, C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc. Natl. Acad. Sci. USA 1991, 88, 6906–6910. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Morimoto, R.I. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol. Cell. Biol. 1993, 13, 1983–1997. [Google Scholar] [PubMed]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Zhang, J.; Moskophidis, D.; Mivechi, N.F. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J. Cell. Biochem. 2002, 86, 376–393. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.R.; Xiao, X.; Shao, L.; Graves, K.; Benjamin, I.J. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 1998, 273, 7523–7528. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Kawazoe, Y.; Takeda, S.; Morimoto, R.I.; Nagata, K.; Nakai, A. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO J. 1998, 17, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Katsuki, K.; Izu, H.; Fujimoto, M.; Sugahara, K.; Yamada, S.-I.; Shinkai, Y.; Oka, Y.; Katoh, Y.; Nakai, A. Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperatures. Mol. Cell. Biol. 2003, 23, 5882–5895. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Eggers-Schumacher, G.; Wunderlich, M.; Schöffl, F. Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol. Genet. Genom. 2004, 271, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Giardina, C.; Lis, J.T. Dynamic protein-DNA architecture of a yeast heat shock promoter. Mol. Cell. Biol. 1995, 15, 2737–2744. [Google Scholar] [CrossRef]
- Kelley, P.M.; Schlesinger, M.J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell 1978, 15, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Hightower, L.E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J. Cell. Physiol. 1980, 102, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Senisterra, G.A.; Huntley, S.A.; Escaravage, M.; Sekhar, K.R.; Freeman, M.L.; Borrelli, M.; Lepock, J.R. Destabilization of the Ca2+-ATPase of sarcoplasmic reticulum by thiol-specific, heat shock inducers results in thermal denaturation at 37 °C. Biochemistry 1997, 36, 11002–11011. [Google Scholar] [CrossRef]
- McDuffee, A.T.; Senisterra, G.; Huntley, S.; Lepock, J.R.; Sekhar, K.R.; Meredith, M.J.; Borrelli, M.J.; Morrow, J.D.; Freeman, M.L. Proteins containing non-native disulfide bonds generated by oxidative stress can act as signals for the induction of the heat shock response. J. Cell. Physiol. 1997, 171, 143–151. [Google Scholar] [CrossRef]
- Liu, H.; Lightfoot, R.; Stevens, J.L. Activation of Heat Shock Factor by Alkylating Agents Is Triggered by Glutathione Depletion and Oxidation of Protein Thiols. J. Biol. Chem. 1996, 271, 4805–4812. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Borrelli, M.J.; Syed, K.; Senisterra, G.; Stafford, D.M.; Lepock, J.R. Characterization of a signal generated by oxidation of protein thiols that activates the heat shock transcription factor. J. Cell. Physiol. 1995, 164, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Latchman, D.S. Transcriptional modulation of heat-shock protein gene expression. Biochem. Res. Int. 2011, 2011, 238601. [Google Scholar] [CrossRef] [PubMed]
- Goodson, M.L.; Park-Sarge, O.-K.; Sarge, K.D. Tissue-dependent expression of heat shock factor 2 isoforms with distinct transcriptional activities. Mol. Cell. Biol. 1995, 15, 5288–5293. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, J.; Ichikawa-Iwata, E.; Kanei-Ishii, C.; Nakai, A.; Matsuzawa, S.-I.; Reed, J.C.; Ishii, S. p53 suppresses the c-Myb-induced activation of heat shock transcription factor 3. J. Biol. Chem. 2000, 275, 15578–15585. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Sasai, N.; Nagata, K.; Liu, X.-D.; Liu, P.C.; Thiele, D.J.; Nakai, A. The mammalian HSF4Gene generates both an activator and a repressor of heat shock genes by alternative splicing. J. Biol. Chem. 1999, 274, 27845–27856. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Izu, H.; Seki, K.; Fukuda, K.; Nishida, T.; Yamada, S.-I.; Kato, K.; Yonemura, S.; Inouye, S.; Nakai, A. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004, 23, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bharadwaj, S.; O’Carroll, R.; Ovsenek, N. HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol. Cell. Biol. 1998, 18, 4949–4960. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guettouche, T.; Fenna, M.; Boellmann, F.; Pratt, W.B.; Toft, D.O.; Smith, D.F.; Voellmy, R. Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J. Biol. Chem. 2001, 276, 45791–45799. [Google Scholar] [CrossRef]
- Thrower, J.S.; Hoffman, L.; Rechsteiner, M.; Pickart, C.M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000, 19, 94–102. [Google Scholar] [CrossRef]
- Ecsedy, J.A.; Michaelson, J.S.; Leder, P. Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity. Mol. Cell. Biol. 2003, 23, 950–960. [Google Scholar] [CrossRef]
- Chang, H.Y.; Nishitoh, H.; Yang, X.; Ichijo, H.; Baltimore, D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1998, 281, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Xavier, I.J.; Mercier, P.A.; McLoughlin, C.M.; Ali, A.; Woodgett, J.R.; Ovsenek, N. Glycogen synthase kinase 3β negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J. Biol. Chem. 2000, 275, 29147–29152. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, S.J.; Sangster, K.; McNally, S.J.; Harrison, E.M.; Ross, J.A.; Fearon, K.C.; Garden, O.J. De-repression of heat shock transcription factor-1 in interleukin-6-treated hepatocytes is mediated by downregulation of glycogen synthase kinase 3β and MAPK/ERK-1. Int. J. Mol. Med. 2007, 19, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Soncin, F.; Zhang, X.; Chu, B.; Wang, X.; Asea, A.; Stevenson, M.A.; Sacks, D.B.; Calderwood, S.K. Transcriptional activity and DNA binding of heat shock factor-1 involve phosphorylation on threonine 142 by CK2. Biochem. Biophys. Res. Commun. 2003, 303, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zuo, X.; Davis, A.A.; McMillan, D.R.; Curry, B.B.; Richardson, J.A.; Benjamin, I.J. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999, 18, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, C.; Stevenson, M.A.; Auron, P.E.; Calderwood, S.K. Heat shock factor 1 represses transcription of the IL-1β gene through physical interaction with the nuclear factor of interleukin 6. J. Biol. Chem. 2002, 277, 11802–11810. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Stevenson, M.A.; Auron, P.E.; Calderwood, S.K. Heat shock factor 1 represses Ras-induced transcriptional activation of the c-fos gene. J. Biol. Chem. 1997, 272, 26803–26806. [Google Scholar] [CrossRef]
- Reinke, H.; Saini, C.; Fleury-Olela, F.; Dibner, C.; Benjamin, I.J.; Schibler, U. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008, 22, 331–345. [Google Scholar] [CrossRef]
- Chang, Y.; Östling, P.; Åkerfelt, M.; Trouillet, D.; Rallu, M.; Gitton, Y.; El Fatimy, R.; Fardeau, V.; Le Crom, S.; Morange, M. Role of heat-shock factor 2 in cerebral cortex formation and as a regulatorof p35 expression. Genes Dev. 2006, 20, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, D.C.; Skaggs, H.S.; Sarge, K.D. HSF2 binds to the Hsp90, Hsp27, and c-Fos promoters constitutively and modulates their expression. Cell Stress Chaperones 2007, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Tanabe, M.; Kawazoe, Y.; Inazawa, J.; Morimoto, R.I.; Nagata, K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 1997, 17, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Feige, U.; Polla, B.S. Hsp70—A multi-gene, multi-structure, multi-function family with potential clinical applications. Experientia 1994, 50, 979–986. [Google Scholar] [CrossRef]
- Amin, V.; Cumming, D.V.; Latchman, D.S. Over-expression of heat shock protein 70 protects neuronal cells against both thermal and ischaemic stress but with different efficiencies. Neurosci. Lett. 1996, 206, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R.; Wispe, J. The stress response and the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997, 273, L1–L9. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Tiwari, P. Chromosomal responses of blowfly Lucilia cuprina to heat and heavy metal stress. Genetica 2000, 109, 211–218. [Google Scholar] [CrossRef]
- Garcia, S.; Garcia, N.; Oliveira, L.; Rodrigues, V.; Mello, M. Experimentally induced heat- and cold-shock tolerance in adult Panstrongylus megistus (Burmeister) (Hemiptera, Reduviidae). Braz. J. Biol. 2003, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Jakubowicz, T. Humoral immune response upon mild heat-shock conditions in Galleria mellonella larvae. J. Insect Physiol. 2007, 53, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.; Anderson, A.J.; Roberts, D.W. Evaluating physical and nutritional stress during mycelial growth as inducers of tolerance to heat and UV-B radiation in Metarhizium anisopliae conidia. Mycol. Res. 2008, 112, 1362–1372. [Google Scholar] [CrossRef]
- Wang, H.; Dong, S.-z.; Li, K.; Hu, C.; Ye, G.-y. A heat shock cognate 70 gene in the endoparasitoid, Pteromalus puparum, and its expression in relation to thermal stress. BMB Rep. 2008, 41, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.A.; Michaud, D.; Cloutier, C. A proteomic analysis of the aphid Macrosiphum euphorbiae under heat and radiation stress. Insect Biochem. Mol. Biol. 2009, 39, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao LiMing, Z.L.; Pridgeon, J.; Becnel, J.; Clark, G.; Linthicum, K. Identification of genes differentially expressed during heat shock treatment in Aedes aegypti. J. Med. Entomol. 2009, 46, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Yamashita, J.; Mitsunobu, H.; Uchino, K.; Kobayashi, I.; Sezutsu, H.; Tamura, T.; Nakajima, H.; Miyagawa, Y.; Lee, J.M. Efficient soluble protein production on transgenic silkworms expressing cytoplasmic chaperones. Appl. Microbiol. Biotechnol. 2010, 87, 2147–2156. [Google Scholar] [CrossRef]
- Lyupina, Y.V.; Dmitrieva, S.B.; Timokhova, A.V.; Beljelarskaya, S.N.; Zatsepina, O.G.; Evgen’ev, M.B.; Mikhailov, V.S. An important role of the heat shock response in infected cells for replication of baculoviruses. Virology 2010, 406, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. Expression of AeaHsp26 and AeaHsp83 in Aedes aegypti (Diptera: Culicidae) larvae and pupae in response to heat shock stress. J. Med. Entomol. 2010, 47, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mahadav, A.; Kontsedalov, S.; Czosnek, H.; Ghanim, M. Thermotolerance and gene expression following heat stress in the whitefly Bemisia tabaci B and Q biotypes. Insect Biochem. Mol. Biol. 2009, 39, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Denlinger, D.L. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol. Biol. 2000, 9, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.; Pavlides, S.; Tammariello, S.; Rinehart, J.; Denlinger, D. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J. Insect Physiol. 2005, 51, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, T.; Perezgasga, L.; Reynaud, E.; Zurita, M. The Drosophila melanogaster homologue of the hsp60 gene is encoded by the essential locus l(1)10Ac and is differentially expressed during fly development. Dev. Genes Evol. 1997, 207, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.; Heikkila, J.J.; Tanguay, R.M. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones 2006, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Bedulina, D.; Evgen’Ev, M.; Timofeyev, M.; Protopopova, M.; Garbuz, D.; Pavlichenko, V.; Luckenbach, T.; Shatilina, Z.M.; Axenov-Gribanov, D.; Gurkov, A. Expression patterns and organization of the hsp70 genes correlate with thermotolerance in two congener endemic amphipod species (Eulimnogammarus cyaneus and E. verrucosus) from Lake Baikal. Mol. Ecol. 2013, 22, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, M.; Pepper, A.N.; Wu, Y.; Kasimer, D.; Westwood, T.; Campos, A.R.; Bedard, P.-A. Menin links the stress response to genome stability in Drosophila melanogaster. PLoS ONE 2010, 5, e14049. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, E.B.; Dretzen, G.; Berger, E.M. The Broad-Complex gene is a tissue-specific modulator of the ecdysone response of the Drosophila hsp23 gene. Mol. Cell. Biol. 1996, 16, 6542. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J.; Hartl, F.U. Protein folding in the cell: Competing models of chaperonin function. FASEB J. 1996, 10, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.M.; Lin, B.; Lian, I.Y.; Mestril, R.; Scheffler, I.E.; Dillmann, W.H. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation 2001, 103, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, S.; Gupta, S.; Knowlton, A. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 2002, 105, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, U.; Singh, M.K.; Abhijnya, K.V.V.; Reddy, L.P.A.; Prasad, B.S.; Pitke, V.V.; Paithankar, K.; Sreedhar, A.S. Hsp60 chaperonin acts as barrier to pharmacologically induced oxidative stress mediated apoptosis in tumor cells with differential stress response. Drug Target Insights 2013, 7, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Dziewanowska, K.; Carson, A.R.; Patti, J.M.; Deobald, C.F.; Bayles, K.W.; Bohach, G.A. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: Role in internalization by epithelial cells. Infect. Immun. 2000, 68, 6321–6328. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zeng, Y.; Graner, M.W.; Katsanis, E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood J. Am. Soc. Hematol. 2002, 100, 4108–4115. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, A.; Meier-Stiegen, F.; Veit, A.; Fleischer, B.; von Bonin, A.; Breloer, M. Lipopolysaccharide-free heat shock protein 60 activates T cells. J. Biol. Chem. 2004, 279, 47906–47911. [Google Scholar] [CrossRef] [PubMed]
- Orme, I.M.; Roberts, A.D.; Griffin, J.P.; Abrams, J. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 1993, 151, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Doerfler, M.; Lee, T.; Guillemin, B.; Rom, W. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J. Clin. Investig. 1993, 91, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Peetermans, W.E.; Raats, C.; van Furth, R.; Langermans, J. Mycobacterial 65-kilodalton heat shock protein induces tumor necrosis factor alpha and interleukin 6, reactive nitrogen intermediates, and toxoplasmastatic activity in murine peritoneal macrophages. Infect. Immun. 1995, 63, 3454–3458. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.C.; Michels, A.; Song, J.; Kampinga, H.H.; Morimoto, R.I. Analysis of molecular chaperone activities using in vitro and in vivo approaches. In Stress Response: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; pp. 393–419. [Google Scholar]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Molecular chaperones: Avoiding the crowd. Curr. Biol. 1997, 7, R531–R533. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Knoflach, M.; Xu, Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu. Rev. Immunol. 2004, 22, 361–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Whittall, T.; McGowan, E.; Younson, J.; Kelly, C.; Bergmeier, L.A.; Singh, M.; Lehner, T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J. Immunol. 2005, 174, 3306–3316. [Google Scholar] [CrossRef] [PubMed]
- Rylander, M.N.; Feng, Y.; Zhang, Y.; Bass, J.; Jason Stafford, R.; Volgin, A.; Hazle, J.D.; Diller, K.R. Optimizing heat shock protein expression induced by prostate cancer laser therapy through predictive computational models. J. Biomed. Opt. 2006, 11, 041113–041116. [Google Scholar] [CrossRef]
- Ranson, N.A.; White, H.; Saibil, H.R. Chaperonins. Biochem. J. 1998, 333, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mamady, H.; Storey, K.B. Up-regulation of the endoplasmic reticulum molecular chaperone GRP78 during hibernation in thirteen-lined ground squirrels. Mol. Cell. Biochem. 2006, 292, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Neuwald, A.F.; Aravind, L.; Spouge, J.L.; Koonin, E.V. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999, 9, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Wah, D.A.; Levchenko, I.; Baker, T.A.; Sauer, R.T. Characterization of a specificity factor for an AAA+ ATPase: Assembly of SspB dimers with ssrA-tagged proteins and the ClpX hexamer. Chem. Biol. 2002, 9, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, E.C.; Glover, J.R.; Singer, M.A.; Lindquist, S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hartling, J.A.; Flanagan, J.M. The structure of ClpP at 2.3 Å resolution suggests a model for ATP-dependent proteolysis. Cell 1997, 91, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, R.; Kessel, M.; Beuron, F.; Steven, A.C.; Maurizi, M.R. Enzymatic and Structural Similarities between the Escherichia coli ATP-dependent Proteases, ClpXP and ClpAP. J. Biol. Chem. 1998, 273, 12476–12481. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.R.; Pak, M.; Maurizi, M.R.; Wickner, S. The role of the ClpA chaperone in proteolysis by ClpAP. Proc. Natl. Acad. Sci. USA 1998, 95, 12135–12140. [Google Scholar] [CrossRef]
- Pak, M.; Hoskins, J.R.; Singh, S.K.; Maurizi, M.R.; Wickner, S. Concurrent chaperone and protease activities of ClpAP and the requirement for the N-terminal ClpA ATP binding site for chaperone activity. J. Biol. Chem. 1999, 274, 19316–19322. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; Trame, C.B.; Tsuruta, H.; Wilbanks, S.M.; Reddy, V.S.; McKay, D.B. Crystal and solution structures of an HslUV protease–chaperone complex. Cell 2000, 103, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, E.; Jeong, M.S.; Jeon, Y.J.; Eom, S.H.; Seol, J.H.; Chung, C.H. HslVU ATP-dependent protease utilizes maximally six among twelve threonine active sites during proteolysis. J. Biol. Chem. 2009, 284, 33475–33484. [Google Scholar] [CrossRef] [PubMed]
- Hodson, S.; Marshall, J.J.; Burston, S.G. Mapping the road to recovery: The ClpB/Hsp104 molecular chaperone. J. Struct. Biol. 2012, 179, 161–171. [Google Scholar] [CrossRef]
- Kędzierska-Mieszkowska, S.; Zolkiewski, M. Hsp100 Molecular Chaperone ClpB and Its Role in Virulence of Bacterial Pathogens. Int. J. Mol. Sci. 2021, 22, 5319. [Google Scholar] [CrossRef] [PubMed]
- Krzewska, J.; Langer, T.; Liberek, K. Mitochondrial Hsp78, a member of the Clp/Hsp100 family in Saccharomyces cerevisiae, cooperates with Hsp70 in protein refolding. FEBS Lett. 2001, 489, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.R.; Lindquist, S. Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 1998, 94, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chamera, T.; Kłosowska, A.; Janta, A.; Wyszkowski, H.; Obuchowski, I.; Gumowski, K.; Liberek, K. Selective Hsp70-dependent docking of Hsp104 to protein aggregates protects the cell from the toxicity of the disaggregase. J. Mol. Biol. 2019, 431, 2180–2196. [Google Scholar] [CrossRef]
- Erives, A.J.; Fassler, J.S. Metabolic and chaperone gene loss marks the origin of animals: Evidence for Hsp104 and Hsp78 chaperones sharing mitochondrial enzymes as clients. PLoS ONE 2015, 10, e0117192. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Schnaider, T.; So, C.; Prohászka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef] [PubMed]
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. CMLS 2002, 59, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Prodromou, C.; Hu, B.; Vaughan, C.; Roe, S.M.; Panaretou, B.; Piper, P.W.; Pearl, L.H. Structural and functional analysis of the middle segment of hsp90: Implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 2003, 11, 647–658. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Buchner, J. Hsp90 & Co.—A holding for folding. Trends Biochem. Sci. 1999, 24, 136–141. [Google Scholar] [PubMed]
- Sreedhar, A.S.; Söti, C.; Csermely, P. Inhibition of Hsp90: A new strategy for inhibiting protein kinases. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2004, 1697, 233–242. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar] [CrossRef]
- Young, J.C.; Schneider, C.; Hartl, F.U. In vitro evidence that hsp90 contains two independent chaperone sites. FEBS Lett. 1997, 418, 139–143. [Google Scholar] [CrossRef]
- Chadli, A.; Bouhouche, I.; Sullivan, W.; Stensgard, B.; McMahon, N.; Catelli, M.G.; Toft, D.O. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc. Natl. Acad. Sci. USA 2000, 97, 12524–12529. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 1997, 90, 65–75. [Google Scholar] [CrossRef]
- Stebbins, C.E.; Russo, A.A.; Schneider, C.; Rosen, N.; Hartl, F.U.; Pavletich, N.P. Crystal structure of an Hsp90–geldanamycin complex: Targeting of a protein chaperone by an antitumor agent. Cell 1997, 89, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T.; Weikl, T.; Buchner, J. Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc. Natl. Acad. Sci. USA 1998, 95, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef]
- Bardwell, J.; Craig, E.A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc. Natl. Acad. Sci. USA 1987, 84, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Ohara-Nemoto, Y.; Ota, M.; Takagi, T.; Yokoyama, K. Mechanism of dimer formation of the 90-kDa heat-shock protein. Eur. J. Biochem. 1995, 233, 1–8. [Google Scholar] [CrossRef]
- Minami, M.; Nakamura, M.; Emori, Y.; Minami, Y. Both the N-and C-terminal chaperone sites of Hsp90 participate in protein refolding. Eur. J. Biochem. 2001, 268, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Chadli, A.; Felts, S.J.; Bouhouche, I.; Catelli, M.G.; Toft, D.O. Hsp90 chaperone activity requires the full-length protein and interaction among its multiple domains. J. Biol. Chem. 2000, 275, 32499–32507. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, H. BIIB021, an Hsp90 inhibitor: A promising therapeutic strategy for blood malignancies. Oncol. Rep. 2018, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell Biol. 2006, 18, 609–615. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Yu, Y.; Zou, P.; Jiang, Y.; Sun, D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J. Biol. Chem. 2009, 284, 35381–35389. [Google Scholar] [CrossRef]
- Yu, Y.; Hamza, A.; Zhang, T.; Gu, M.; Zou, P.; Newman, B.; Li, Y.; Gunatilaka, A.L.; Zhan, C.-G.; Sun, D. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 2010, 79, 542–551. [Google Scholar] [CrossRef]
- Gracia, L.; Lora, G.; Blair, L.J.; Jinwal, U.K. Therapeutic potential of the Hsp90/Cdc37 interaction in neurodegenerative diseases. Front. Neurosci. 2019, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Gebru, N.T.; Blazier, D.M.; Gould, L.A.; Baker, J.D.; Beaulieu-Abdelahad, D.; Blair, L.J. Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice. Acta Neuropathol. Commun. 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Shelton, L.B. Targeting the Hsp90/Aha1 Complex for the Treatment of Tauopathies. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2018. [Google Scholar]
- Lackie, R.E.; de Miranda, A.S.; Lim, M.P.; Novikov, V.; Madrer, N.; Karunatilleke, N.C.; Rutledge, B.S.; Tullo, S.; Brickenden, A.; Maitland, M.E. Stress-inducible phosphoprotein 1 (HOP/STI1/STIP1) regulates the accumulation and toxicity of α-synuclein in vivo. Acta Neuropathol. 2022, 144, 881–910. [Google Scholar] [CrossRef] [PubMed]
- Lackie, R.E.; Marques-Lopes, J.; Ostapchenko, V.G.; Good, S.; Choy, W.-Y.; van Oosten-Hawle, P.; Pasternak, S.H.; Prado, V.F.; Prado, M.A. Increased levels of Stress-inducible phosphoprotein-1 accelerates amyloid-β deposition in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W. Chronic treatment with novel small molecule Hsp90 inhibitors rescues striatal dopamine levels but not α-synuclein-induced neuronal cell loss. PLoS ONE 2014, 9, e86048. [Google Scholar] [CrossRef]
- Silvestro, S.; Raffaele, I.; Mazzon, E. Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 16233. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Dai, X.-Y.; Wei, B.; Weng, Q.-J.; Chen, X.; Ouyang, D.-F.; Yan, R.; Huang, Z.-J.; Jiang, H.-L. Novel Hsp90 inhibitor platycodin D disrupts Hsp90/Cdc37 complex and enhances the anticancer effect of mTOR inhibitor. Toxicol. Appl. Pharmacol. 2017, 330, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, C.; Li, L.; Chen, S.; Wang, L.; Li, Q.; Wang, X.; Lei, X.; Shen, Z. Natural product kongensin A is a non-canonical HSP90 inhibitor that blocks RIP3-dependent necroptosis. Cell Chem. Biol. 2016, 23, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Chiosis, G.; Digwal, C.S.; Trepel, J.B.; Neckers, L. Structural and functional complexity of HSP90 in cellular homeostasis and disease. Nat. Rev. Mol. Cell Biol. 2023, 24, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Forreiter, C.; Nover, L. Heat induced stress proteins and the concept of molecular chaperones. J. Biosci. 1998, 23, 287–302. [Google Scholar] [CrossRef]
- McCarty, J.S.; Buchberger, A.; Reinstein, J.; Bukau, B. The role of ATP in the functional cycle of the DnaK chaperone system. J. Mol. Biol. 1995, 249, 126–137. [Google Scholar] [CrossRef]
- Szabo, A.; Langer, T.; Schröder, H.; Flanagan, J.; Bukau, B.; Hartl, F.U. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system DnaK, DnaJ, and GrpE. Proc. Natl. Acad. Sci. USA 1994, 91, 10345–10349. [Google Scholar] [CrossRef]
- Vogel, M.; Mayer, M.P.; Bukau, B. Allosteric regulation of Hsp70 chaperones involves a conserved interdomain linker. J. Biol. Chem. 2006, 281, 38705–38711. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Maes, E.G.; Taylor, A.B.; Wang, L.; Hinck, A.P.; Lafer, E.M.; Sousa, R. Structural basis of J cochaperone binding and regulation of Hsp70. Mol. Cell 2007, 28, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Zuiderweg, E.R.; Bertelsen, E.B.; Rousaki, A.; Mayer, M.P.; Gestwicki, J.E.; Ahmad, A. Allostery in the Hsp70 chaperone proteins. In Molecular Chaperones; Springer: Berlin/Heidelberg, Germany, 2013; pp. 99–153. [Google Scholar]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Kluger, M.J.; Rudolph, K.; Soszynski, D.; Conn, C.A.; Leon, L.R.; Kozak, W.; Wallen, E.S.; Moseley, P.L. Effect of heat stress on LPS-induced fever and tumor necrosis factor. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R858–R863. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Oberley, T.; Moseley, P.; Buettner, G.; Oberley, L.; Weindruch, R.; Kregel, K. Caloric restriction improves thermotolerance and reduces hyperthermia-induced cellular damage in old rats. FASEB J. 2000, 14, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Xu, L.; Drake, V.; Oberley, L.; Oberley, T.; Moseley, P.; Kregel, K. Aging reduces adaptive capacity and stress protein expression in the liver after heat stress. J. Appl. Physiol. 2000, 89, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Udono, H.; Srivastava, P.K. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 1993, 178, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P. Interaction of heat shock proteins with peptides and antigen presenting cells: Chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 2002, 20, 395–425. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, P.; Zorzi, E.; Mussolin, L.; Monaco, G.; Pigazzi, M.; Basso, G.; Rosolen, A. The effect of the cyclin-dependent kinase inhibitor flavopiridol on anaplastic large cell lymphoma cells and relationship with NPM-ALK kinase expression and activity. haematologica 2009, 94, 944. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, C.; Huang, H.; Xin, Y.; Xu, Y.; Luo, L.; Yin, Z. Heat shock protein 70 together with its co-chaperone CHIP inhibits TNF-α induced apoptosis by promoting proteasomal degradation of apoptosis signal-regulating kinase1. Apoptosis 2010, 15, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Jia, H.; Wang, X.; He, X.; Jin, Q.; Cao, J.; Jing, Z. Ectromelia virus upregulates the expression of heat shock protein 70 to promote viral replication. Int. J. Mol. Med. 2018, 42, 1044–1053. [Google Scholar] [PubMed]
- Peng, C.-W.; Zhao, B.; Chen, H.-C.; Chou, M.-L.; Lai, C.-Y.; Lin, S.-Z.; Hsu, H.-Y.; Kieff, E. Hsp72 up-regulates Epstein-Barr virus EBNALP coactivation with EBNA2. Blood J. Am. Soc. Hematol. 2007, 109, 5447–5454. [Google Scholar] [CrossRef]
- Brown, G.; Rixon, H.W.M.; Steel, J.; McDonald, T.P.; Pitt, A.R.; Graham, S.; Sugrue, R.J. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology 2005, 338, 69–80. [Google Scholar] [CrossRef]
- Guerrero, C.A.; Bouyssounade, D.; Zárate, S.; Iša, P.; López, T.; Espinosa, R.; Romero, P.; Méndez, E.; López, S.; Arias, C.F. Heat shock cognate protein 70 is involved in rotavirus cell entry. J. Virol. 2002, 76, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Garbuz, D.; Zatsepina, O.; Evgen’ev, M. The major human stress protein Hsp70 as a factor of protein homeostasis and a cytokine-like regulator. Mol. Biol. 2019, 53, 176–191. [Google Scholar] [CrossRef]
- Morimoto, R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008, 22, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Peinado-Ruiz, I.C.; Burgos-Molina, A.M.; Sendra-Portero, F.; Ruiz-Gómez, M.J. Relationship between heat shock proteins and cellular resistance to drugs and ageing. Exp. Gerontol. 2022, 167, 111896. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-C.; Lee, C.-H.; Yang, R.-C.; Chen, J.-Y.; Su, T.-C.; Chang, Y.-J.; Lin, C.-Y.; Tsai, Y.-G. Heat shock protein-70 levels are associated with a state of oxidative damage in the development of bronchopulmonary dysplasia. Front. Pediatr. 2021, 9, 616452. [Google Scholar] [CrossRef] [PubMed]
- Mulyani, W.R.W.; Sanjiwani, M.I.D.; Sandra; Prabawa, I.P.Y.; Lestari, A.A.W.; Wihandani, D.M.; Suastika, K.; Saraswati, M.R.; Bhargah, A.; Manuaba, I.B.A.P. Chaperone-based therapeutic target innovation: Heat shock protein 70 (HSP70) for Type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Sellares, J.; Veraldi, K.L.; Thiel, K.J.; Cárdenes, N.; Alvarez, D.; Schneider, F.; Pilewski, J.M.; Rojas, M.; Feghali-Bostwick, C.A. Intracellular heat shock protein 70 deficiency in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2019, 60, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Devi, S.S.; Oswalia, J.; Ramalingam, S.; Arya, R. Role of HSP70 chaperone in protein aggregate phenomenon of GNE mutant cells: Therapeutic lead for GNE Myopathy. Int. J. Biochem. Cell Biol. 2022, 149, 106258. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.D.; Glickman, M.G.; Rapoport, S.; Ferrannini, E.; DeFronzo, R.A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes 1983, 32, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Kaji, H. Thermal Effect on Heat Shock Protein 70 Family to Prevent Atherosclerotic Cardiovascular Disease. Biomolecules 2023, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, H.; Liu, J.; DiSanto, M.E.; Zhang, X. The role of heat shock protein 70 subfamily in the hyperplastic prostate: From molecular mechanisms to therapeutic opportunities. Cells 2022, 11, 2052. [Google Scholar] [CrossRef] [PubMed]
- Bobkova, N.V.; Evgen’ev, M.; Garbuz, D.G.; Kulikov, A.M.; Morozov, A.; Samokhin, A.; Velmeshev, D.; Medvinskaya, N.; Nesterova, I.; Pollock, A. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc. Natl. Acad. Sci. USA 2015, 112, 16006–16011. [Google Scholar] [CrossRef]
- Tytell, M.; Davis, A.T.; Giles, J.; Snider, L.C.; Xiao, R.; Dozier, S.G.; Presley, T.D.; Kavanagh, K. Alfalfa-derived HSP70 administered intranasally improves insulin sensitivity in mice. Cell Stress Chaperones 2018, 23, 189–194. [Google Scholar] [CrossRef]
- Sha, G.; Jiang, Z.; Zhang, W.; Jiang, C.; Wang, D.; Tang, D. The multifunction of HSP70 in cancer: Guardian or traitor to the survival of tumor cells and the next potential therapeutic target. Int. Immunopharmacol. 2023, 122, 110492. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Kim, S.-C.; Wang, Y.; Gupta, S.; Davis, B.; Simon, S.I.; Torre-Amione, G.; Knowlton, A.A. HSP60 in heart failure: Abnormal distribution and role in cardiac myocyte apoptosis. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H2238–H2247. [Google Scholar] [CrossRef] [PubMed]
- Soltys, B.J.; Gupta, R.S. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp. Cell Res. 1996, 222, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Hartl, F.U. Chaperone-assisted protein folding. Curr. Opin. Struct. Biol. 1997, 7, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Evolution of the chaperonin families (HSP60, HSP 10 and TCP-1) of proteins and the origin of eukaryotic cells. Mol. Microbiol. 1995, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krebs, R.A.; Feder, M.E. Deleterious consequences of Hsp70 overexpression in Drosphilla melanogaster larvae. Cell Stress Chaperones 1997, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Netzer, W.J.; Hartl, F.U. Protein folding in the cytosol: Chaperonin-dependent and-independent mechanisms. Trends Biochem. Sci. 1998, 23, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rommelaere, H.; Van Troys, M.; Gao, Y.; Melki, R.; Cowan, N.J.; Vandekerckhove, J.; Ampe, C. Eukaryotic cytosolic chaperonin contains t-complex polypeptide 1 and seven related subunits. Proc. Natl. Acad. Sci. USA 1993, 90, 11975–11979. [Google Scholar] [CrossRef] [PubMed]
- Llorca, O.; McCormack, E.A.; Hynes, G.; Grantham, J.; Cordell, J.; Carrascosa, J.L.; Willison, K.R.; Fernandez, J.J.; Valpuesta, J.M. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 1999, 402, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.L.; Schmidt, G.W. Pervasive migration of organellar DNA to the nucleus in plants. J. Mol. Evol. 1995, 41, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.G.; Zomorodipour, A.; Andersson, J.O.; Sicheritz-Pontén, T.; Alsmark, U.C.M.; Podowski, R.M.; Näslund, A.K.; Eriksson, A.-S.; Winkler, H.H.; Kurland, C.G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 1998, 396, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; Pfeifer, G.; Martin, J.; Baumeister, W.; Hartl, F.-U. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992, 11, 4757–4765. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.F.; Weaver, A.J.; Landry, S.J.; Gierasch, L.; Deisenhofer, J. The crystal structure of the GroES co-chaperonin at 2.8 Å resolution. Nature 1996, 379, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sigler, P.B.; Xu, Z.; Rye, H.S.; Burston, S.G.; Fenton, W.A.; Horwich, A.L. Structure and function in GroEL-mediated protein folding. Annu. Rev. Biochem. 1998, 67, 581–608. [Google Scholar] [CrossRef] [PubMed]
- Llorca, O.; Galán, A.; Carrascosa, J.L.; Muga, A.; Valpuesta, J.M. GroEL under heat-shock: Switching from a folding to a storing function. J. Biol. Chem. 1998, 273, 32587–32594. [Google Scholar] [CrossRef] [PubMed]
- Szpikowska, B.K.; Sherman, M.A.; Mas, M.T.; Swiderek, K.M. MgATP binding to the nucleotide-binding domains of the eukaryotic cytoplasmic chaperonin induces conformational changes in the putative substrate-binding domains. Protein Sci. 1998, 7, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, L.; Löwe, J.; Stock, D.; Stetter, K.-O.; Huber, H.; Huber, R.; Steinbacher, S. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell 1998, 93, 125–138. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Gadd, G. Inhibition of H+ efflux and K+ uptake, and induction of K+ efflux in yeast by heavy metals. Toxic. Assess. 1987, 2, 437–447. [Google Scholar] [CrossRef]
- Karlin, S.; Brocchieri, L. Significant Segment Alignment of Pairs of Protein Sequences from Animals, Plants and Fungi. In Evolutionary Theory and Processes: Modern Perspectives: Papers in Honour of Eviatar Nevo; Springer: Berlin/Heidelberg, Germany, 1998; pp. 213–221. [Google Scholar]
- Yamaguchi, H.; Osaki, T.; Taguchi, H.; Hanawa, T.; Yamamoto, T.; Kamiya, S. Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J. Med. Microbiol. 1996, 45, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Reddy, P.J.; Sreedhar, A.; Tiwari, P. Molecular characterization and expression analysis of hsp60 gene homologue of sheep blowfly, Lucilia cuprina. J. Therm. Biol. 2015, 52, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.E.; Schoel, B.; Modrow, S.; Karr, R.W.; Young, R.; Kaufmann, S. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J. Immunol. 1989, 143, 2844–2849. [Google Scholar] [CrossRef] [PubMed]
- Anderton, S.M.; Van der Zee, R.; Prakken, B.; Noordzij, A.; Van Eden, W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J. Exp. Med. 1995, 181, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.; van Kooten, P.J.; van Eden, W.; van der Zee, R. Highly autoproliferative T cells specific for 60-kDa heat shock protein produce IL-4/IL-10 and IFN-γ and are protective in adjuvant arthritis. J. Immunol. 2000, 165, 7270–7277. [Google Scholar] [CrossRef] [PubMed]
- De Kleer, I.; Kamphuis, S.; Rijkers, G.; Scholtens, L.; Gordon, G.; De Jager, W.; Häfner, R.; Van De Zee, R.; Van Eden, W.; Kuis, W. The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wick, G.; Willeit, J.; Marosi, M.; Kiechl, S.; Luef, G.; Kleindienst, R.; Stulnig, T.; Oberhollenzer, F. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet 1993, 341, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Benke, D.; Eitner, F.; Engel, D.; Ehrlich, S.; Breloer, M.; Hamilton-Williams, E.; Specht, S.; Hoerauf, A.; von Bonin, A. Heat Shock Protein 60 Is Released in Immune-Mediated Glomerulonephritis and Aggravates Disease: In Vivo: Evidence for an Immunologic Danger Signal. J. Am. Soc. Nephrol. 2005, 16, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.; Gupta, S. HSP60, Bax, and cardiac apoptosis. Cardiovasc. Toxicol. 2003, 3, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sigala, J.L.D.; Bottero, V.; Young, D.B.; Shevchenko, A.; Mercurio, F.; Verma, I.M. Activation of transcription factor NF-κB requires ELKS, an IκB kinase regulatory subunit. Science 2004, 304, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.N.; Choi, B.; Lee, K.W.; Lee, D.J.; Kang, D.H.; Lee, J.Y.; Song, I.S.; Kim, H.I.; Lee, S.-H.; Kim, H.S. Cytosolic Hsp60 is involved in the NF-κB-dependent survival of cancer cells via IKK regulation. PLoS ONE 2010, 5, e9422. [Google Scholar] [CrossRef]
- Grundtman, C.; Kreutmayer, S.B.; Almanzar, G.; Wick, M.C.; Wick, G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, C.; Cornut, E.; Poirier, F.; Pons, S.; Beaulieu, C.; Charrier, J.P.; Haidous, H.; Cotte, E.; Lambert, C.; Piard, F. Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer. FEBS J. 2011, 278, 4845–4859. [Google Scholar] [CrossRef]
- Rizzo, M.; JL Macario, A.; Conway de Macario, E.; Gouni-Berthold, I.; K Berthold, H.; Battista Rini, G.; Zummo, G.; Cappello, F. Heat shock protein-60 and risk for cardiovascular disease. Curr. Pharm. Des. 2011, 17, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Schett, G.; Perschinka, H.; Mayr, M.; Egger, G.; Oberhollenzer, F.; Willeit, J.; Kiechl, S.; Wick, G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 2000, 102, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Lauritzen, H.P.; Ussar, S.; Christensen, J.H.; Mori, M.A.; Bross, P.; Kahn, C.R. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J. Clin. Investig. 2013, 123, 4667–4680. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.-Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hollestelle, S.C.; De Vries, M.R.; Van Keulen, J.K.; Schoneveld, A.H.; Vink, A.; Strijder, C.F.; Van Middelaar, B.J.; Pasterkamp, G.; Quax, P.H.; De Kleijn, D.P. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation 2004, 109, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Wei, X.; Li, P.; Cui, Y.; Qin, Y.; Wei, X.; Jin, M.; Kohama, K.; Gao, Y. Heat shock protein 60 stimulates the migration of vascular smooth muscle cells via Toll-like receptor 4 and ERK MAPK activation. Sci. Rep. 2015, 5, 15352. [Google Scholar] [CrossRef]
- Sun, B.; Li, G.; Yu, Q.; Liu, D.; Tang, X. HSP60 in cancer: A promising biomarker for diagnosis and a potentially useful target for treatment. J. Drug Target. 2022, 30, 31–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, Y.; Luo, J.; Yang, Y.; Zang, H.; Ma, J.; Fan, S.; Wen, Q. High expression of HSP60 and survivin predicts poor prognosis for oral squamous cell carcinoma patients. BMC Oral Health 2023, 23, 629. [Google Scholar] [CrossRef] [PubMed]
- Märker, T.; Sell, H.; Zilleßen, P.; Glöde, A.; Kriebel, J.; Ouwens, D.M.; Pattyn, P.; Ruige, J.; Famulla, S.; Roden, M. Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes 2012, 61, 615–625. [Google Scholar] [CrossRef]
- Vercoulen, Y.; van Teijlingen, N.H.; de Kleer, I.M.; Kamphuis, S.; Albani, S.; Prakken, B.J. Heat shock protein 60 reactive T cells in juvenile idiopathic arthritis: What is new? Arthritis Res. Ther. 2009, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Fouani, M.; Basset, C.A.; Mangano, G.D.; Leone, L.G.; Lawand, N.B.; Leone, A.; Barone, R. Heat shock proteins alterations in rheumatoid arthritis. Int. J. Mol. Sci. 2022, 23, 2806. [Google Scholar] [CrossRef] [PubMed]
- Walls, K.C.; Coskun, P.; Gallegos-Perez, J.L.; Zadourian, N.; Freude, K.; Rasool, S.; Blurton-Jones, M.; Green, K.N.; LaFerla, F.M. Swedish Alzheimer mutation induces mitochondrial dysfunction mediated by HSP60 mislocalization of amyloid precursor protein (APP) and beta-amyloid. J. Biol. Chem. 2012, 287, 30317–30327. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; Pace, A.; Caruso Bavisotto, C.; Marzullo, P.; Marino Gammazza, A.; Buscemi, S.; Palumbo Piccionello, A. Heat shock proteins in Alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018, 19, 2603. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Duan, Y.; Yang, F.; Trexler, C.; Wang, H.; Huang, L.; Li, Y.; Tang, H.; Wang, G.; Fang, X. Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ. 2020, 27, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Vilasi, S.; Carrotta, R.; Ricci, C.; Rappa, G.C.; Librizzi, F.; Martorana, V.; Ortore, M.G.; Mangione, M.R. Inhibition of Aβ1–42 fibrillation by chaperonins: Human Hsp60 is a stronger inhibitor than its bacterial homologue GroEL. ACS Chem. Neurosci. 2019, 10, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Mangione, M.R.; Vilasi, S.; Marino, C.; Librizzi, F.; Canale, C.; Spigolon, D.; Bucchieri, F.; Fucarino, A.; Passantino, R.; Cappello, F. Hsp60, amateur chaperone in amyloid-beta fibrillogenesis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Stromer, T.; Ehrnsperger, M.; Gaestel, M.; Buchner, J. Analysis of the interaction of small heat shock proteins with unfolding proteins. J. Biol. Chem. 2003, 278, 18015–18021. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.-P. Expression and function of the low molecular-weight heat shock proteins. In The Biology of Heat Shock Proteins and Molecular Chaperones; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1994; pp. 335–373. [Google Scholar]
- Pfoh, R.; Lacdao, I.K.; Saridakis, V. Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr.-Relat. Cancer 2015, 22, T35–T54. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.; Heikkila, J.J. Identification of members of the HSP30 small heat shock protein family and characterization of their developmental regulation in heat-shocked xenopus laevis embryos. Dev. Genet. 1995, 17, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Helbing, C.; Gallimore, C.; Atkinson, B.G. Characterization of Rana catesbeiana HSP30 gene and its expression in the liver of this amphibian during both spontaneous and thyroid hormone-induced metamorphosis. Dev. Genet. 1996, 18, 223–233. [Google Scholar] [CrossRef]
- Heikkila, J.; Ohan, N.; Tam, Y.; Ali, A. Heat shock protein gene expression during Xenopus development. Cell. Mol. Life Sci. CMLS 1997, 53, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Charette, S.J.; Landry, J. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann. N. Y. Acad. Sci. 2000, 926, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.-M.; Rest, J.S.; Welsh, M.J.; Benndorf, R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones 2003, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Kappé, G.; Franck, E.; Verschuure, P.; Boelens, W.C.; Leunissen, J.A.; de Jong, W.W. The human genome encodes 10 α-crystallin–related small heat shock proteins: HspB1–10. Cell Stress Chaperones 2003, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Voellmy, R.; Goldschmidt-Clermont, M.; Southgate, R.; Tissières, A.; Levis, R.; Gehring, W. A DNA segment isolated from chromosomal site 67B in D. melanogaster contains four closely linked heat-shock genes. Cell 1981, 23, 261–270. [Google Scholar] [CrossRef]
- Southgate, R.; Ayme, A.; Voellmy, R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J. Mol. Biol. 1983, 165, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Narberhaus, F. α-Crystallin-type heat shock proteins: Socializing minichaperones in the context of a multichaperone network. Microbiol. Mol. Biol. Rev. 2002, 66, 64–93. [Google Scholar] [CrossRef]
- Laksanalamai, P.; Robb, F.T. Small heat shock proteins from extremophiles: A review. Extremophiles 2004, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brahma, S.; McDevitt, D.; DeFize, L. Ontogeny of αA and αB crystallin polypeptides during Rana temporaria lens development. Exp. Eye Res. 1987, 45, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lindner, R.A.; Kapur, A.; Mariani, M.; Titmuss, S.J.; Carver, J.A. Structural alterations of α-crystallin during its chaperone action. Eur. J. Biochem. 1998, 258, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.; van den IJssel, P. Fatal attraction: When chaperone turns harlot. Nat. Med. 1999, 5, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Heikkila, J.J. Expression and function of small heat shock protein genes during Xenopus development. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 259–266. [Google Scholar]
- Wieske, M.; Benndorf, R.; Behlke, J.; Dölling, R.; Grelle, G.; Bielka, H.; Lutsch, G. Defined sequence segments of the small heat shock proteins HSP25 and αB-crystallin inhibit actin polymerization. Eur. J. Biochem. 2001, 268, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Barral, J.M.; Hartl, F.U. More than folding: Localized functions of cytosolic chaperones. Trends Biochem. Sci. 2003, 28, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Jakob, U.; Buchner, J. Assisting spontaneity: The role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem. Sci. 1994, 19, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Oesterreich, S.; Chamness, G.C.; MCGuire, W.L.; Fuqua, S.A. Biological and clinical implications of heat shock protein 27000 (Hsp27): A review. JNCI J. Natl. Cancer Inst. 1993, 85, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Preville, X.; Chareyron, P.; Briolay, J.; Klemenz, R.; Arrigo, A.-P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF-and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J. Immunol. 1995, 154, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Samali, A.; Cotter, T.G. Heat shock proteins increase resistance to apoptosis. Exp. Cell Res. 1996, 223, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Champagne, M.-J.e.; Dumas, P.; Orlov, S.N.; Bennett, M.R.; Hamet, P.; Tremblay, J. Protection against necrosis but not apoptosis by heat-stress proteins in vascular smooth muscle cells: Evidence for distinct modes of cell death. Hypertension 1999, 33, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Bruey, J.-M.; Paul, C.; Fromentin, A.; Hilpert, S.; Arrigo, A.-P.; Solary, E.; Garrido, C. Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene 2000, 19, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Concannon, C.; Gorman, A.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Thanner, J.; Bekos, C.; Veraar, C.; Janik, S.; Laggner, M.; Boehm, P.M.; Schiefer, A.-I.; Müllauer, L.; Klepetko, W.; Ankersmit, H.J. Heat shock protein 90α in thymic epithelial tumors and non-thymomatous myasthenia gravis. Oncoimmunology 2020, 9, 1756130. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, P.; Hu, P.; Liu, Z.; Diaz, L.A.; Enghild, J.J.; Chua, M.P.; Rubenstein, D.S. Desmosome signaling: Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J. Biol. Chem. 2005, 280, 23778–23784. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mansour, M.; Crack, J.A.; Gass, G.L.; MacRae, T.H. Oligomerization, chaperone activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J. Biol. Chem. 2004, 279, 39999–40006. [Google Scholar] [CrossRef] [PubMed]
- Kappé, G.; Leunissen, J.A.; de Jong, W.W. Evolution and diversity of prokaryotic small heat shock proteins. In Small Stress Proteins; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–17. [Google Scholar]
- Feil, I.K.; Malfois, M.; Hendle, J.r.; van der Zandt, H.; Svergun, D.I. A novel quaternary structure of the dimeric α-crystallin domain with chaperone-like activity. J. Biol. Chem. 2001, 276, 12024–12029. [Google Scholar] [CrossRef] [PubMed]
- Stromer, T.; Fischer, E.; Richter, K.; Haslbeck, M.; Buchner, J. Analysis of the regulation of the molecular chaperone Hsp26 by temperature-induced dissociation: The N-terminal domain is important for oligomer assembly and the binding of unfolding proteins. J. Biol. Chem. 2004, 279, 11222–11228. [Google Scholar] [CrossRef] [PubMed]
- Van den Oetelaar, P.J.; Van Someren, P.F.; Thomson, J.A.; Siezen, R.J.; Hoenders, H.J. A dynamic quaternary structure of bovine. alpha.-crystallin as indicated from intermolecular exchange of subunits. Biochemistry 1990, 29, 3488–3493. [Google Scholar] [CrossRef]
- Haley, D.A.; Horwitz, J.; Stewart, P.L. The small heat-shock protein, αB-crystallin, has a variable quaternary structure. J. Mol. Biol. 1998, 277, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Roseman, A.M.; Saibil, H.R.; Vierling, E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997, 16, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Knauf, U.; Jakob, U.; Engel, K.; Buchner, J.; Gaestel, M. Stress-and mitogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J. 1994, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.N.; Lambert, H.; Hickey, E.; Weber, L.A.; Landry, J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell. Biol. 1995, 15, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Mehlen, A.; Godet, J.; Arrigo, A.-P. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J. Biol. Chem. 1997, 272, 31657–31665. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Tsujimoto, N.; Nakagawa, H.; Iwaki, T.; Fukumaki, Y.; Iwaki, A. Association of HSPB2, a member of the small heat shock protein family, with mitochondria. Exp. Cell Res. 2001, 271, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Nédellec, P.; Edling, Y.; Perret, E.; Fardeau, M.; Vicart, P. Glucocorticoid treatment induces expression of small heat shock proteins in human satellite cell populations: Consequences for a desmin-related myopathy involving the R120G alpha B-crystallin mutation. Neuromuscul. Disord. 2002, 12, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-C.; Liu, T.-J.; Ting, C.-T.; Yang, J.-Y.; Huang, L.; Wallace, D.; Kaiser, P.; Wang, P.H. Regulation of IGF-I receptor signaling in diabetic cardiac muscle: Dysregulation of cytosolic and mitochondria HSP60. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E292–E297. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Mehlen, P.; Fromentin, A.; Hammann, A.; Assem, M.; Arrigo, A.P.; Chauffert, B. Inconstant Association between 27-kDa Heat-Shock Protein (Hsp27) Content and Doxorubicin Resistance in Human Colon Cancer Cells: The Doxorubicin-Protecting Effect of Hsp27. Eur. J. Biochem. 1996, 237, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.; Saville, B.; Hoivik, D.; Safe, S. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol. Endocrinol. 1997, 11, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.K.; Parra, I.; Lemieux, P.; Oesterreich, S.; Hilsenbeck, S.G.; Fuqua, S.A. Hsp27 overexpression inhibits doxorubicin–induced apoptosis in human breast cancer cells. Breast Cancer Res. Treat. 1999, 56, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.A.; Dodson, A.R.; Parsons, K.F.; Desmond, A.D.; Woolfenden, A.; Fordham, M.; Neoptolemos, J.P.; Ke, Y.; Foster, C.S. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000, 60, 7099–7105. [Google Scholar] [PubMed]
- Rocchi, P.; So, A.; Kojima, S.; Signaevsky, M.; Beraldi, E.; Fazli, L.; Hurtado-Coll, A.; Yamanaka, K.; Gleave, M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004, 64, 6595–6602. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; MacRae, T.H. The Synthesis of a Small Heat Shock/α-Crystallin Protein inArtemiaand Its Relationship to Stress Tolerance during Development. Dev. Biol. 1999, 207, 445–456. [Google Scholar] [CrossRef]
- Parcellier, A.; Schmitt, E.; Gurbuxani, S.; Seigneurin-Berny, D.; Pance, A.; Chantôme, A.; Plenchette, S.; Khochbin, S.; Solary, E.; Garrido, C. HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol. Cell. Biol. 2003, 23, 5790–5802. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.V.; Chegão, A.; Oliveira, M.; Gomes, B.F.; Enguita, F.J.; Outeiro, T.F. Hsp27 reduces glycation-induced toxicity and aggregation of α-synuclein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Navarro-Zaragoza, J.; Cuenca-Bermejo, L.; Almela, P.; Laorden, M.-L.; Herrero, M.-T. Could small heat shock protein HSP27 be a first-line target for preventing protein aggregation in Parkinson’s disease? Int. J. Mol. Sci. 2021, 22, 3038. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M. Targeting of substrates to the 26S proteasome. FASEB J. 1997, 11, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.A.; Lawson, T.G.; Velayutham, M.; Zweier, J.L.; Pickart, C.M. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 2002, 416, 763–767. [Google Scholar] [CrossRef]
- Pickart, C.M.; Fushman, D. Polyubiquitin chains: Polymeric protein signals. Curr. Opin. Chem. Biol. 2004, 8, 610–616. [Google Scholar] [CrossRef]
- Maxwell, B.A.; Gwon, Y.; Mishra, A.; Peng, J.; Nakamura, H.; Zhang, K.; Kim, H.J.; Taylor, J.P. Ubiquitination is essential for recovery of cellular activities after heat shock. Science 2021, 372, eabc3593. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Urbé, S.; Komander, D. Breaking the chains: Deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chung, H.J.; Vogt, M.; Jin, Y.; Malide, D.; He, L.; Dundr, M.; Levens, D. JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J. 2011, 30, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Lee, C.S.; Young, P.; Beskow, A.; Chan, J.Y.; Deshaies, R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell 2010, 38, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Park, S.; Lee, M.J.; Huck, B.; McAllister, F.; Hill, C.P.; Gygi, S.P.; Finley, D. An asymmetric interface between the regulatory and core particles of the proteasome. Nat. Struct. Mol. Biol. 2011, 18, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Wu, H. Ubiquitination-proteasome system (UPS) and autophagy two main protein degradation machineries in response to cell stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [PubMed]
- Hyrskyluoto, A.; Bruelle, C.; Lundh, S.H.; Do, H.T.; Kivinen, J.; Rappou, E.; Reijonen, S.; Waltimo, T.; Petersén, Å.; Lindholm, D. Ubiquitin-specific protease-14 reduces cellular aggregates and protects against mutant huntingtin-induced cell degeneration: Involvement of the proteasome and ER stress-activated kinase IRE1α. Hum. Mol. Genet. 2014, 23, 5928–5939. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Z.; Hu, Y.; Lu, Y.; Li, D.; Liu, J.; Liao, S.; Hu, M.; Wang, Y.; Zhang, D. Sustained ER stress promotes hyperglycemia by increasing glucagon action through the deubiquitinating enzyme USP14. Proc. Natl. Acad. Sci. USA 2019, 116, 21732–21738. [Google Scholar] [CrossRef] [PubMed]
- Wasner, K.; Grünewald, A.; Klein, C. Parkin-linked Parkinson’s disease: From clinical insights to pathogenic mechanisms and novel therapeutic approaches. Neurosci. Res. 2020, 159, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, S.; Lee, S.; Kim, S.; Yuan, C.; Karuppagounder, S.S.; Ge, P.; Shi, R.; Kim, E.J.; Liu, A.; Kim, D. Parkin interacting substrate zinc finger protein 746 is a pathological mediator in Parkinson’s disease. Brain 2019, 142, 2380–2401. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Ao, X.; Liu, Y.; Wang, Y.-Y.; Fa, H.-G.; Wang, M.-Y.; He, Y.-Q.; Wang, J.-X. Post-translational modification of Parkin and its research progress in cancer. Cancer Commun. 2019, 39, 77. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, C.; De Snoo, M.L.; Gondard, E.; Neudorfer, C.; Chau, H.; Ngana, S.G.; O’Hara, D.M.; Brotchie, J.M.; Koprich, J.B.; Lozano, A.M. Early-onset impairment of the ubiquitin-proteasome system in dopaminergic neurons caused by α-synuclein. Acta Neuropathol. Commun. 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Du, X.; Jiao, Q.; Chen, X.; Jiang, H. Expanding the role of proteasome homeostasis in Parkinson’s disease: Beyond protein breakdown. Cell Death Dis. 2021, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Watzlawik, J.O.; Fiesel, F.C.; Springer, W. Autophagy in Parkinson’s disease. J. Mol. Biol. 2020, 432, 2651–2672. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Han, L.; Yu, M.; Cao, Z.; Li, X.; Shao, Y.; Zhu, G. The prognostic value of PERK in cancer and its relationship with immune cell infiltration. Front. Mol. Biosci. 2021, 8, 648752. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rohilla, M.S.; Reddy, P.; Tiwari, P. In vitro induction of 60-kDa and 70-kDa heat shock proteins by endosulphan and monocrotophos in sheep blowfly Lucilia cuprina. Arch. Environ. Contam. Toxicol. 2008, 55, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Andrulis, M.; Stühmer, T.; Müller, E.; Hofmann, C.; Steinbrunn, T.; Heimberger, T.; Schraud, H.; Kressmann, S.; Einsele, H. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 2013, 98, 1132. [Google Scholar] [CrossRef] [PubMed]
- Koziol, C.; Wagner-Hülsmann, C.; Mikoc, A.; Gamulin, V.; Kruse, M.; Pancer, Z.; Schäcke, H.; Müller, W.E. Cloning of a heat-inducible biomarker, the cDNA encoding the 70 kDa heat shock protein, from the marine sponge Geodia cydonium: Response to natural stressors. Mar. Ecol. Prog. Ser. 1996, 136, 153–161. [Google Scholar] [CrossRef]
- Sanders, B.M. Stress proteins in aquatic organisms: An environmental perspective. Crit. Rev. Toxicol. 1993, 23, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Nunamaker, R.; Dean, V.; Murphy, K.; Lockwood, J. Stress proteins elicited by cold shock in the biting midge, Culicoides variipennis sonorensis Wirth and Jones. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 113, 73–77. [Google Scholar] [CrossRef]
- Köhler, H.-R.; Triebskorn, R.; Stöcker, W.; Kloetzel, P.-M.; Alberti, G. The 70 kD heat shock protein (hsp 70) in soil invertebrates: A possible tool for monitoring environmental toxicants. Arch. Environ. Contam. Toxicol. 1992, 22, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Brown, B.E.; Sharp, V.A.; Nganro, N. Changes in the expression of soluble proteins extracted from the symbiotic anemone Anemonia viridis accompany bleaching induced by hyperthermia and metal stressors. J. Therm. Biol. 1992, 17, 217–223. [Google Scholar] [CrossRef]
- Vedel, G.R.; Depledge, M.H. Stress-70 levels in the gills of Carcinus maenas exposed to copper. Mar. Pollut. Bull. 1995, 31, 84–86. [Google Scholar] [CrossRef]


| Name | Size (kDa) | Bacterial Homolog | Location | Functions |
|---|---|---|---|---|
| HSP 100 | 104/110 | Clp | Cytosol, nucleus | Mitigate severe stress |
| HSP 90 | 90 | HtpG | Cytosol, nucleus | Part of the steroid hormone receptor complex; stabilize substrate proteins; and inhibit protein aggregation |
| HSP 70 | 72 | Dna K | Cytosol, nucleus | Highly stress inducible |
| HSC 70 | 73 | Dna K | cytosol | Constitutively expressed |
| HSP 60 | 60 | GroEL | Mitochondria, chloroplast, and nucleus | Assists protein folding |
| HSP 40 | 47 | Dna J | Endoplasmic reticulum | Co-chaperone of Dna K; protein folding and refolding |
| Small HSPs | 20–34 | IbpA/B | Cytosol, nucleus | Prevent aggregation of proteins |
| HSP10 | 10 | GroES | mitochondria, chloroplast | Assist as a co-chaperone |
| Ubiquitin | 8 | Cytosol, nucleus | Involved in non-lysosomal protein degradation |
| Chaperonin | Organism | Chaperone | Co-Chaperone | Localization | Functions |
|---|---|---|---|---|---|
| HSP60/ HSPD1 | Bacterial | GroEL | GroES | Cytosol | Assist folding and refolding of denatured proteins |
| Mammalian | mHSP60 (HSPD)/ TriC/CCT | HSP10 (HSPE) | Mitochondria, cytosol | Folding of nascent proteins and mitochondria proteostasis | |
| HSP40/HSPF | Bacterial | DnaJ | DnaK/GrpE | Cytosol | Modulating activity of DnaK, associated with nascent polypeptides, binds to unfolded proteins |
| Mammalian | Hdj1/2, HSP40, auxilin | HSP70/HIP | Cytosol | Modulating ATPase activity of DnaK, auxilin recruits HSP70 partner HSC70 to uncoat clathrin-coated vesicles | |
| HSP70/HSPA | Bacterial | DnaK | DnaJ/GrpE/ClpB | Cytosol | Folding and export of nascent peptides, disaggregation and degradation of stress-induced folding and translocation |
| Mammalian | Bip/Grp78 | DnaJ-like ER proteins (Grp70, Sil1/sls1) | Endoplasmic reticulum | Involved in calcium homeostasis, translocation, folding, transport and re-translocation of polypeptides, regulation of unfolded protein response | |
| HSC70 (HSP73), HSP70 (HSP72) | HSP40, Hop, Bag1-5, HIP, HSPBP1, CHIP, SGT, HSP110, Homologs to Tom 70, TPR1 | Cytosol | Folding and transportation of nascent polypeptide, inhibits mis-folding and aggregations | ||
| mHSP70/Grp75/mortalin | - | Cytosol | Protein folding and translocation into mitochondria | ||
| HSP90/HSPC | Bacterial | HtpG | - | Cytosol | Stress-responsive protein folding |
| Mammalian | HSP90/83/89, TRAP1/2 | HOP/HIP, HSP70, p50, p23, CHIP, Sgt1/TPR2, Immunophilins | Cytosol, mitochondrial | Folding and conformational regulation of signaling protein, regulation of steroid hormone receptor and kinases | |
| Grp94 | Grp78 | ER | Folding and assembly of secretary proteins | ||
| HSP100/HSPH | Bacterial | ClpA | ClpP, SspB | Cytosol | ATP dependent protein unfolding and proteolysis |
| ClpB | Dnak, DnaJ, GrpE | Cytosol | ATP dependent processing of aggregated proteins. | ||
| Small HSPs (HSPB) | Bacterial | IbpA/IbpB | - | Cytosol | Associated with inclusion bodies, prevent heat denatured protein aggregation |
| Mammalian | α-crystallin, HSP27 | - | Cytosol | Prevent heat denatured protein aggregation, regulate microfilament polymerization | |
| Chaperones | Bacterial | HSP33, SecB | SecA | Cytosol | Prevent aggregation of oxidative damage proteins. Shuttling of secretory proteins SecA/B, maintenance of periplasm proteins, Pili assembly |
| SKP/PapD/FimC | PapC, FimD | Periplasm | |||
| Mammalian | Calnexin, calreticulin, PDI, HSP47 (collagen) | ERp57, Cnx/Crt | Endoplasmic reticulum (ER) | Folding of ER glycosylated proteins (Cnx/Crt); collagen biosynthesis (HSP47), assist di-sulfide bond formation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.K.; Shin, Y.; Ju, S.; Han, S.; Choe, W.; Yoon, K.-S.; Kim, S.S.; Kang, I. Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. Int. J. Mol. Sci. 2024, 25, 4209. https://doi.org/10.3390/ijms25084209
Singh MK, Shin Y, Ju S, Han S, Choe W, Yoon K-S, Kim SS, Kang I. Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. International Journal of Molecular Sciences. 2024; 25(8):4209. https://doi.org/10.3390/ijms25084209
Chicago/Turabian StyleSingh, Manish Kumar, Yoonhwa Shin, Songhyun Ju, Sunhee Han, Wonchae Choe, Kyung-Sik Yoon, Sung Soo Kim, and Insug Kang. 2024. "Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases" International Journal of Molecular Sciences 25, no. 8: 4209. https://doi.org/10.3390/ijms25084209
APA StyleSingh, M. K., Shin, Y., Ju, S., Han, S., Choe, W., Yoon, K.-S., Kim, S. S., & Kang, I. (2024). Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. International Journal of Molecular Sciences, 25(8), 4209. https://doi.org/10.3390/ijms25084209







