Green Approach for Synthesis of Silver Nanoparticles with Antimicrobial and Antioxidant Properties from Grapevine Waste Extracts
Abstract
1. Introduction
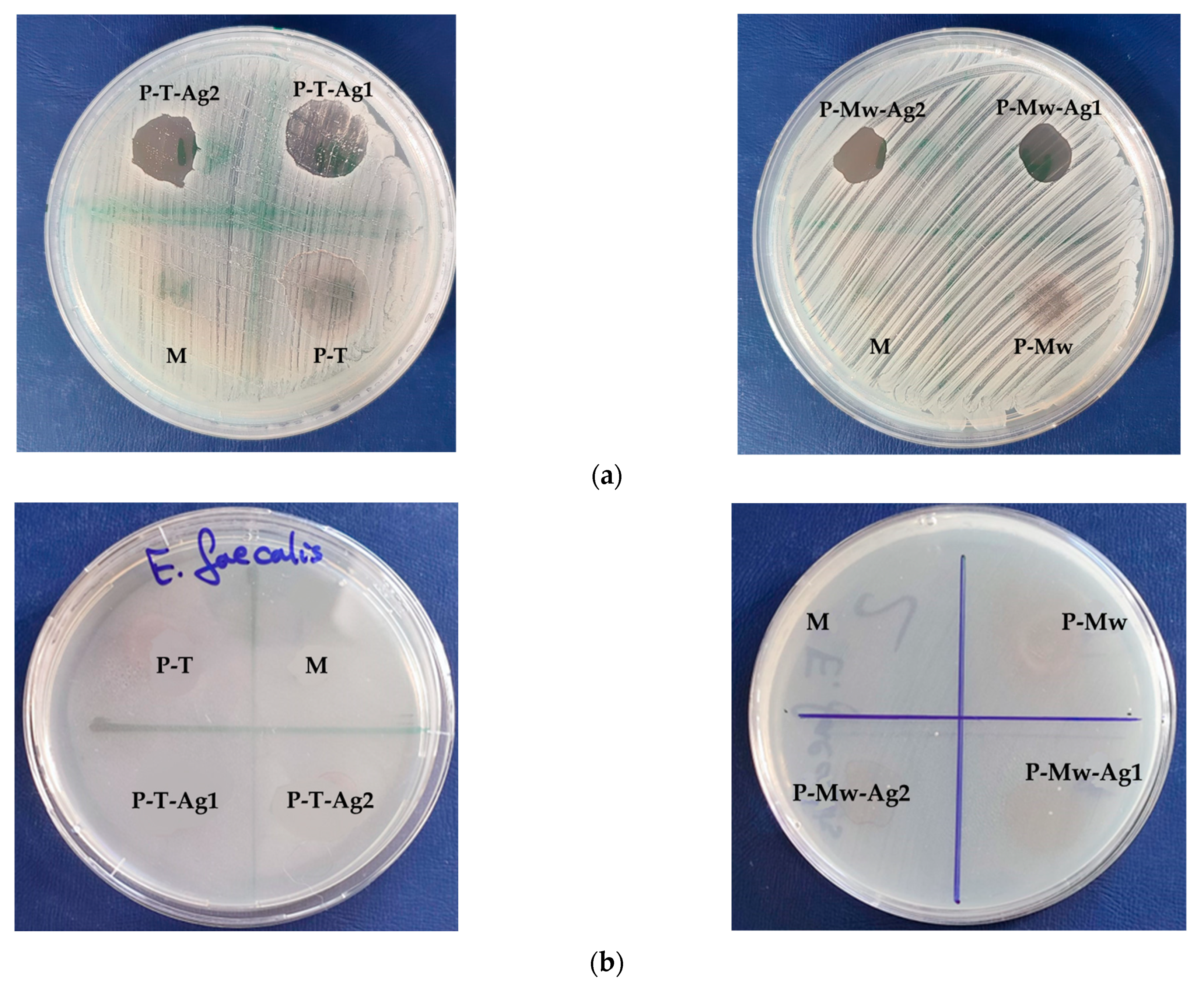
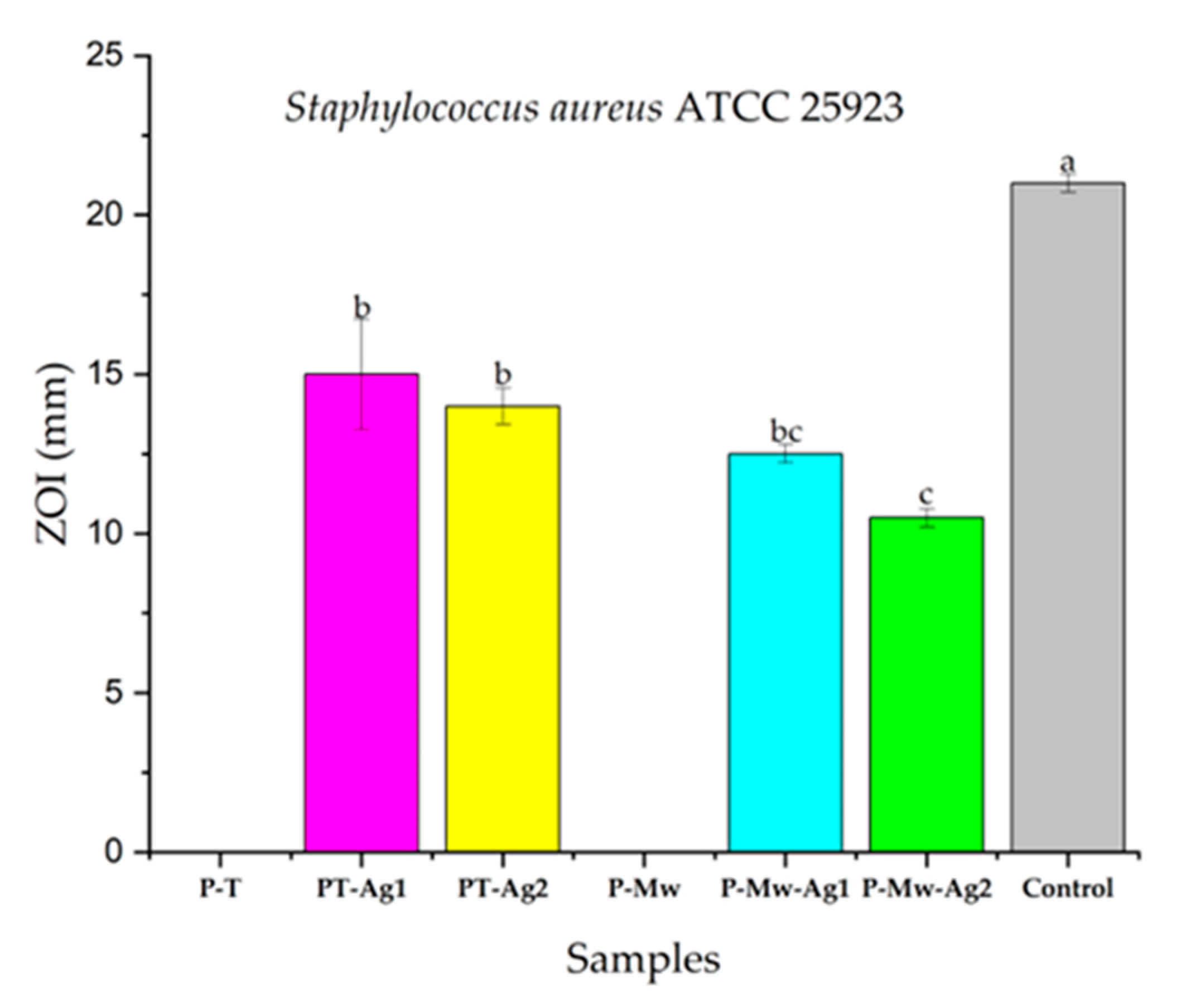
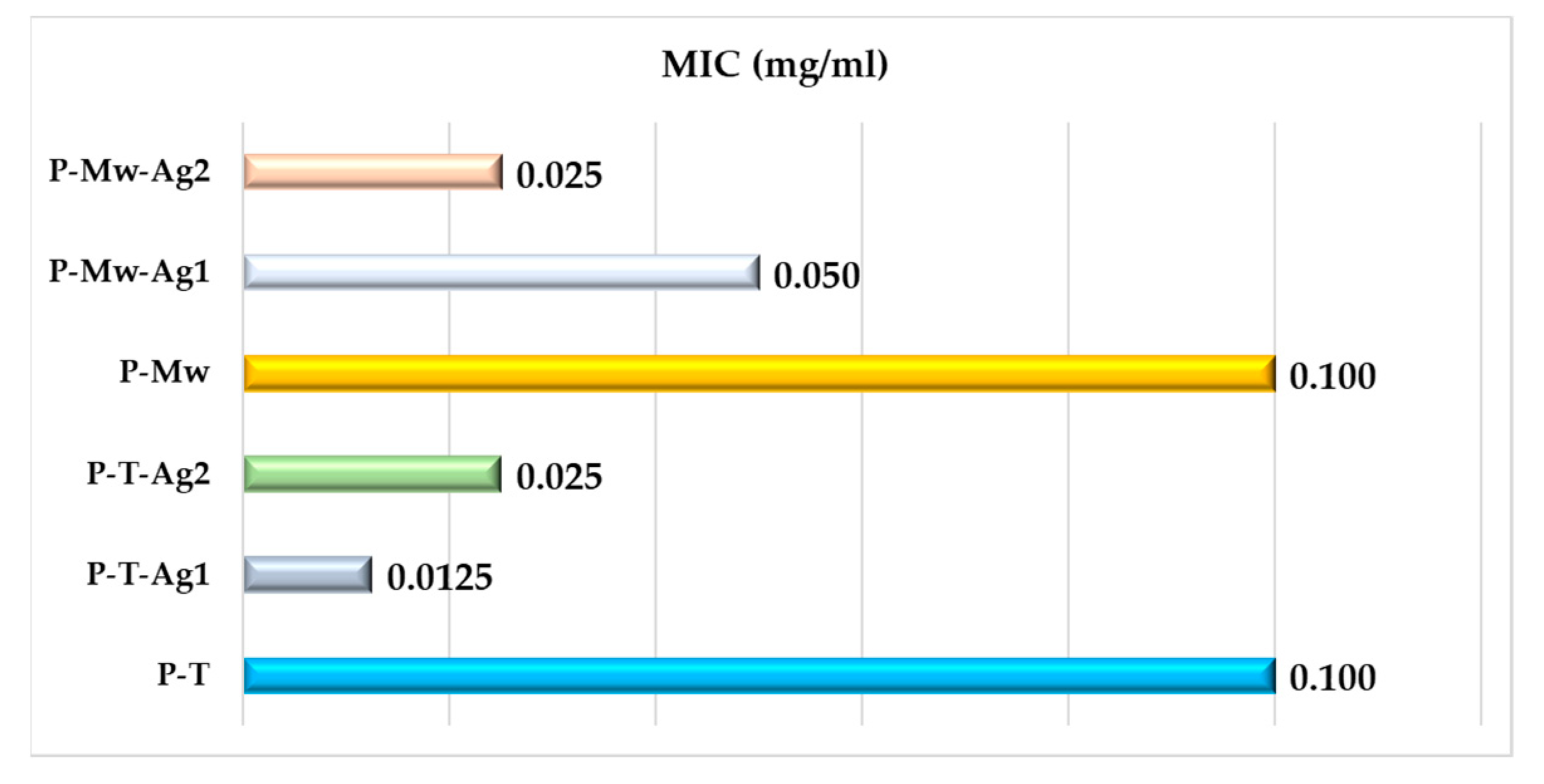
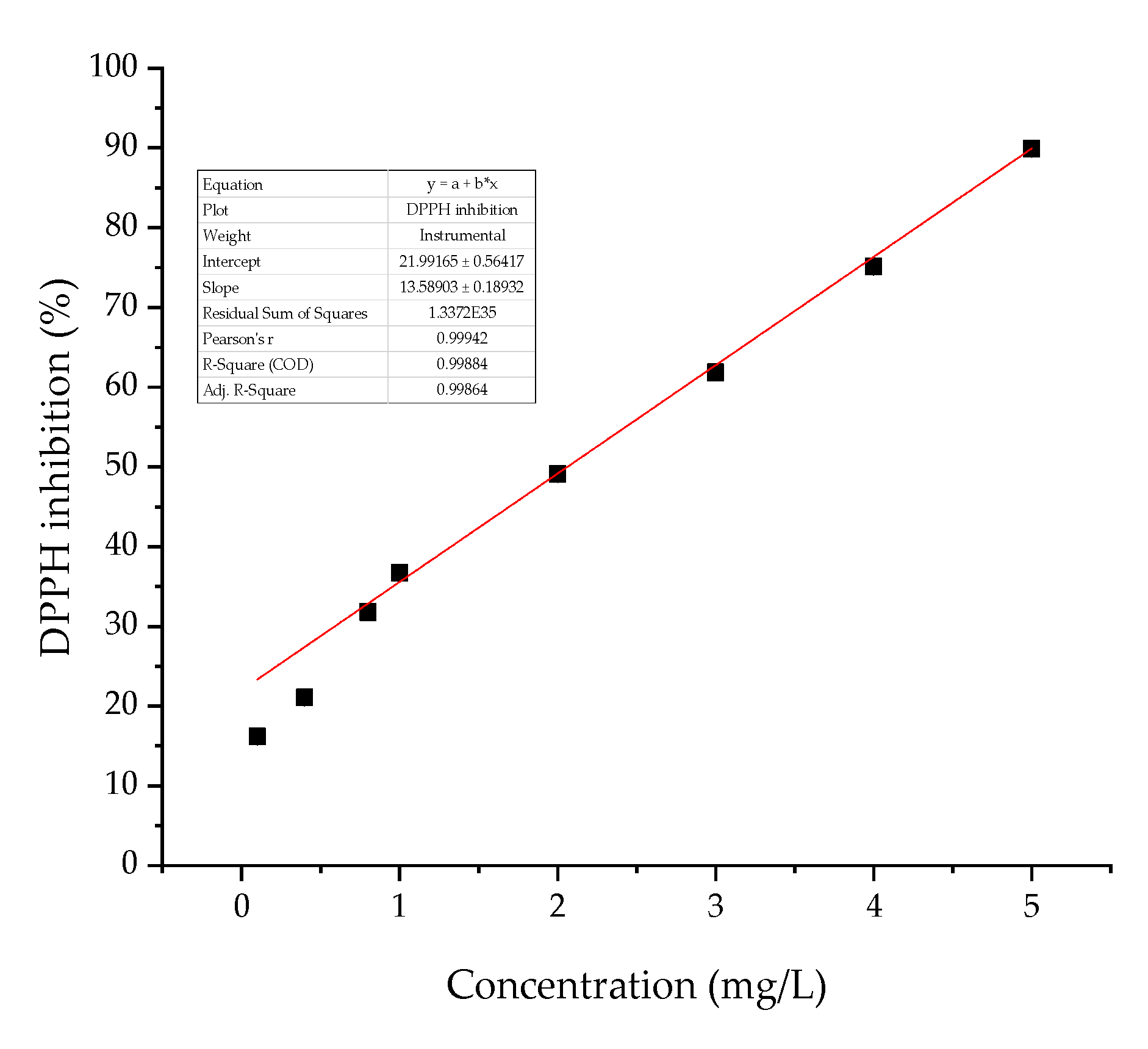
2. Results
3. Discussion
- Cell membrane damage. AgNPs can interact with the bacterial cell membrane, leading to a disruption of the membrane’s integrity. This disruption causes a leakage of intracellular components, loss of membrane potential, and eventual cell death. AgNPs can penetrate the bacterial cell membrane, disrupting its structure and function. This penetration disturbs membrane-bound proteins and enzymes, affecting essential cellular processes [47,48].
- Generation of reactive oxygen species (ROS). AgNPs can generate ROS, such as superoxide radicals (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−). These ROS induce oxidative stress within bacterial cells, damaging proteins, lipids, and DNA. ROS-mediated damage to cellular components disrupts bacterial metabolism, impairs cellular respiration, and leads to cell death [48].
- Inhibition of DNA replication and protein synthesis. AgNPs can bind to bacterial DNA, interfering with DNA replication and transcription processes. This binding disrupts DNA integrity, leading to DNA strand breaks, replication errors, and inhibition of protein synthesis. AgNPs can also interact with ribosomal subunits, inhibiting protein synthesis and impairing bacterial growth and proliferation [48,49].
- Disruption of electron transport chain (ETC). AgNPs can interfere with the electron transport chain (ETC) in bacterial cells, disrupting ATP synthesis and energy metabolism. This disruption leads to a depletion of cellular energy stores, metabolic dysfunction, and, ultimately, cell death. The inhibition of ETC components such as cytochromes and other electron carriers by AgNPs impairs bacterial respiration and ATP production [48].
- Induction of apoptosis-like cell death. AgNPs can trigger apoptosis-like cell-death pathways in bacteria, leading to programmed cell death. This process involves the activation of caspase-like enzymes, DNA fragmentation, and morphological changes characteristic of apoptosis. Apoptosis-like cell death induced by AgNPs prevents the release of inflammatory mediators and reduces the risk of inflammatory responses associated with bacterial infections [50].
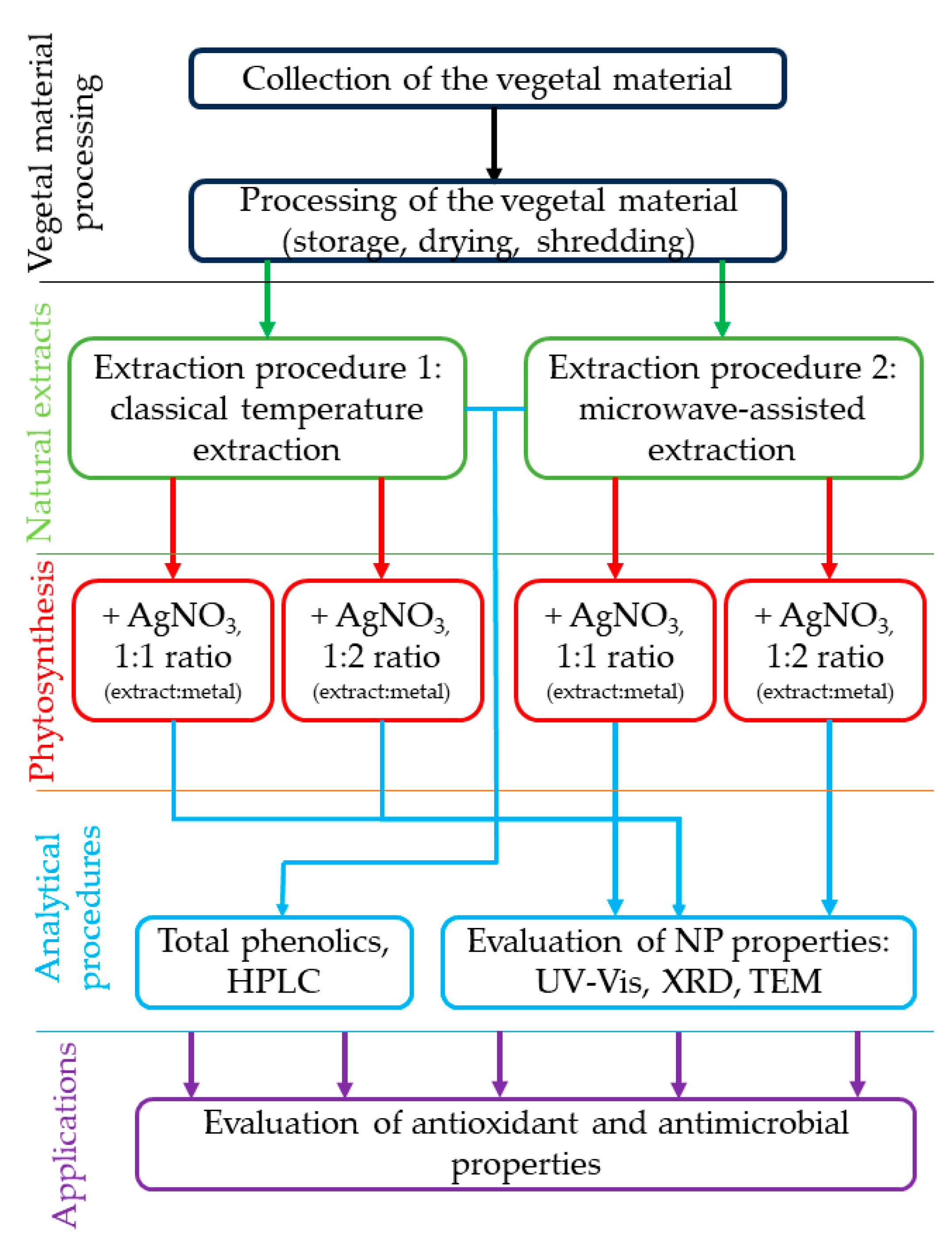
4. Materials and Methods
4.1. Collection and Processing of Vegetal Waste Material
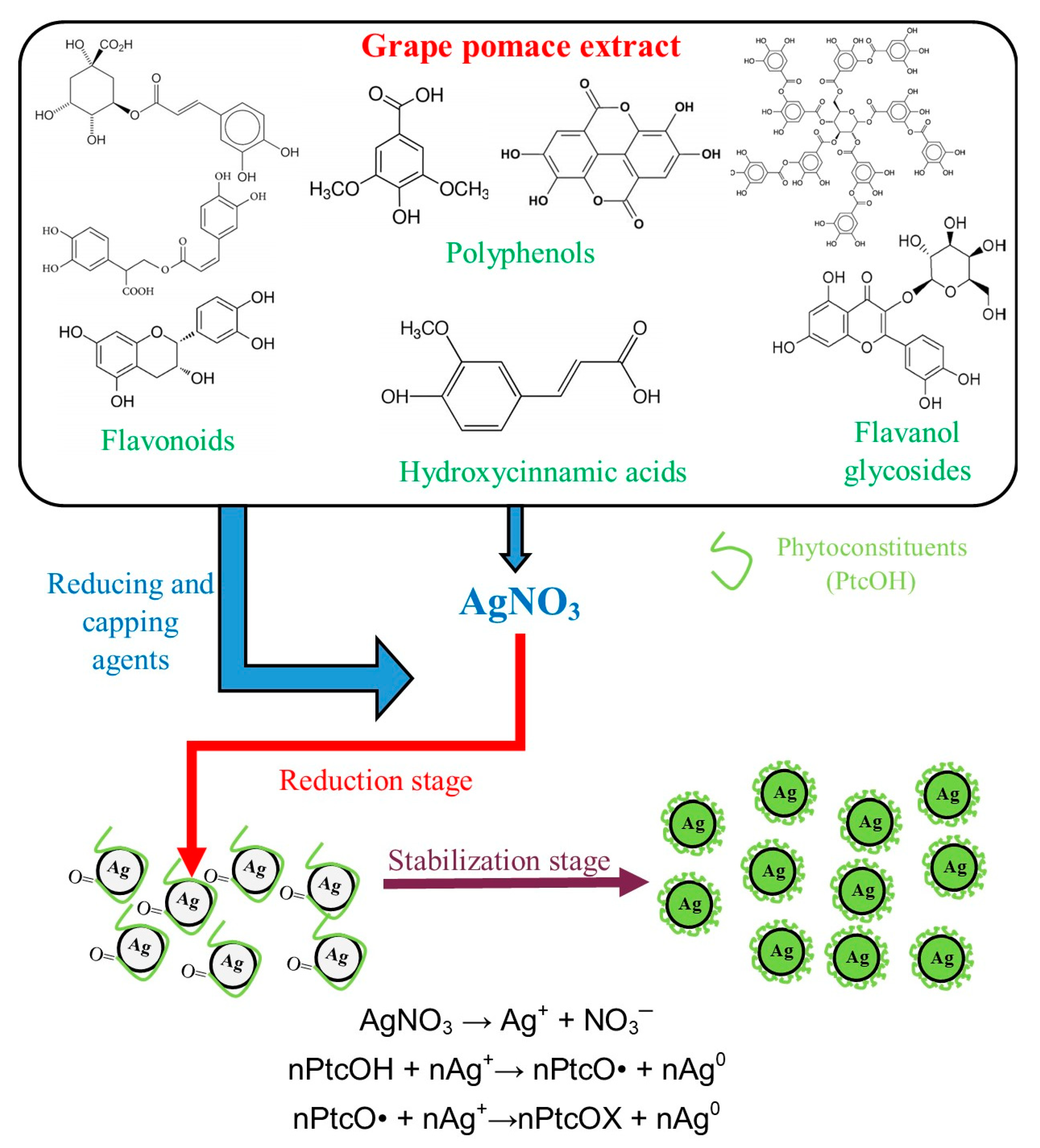
4.2. Preparation and Characterization of Grape Pomace (P) Extracts and Nanoparticle Phytosynthesis
- Classical temperature extraction (T). The grapevine waste material was mixed with the solvent, and the extraction was carried out using a BOV-T70C oven (Biobase, Shandong, China) with an extraction time of 60 min and at a temperature of 60 °C; the obtained extract was encoded as P-T.
- Microwave-assisted extraction (Mw). The weighed vegetal material was placed in Teflon tubes, to which the solvent (in the previously presented ratio) was added. The extraction was performed using an Ethos Easy Advanced Microwave Digestion System (Milestone Srl, Sorisole, Italy). The extraction parameters were as follows: extraction time: 30 min, extraction temperature: 60 °C, maximum microwave power: 1000 W; the obtained extract was encoded P-Mw.
4.3. Evaluation of Antioxidant and Antimicrobial Properties
4.4. Statistical Interpretation and Data Representation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garrido, R.A.; Manrique, R.; Fredes, J.; Rodriguez, P.; Rodríguez, A.; Serafini, D.; Mena, M.; Masip, Y.; Díaz, I. Evaluating hydrogen production from grape pomace gasification: Unveiling the potential for Chile’s wine industry and its solid waste recovery as energy source. Renew. Energy 2024, 223, 119953. [Google Scholar] [CrossRef]
- Duarte, H.; Aliaño-González, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable extraction of polyphenols from vine shoots using deep eutectic solvents: Influence of the solvent, Vitis sp., and extraction technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Smith, D.; Shahabi, J.; Gee, T.; Nejati, M.; McGuinness, B.; Black, K.; Tobias, J.; Jangali, R.; Lim, H.; et al. Modelling wine grapevines for autonomous robotic cane pruning. Biosyst. Eng. 2023, 235, 31–49. [Google Scholar] [CrossRef]
- Ginni, G.; Kavith, S.; Yukesh Kannah, R.; Kant Bhatia, S.; Adish Kumar, S.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Richard, T.; Cantos-Villar, E. Grapevine Cane extracts: Raw plant material, extraction methods, quantification, and applications. Biomolecules 2020, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Enes Dapkevicius, M.d.L.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Cheng, T.M.; Chu, H.L.; Tan, S.H.; Kuo, J.C.; Hsu, P.H.; Su, C.Y.; Chen, H.M.; Lee, C.M.; Kuo, T.R. Metabolic Mechanism Investigation of Antibacterial Active Cysteine-Conjugated Gold Nanoclusters in Escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Turner, S.; Raisley, B.; Roach, K.; Bajaña, S.; Munroe, M.E.; James, J.A.; Coggeshall, K.M.; Kovats, S. Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1β and Promote TH17 Cell Differentiation. Microorganisms 2023, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishnan, M.; Haryini, S.; Priya Doss, G. Emerging strategies and therapeutic innovations for combating drug resistance in Staphylococcus aureus strains: A comprehensive review. J. Basic Microbiol. 2024, 1–33. [Google Scholar] [CrossRef]
- Zilong, D.; Binbin, L.; Fan, L.; Wanghong, Z. Role of Enterococcus faecalis in refractory apical periodontitis: From pathogenicity to host cell response. J. Oral Microbiol. 2023, 15, 1. [Google Scholar]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; Saavedra, M.J.; Barros, A.; Gouvinhas, I. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Lores, M.; Rama, J.L.R.; Villarino, R.-A.; Calvo, L.G.; Castillo, A.; Celeiro, M.; de Miguel, T. Antibacterial Activity against Clinical Strains of a Natural Polyphenolic Extract from Albariño White Grape Marc. Pharmaceuticals 2023, 16, 950. [Google Scholar] [CrossRef] [PubMed]
- El-Sawi, S.A.; Ragab, N.A.; Sleem, A.A.; Farghaly, A.A.; Awad, G.E.A.; Maamoun, M.A.I. Principal component analysis of phenolic compounds of grape waste parts and correlations to their bioassays, Biocatal. Agric. Biotechnol. 2023, 51, 102780. [Google Scholar]
- Muddassir, M.; Raza, A.; Munir, S.; Basirat, A.; Ahmed, M.; Butt, M.S.; Dar, O.A.; Ahmed, S.S.; Shamim, S.; Naqvi, S.Z.H. Antibacterial efficacy of silver nanoparticles (AgNPs) against metallo-β-lactamase and extended spectrum β-lactamase producing clinically procured isolates of Pseudomonas aeruginosa. Sci. Rep. 2022, 12, 20685. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Singh Dhanjal, D.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Singh, P.; Kumar, S.; Kumar, P.; Bhankar, V.; Kumar, K. Plant-mediated synthesis of nanoparticles and their applications: A review. Mat. Res. Bull. 2023, 163, 112233. [Google Scholar] [CrossRef]
- Fierascu, I.C.; Fierascu, I.; Baroi, A.M.; Ungureanu, C.; Spinu, S.; Avramescu, S.M.; Somoghi, R.; Fierascu, R.C.; Dinu-Parvu, C.E. Phytosynthesis of Silver Nanoparticles Using Leonurus cardiaca L. Extracts. Materials 2023, 16, 3472. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Fierascu, I.C.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C. Phytosynthesized Metallic Nanoparticles—Between Nanomedicine and Toxicology. A Brief Review of 2019′s Findings. Materials 2020, 13, 574. [Google Scholar] [CrossRef]
- Shiraz, M.; Imtiaz, H.; Azam, A.; Hayat, S. Phytogenic nanoparticles: Synthesis, characterization, and their roles in physiology and biochemistry of plants. Biometals 2023, 37, 23–70. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.; Naeem, I.; Sahi, S.T.; Rajput, N.A.; Haider, E.; Usman, M.; Shahbaz, H.; Fatima, K.; Arif, E.; Qayyum, A. Nanoparticles: A safe way towards fungal diseases. Arch. Phytopathol. Plant Prot. 2020, 53, 781–792. [Google Scholar] [CrossRef]
- Onache, P.A.; Geana, E.I.; Ciucure, C.T.; Florea, A.; Sumedrea, D.I.; Ionete, R.E.; Tița, O. Bio-active Phytochemical Composition of Grape Pomace Resulted from Different White and Red Grape Cultivars. Separations 2022, 9, 395. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A comparative analysis of the “green” techniques applied for polyphenols extraction from bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
- Balea, S.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera L. var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.M.; Dabić Zagorac, D.C.; Ćirić, I.Z.; Pergal, M.V.; Relić, D.J.; Todić, S.R.; Natić, M.M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Jančářová, I.; Jančář, L.; Náplavová, A.; Kubáň, V. Changes of organic acids and phenolic compounds contents in grapevine berries during their ripening. Open Chem. 2013, 11, 1575–1582. [Google Scholar] [CrossRef]
- Daskalakis, I.; Stavrakaki, M.; Sotirakoglou, K.; Biniari, K. Variations in the Levels of Individual Phenolic Compounds in Grapevine Latent Buds during Eco-Dormancy, Following Chemically-Induced Stress Conditions. Agronomy 2021, 11, 1798. [Google Scholar] [CrossRef]
- Akbar, A.; Gul, Z.; Hussain, N.; Al Haddad, A.H.I.; Khan, N.A.; Sadiq, M.B.; Sher, H. High throughput biochemical profiling, and functional potential analysis for valorization of grape peduncles. Sci. Rep. 2023, 13, 8328. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Sultana, M.S.; Kurnianta, H. Polyphenolic Contents and Antioxidant Activities of Underutilized Grape (Vitis vinifera L.) Pomace Extracts. Prev. Nutr. Food Sci. 2015, 20, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Untea, A.E.; Varzaru, I.; Vlaicu, P.A.; Turcu, R.P.; Panaite, T.D. Studies on antioxidant activities of grape pomace using in vitro, ex vivo, and in vivo models. Food Meas. 2023, 17, 121–128. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Singh, R.P.; Jayaprakasha, G.K. Antioxidant Activities of Grape (Vitis vinifera) Pomace Extracts. J. Agric. Food Chem. 2002, 50, 5909–5914. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly)phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Tyagi, P.K.; Bhatnagar, S.; Bachheti, R.K.; Bachheti, A.; Ghorbanpour, M. Biosynthesis and assessment of antibacterial and antioxidant activities of silver nanoparticles utilizing Cassia occidentalis L. seed. Sci. Rep. 2024, 14, 7243. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Silva, C.S.; Tonelli, F.M.P.; Delgado, V.M.S.; Lourenço, V.d.O.; Pinto, G.d.C.; Azevedo, L.S.; Lima, L.A.R.d.S.; Furtado, C.A.; Ferreira, D.R.C.; Tonelli, F.C.P.; et al. Nanoremediation and Antioxidant Potential of Biogenic Silver Nanoparticles Synthesized Using Leucena’s Leaves, Stem, and Fruits. Int. J. Mol. Sci. 2024, 25, 3993. [Google Scholar] [CrossRef]
- Bailey, R.G.; Turner, R.D.; Mullin, N.; Clarke, N.; Foster, S.J.; Hobbs, J.K. The Interplay between Cell Wall Mechanical Properties and the Cell Cycle in Staphylococcus aureus. Biophys. J. 2014, 107, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.D.; Wallace, A.G.; Foster, E.E.; Kim, S.J. Peptidoglycan Compositional Analysis of Enterococcus faecalis Biofilm by Stable Isotope Labeling by Amino Acids in a Bacterial Culture. Biochemistry 2018, 57, 1274–1283. [Google Scholar] [CrossRef]
- Snowden, M.A.; Perkins, H.R. Peptidoglycan cross-linking in Staphylococcus aureus. Eur. J. Biochem. 1990, 191, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Singh, M.; Kim, S.J.; Schaefer, J. Characterization of the tertiary structure of the peptidoglycan of Enterococcus faecalis. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Choo, P.Y.; Wang, C.Y.; VanNieuwenhze, M.S.; Kline, K.A. Spatial and temporal localization of cell wall associated pili in Enterococcus faecalis. Mol. Microbiol. 2023, 119, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram-positive and Gram-negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Swolana, D.; Wojtyczka, R.D. Activity of Silver Nanoparticles against Staphylococcus spp. Int. J. Mol. Sci. 2022, 23, 4298. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha Piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Bokhari, N.A.; Soliman, D.A. Antibacterial activity of Phoenix dactylifera L. leaf and pit extracts against selected Gram negative and Gram positive pathogenic bacteria. J. Med. Plants Res. 2012, 6, 296–300. [Google Scholar] [CrossRef]
- Lee, E.W.; Huda, M.N.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob. Agents Chemother. 2003, 47, 3733–3738. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.G. Silver nanoparticles-induced H2O2 triggers apoptosis-like death and is associated with dinF in Escherichia coli. Free Radic. Res. 2021, 55, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. Diet. Phytochem. Microbes 2012, 18, 1–32. [Google Scholar] [CrossRef]
- de Melo, A.L.F.; Rossato, L.; Barbosa, M.D.S.; Palozi, R.A.C.; Alfredo, T.M.; Antunes, K.A.; Eduvirgem, J.; Ribeiro, S.M.; Simionatto, S. From the environment to the hospital: How plants can help to fight bacteria biofilm. Microbiol. Res. 2022, 261, 127074. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomat. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef] [PubMed]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A.S. Size, Shape, Stability, and Color of Plasmonic Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
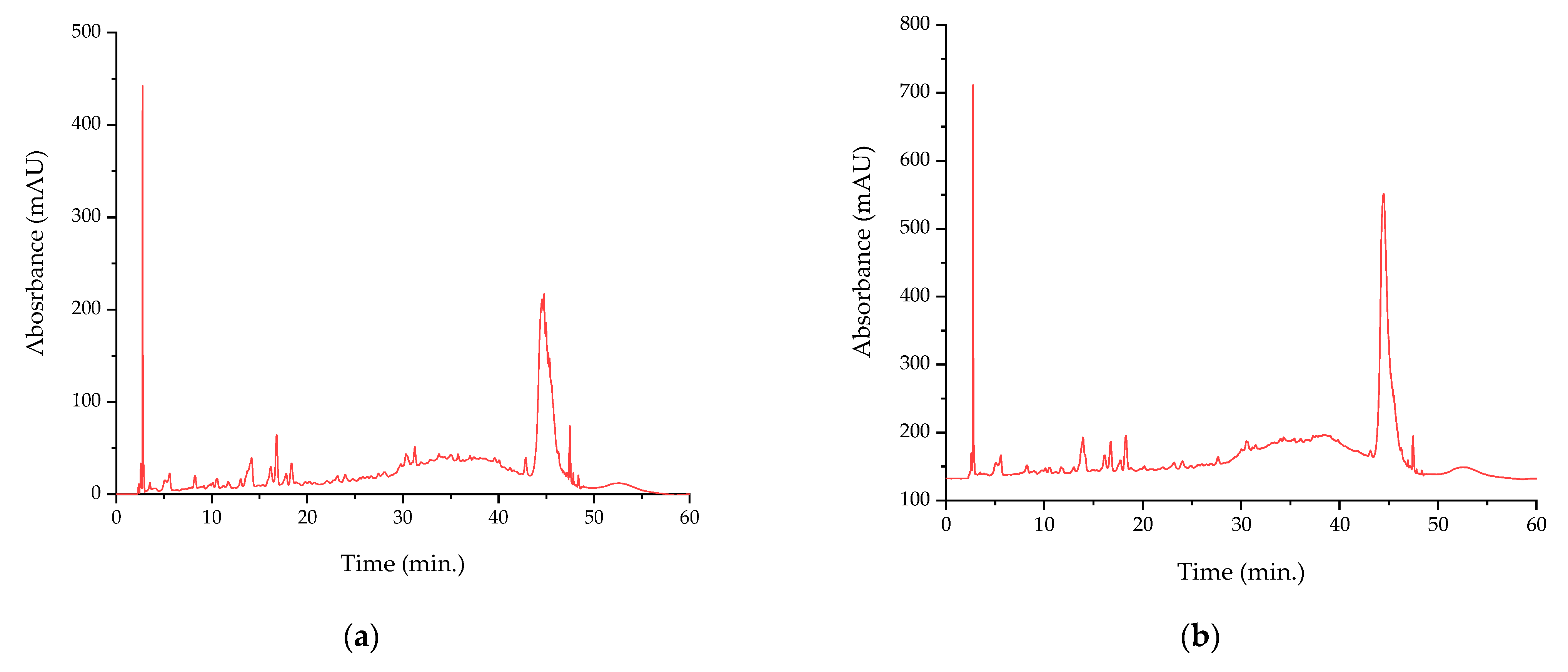
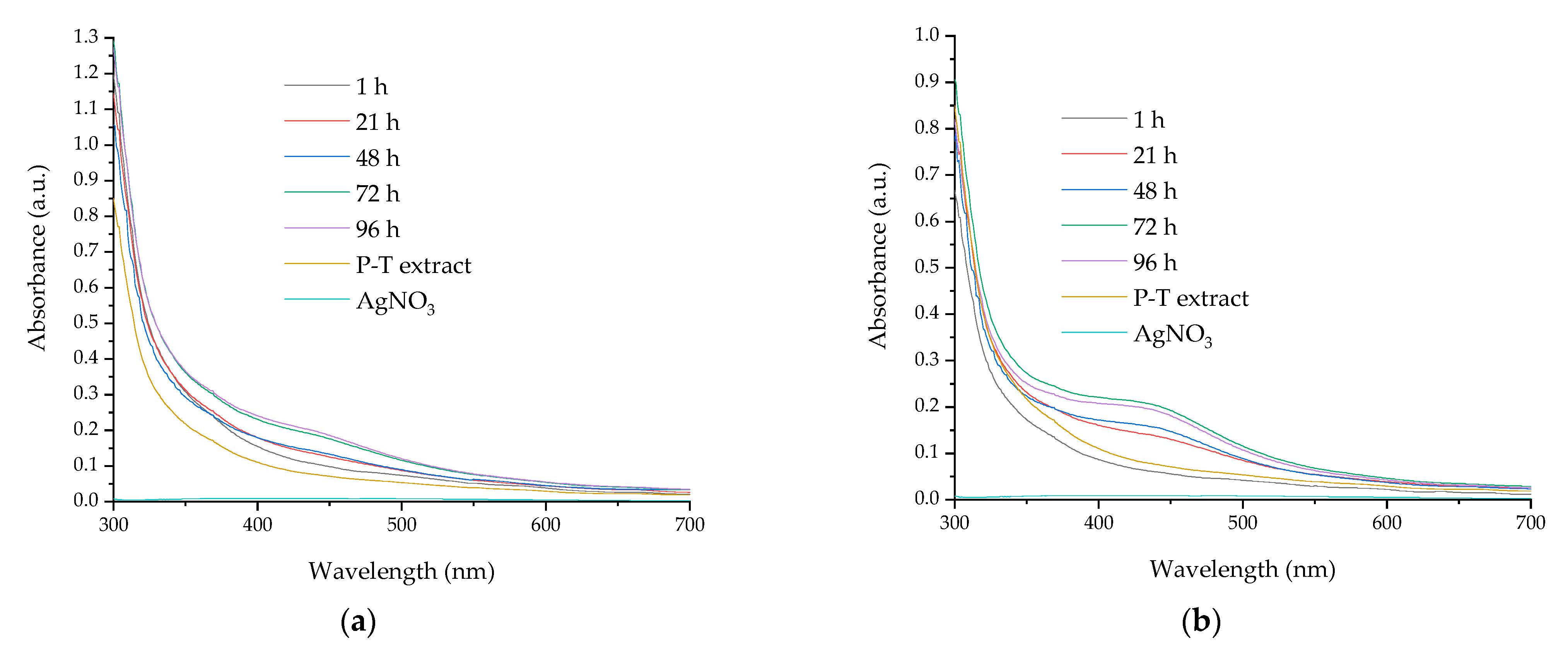

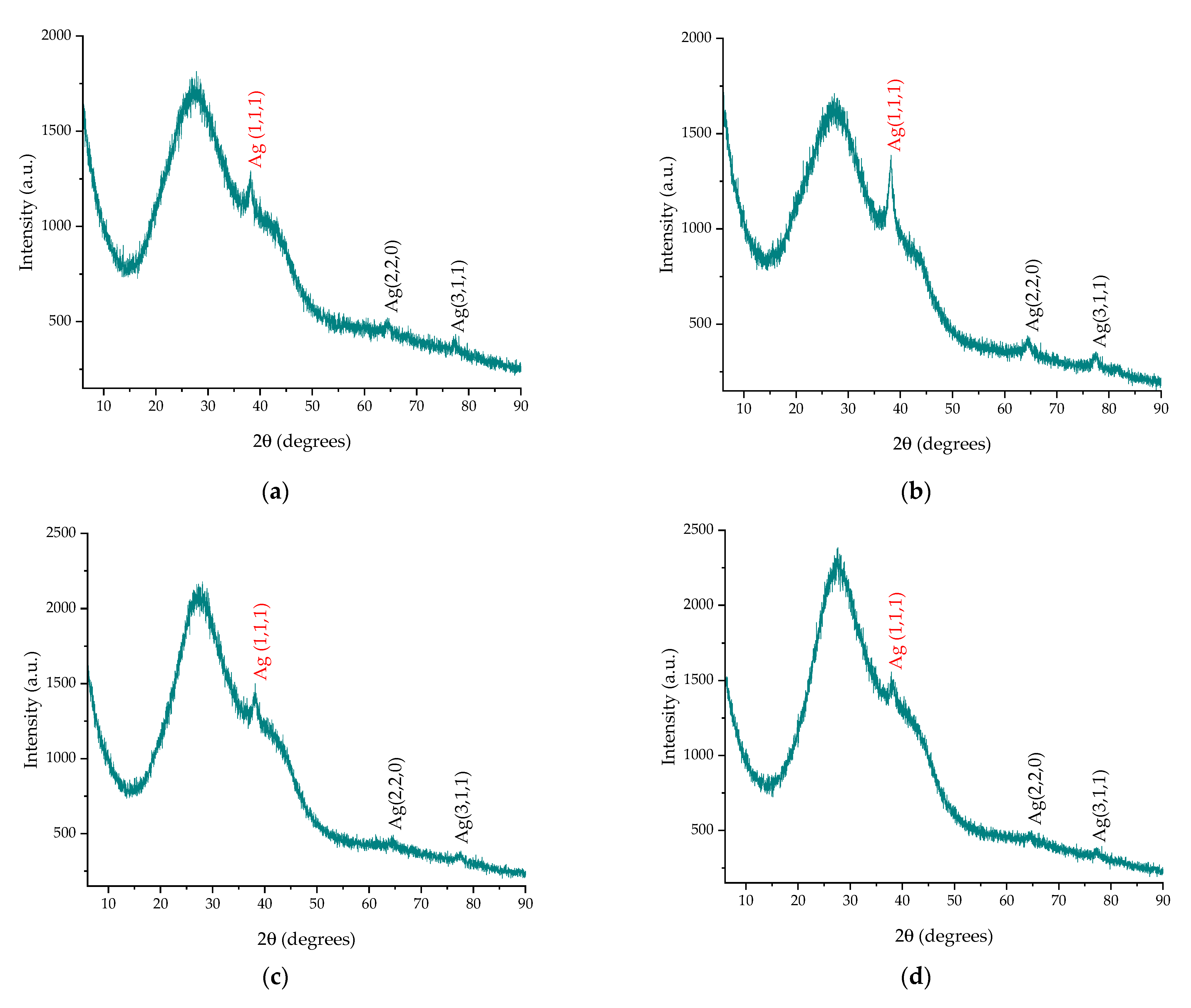
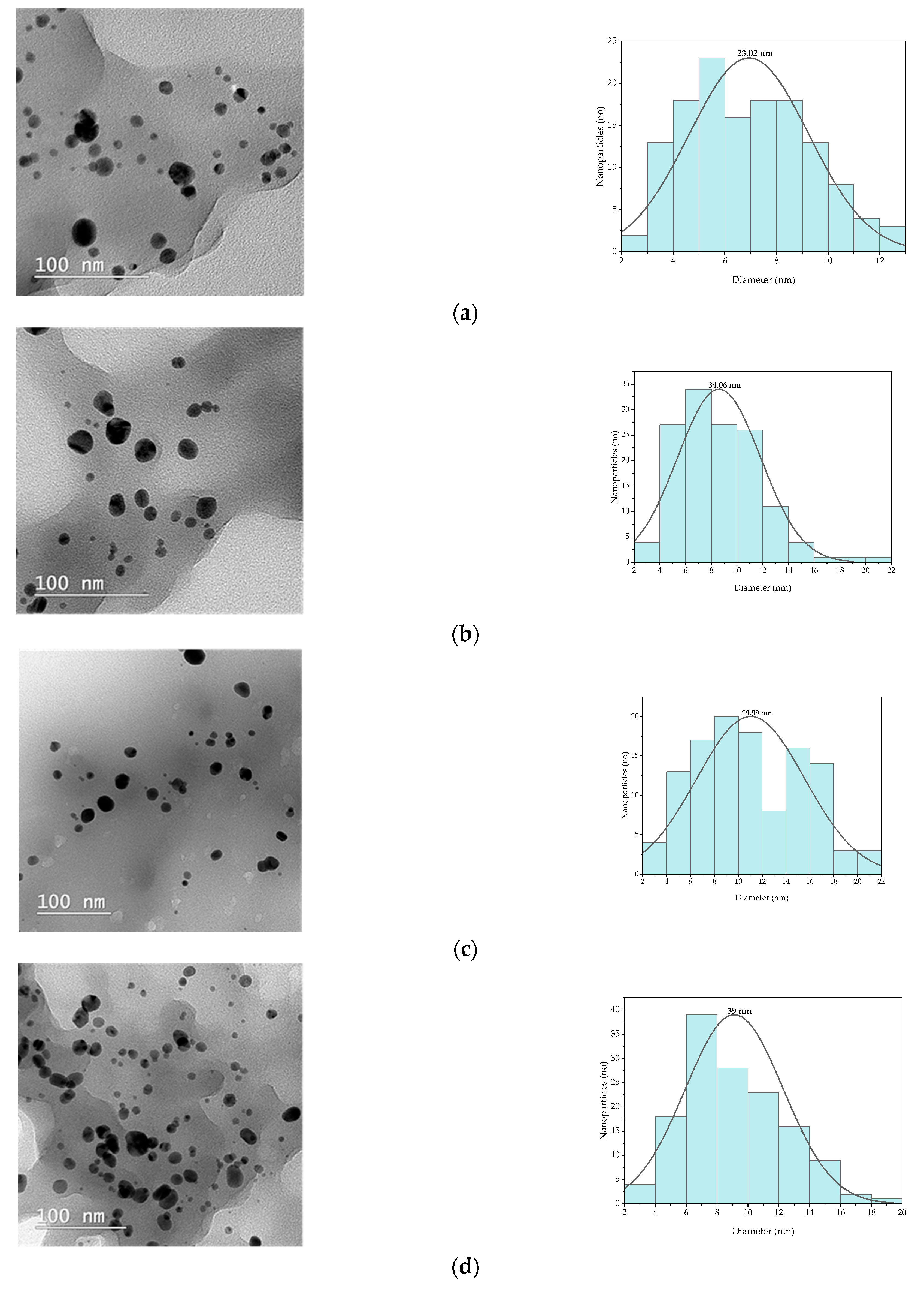



| Compound | Retention Time (min.) | Extract P-T | Extract P-Mw |
|---|---|---|---|
| Total phenolic content (mg GAE/g dry weight) | - | 48.9 ± 0.00508 b | 101 ± 0.008 a |
| Tannic acid (mg/L) | 2.6 | 13.3 ± 0.119 b | 15.1 ± 0.135 a |
| Gallic acid (mg/L) | 5.587 | 1.37 ± 0.0121 b | 1.68 ± 0.015 a |
| Catechin (mg/L) | 13.987 | N.D. | 19.7 ± 0.177 a |
| Vanillic acid (mg/L) | 14.207 | 13.3 ± 0.118 b | 26.6 ± 0.238 a |
| Chlorogenic acid (mg/L) | 15.7 | 5.18 ± 0.0462 b | 7.02 ± 0.0629 a |
| Syringic acid (mg/L) | 15.92 | 14.8 ± 0.132 a | 13.4 ± 0.12 b |
| Epicatechin (mg/L) | 16.893 | 48.6 ± 0.434 b | 100 ± 0.897 a |
| Ferulic acid (mg/L) | 20.743 | 5.37 ± 0.0479 b | 8.15 ± 0.0727 a |
| Ellagic acid (mg/L) | 26.387 | 3.47 ± 0.0312a | 1.16 ± 0.0104 b |
| Isoquercetin (mg/L) | 30.813 | N.D. | 57.2 ± 0.511 a |
| Rutin (mg/L) | 30.953 | N.D. | 25 ± 0.223 a |
| Luteolin-O-glucoside (mg/L) | 31.237 | 158 ± 1.41 a | N.D. |
| Hyperoside (mg/L) | 31.423 | 12.9 ± 0.115 b | 25.1 ± 0.224 a |
| Rosmarinic acid (mg/L) | 33.993 | 0.559 ± 0.0052 b | 1.11 ± 0.00981 a |
| Time | NPs Obtained by Classical Temperature Pomace Extract | NPs Obtained by Microwave-Assisted Pomace Extract | ||
|---|---|---|---|---|
| P-T-Ag1Pmax | P-T-Ag2Pmax | P-Mw-Ag1Pmax | P-Mw-Ag2Pmax | |
| 1 h | 448 | - | - | - |
| 21 h | 446 | 440 | 435 | 444 |
| 48 h | 446 | 436 | 443 | 442 |
| 72 h | 442 | 436 | 441 | 441 |
| 96 h | 439 | 434 | 443 | 433 |
| Sample | Peak Position (1,1,1), ° | Peak Position (2,2,0), ° | Peak Position (3,1,1), ° | FWHM, ° 1 | Crystallite Size (nm) 1 |
|---|---|---|---|---|---|
| P-T-Ag1 | 38.17 | 64.30 | 77.16 | 1.7084 | 5.14 |
| P-T-Ag2 | 38.25 | 64.43 | 77.32 | 1.0163 | 8.64 |
| P-Mw-Ag1 | 38.09 | 64.57 | 77.69 | 1.9154 | 4.58 |
| P-Mw-Ag2 | 38.09 | 64.12 | 77.25 | 0.912 | 9.62 |
| Type of Extract Used | NPs Encoding | |
|---|---|---|
| Extract:AgNO3 Ratio: 1:1 (v/v) | Extract:AgNO3 Ratio: 1:2 (v/v) | |
| Classical temperature grape pomace extract (P-T) | P-T-Ag1 | P-T-Ag2 |
| Microwave-assisted grape pomace extract (P-Mw) | P-Mw-Ag1 | P-Mw-Ag2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroi, A.M.; Fierascu, I.; Ghizdareanu, A.-I.; Trica, B.; Fistos, T.; Matei, R.I.; Fierascu, R.C.; Firinca, C.; Sardarescu, I.D.; Avramescu, S.M. Green Approach for Synthesis of Silver Nanoparticles with Antimicrobial and Antioxidant Properties from Grapevine Waste Extracts. Int. J. Mol. Sci. 2024, 25, 4212. https://doi.org/10.3390/ijms25084212
Baroi AM, Fierascu I, Ghizdareanu A-I, Trica B, Fistos T, Matei RI, Fierascu RC, Firinca C, Sardarescu ID, Avramescu SM. Green Approach for Synthesis of Silver Nanoparticles with Antimicrobial and Antioxidant Properties from Grapevine Waste Extracts. International Journal of Molecular Sciences. 2024; 25(8):4212. https://doi.org/10.3390/ijms25084212
Chicago/Turabian StyleBaroi, Anda Maria, Irina Fierascu, Andra-Ionela Ghizdareanu, Bogdan Trica, Toma Fistos, Roxana Ioana Matei (Brazdis), Radu Claudiu Fierascu, Cristina Firinca, Ionela Daniela Sardarescu, and Sorin Marius Avramescu. 2024. "Green Approach for Synthesis of Silver Nanoparticles with Antimicrobial and Antioxidant Properties from Grapevine Waste Extracts" International Journal of Molecular Sciences 25, no. 8: 4212. https://doi.org/10.3390/ijms25084212
APA StyleBaroi, A. M., Fierascu, I., Ghizdareanu, A.-I., Trica, B., Fistos, T., Matei, R. I., Fierascu, R. C., Firinca, C., Sardarescu, I. D., & Avramescu, S. M. (2024). Green Approach for Synthesis of Silver Nanoparticles with Antimicrobial and Antioxidant Properties from Grapevine Waste Extracts. International Journal of Molecular Sciences, 25(8), 4212. https://doi.org/10.3390/ijms25084212










