Unraveling the Anti-Aging Properties of Phycocyanin from the Cyanobacterium Spirulina (Arthrospira platensis)
Abstract
1. Introduction
2. Results and Discussion
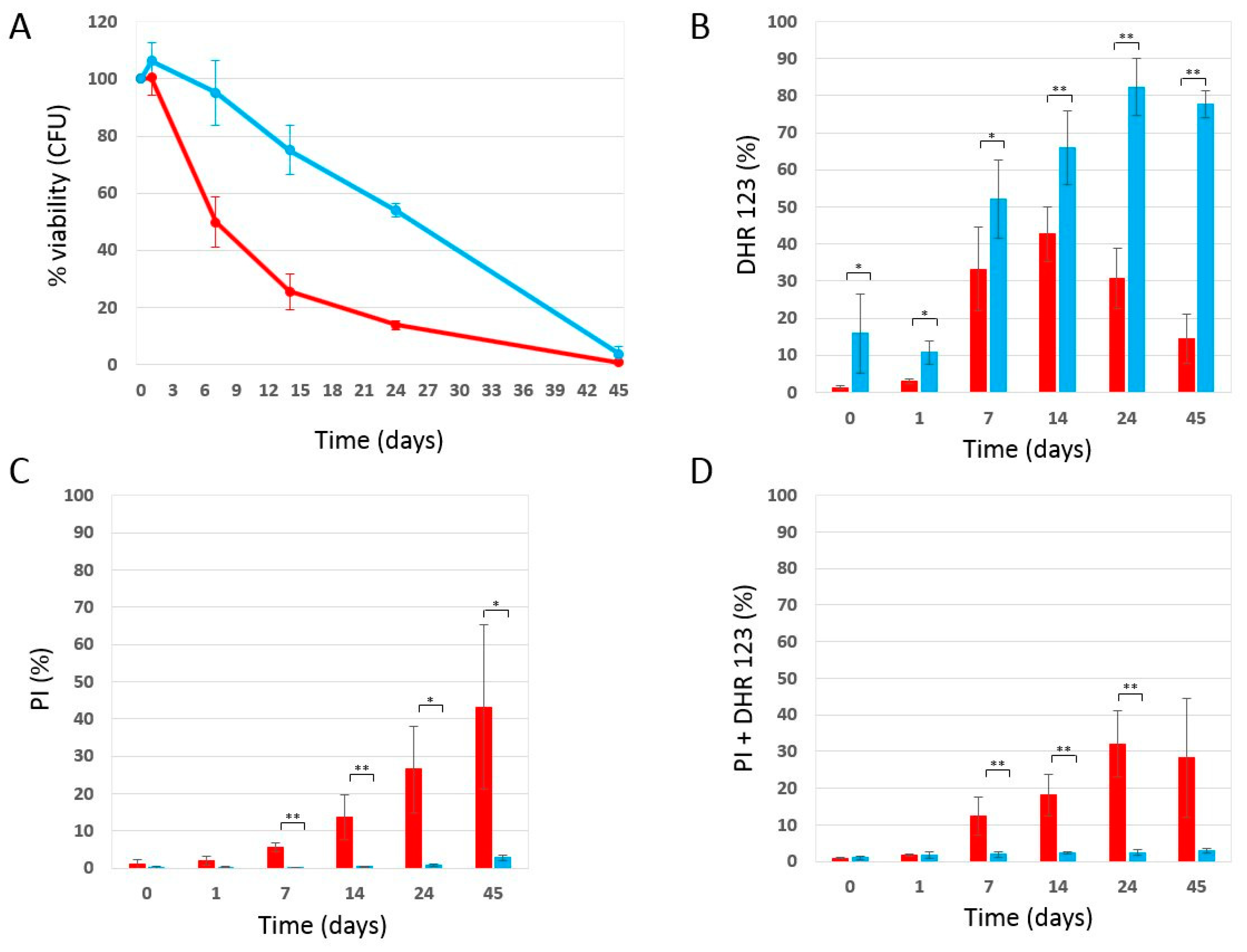
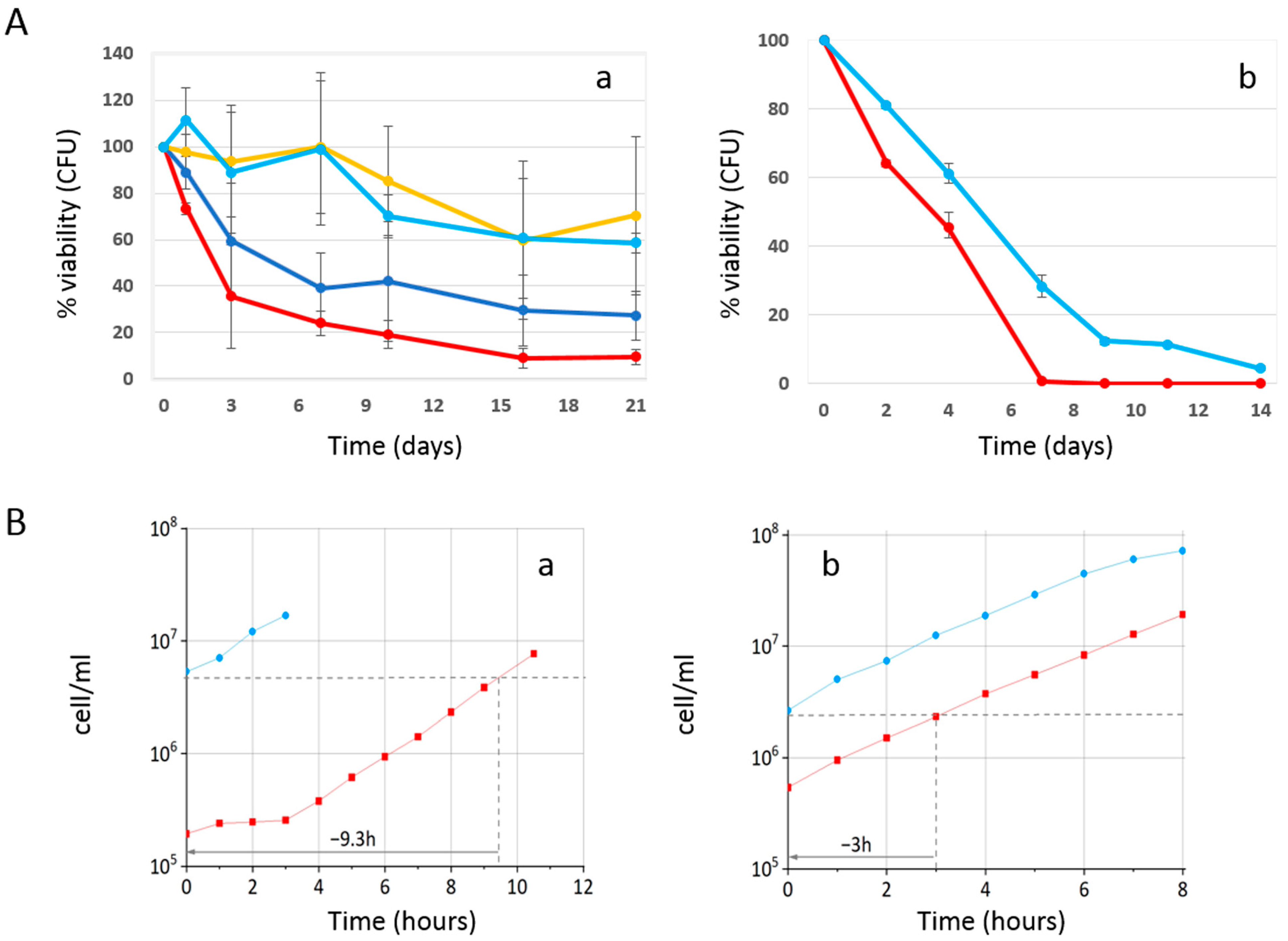
2.1. Phycocyanin Delays the Chronological Aging of Yeast Cells
2.2. Effect of Phycocyanin on Reactive Oxygen Species Accumulation
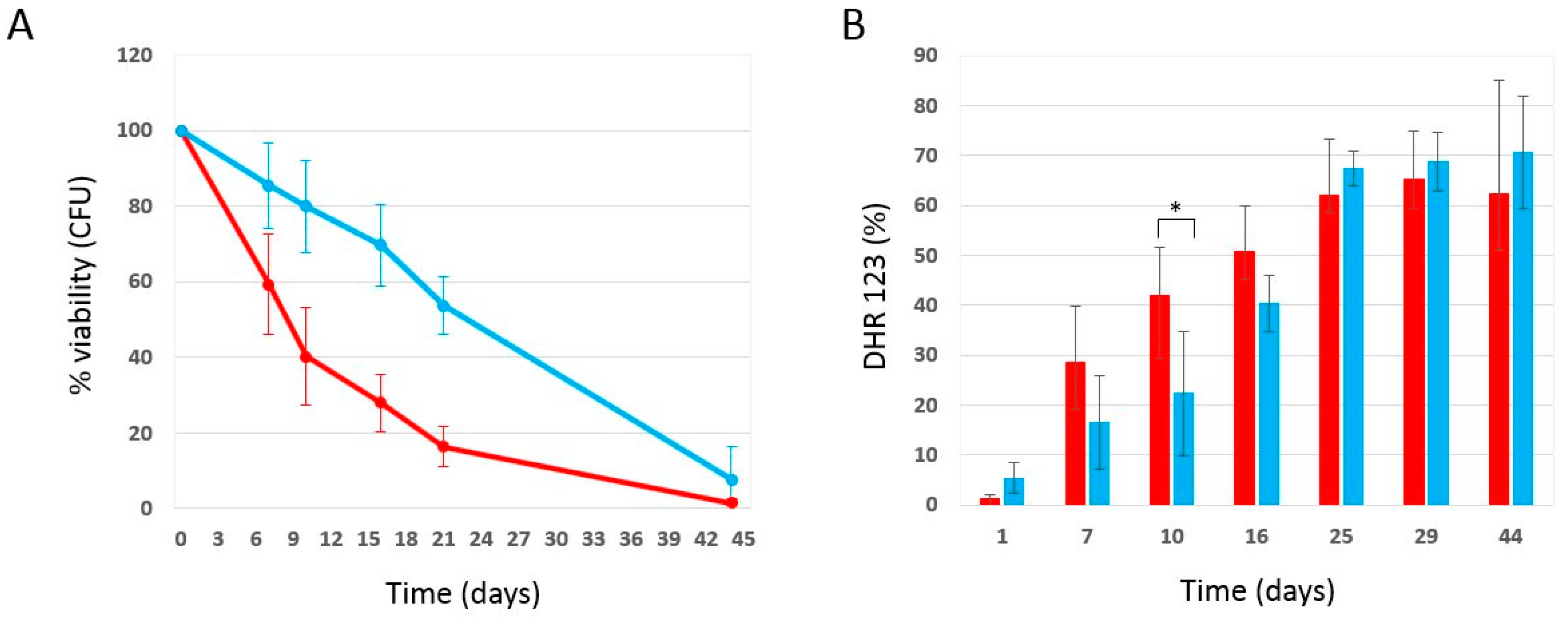
2.3. Effect of Phycocyanin Added on Day 0 of CLS, When Maximum Cell Density Is Reached
3. Materials and Methods
3.1. Yeast Strains and Media
3.2. Aging Experiments and Cell Viability
3.3. Dihydrorhodamine 123 (DHR123) and Propidium Iodide (PI) Staining
3.4. Phycocyanin Characterisation
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekaran, A.; Sosa Idelchik, M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Shaden, A.M.; Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jin, Y.; Hou, X.; Zhu, Y.; Chen, D.; Tai, J.; Chen, Q.; Shi, C.; Ye, J.; Wu, M.; et al. Application of Marine Natural Products against Alzheimer’s Disease: Past, Present and Future. Mar. Drugs 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Alfredo Fernández-Quintela, A.; Portillo, M.P. Anti-Obesity Effects of Microalgae. Int. J. Mol. Sci. 2020, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, D.L.; Venkataraman, G.S.; Duggal, S.K.; Eggum, B.O. Nutritional quality of the blue-green alga Spirulina platensis Geitler. J. Sci. Food Agric. 1982, 33, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Sotiroudis, T.G.; Sotiroudis, G.T. Health aspects of Spirulina (Arthrospira) microalga food supplement. J. Serb. Chem. Soc. 2013, 78, 395–405. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef]
- Bannu, S.A.; Lomada, D.; Gulla, S.; Chandrasekhar, T.; Reddanna, P.; Reddy, M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr. Drug Metab. 2019, 20, 967–976. [Google Scholar] [CrossRef]
- Kuddus, M.; Singh, P.; Thomas, G.; Al Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. BioMed Res. Int. 2013, 2013, 742859. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Armesto, J.; Remirez, D.; Gonzalez, R.; Ledon, N.; Garcıa, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef] [PubMed]
- Rogov, A.G.; Sergeeva, Y.E.; Sukhinov, D.V.; Ivaschenko, M.V.; Kuvyrchenkova, A.P.; Vasilov, R.G. The Effect of Phycocyanin Isolated from Arthrospira platensis on the Oxidative Stress in Yeasts. Nanobiotechnol. Rep. 2023, 18, 126–131. [Google Scholar] [CrossRef]
- Pleonsila, P.; Soogarunb, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, P.; Pinero, E.; Villar, A.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008, 110, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-inflammatory effect and mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.; Yang, F.; Qi, J.; Shang, L.; Zhang, H.; Xu, F.; Li, L.; Yu, H.; Li, Y.; et al. C-Phycocyanin Ameliorates the Senescence of Mesenchymal Stem Cells through ZDHHC5-Mediated Autophagy via PI3K/AKT/mTOR Pathway. Aging Dis. 2023, 14, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, F.; Li, Q.; Yang, Q.; Zhang, W. Phycocyanin Protects against High Glucose High Fat Diet Induced Diabetes in Mice and Participates in AKT and AMPK Signaling. Foods 2022, 11, 3183. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; de Magalhães, J.P. The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Res. Rev. 2021, 70, 101407. [Google Scholar] [CrossRef] [PubMed]
- Gems, D. The hyperfunction theory: An emerging paradigm for the biology of aging. Ageing Res. Rev. 2022, 74, 101557. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Fabrizio, P. Chronological aging in Saccharomyces cerevisiae. Subcell Biochem. 2012, 57, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Marques, B.; Burhans, W.C.; Ludovico, P. Yeast at the forefront of research on ageing and age-related diseases. Prog. Mol. Subcell Biol. 2019, 58, 217–242. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Mohammad, T.; Alajmi, M.F.; Rehman, M.T.; Hasan, G.M.; Hussain, A.; Hassan, M.I. Insights into the Conserved Regulatory Mechanisms of Human and Yeast Aging. Biomolecules 2020, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free. Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Burhans, W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014, 14, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Nascarella, M.; Pressman, P.; Hayes, A.W.; Dhawan, G.; Kapoor, R.; Calabrese, V.; Agathokleous, E. Hormesis determines lifespan. Ageing Res. Rev. 2024, 94, 102181. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants 2022, 11, 1613. [Google Scholar] [CrossRef]
- Macedo, D.; Bertolin, T.E.; Oro, T.; Taís Hartmann Backes, L.; Caldeira Brás, I.; Nunes Santos, C.; Tenreiro, S.; Fleming Outeiro, T. Phycocyanin protects against Alpha-Synuclein toxicity in yeast. J. Funct. Foods 2017, 38, 553–560. [Google Scholar] [CrossRef]
- Fabrizio, P.; Longo, V.D. The chronological life span of Saccharomyces cerevisiae. Methods Mol. Biol. 2007, 371, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Butler Gralla, E.; Selverstone Valentine, J. Superoxide Dismutase Activity Is Essential for Stationary Phase Survival in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 12275–12280. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life Span Extension by Calorie Restriction Depends on Rim15 and Transcription Factors Downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008, 4, e13. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Ruiz, M.A.; Pujol, N.; Sundaran, V. Coping with oxidative stress. The yeast model. Curr. Drug Targets 2015, 16, 2–12. [Google Scholar] [CrossRef]
- Burstein, M.T.; Kyryakov, P.; Beach, A.; Richard, V.R.; Koupaki, O.; Gometz-Perez, A.; Leonov, A.; Levy, S.; Noohi, F.; Titorenko, V.I. Lithocholic acid extends longevity of chronologically aging yeast only if added at certain critical periods of their lifespan. Cell Cycle 2012, 11, 3443–3462. [Google Scholar] [CrossRef] [PubMed]
- Baroni, M.D.; Colombo, S.; Libens, O.; Pallavi, R.; Giorgio, M.; Martegani, E. In S. cerevisiae hydroxycitric acid antagonizes chronological aging and apoptosis regardless of citrate lyase. Apoptosis 2020, 25, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.J.; Rothstein, R. Elevated recombination rates in transcriptionally active DNA. Cell 1989, 56, e619–e630. [Google Scholar] [CrossRef]
- Amigoni, L.; Colombo, S.; Belotti, F.; Alberghina, L.; Martegani, E. The transcription factor Swi4 is target for PKA regulation of cell size at the G1 to S transition in Saccharomyces cerevisiae. Cell Cycle 2015, 14, 2429–2438. [Google Scholar] [CrossRef]
- Madeo, F.; Fröhlich, E.; Ligr, M.; Grey, M.; Sigrist, S.J.; Wolf, D.H.; Fröhlich, K.-U. Oxygen stress: A regulator of apoptosis in yeast. J. Cell Biol. 1999, 145, 757–767. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nova, M.; Citterio, S.; Martegani, E.; Colombo, S. Unraveling the Anti-Aging Properties of Phycocyanin from the Cyanobacterium Spirulina (Arthrospira platensis). Int. J. Mol. Sci. 2024, 25, 4215. https://doi.org/10.3390/ijms25084215
Nova M, Citterio S, Martegani E, Colombo S. Unraveling the Anti-Aging Properties of Phycocyanin from the Cyanobacterium Spirulina (Arthrospira platensis). International Journal of Molecular Sciences. 2024; 25(8):4215. https://doi.org/10.3390/ijms25084215
Chicago/Turabian StyleNova, Mariachiara, Stefania Citterio, Enzo Martegani, and Sonia Colombo. 2024. "Unraveling the Anti-Aging Properties of Phycocyanin from the Cyanobacterium Spirulina (Arthrospira platensis)" International Journal of Molecular Sciences 25, no. 8: 4215. https://doi.org/10.3390/ijms25084215
APA StyleNova, M., Citterio, S., Martegani, E., & Colombo, S. (2024). Unraveling the Anti-Aging Properties of Phycocyanin from the Cyanobacterium Spirulina (Arthrospira platensis). International Journal of Molecular Sciences, 25(8), 4215. https://doi.org/10.3390/ijms25084215






