Harnessing the Cross-Neutralisation Potential of Existing Antivenoms for Mitigating the Outcomes of Snakebite in Sub-Saharan Africa
Abstract
1. Introduction
2. Results
2.1. Protein Concentration
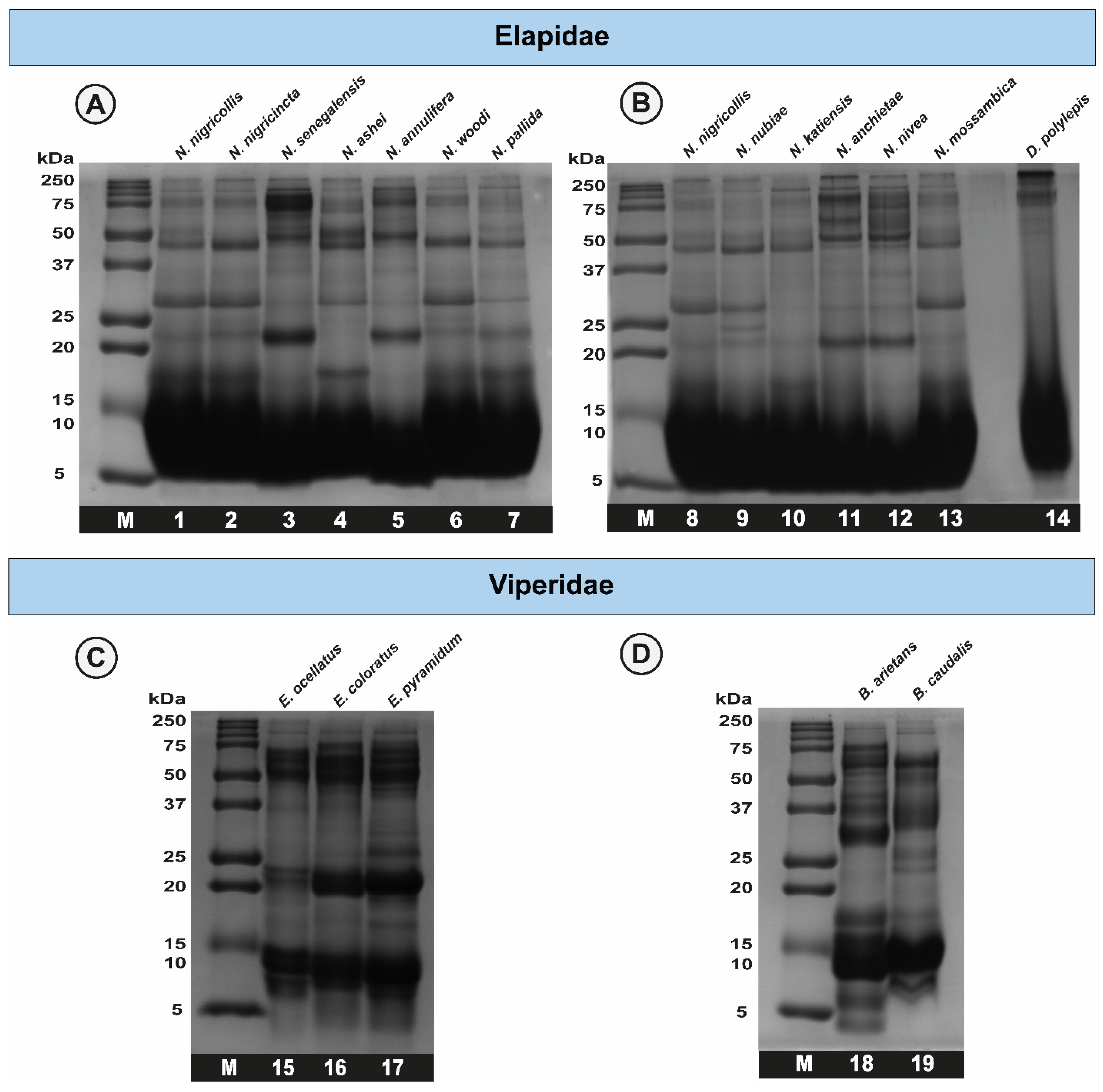
2.2. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) Profiles of African Snake Venoms
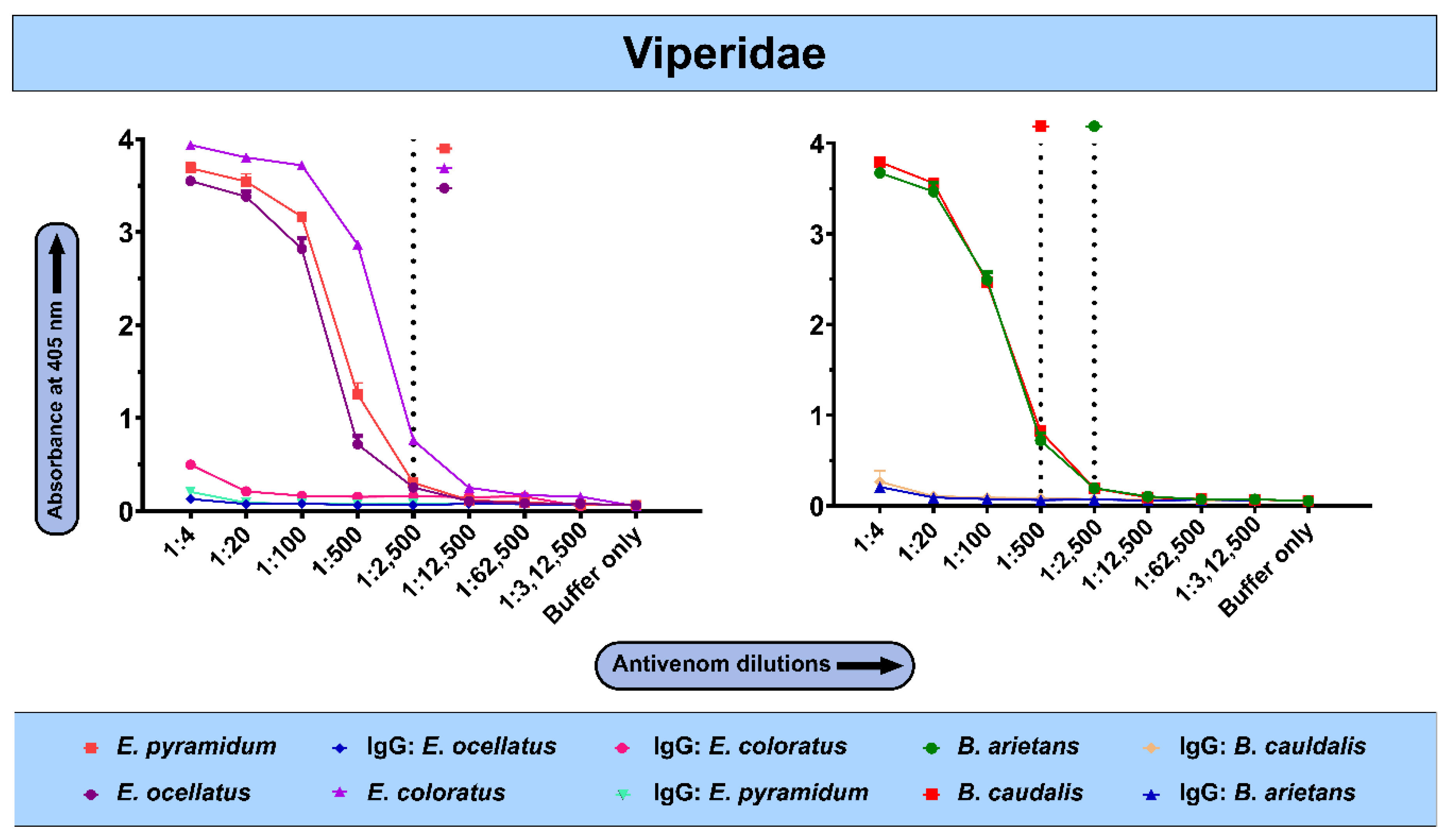
2.3. The In Vitro Binding Potential of PANAF-Premium Antivenom
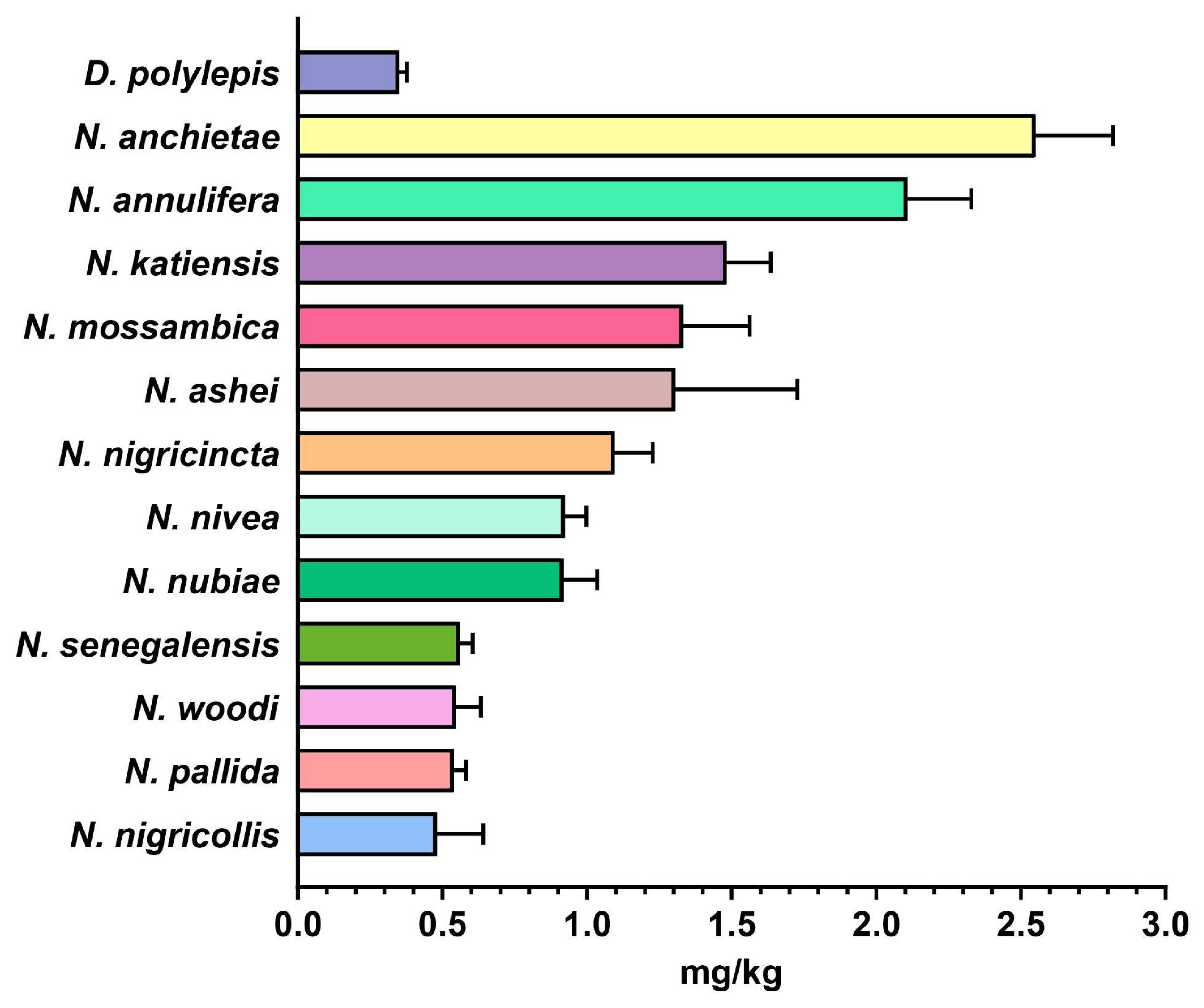
2.4. In Vivo Toxicity Evaluation of Snake Venoms from sSA
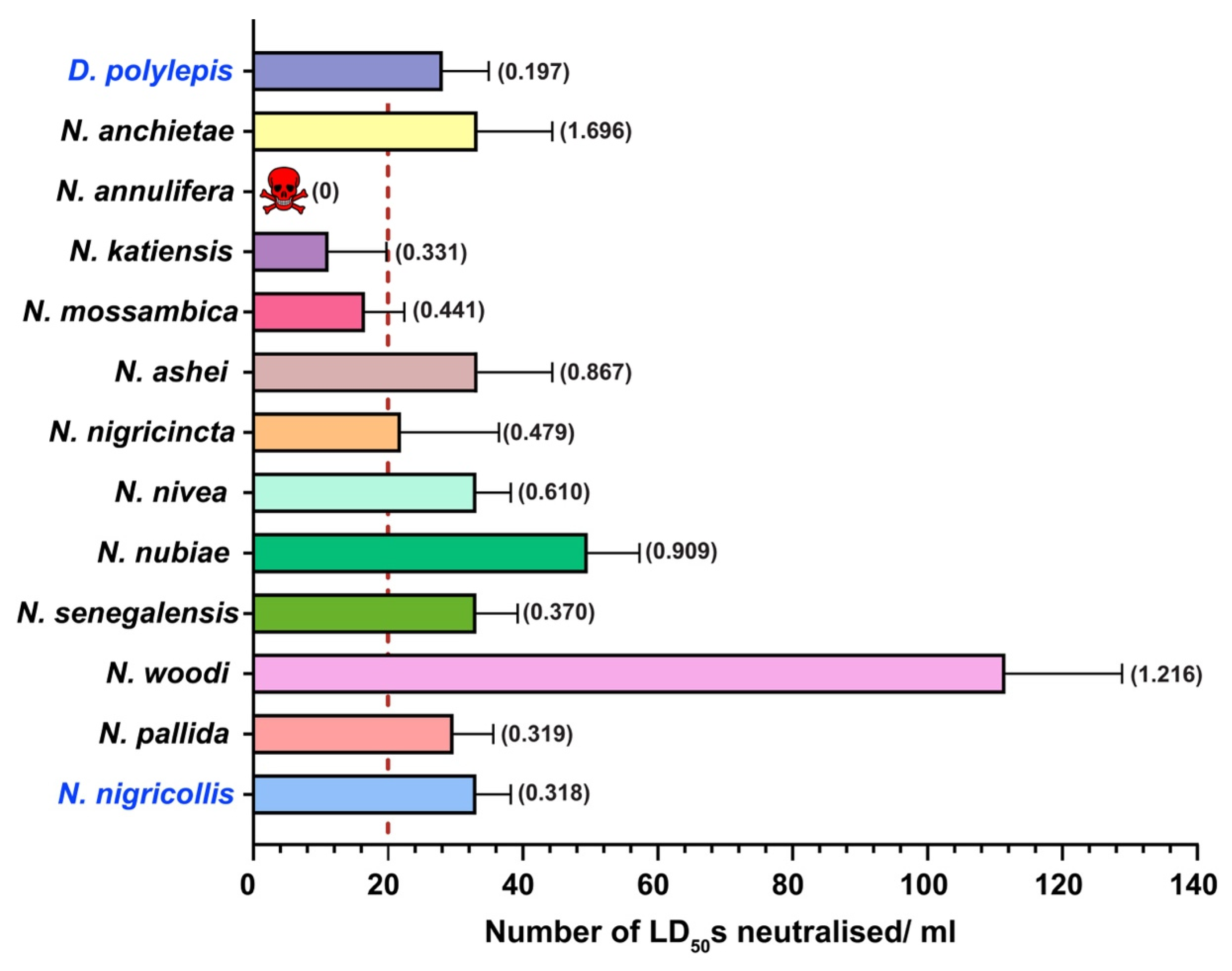
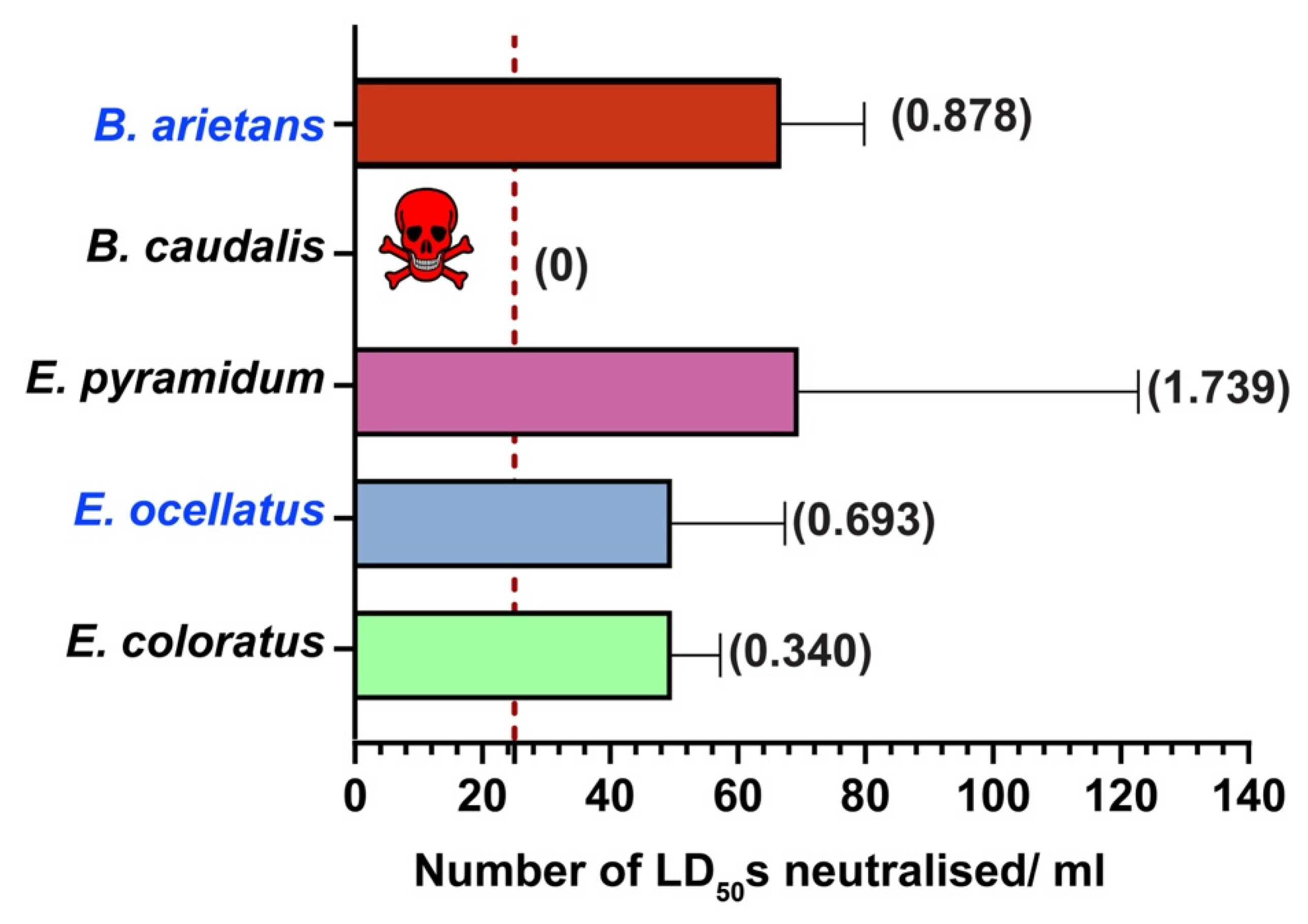
2.5. In Vivo Neutralisation Potential of PANAF-Premium Antivenom
3. Discussion
3.1. Variations in Toxicity Profiles of African Snake Venoms
3.2. Cross-Neutralisation Potential of PANAF-Premium Antivenom
4. Materials and Methods
4.1. Sample Details
4.2. Ethical Statements
4.3. Protein Estimation
4.4. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Median Lethal Dose (LD50)
4.7. Median Effective Dose (ED50)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 5 February 2024).
- Appiah, B. Snakebite neglect rampant in Africa. CMAJ 2012, 184, E27–E28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Attarde, S.; Iyer, A.; Khochare, S.; Shaligram, U.; Vikharankar, M.; Sunagar, K. The Preclinical Evaluation of a Second-Generation Antivenom for Treating Snake Envenoming in India. Toxins 2022, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Iyer, A.; Sunagar, K. Evolution Bites—Timeworn Inefficacious Snakebite Therapy in the Era of Recombinant Vaccines. Indian Pediatr. 2021, 58, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Tan, C.H. Quantitative proteomics of Naja annulifera (sub-Saharan snouted cobra) venom and neutralization activities of two antivenoms in Africa. Int. J. Biol. Macromol. 2020, 158, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Oluoch, G.O.; Ainsworth, S.; Alsolaiss, J.; Bolton, F.; Arias, A.S.; Gutierrez, J.M.; Rowley, P.; Kalya, S.; Ozwara, H.; et al. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl. Trop. Dis. 2017, 11, e0005969. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.; Menzies, S.K.; Casewell, N.R.; Harrison, R.A. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Negl. Trop. Dis. 2020, 14, e0008579. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Alomran, N.; Blundell, P.; Alsolaiss, J.; Crittenden, E.; Ainsworth, S.; Dawson, C.A.; Edge, R.J.; Hall, S.R.; Harrison, R.A.; Wilkinson, M.C.; et al. Exploring the Utility of Recombinant Snake Venom Serine Protease Toxins as Immunogens for Generating Experimental Snakebite Antivenoms. Toxins 2022, 14, 443. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Laustsen, A.H.; Pus, U.; Wade, J.; Villar, P.; Boddum, K.; Slavny, P.; Masters, E.W.; Arias, A.S.; Oscoz, S.; et al. In vitro discovery of a human monoclonal antibody that neutralizes lethality of cobra snake venom. MAbs 2022, 14, 2085536. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease? PLoS Negl. Trop. Dis. 2017, 11, e0005361. [Google Scholar] [CrossRef] [PubMed]
- Khalek, I.S.; Senji Laxme, R.R.; Nguyen, Y.T.K.; Khochare, S.; Patel, R.N.; Woehl, J.; Smith, J.M.; Saye-Francisco, K.; Kim, Y.; Misson Mindrebo, L.; et al. Synthetic development of a broadly neutralizing antibody against snake venom long-chain alpha-neurotoxins. Sci. Transl. Med. 2024, 16, eadk1867. [Google Scholar] [CrossRef] [PubMed]
- Urs, N.A.N.; Yariswamy, M.; Joshi, V.; Nataraju, A.; Gowda, T.V.; Vishwanath, B.S. Implications of phytochemicals in snakebite management: Present status and future prospective. Toxin Rev. 2014, 33, 60–83. [Google Scholar] [CrossRef]
- Titus, J.K.; Kay, M.K.; Glaser, C.J.J. Application of phage display for the development of a novel inhibitor of PLA(2) activity in Western cottonmouth venom. J. Venom. Res. 2017, 8, 19–24. [Google Scholar] [PubMed]
- Hall, S.R.; Rasmussen, S.A.; Crittenden, E.; Dawson, C.A.; Bartlett, K.E.; Westhorpe, A.P.; Albulescu, L.-O.; Kool, J.; Gutiérrez, J.M.; Casewell, N.R. Repurposed drugs and their combinations prevent morbidity-inducing dermonecrosis caused by diverse cytotoxic snake venoms. Nat. Commun. 2023, 14, 7812. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.M.; Abo, B.N.; Brandehoff, N. Snake envenomation in Africa. Curr. Trop. Med. Rep. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Spawls, S.; Branch, B. The Dangerous Snakes of Africa; Bloomsbury Publishing: London, UK, 2020. [Google Scholar]
- Deikumah, J.P.; Biney, R.P.; Awoonor-Williams, J.K.; Gyakobo, M.K. Compendium of medically important snakes, venom activity and clinical presentations in Ghana. PLoS Negl. Trop. Dis. 2023, 17, e0011050. [Google Scholar] [CrossRef] [PubMed]
- de Silva, H.A.; Ryan, N.M.; de Silva, H.J. Adverse reactions to snake antivenom, and their prevention and treatment. Br. J. Clin. Pharmacol. 2016, 81, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Smith, B.J. SDS polyacrylamide gel electrophoresis for N-terminal protein sequencing. Methods Mol. Biol. 1997, 64, 17–24. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Cook, D.A.; Wagstaff, S.C.; Nasidi, A.; Durfa, N.; Wuster, W.; Harrison, R.A. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl. Trop. Dis. 2010, 4, e851. [Google Scholar] [CrossRef] [PubMed]
- Saganuwan, S.A. The new algorithm for calculation of median lethal dose (LD(50)) and effective dose fifty (ED(50)) of Micrarus fulvius venom and anti-venom in mice. Int. J. Vet. Sci. Med. 2016, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Finney, D. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Araujo, H.P.; Bourguignon, S.C.; Boller, M.A.; Dias, A.A.; Lucas, E.P.; Santos, I.C.; Delgado, I.F. Potency evaluation of antivenoms in Brazil: The national control laboratory experience between 2000 and 2006. Toxicon 2008, 51, 502–514. [Google Scholar] [CrossRef]


| Sr. No. | Species | Location | Venom Concentration (mg/mL) |
|---|---|---|---|
| Naja spp. | |||
| 1 | N. nigricollis | Togo, Tanzania, Cameroon | 1.39 |
| 2 | N. nubiae | Egypt | 1.32 |
| 3 | N. katiensis | Togo | 1.26 |
| 4 | N. anchietae | Botswana | 1.05 |
| 5 | N. nivea | Western Cape | 1.33 |
| 6 | N. mossambica | RSA | 1.50 |
| 7 | N. nigricincta | Namibia | 1.39 |
| 8 | N. senegalensis | West Africa | 1.50 |
| 9 | N. ashei | Northern Cape | 1.23 |
| 10 | N. annulifera | RSA | 1.49 |
| 11 | N. woodi | Northern Cape | 1.42 |
| 12 | N. pallida | Tanzania | 1.39 |
| Bitis spp. | |||
| 13 | B. arietans | Zimbabwe | 2.43 |
| 14 | B. caudalis | Limpopo | 1.41 |
| Echis spp. | |||
| 15 | E. ocellatus | Benin | 2.09 |
| 16 | E. coloratus | Egypt | 2.58 |
| 17 | E. pyramidum | Egypt | 2.06 |
| Dendroaspis spp. | |||
| 18 | D. polylepis | Zimbabwe | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khochare, S.; Jaglan, A.; Rashmi, U.; Dam, P.; Sunagar, K. Harnessing the Cross-Neutralisation Potential of Existing Antivenoms for Mitigating the Outcomes of Snakebite in Sub-Saharan Africa. Int. J. Mol. Sci. 2024, 25, 4213. https://doi.org/10.3390/ijms25084213
Khochare S, Jaglan A, Rashmi U, Dam P, Sunagar K. Harnessing the Cross-Neutralisation Potential of Existing Antivenoms for Mitigating the Outcomes of Snakebite in Sub-Saharan Africa. International Journal of Molecular Sciences. 2024; 25(8):4213. https://doi.org/10.3390/ijms25084213
Chicago/Turabian StyleKhochare, Suyog, Anurag Jaglan, U. Rashmi, Paulomi Dam, and Kartik Sunagar. 2024. "Harnessing the Cross-Neutralisation Potential of Existing Antivenoms for Mitigating the Outcomes of Snakebite in Sub-Saharan Africa" International Journal of Molecular Sciences 25, no. 8: 4213. https://doi.org/10.3390/ijms25084213
APA StyleKhochare, S., Jaglan, A., Rashmi, U., Dam, P., & Sunagar, K. (2024). Harnessing the Cross-Neutralisation Potential of Existing Antivenoms for Mitigating the Outcomes of Snakebite in Sub-Saharan Africa. International Journal of Molecular Sciences, 25(8), 4213. https://doi.org/10.3390/ijms25084213








