Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production
Abstract
:1. Introduction
2. Results
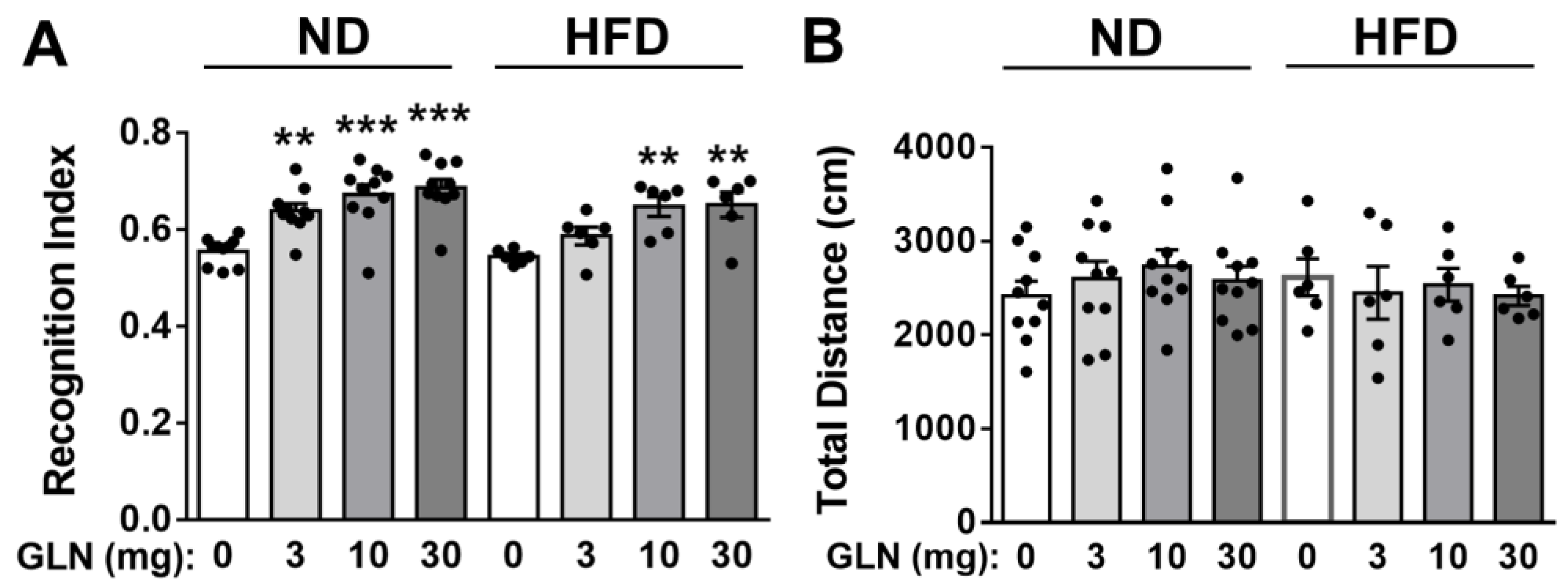
2.1. GLN’s Enhancement of Learning and Memory Functions in Normal Diet (ND)- and High-Fat Diet (HFD)-Fed Mice
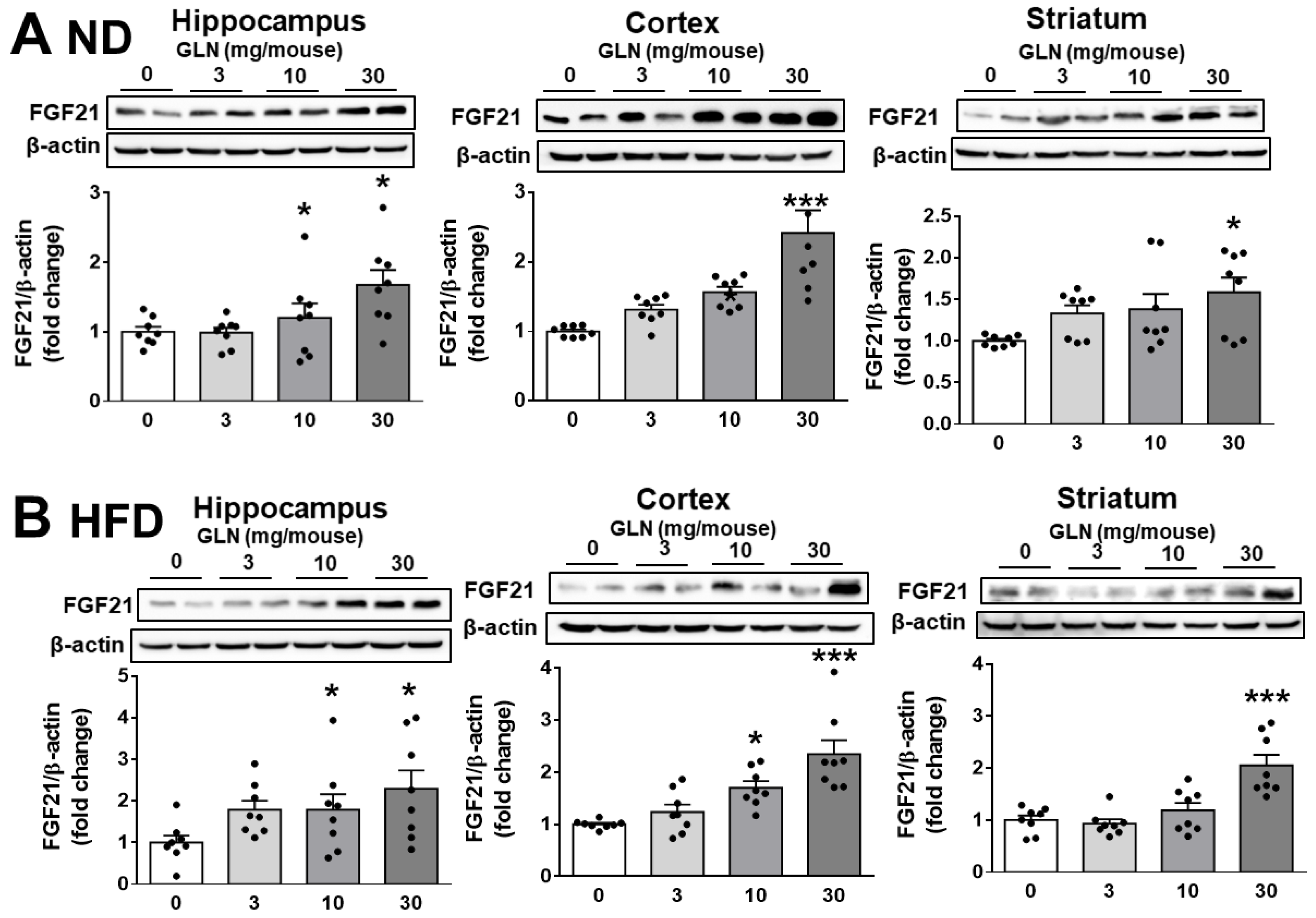
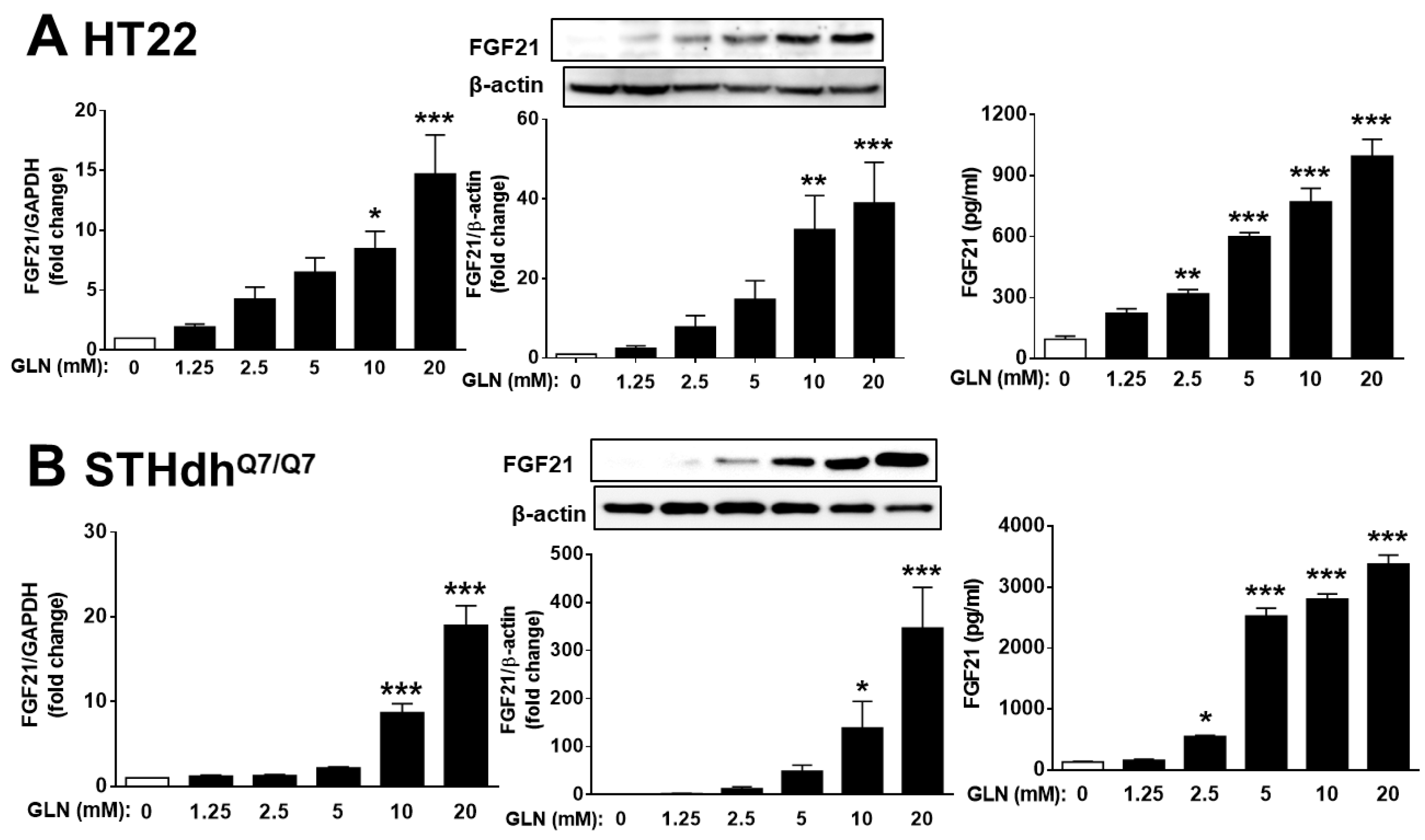
2.2. GLN’s Induction of FGF21 Expression in Brain Tissues and Nerve Cells
2.3. Potential Involvement of FGF21 in the GLN-induced Enhancement of Learning and Memory Functions
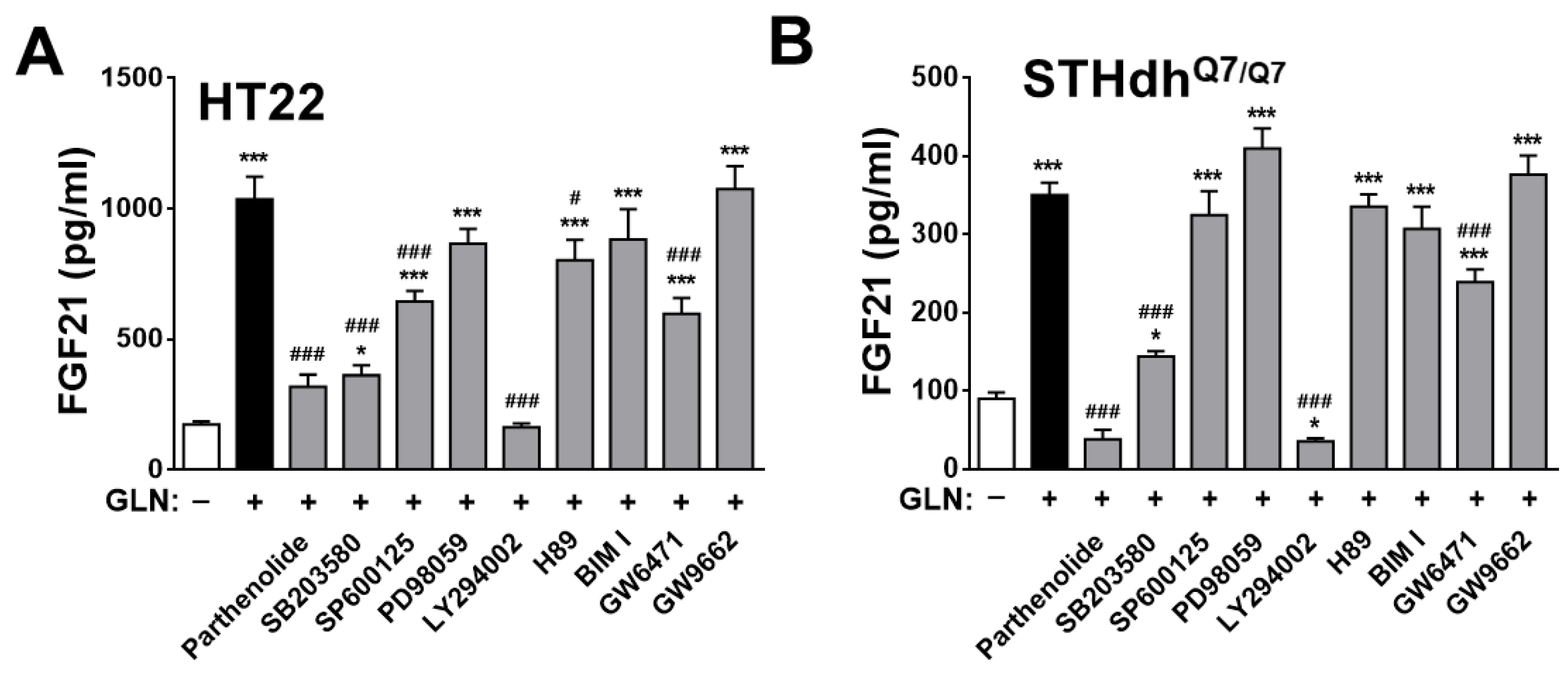
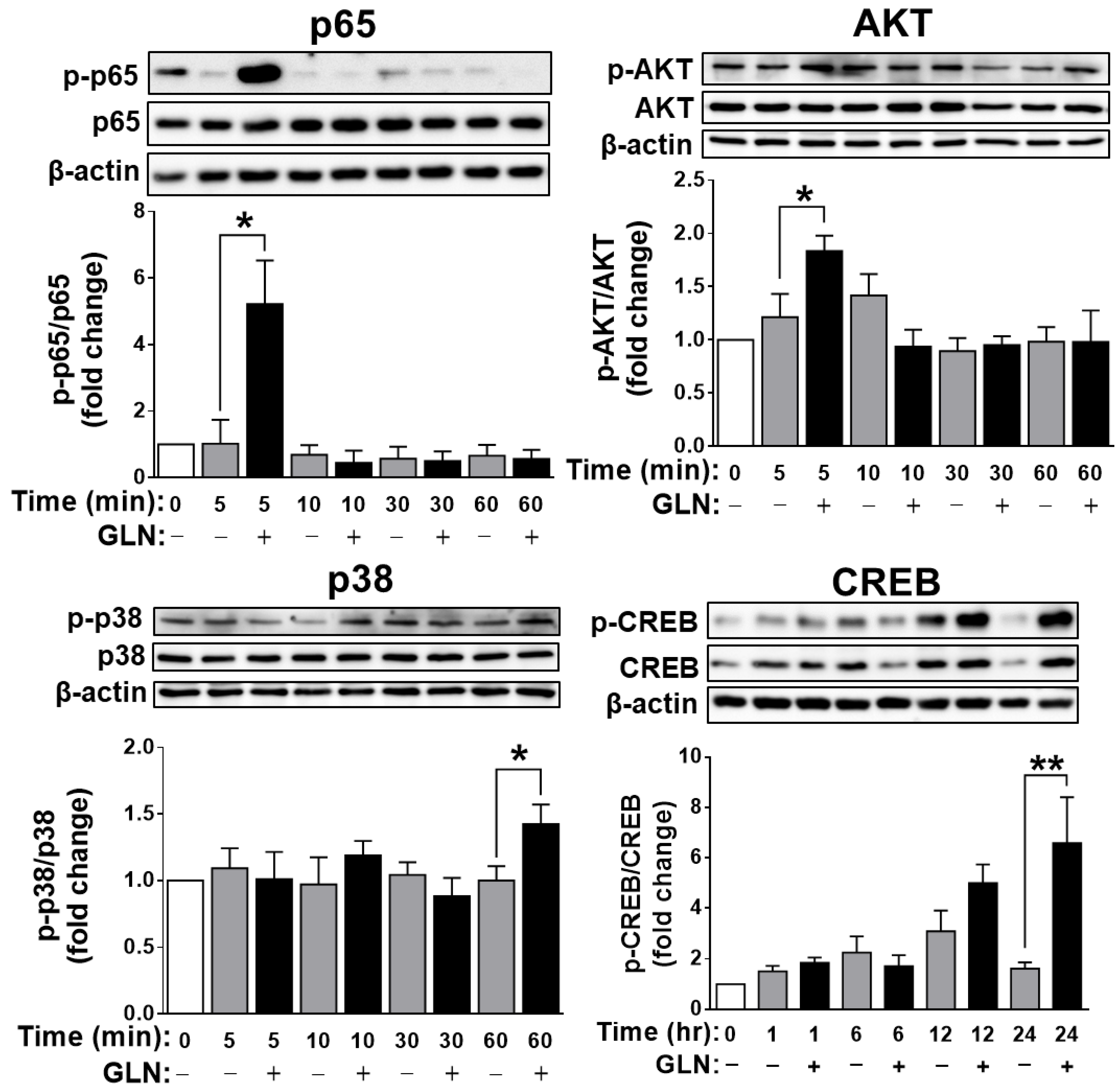
2.4. Identification of the Potential Target Signaling Molecules of GLN for Regulating FGF21 Production
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Ethics
4.3. Animal Experiments
4.4. Cell Culture
4.5. Western Blotting Analysis
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Quantitative Reverse Transcription Polymerase Chain Reaction (Q-RT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef] [PubMed]
- Yavas, E.; Gonzalez, S.; Fanselow, M.S. Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Hebisch, M.; Iefremova, V.; Flitsch, L.J.; Breitkreuz, Y.; Tanzi, R.E.; Kim, D.Y.; Peitz, M.; Brüstle, O. Dissecting Alzheimer’s disease pathogenesis in human 2D and 3D models. Mol. Cell. Neurosci. 2021, 110, 103568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, H.; Ni, Z.; Shen, Y.; Wang, D.; Li, W.; Zhao, L.; Li, C.; Gao, H. Fibroblast growth factor 21 alleviates diabetes-induced cognitive decline. Cereb. Cortex 2024, 34, bhad502. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, K.; Sadeghnia, H.R.; Mohammadi, G.; Kazemabad, A.M.; Hosseini, M. Glucosamine alleviates scopolamine-induced spatial learning and memory deficits in rats. Pathophysiology 2013, 20, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as modulator of metabolism in health and disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Y.B.; Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 2017, 6, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.; Tselykh, T.V.; Maiorana, F.; Eriksson, O.; Do, H.T.; Mudò, G.; Korhonen, L.T.; Belluardo, N.; Lindholm, D. Fibroblast growth factor-21 enhances mitochondrial functions and increases the activity of PGC-1α in human dopaminergic neurons via sirtuin-1. Springerplus 2014, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Prida, E.; Álvarez-Delgado, S.; Pérez-Lois, R.; Soto-Tielas, M.; Estany-Gestal, A.; Fernø, J.; Seoane, L.M.; Quiñones, M.; Al-Massadi, O. Liver brain interactions: Focus on FGF21 a systematic review. Int. J. Mol. Sci. 2022, 23, 13318. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, J.; Yu, Z.; Lin, L.; Jiang, Y.; Cao, Z.; Zhuang, P.; Whalen, M.J.; Song, B.; Wang, X.J.; et al. FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol. Neurobiol. 2018, 55, 4702–4717. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuroo, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lin, H.W.K.; Zhang, Q.; Zong, X. Targeting Alzheimer’s disease: The critical crosstalk between the liver and brain. Nutrients 2022, 14, 4298. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Li, T.; Yu, L.; Li, Y.; Li, M.; Guo, M.; Zhao, J.; Zhai, Q.; Tian, F.; Chen, W. A low-protein, high-carbohydrate diet exerts a neuroprotective effect on mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease by regulating the microbiota-metabolite-brain axis and fibroblast growth factor 21. J. Agric. Food Chem. 2023, 71, 8877–8893. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Zhang, Z.; Ma, B.; Sun, S.; Gao, L.; Gao, G. FGF21 alleviates endothelial mitochondrial damage and prevents BBB from disruption after intracranial hemorrhage through a mechanism involving SIRT6. Mol. Med. 2023, 29, 165. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Xu, P.; Wang, M.; Chunyu, J.; Sun, X.; Ren, G.; Xiao, W.; Li, D. FGF21 attenuates neurodegeneration through modulating neuroinflammation and oxidant-stress. Biomed. Pharmacother. 2020, 129, 110439. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wang, L.; Shi, L.; Jin, X.; Liu, Y.; Liu, R.H.; Yin, F.; Cadenas, E.; Dai, X.; Liu, Z.; et al. Methionine restriction alleviates age-associated cognitive decline via fibroblast growth factor 21. Redox Biol. 2021, 41, 101940. [Google Scholar] [CrossRef] [PubMed]
- Rühlmann, C.; Dannehl, D.; Brodtrück, M.; Adams, A.C.; Stenzel, J.; Lindner, T.; Krause, B.J.; Vollmar, B.; Kuhla, A. Neuroprotective effects of the FGF21 analogue LY2405319. J. Alzheimers Dis. 2021, 80, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Bieberich, E. Synthesis, processing, and function of N-Glycans in N-glycoproteins. Adv. Neurobiol. 2023, 29, 65–93. [Google Scholar] [PubMed]
- Meng, Z.; Liu, J.; Zhou, N. Efficacy and safety of the combination of glucosamine and chondroitin for knee osteoarthritis: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2023, 143, 409–421. [Google Scholar] [CrossRef]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef]
- Kwoh, C.K.; Roemer, F.W.; Hannon, M.J.; Moore, C.E.; Jakicic, J.M.; Guermazi, A.; Green, S.M.; Evans, R.W.; Boudreau, R. Effect of oral glucosamine on joint structure in individuals with chronic knee pain: A randomized, placebo-controlled clinical trial. Arthritis Rheumatol. 2014, 66, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms of anticancer effects of glucosamine. Biomed. Pharmacother. 2017, 95, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, K.; Arasteh, O.; Riahi, M.M.; Mehri, S.; Riahi-Zanjani, B.; Karimi, G. Protective effects of glucosamine hydrochloride against free radical-induced erythrocytes damage. Environ. Toxicol. Pharmacol. 2014, 38, 212–219. [Google Scholar] [CrossRef]
- Jung, A.Y.; Heo, M.J.; Kim, Y.H. Glucosamine has an antiallergic effect in mice with allergic asthma and rhinitis. Int. Forum Allergy Rhinol. 2017, 7, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.Y.; Chuang, K.H.; Sun, D.; Lee, Y.H.; Kao, P.H.; Lin, Y.Y.; Wang, H.W.; Wu, Y.L. Inhibition of PKC-induced COX-2 and IL-8 expression in human breast cancer cells by glucosamine. J. Cell. Physiol. 2015, 230, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Popov, N. Effects of D-galactosamine and D-glucosamine on retention performance of a brightness discrimination task in rats. Biomed. Biochim. Acta 1985, 44, 611–622. [Google Scholar] [PubMed]
- Zhang, G.X.; Yu, S.; Gran, B.; Rostami, A. Glucosamine abrogates the acute phase of experimental autoimmune encephalomyelitis by induction of Th2 response. J. Immunol. 2005, 175, 7202–7208. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Shin, J.H.; Hwang, J.S.; Kim, S.Y.; Shin, J.A.; Oh, E.S.; Oh, S.; Kim, J.B.; Lee, J.K.; Han, I.O. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia 2010, 58, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, C.F.; Vera, A.; Quintana-Peña, V.; Arango-Davila, C.A.; Rengifo, J. Regulation of Tau protein phosphorylation by glucosamine-induced O-GlcNAcylation as a neuroprotective mechanism in a brain ischemia-reperfusion model. Int. J. Neurosci. 2023, 133, 194–200. [Google Scholar] [CrossRef]
- Jhelum, P.; Radhakrishnan, M.; Paul, A.R.S.; Dey, S.K.; Kamle, A.; Kumar, A.; Sharma, A.; Chakravarty, S. Neuroprotective and proneurogenic effects of glucosamine in an internal carotid artery occlusion model of ischemia. Neuromol. Med. 2022, 24, 268–273. [Google Scholar] [CrossRef]
- Marshall, S.; Nadeau, O.; Yamasaki, K. Glucosamine-induced activation of glycogen biosynthesis in isolated adipocytes. Evidence for a rapid allosteric control mechanism within the hexosamine biosynthesis pathway. J. Biol. Chem. 2005, 280, 11018–11024. [Google Scholar] [CrossRef] [PubMed]
- Dostrovsky, N.R.; Towheed, T.E.; Hudson, R.W.; Anastassiades, T.P. The effect of glucosamine on glucose metabolism in humans: A systematic review of the literature. Osteoarthr. Cartil. 2011, 19, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Nicolosi, R.J.; Borzelleca, J.F. Glucosamine effects in humans: A review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem. Toxicol. 2005, 43, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.R.; Marks, V.; Leeds, A.R.; Anderson, J.W. A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab. Res. Rev. 2011, 27, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver plays a major role in FGF-21 mediated glucose homeostasis. Cell. Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- Sarruf, D.A.; Thaler, J.P.; Morton, G.J.; German, J.; Fischer, J.D.; Ogimoto, K.; Schwartz, M.W. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 2010, 59, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Park, J.W.; Nam, M.S.; Cho, H.; Han, I.O. Glucosamine enhances body weight gain and reduces insulin response in mice fed chow diet but mitigates obesity, insulin resistance and impaired glucose tolerance in mice high-fat diet. Metabolism 2015, 64, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Fibroblast growth factor 21 in metabolic syndrome. Front. Endocrinol. 2023, 14, 1220426. [Google Scholar] [CrossRef]
- Chou, L.Y.; Chao, Y.M.; Peng, Y.C.; Lin, H.C.; Wu, Y.L. Glucosamine enhancement of BDNF expression and animal cognitive function. Molecules 2020, 25, 3667. [Google Scholar] [CrossRef]
- Chen, T.Y.; Sun, D.; Lin, W.S.; Lin, Y.L.; Chao, Y.M.; Chen, S.Y.; Chen, Y.R.; Wu, Y.L. Glucosamine regulation of fibroblast growth factor 21 expression in liver and adipose tissues. Biochem. Biophys. Res. Commun. 2020, 529, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Nao, J. Focus on Alzheimer’s disease: The role of fibroblast growth factor 21 and autophagy. Neuroscience 2023, 511, 13–28. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Deng, P.; Wang, X.; Zhu, L.; Zhao, L.; Li, C.; Gao, H. Fibroblast growth factor 21 ameliorates behavior deficits in Parkinson’s disease mouse model via modulating gut microbiota and metabolic homeostasis. CNS Neurosci. Ther. 2023, 29, 3815–3828. [Google Scholar] [CrossRef]
- Pardo, O.E.; Latigo, J.; Jeffery, R.E.; Nye, E.; Poulsom, R.; Spencer-Dene, B.; Lemoine, N.R.; Stamp, G.W.; Aboagye, E.O.; Seckl, M.J. The fibroblast growth factor receptor inhibitor PD173074 blocks small cell lung cancer growth in vitro and in vivo. Cancer Res. 2009, 69, 8645–8651. [Google Scholar] [CrossRef] [PubMed]
- Arias-Calderón, M.; Casas, M.; Balanta-Melo, J.; Morales-Jiménez, C.; Hernández, N.; Llanosm, P.; Jaimovich, E.; Buvinic, S. Fibroblast growth factor 21 is expressed and secreted from skeletal muscle following electrical stimulation via extracellular ATP activation of the PI3K/Akt/mTOR signaling pathway. Front. Endocrinol. 2023, 14, 1059020. [Google Scholar] [CrossRef]
- Bae, K.H.; Kim, J.G.; Park, K.G. Transcriptional regulation of fibroblast growth factor 21 expression. Endocrinol. Metab. 2014, 29, 105–111. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A.; Marty, M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res. Ther. 2012, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.G.; Samarasinghe, R.K. Review article: Glucosamine. J. Orthop. Surg. 2009, 17, 72–76. [Google Scholar] [CrossRef]
- Kim, C.H.; Cheong, K.A.; Park, C.D.; Lee, A.Y. Glucosamine improved atopic dermatitis-like skin lesions in NC/Nga mice by inhibition of Th2 cell development. Scand. J. Immunol. 2011, 73, 536–545. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef]
- Pedditzi, E.; Peters, R.; Beckett, N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.D.; Williamson, R.; Balfour, D.J.; Stewart, C.A.; Sutherland, C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia 2012, 55, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Gainey, S.J.; Kwakwa, K.A.; Bray, J.K.; Pillote, M.M.; Tir, V.L.; Towers, A.E.; Freund, G.G. Short term high-fat diet (HFD) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front. Behav. Neurosci. 2016, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Satjaritanun, P.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Pratchayasakul, W.; Chattipakorn, N.; et al. FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm. Behav. 2016, 85, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease. Redox Biol. 2019, 22, 101133. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bai, F.; Wang, W.; Liu, Y.; Yuan, Q.; Qu, S.; Zhang, T.; Tian, G.; Li, S.; Li, D.; et al. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol. Biochem. Behav. 2015, 133, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Froum, S.; Hamby, J.M.; Schroeder, M.C.; Panek, R.L.; Lu, G.H.; Eliseenkova, A.V.; Green, D.; Schlessinger, J.; Hubbard, S.R. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998, 17, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Wei, X.; Yin, T.; Xia, Y.; Li, D.; Shao, B.; Song, X.; He, S.; Luo, M.; Gao, X.; et al. Inhibition of FGFR signaling by PD173074 improves antitumor immunity and impairs breast cancer metastasis. Breast Cancer Res. Treat. 2014, 143, 435–446. [Google Scholar] [CrossRef]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef]
- Chen, P.; Tang, H.; Zhang, Q.; Xu, L.; Zhou, W.; Hu, X.; Deng, Y.; Zhang, L. Basic fibroblast growth factor (bFGF) protects the blood-brain barrier by binding of FGFR1 and activating the ERK signaling pathway after intra-abdominal hypertension and traumatic brain injury. Med. Sci. Monit. 2020, 26, e922009. [Google Scholar] [CrossRef]
- Hynes, N.E.; Dey, J.H. Potential for targeting the fibroblast growth factor receptors in breast cancer. Cancer Res. 2010, 70, 5199–5202. [Google Scholar] [CrossRef] [PubMed]
- Jeanson, Y.; Ribas, F.; Galinier, A.; Arnaud, E.; Ducos, M.; André, M.; Chenouard, V.; Villarroya, F.; Casteilla, L.; Carrière, A.; et al. Lactate induces FGF21 expression in adipocytes through a P38-MAPK pathway. Biochem. J. 2016, 473, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, H.A.; Alonge, K.M.; Ippagunta, S.M.; Hillgartner, F.B. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS ONE 2014, 29, e94996. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hirata, M.; Aoki, Y.; Iwase, M.; Takahashi, H.; Kim, M.; Li, Y.; Jheng, H.F.; Nomura, W.; Takahashi, N. The hepatokine FGF21 is crucial for peroxisome proliferator-activated receptor-alpha agonist-induced amelioration of metabolic disorders in obese mice. J. Biol. Chem. 2017, 292, 9175–9190. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.; Moreau, R. The regulation of FGF21 gene expression by metabolic factors and nutrients. Horm Mol. Biol. Clin. Investig. 2016, 30, 20160016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Wang, X.; Deng, P.; He, W.; Zheng, H.; Zhao, L.; Gao, H. Integration of FGF21 signaling and metabolomics in high-fat diet-induced obesity. J. Proteome Res. 2021, 20, 3900–3912. [Google Scholar] [CrossRef]
- Hua, L.; Li, J.; Yang, Y.; Jiang, D.; Jiang, X.; Han, X.; Chao, J.; Feng, B.; Che, L.; Xu, S.; et al. Liver-derived FGF21 is required for the effect of time-restricted feeding on high-fat diet-induced fatty liver in mice. FASEB J. 2023, 37, e22898. [Google Scholar] [CrossRef]
- Shi, X.; Bai, H.; Wang, J.; Wang, J.; Huang, L.; He, M.; Zheng, X.; Duan, Z.; Chen, D.; Zhang, J.; et al. Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Front. Neurol. 2021, 12, 667511. [Google Scholar] [CrossRef]
- Degirmenci, Y.; Angelopoulou, E.; Georgakopoulou, V.E.; Bougea, A. Cognitive impairment in Parkinson’s disease: An updated overview focusing on emerging pharmaceutical treatment approaches. Medicina 2023, 59, 1756. [Google Scholar] [CrossRef]
- Ibrahim, A.; Gilzad-kohan, M.H.; Aghazadeh-Habashi, A.; Jamali, F. Absorption and bioavailability of glucosamine in the rat. J. Pharm. Sci. 2012, 101, 2574–2583. [Google Scholar] [CrossRef]
- Asthana, C.; Peterson, G.M.; Shastri, M.D.; Jones, G.; Patel, R.P. Variation in plasma levels of glucosamine with chronic dosing: A possible reason for inconsistent clinical outcomes in osteoarthritis. Clin. Ther. 2020, 42, e140–e149. [Google Scholar] [CrossRef] [PubMed]
- Asthana, C.; Peterson, G.M.; Shastri, M.D.; Patel, R.P. Variation in the pharmacokinetics of glucosamine in healthy individuals. Rheumatology 2021, 60, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Dhuli, K.; Medori, M.C.; Caruso, P.; Manganotti, P.; Chiurazzi, P.; Bertelli, M. Dietary supplements in neurological diseases and brain aging. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E174–E188. [Google Scholar]
- Petersen, M.J.; Bergien, S.O.; Staerk, D. A systematic review of possible interactions for herbal medicines and dietary supplements used concomitantly with disease-modifying or symptom-alleviating multiple sclerosis drugs. Phytother. Res. 2021, 35, 3610–3631. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, S.; Gan, L.; Wang, J.; Hu, B.; Xu, H.; Tong, R.; Yang, H.; Cristina, I.; Xue, J.; et al. FGFR1 signaling potentiates tumor growth and predicts poor prognosis in esophageal squamous cell carcinoma patients. Cancer Biol. Ther. 2018, 19, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, X.; Cai, C.; Zeng, S.; Bai, J.; Guo, K.; Fang, M.; Hu, J.; Liu, H.; Zhu, L.; et al. FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/beta-klotho. Exp. Neurol. 2019, 317, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Krafft, P.R.; Ma, Q.; Rolland, W.B.; Caner, B.; Lekic, T.; Manaenko, A.; Le, M.; Tang, J.; Zhang, J.H. Fibroblast growth factors preserve blood brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol. Dis. 2012, 46, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, L.; Hu, J.; Guo, R.; Ye, S.; Liu, F.; Wang, D.; Zhao, Y.; Hu, A.; Wang, X.; et al. FGF21 attenuated LPS-Induced depressive-like behavior via inhibiting the inflammatory pathway. Front. Pharmacol. 2020, 11, 154. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Q.; Huang, Q.; Gu, Q.; Zhu, Y.; Tang, M.; Tian, S.; Wang, L.; Yan, F.; Ge, J.; et al. FGF21 prevents neuronal cell ferroptosis after spinal cord injury by activating the FGFR1/beta-Klotho pathway. Brain Res. Bull. 2023, 202, 110753. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar]
- Davis, J.B.; Maher, P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994, 652, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Trettel, F.; Rigamonti, D.; Hilditch-Maguire, P.; Wheeler, V.C.; Sharp, A.H.; Persichetti, F.; Cattaneo, E.; MacDonald, M.E. Dominant phenotypes produced by the Hd mutation in Sthdh(Q111) striatal cells. Hum. Mol. Genet. 2000, 9, 2799–2809. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, Y.-M.; Wu, H.-Y.; Yeh, S.-H.; Yang, D.-I.; Her, L.-S.; Wu, Y.-L. Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production. Int. J. Mol. Sci. 2024, 25, 4211. https://doi.org/10.3390/ijms25084211
Chao Y-M, Wu H-Y, Yeh S-H, Yang D-I, Her L-S, Wu Y-L. Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production. International Journal of Molecular Sciences. 2024; 25(8):4211. https://doi.org/10.3390/ijms25084211
Chicago/Turabian StyleChao, Yu-Ming, Hon-Yen Wu, Sin-Huei Yeh, Ding-I Yang, Lu-Shiun Her, and Yuh-Lin Wu. 2024. "Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production" International Journal of Molecular Sciences 25, no. 8: 4211. https://doi.org/10.3390/ijms25084211
APA StyleChao, Y.-M., Wu, H.-Y., Yeh, S.-H., Yang, D.-I., Her, L.-S., & Wu, Y.-L. (2024). Glucosamine Enhancement of Learning and Memory Functions by Promoting Fibroblast Growth Factor 21 Production. International Journal of Molecular Sciences, 25(8), 4211. https://doi.org/10.3390/ijms25084211




