Compilation of Evidence Supporting the Role of a T Helper 2 Reaction in the Pathogenesis of Acute Appendicitis
Abstract
1. Introduction
1.1. Immune Defenses
1.2. Appendicitis
2. T-Lymphocytes: IL-4, IL-5, IL-9 and IL-13
3. B-Lymphocytes IgA, IgE, IgG e IgM
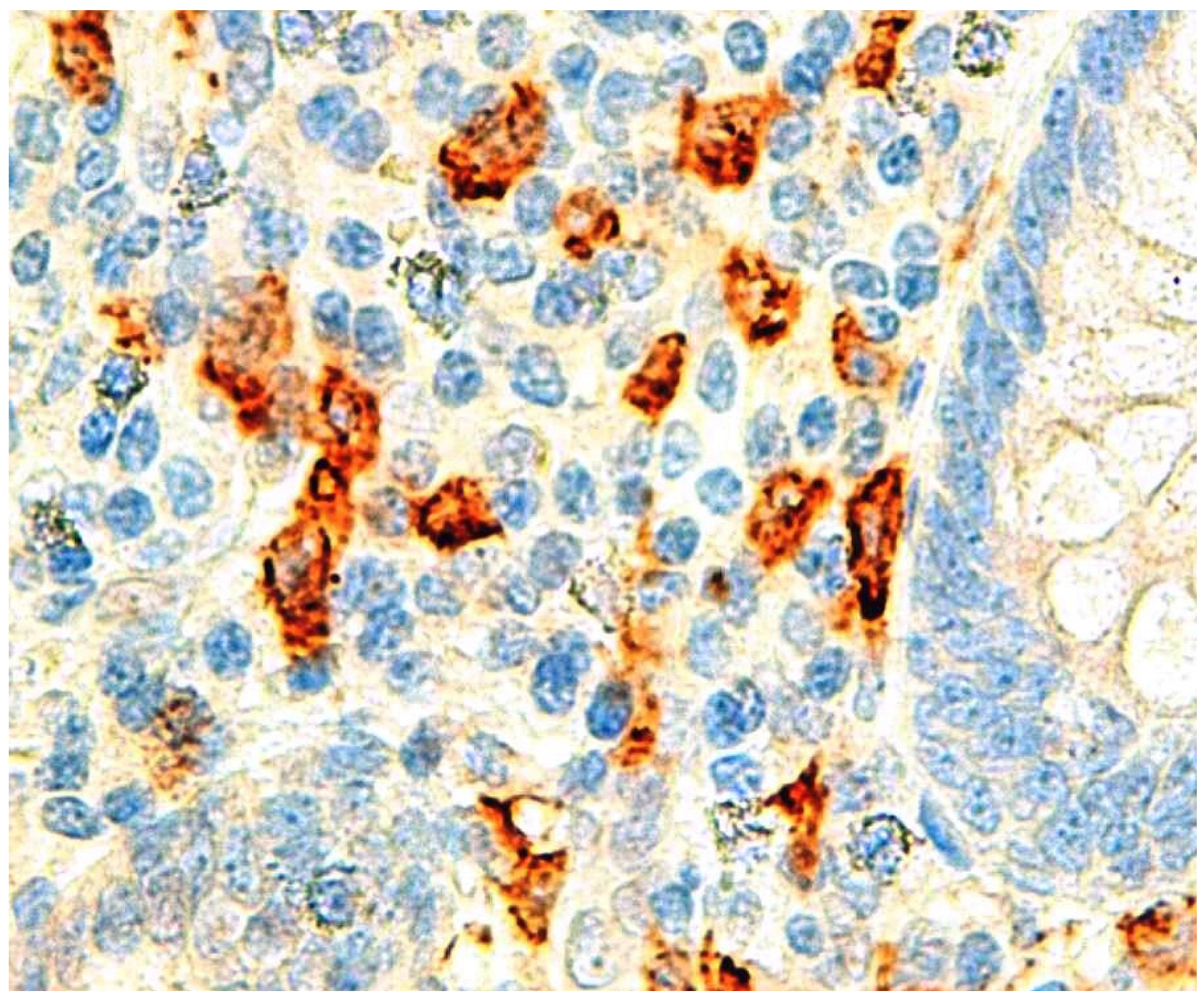
4. Eosinophils: Eosinophil-Derived Neurotoxin, Eosinophilic Cationic Protein, Eosinophilic Peroxidase
5. Mast Cells: Tryptase, Histamine, Serotonin
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
References
- Clark, R.; Kupper, T. Old meets new: The interaction between innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Janefjord, C. Th1, Th2 and Treg Associated Factors in Relation to Allergy. Ph.D. Thesis, Linköping University, Linköping, Sweden, 2006. [Google Scholar]
- Powell, R.J.; Jenkins, J.S. Lymphocyte subpopulations. Postgrad. Med. J. 1987, 63, 931–935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenkins, J.S. Lymphocyte subpopulations. Diabet. Med. 1987, 4, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Sampson, H.A. Mucosal immunology of food allergy. Curr. Biol. 2013, 23, R389–R400. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Principles of Innate and Adaptive Immunity. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Kobayashi, S.D.; Voyich, J.M.; Burlak, C.; DeLeo, F.R. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. 2005, 53, 505–517. [Google Scholar]
- Lamps, L.W. Beyond acute inflammation: A review of appendicitis and infections of the appendix. Diagn. Histopathol. 2008, 14, 68–77. [Google Scholar] [CrossRef]
- Rahn, S.J. Allergy Models and Related Assays to Test the Allergic Qualities of Escherichia coli Heat Labile Toxin Subunit B. Master’s Thesis, Iowa State University, Ames, IA, USA, 2009. [Google Scholar]
- Daschner, A.; Cuéllar, C. The hidden sense of symptoms: Urticaria can be beneficial. Med. Hypotheses 2010, 75, 623–626. [Google Scholar] [CrossRef]
- Butcher, M.J.; Zhu, J. Recent advances in understanding the Th1/Th2 effector choice. Fac. Rev. 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Kampe, M. Eosinophil Inflammation in Allergic Disease. Clínical and experimental studies in allergic asthma and allergic rhinitis; Acta Universitatis Upsaliensis; Digital comprehensive summaries of Uppsala dissertations from the faculty of medicine. Ph.D. Thesis, Uppsala Universitet, Uppsala, Sweden, 2010. [Google Scholar]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006, 6, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, D.; Nakamura, T.; Ohbayashi, M.; Kuo, C.H.; Komatsu, N.; Yakura, K.; Tominaga, T.; Inoue, Y.; Higashi, H.; Murata, M.; et al. Ablation of type I hypersensitivity in experimental allergic conjunctivitis by eotaxin-1/CCR3 blockade. Int. Immunol. 2009, 21, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.V. The Gell-Coombs classification of hypersensitivity reactions: A re-interpretation. Trends Immunol. 2003, 24, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.B.; Cyr, S.L.; Arima, K.; McDonald, R.A.; Levit, N.A.; Nestle, F.O. Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis. Dermatol. Ther. 2022, 12, 1501–1533. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, M.; van den Biggelaar, A.; Maizels, R.M. Th2 responses without atopy: Immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001, 22, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Carolino, E.; Coelho, H.; Cóias, A.; Trindade, M.; Vaz, J.; Cismasiu, B.; Moita, C.; Moita, L.; Costa, P.M. IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis. Int. J. Mol. Sci. 2022, 23, 15086. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Pathophysiology of asthma. Eur. Respir. Mon. 2003, 23, 84–113. [Google Scholar]
- Renauld, J.C. New insights into the role of cytokines in asthma. J. Clin. Pathol. 2001, 54, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Barrios, R.J.; Kheradmand, F.; Batts, L.; Corry, D.B. Asthma: Pathology and pathophysiology. Arch. Pathol. Lab. Med. 2006, 130, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Shakib, F.; Ghaemmaghami, A.M.; Sewell, H.F. The molecular basis of allergenicity. Trends Immunol. 2008, 29, 633–642. [Google Scholar] [CrossRef]
- Jutel, M.; Blaser, K.; Akdis, C.A. Histamine in allergic inflammation and immune modulation. Int. Arch. Allergy Immunol. 2005, 137, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Rolli-Derkinderen, M.; Arock, M.; Dy, M. Trends in histamine research: New functions during immune responses and hematopoiesis. Trends Immunol. 2002, 23, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Blaser, K. Histamine in the immune regulation of allergic inflammation. J. Allergy Clin. Immunol. 2003, 112, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lechin, F.; van der Dijs, B.; Orozco, B.; Lechin, M.; Lechin, A.E. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann. Allergy Asthma Immunol. 1996, 77, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, H.; Zeng, X.; Guo, L.; Sun, X.; He, S. Subsets of regulatory T cells and their roles in allergy. J. Transl. Med. 2014, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.F.; Guernsey, L.A.; Singh, A.; Vella, A.T.; Schramm, C.M.; Thrall, R.S. Accumulation of regulatory T cells in local draining lymph nodes of the lung correlates with spontaneous resolution of chronic asthma in a murine model. Int. Arch. Allergy Immunol. 2008, 145, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Vartala, S. From allergen genes to new forms of allergy diagnosis and treatment. Allergy 2008, 63, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Borges, M.; Martin, B.L.; Muraro, A.M.; Wood, R.A.; Agache, I.O.; Ansotegui, I.J.; Casale, T.B.; Fleisher, T.A.; Hellings, P.W.; Papadopoulos, N.G.; et al. The importance of allergic disease in public health: An iCAALL statement. World Allergy Organ. J. 2018, 11, 8. [Google Scholar] [CrossRef]
- Dixon, F.; Singh, A. Acute appendicitis. Surgery 2023, 41, 418–425. [Google Scholar] [CrossRef]
- Guan, L.; Liu, Z.; Pan, G.; Zhang, B.; Wu, Y.; Gan, T.; Ouyang, G. The global, regional, and national burden of appendicitis in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. BMC Gastroenterol. 2023, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, A.; Villar Barroso, T.V. Pathophysiology of Acute Appendicitis. JSM Gastroenterol. Hepatol. 2016, 4, 4–7. [Google Scholar]
- Sallinen, V.; Akl, E.A.; You, J.J.; Agarwal, A.; Shoucair, S.; Vandvik, P.O.; Agoritsas, T.; Heels-Ansdell, D.; Guyatt, G.H.; Tikkinen, K.A. Meta-analysis of antibiotics versus appendicectomy for non-perforated acute appendicitis. Br. J. Surg. 2016, 103, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Engin, O.; Yildirim, M.; Yakan, S.; Coskun, G.A. Can fruit seeds and undigested plant residuals cause acute appendicitis. Asian Pac. J. Trop. Biomed. 2011, 1, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Pahissa, R.A.; Lin-Hurtubise, K.M. Giant Appendicolith: A Case Report and Review of the Literature. Mil. Med. 2020, 185, e1851–e1853. [Google Scholar] [CrossRef] [PubMed]
- Kooij, I.A.; Sahami, S.; Meijer, S.L.; Buskens, C.J.; Te Velde, A.A. The immunology of the vermiform appendix: A review of the literature. Clin. Exp. Immunol. 2016, 186, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Brower-Sinning, R.; Firek, B.; Zhong, D.; Morowitz, M.J. Acute Appendicitis in Children Is Associated with a Local Expansion of Fusobacteria. Clin. Infect. Dis. 2016, 63, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, J.; Rubér, M.; Skarstedt, M.; Andersson, M.; Andersson, R.E. Genetic polymorphism patterns suggest a genetic driven inflammatory response as pathogenesis in appendicitis. Int. J. Color. Dis. 2020, 35, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.T.; Mongodin, E.F.; Davenport, K.P.; Fraser, C.M.; Sandler, A.D.; Zeichner, S.L. Culture-independent evaluation of the appendix and rectum microbiomes in children with and without appendicitis. PLoS ONE 2014, 9, e95414. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; McMahon, G.; Reen, D.; Puri, P. New insights into the pathogenesis of appendicitis based on immunocytochemical analysis of early immune response. J. Pediatr. Surg. 1990, 25, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Puri, P.; Reen, D.J. Characterization of the local inflammatory response in appendicitis. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, K. Eosinophils in acute appendicitis: Possible significance. Indian J. Pathol. Microbiol. 1997, 40, 491–498. [Google Scholar] [PubMed]
- Carvalho, N.; Borges, F.; Costa, B.; Costa, P. A etiologia da apendicite aguda. Será a alergia o elo perdido? Uma revisão narrativa. (The aetiology of acute appendicitis. Is allergy the missing link? A narrative review). Rev. Port. Cir. 2022, 52, 1–10. [Google Scholar]
- Carvalho, N.; Barros, A.; Coelho, H.O.; Moita, C.F.; Neves-Costa, A.; Pedroso, D.; Borges, F.C.; Moita, L.F.; Costa, P.M. A Th2 Cytokine Profile in Appendicular Lavage Fluid Suggests Allergy as a Possible Etiology for Acute Appendicitis. Mediat. Inflamm. 2019, 2019, 8146257. [Google Scholar] [CrossRef] [PubMed]
- Echevarria, S.; Rauf, F.; Hussain, N.; Zaka, H.; Farwa, U.E.; Ahsan, N.; Broomfield, A.; Akbar, A.; Khawaja, U.A. Typical and Atypical Presentations of Appendicitis and Their Implications for Diagnosis and Treatment: A Literature Review. Cureus 2023, 15, e37024. [Google Scholar] [CrossRef] [PubMed]
- Salö, M.; Gudjonsdottir, J.; Omling, E.; Hagander, L.; Stenström, P. Association of IgE-Mediated Allergy with Risk of Complicated Appendicitis in a Pediatric Population. JAMA Pediatr. 2018, 172, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Barros, A.; Coelho, H.; Cóias, A.; Botelho, P.; Cismasiu, B.; Moita, L.; Costa, P. Increased IgE Deposition in Appendicular Tissue Specimens Is Compatible with a Type I Hypersensitivity Reaction in Acute Appendicitis. Mediat. Inflamm. 2021, 2021, 4194859. [Google Scholar] [CrossRef]
- Pan, Z.B.; Zhang, T.; Yuan Cheng, M.M.; Yu-Liang Zhou, M.M.; Zhen-Qiang Zhang, M.M. Analysis of type I hypersensitivity-induced inflammatory response in children of different age groups with acute appendicitis. Mol. Immunol. 2023, 158, 103–106. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, Y.; Zhou, Y.; Zhang, Z.; Qi, S.; Pan, Z. Diagnostic performance of type I hypersensitivity-specific markers combined with CRP and IL-6 in complicated acute appendicitis in pediatric patients. Int. Immunopharmacol. 2023, 124, 110977. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Carolino, E.; Coelho, H.; Barreira, A.L.; Moreira, L.; André, M.; Henriques, S.; Cardoso, C.; Moita, L.; Costa, P.M. Eosinophil Granule Proteins Involvement in Acute Appendicitis-An Allergic Disease? Int. J. Mol. Sci. 2023, 24, 9091. [Google Scholar] [CrossRef]
- Carvalho, N.; Carolino, E.; Ferreira, M.; Coelho, H.; Santos, C.R.; Barreira, A.L.; Henriques, S.; Cardoso, C.; Moita, L.; Costa, P.M. Tryptase in Acute Appendicitis: Unveiling Allergic Connections through Compelling Evidence. Int. J. Mol. Sci. 2024, 25, 1645. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsdottir, J.; Roth, B.; Lovén, G.; Ohlsson, B.; Hagander, L.; Salö, M. An Evaluation of Serum IgE and Th2-Associated Interleukins in Children with Uncomplicated and Complicated Appendicitis. Front. Pediatr. 2022, 10, 884138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Carolino, E.; Carvalho, I.; Lisboa, V.; Coelho, H.; Trindade, M.; Vaz, J.; Moita, L.; Costa, P. Immunoglobulin sérum concentration do not correlate with appendicitis. Rev. Port. De Cir. 2022, 53, 37–45. [Google Scholar] [CrossRef]
- Das, N.M.; Thomas, S.; Aravindan, K.P. Morphology and serum IgE levels in recurrent acute appendicitis. Int. J. Adv. Res. 2016, 4, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Hasassri, M.E.; Jackson, E.R.; Ghawi, H.; Ryoo, E.; Wi, C.I.; Bartlett, M.G.; Volcheck, G.W.; Moir, C.R.; Ryu, E.; Juhn, Y.J. Asthma and Risk of Appendicitis in Children: A Population-Based Case-Control Study. Acad. Pediatr. 2017, 17, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, D.J.; Park, B.; Park, I.S.; Choi, H.G. Increased risk of appendectomy in patients with asthma: A nested case-control study using a national sample cohort. Medicine 2019, 98, e17203. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37 (Suppl. S1), S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. Bridging innate and adaptive immunity. Cell 2011, 147, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Larosa, D.F.; Orange, J.S. 1. Lymphocytes. J. Allergy Clin. Immunol. 2008, 121 (Suppl. S2), S364–S369. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, A.J.; Motta, A.C. Th1/Th2 paradigm: Not seeing the forest for the trees? Eur. Respir. J. 2005, 25, 591–593. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Osińska, M.; Salińska, A.; Blicharz, L.; Goldust, M.; Olszewska, M.; Rudnicka, L. The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications. Cells 2021, 10, 3397. [Google Scholar] [CrossRef]
- Romagnani, S. Cytokines and chemoattractants in allergic inflammation. Mol. Immunol. 2002, 38, 881–885. [Google Scholar] [CrossRef] [PubMed]
- de Costa, A. The appendix-mucosal immunity and tolerance in the gut: Consequences for the syndromes of appendicitis and its epidemiology. ANZ J. Surg. 2022, 92, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Korn, T.; Kuchroo, V.K. Th17: The third member of the effector T cell trilogy. Curr. Opin. Immunol. 2007, 19, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, K.; Brandtzaeg, P.; Rognum, T.O. Distribution of immunoglobulin producing cells is different in normal human appendix and colon mucosa. Gut 1986, 27, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Finn, T.; Isaacson, P.G. Gut associated lymphoid tissue: A morphological and immunocytochemical study of the human appendix. Gut 1985, 26, 672–679. [Google Scholar] [CrossRef]
- Vanhatalo, S.; Munukka, E.; Kallonen, T.; Sippola, S.; Grönroos, J.; Haijanen, J.; Hakanen, A.J.; Salminen, P. Appendiceal microbiome in uncomplicated and complicated acute appendicitis: A prospective cohort study. PLoS ONE 2022, 17, e0276007. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, A.; Gonzalez-Perez, I.; Arnold, I.C. Intestinal eosinophils, homeostasis and response to bacterial intrusion. Semin. Immunopathol. 2021, 43, 295–306. [Google Scholar] [CrossRef]
- Vandezande, L.M.; Wallaert, B.; Desreumaux, P.; Tsicopoulos, A.; Lamblin, C.; Tonnel, A.B.; Janin, A. Interleukin-5 immunoreactivity and mRNA expression in gut mucosa from patients with food allergy. Clin. Exp. Allergy 1999, 29, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Vernersson, M. The Rise and Fall of IgE; Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology. Ph.D. Thesis, Uppsala Universitet, Uppsala, Sweden, 2002. [Google Scholar]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Meng, G.; Liu, F.; Zhang, Q.; Liu, L.; Wu, H.; Du, H.; Shi, H.; Xia, Y.; Liu, X.; et al. Serum levels of immunoglobulins in an adult population and their relationship with type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 115, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Smurthwaite, L.; Walker, S.N.; Wilson, D.R.; Birch, D.S.; Merrett, T.G.; Durham, S.R.; Gould, H.J. Persistent IgE synthesis in the nasal mucosa of hay fever patients. Eur. J. Immunol. 2001, 31, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- KleinJan, A.; Vinke, J.G.; Severijnen, L.W.; Fokkens, W.J. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur. Respir. J. 2000, 15, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Kawata, R. Diagnosis and Treatment of Local Allergic Rhinitis. Pathogens 2022, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Callaway, Z.; Pawankar, R. Eosinophil granule proteins as a biomarker in managing asthma and allergies. Asia Pac. Allergy 2023, 13, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Salvo-Romero, E.; Rodiño-Janeiro, B.K.; Albert-Bayo, M.; Lobo, B.; Santos, J.; Farré, R.; Martinez, C.; Vicario, M. Eosinophils in the Gastrointestinal Tract: Key Contributors to Neuro-Immune Crosstalk and Potential Implications in Disorders of Brain-Gut Interaction. Cells 2022, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, J.; Amin, K.; Bishop-Bailey, D. Analysing the eosinophil cationic protein—A clue to the function of the eosinophil granulocyte. Respir. Res. 2011, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Howarth, P.; Quirce, S.; Papi, A.; Israel, E.; Mallett, S.; Bates, S.; Yancey, S.; Albers, F.C.; Kwon, N. Eosinophil-derived neurotoxin and clinical outcomes with mepolizumab in severe eosinophilic asthma. Allergy 2020, 75, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef]
- Zhang, P.L.; Qu, Z. Increased eosinophils in the muscularis propria of appendix may indicate early symptomatic appendicitis. Histopathology 2022, 81, 64. [Google Scholar]
- Abidi, K.; Khoudri, I.; Belayachi, J.; Madani, N.; Zekraoui, A.; Zeggwagh, A.A.; Abouqal, R. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit. Care 2008, 12, R59. [Google Scholar] [CrossRef]
- Cunha, L.; Marcelino, G.; Carvalho, N.; Antunes, C.; Brito, M.J. Eosinophils and C-reactive protein are diagnostic and severity markers in acute appendicitis. Rev. Port. Cir. 2018, 19–24. Available online: https://revista.spcir.com/index.php/spcir/article/view/621 (accessed on 30 March 2024).
- Sousa, J.; Hawkins, R.; Shenoy, A.; Petroze, R.; Mustafa, M.; Taylor, J.; Larson, S.; Islam, S. Enterobius vermicularis -associated appendicitis: A 22-year case series and comprehensive review of the literature. J. Pediatr. Surg. 2022, 57, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.T.; Kristiansen, H.P.; Winther-Larsen, A. Short-term biological variation of serum tryptase. Clin. Chem. Lab. Med. 2024, 62, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Kotrba, J.; Gaffal, E.; Katsoulis-Dimitriou, K.; Dudeck, A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int. J. Mol. Sci. 2021, 22, 4589. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Zhang, X.; Pan, Z.; Spechler, S.J.; Souza, R.F. Mast cell effects on esophageal smooth muscle and their potential role in eosinophilic esophagitis and achalasia. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G319–G327. [Google Scholar] [CrossRef]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Pejler, G. Biology of mast cell tryptase. An. Inflamm. Mediat. FEBS J. 2006, 273, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Kalra, U.; Chitkara, N.; Dadoo, R.C.; Singh, G.P.; Gulati, P.; Narula, S. Evaluation of plasma serotonin concentration in acute appendicitis. Indian J. Gastroenterol. 1997, 16, 18–19. [Google Scholar] [PubMed]
- Kuga, T.; Taniguchi, S.; Inoue, T.; Zempo, N.; Esato, K. Immunocytochemical analysis of cellular infiltrates in human appendicitis. Surg. Today 2000, 30, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Sellars, H.; Boorman, P. Acute appendicitis. Surgery 2017, 35, 432–438. [Google Scholar] [CrossRef]
- Andreou, P.; Blain, S.; Du Boulay, C.E. A histopathological study of the appendix at autopsy and after surgical resection. Histopathology 1990, 17, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Carr, N.J. The pathology of acute appendicitis. Ann. Diagn. Pathol. 2000, 4, 46–58. [Google Scholar] [CrossRef]
- Chang, A.R. An analysis of the pathology of 3003 appendices. Aust. N. Z. J. Surg. 1981, 51, 169–178. [Google Scholar] [CrossRef] [PubMed]
- The, S.-M.M.L.; van Amstel, P.; Noordzij, S.M.; Bakx, R.; Bijlsma, T.S.; Derikx, J.P.M.; van Heurn, L.W.E.; van der Kuip, M.; Gorter, R.R. Trends in Simple and Complex Appendicitis in Children and the Potential Correlation to Common Viral Pathogens—A Retrospective Cohort Study between 2010 and 2019 in The Netherlands. Children 2023, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Pogorelić, Z.; Čohadžić, T. A Bizarre Cause of Acute Appendicitis in a Pediatric Patient: An Ingested Tooth. Children 2023, 10, 108. [Google Scholar] [CrossRef]
- Prystowsky, J.B.; Pugh, C.M.; Nagle, A.P. Current problems in surgery. Append. Curr. Probl. Surg. 2005, 42, 688–742. [Google Scholar] [CrossRef] [PubMed]
- Soo, K.S.; Michie, C.A.; Baker, S.R.; Wyllie, J.H.; Beverley, P.C. Selective recruitment of lymphocyte subsets to the inflamed appendix. Clin. Exp. Immunol. 1995, 100, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. The Etiology of acute appendicitis. JAMA 1925, 85, 197. [Google Scholar] [CrossRef][Green Version]

| Component Evaluated | Reference | Study Design | Conclusion |
|---|---|---|---|
| Epidemiology | Salo M [49] | Cohort | IgE-mediated allergy protects from complicated AA |
| Epidemiology | Hasassri ME [58] | Case-control | Increased risk of AA in active asthma |
| Epidemiology | Kim SY [59] | Case-control | Increased risk of appendectomy in asthma |
| Appendicular wall eosinophils | Aravindan K [45] | Prospective | Eosinophils infiltrate appendicular wall in AA |
| Appendicular wall eosinophils | Carvalho N [19] | Retrospective | Eosinophils infiltrate appendicular wall in phlegmonous AA |
| Appendicular wall IgE | Carvalho N [50] | Retrospective | IgE positive cells higher in phlegmonous AA |
| Serum IgE | Das NM [57] | Prospective | IgE elevation in recurrent AA |
| Serum IgE | Carvalho N [56] | Prospective | No differences found between AA and control group |
| ALF IL-4, IL-5 and IL-9 | Carvalho N [47] | Prospective | Th2 cytokine levels higher in AA |
| ALF IgE, IL-4, IL-5, IL-6 and IL-9 | Pan ZB [51] | Prospective | IgE, cytokines elevated in AA |
| ALF/Serum IL-4 and IL-13 | Unpublished | Prospective | Cytokines elevated in AA |
| Serum IgE, IL-4, IL-9, and IL-13 | Gudjonsdottir J [55] | Prospective | IL-13 elevated in complicated AA |
| Serum IgE, IL-4, IL-5, IL-6, IL-9 and IL-13 | Zhang T [52] | Retrospective | IgE, IL-6 and IL-13 elevated in complicated AA |
| ALF/Serum eosinophil granule proteins | Carvalho N [53] | Prospective | ECP and EP elevated in AA |
| ALF/Serum tryptase | Carvalho N [54] | Prospective | Tryptase levels elevated in AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, N.; Barreira, A.L.; Henriques, S.; Ferreira, M.; Cardoso, C.; Luz, C.; Costa, P.M. Compilation of Evidence Supporting the Role of a T Helper 2 Reaction in the Pathogenesis of Acute Appendicitis. Int. J. Mol. Sci. 2024, 25, 4216. https://doi.org/10.3390/ijms25084216
Carvalho N, Barreira AL, Henriques S, Ferreira M, Cardoso C, Luz C, Costa PM. Compilation of Evidence Supporting the Role of a T Helper 2 Reaction in the Pathogenesis of Acute Appendicitis. International Journal of Molecular Sciences. 2024; 25(8):4216. https://doi.org/10.3390/ijms25084216
Chicago/Turabian StyleCarvalho, Nuno, Ana Lúcia Barreira, Susana Henriques, Margarida Ferreira, Carlos Cardoso, Carlos Luz, and Paulo Matos Costa. 2024. "Compilation of Evidence Supporting the Role of a T Helper 2 Reaction in the Pathogenesis of Acute Appendicitis" International Journal of Molecular Sciences 25, no. 8: 4216. https://doi.org/10.3390/ijms25084216
APA StyleCarvalho, N., Barreira, A. L., Henriques, S., Ferreira, M., Cardoso, C., Luz, C., & Costa, P. M. (2024). Compilation of Evidence Supporting the Role of a T Helper 2 Reaction in the Pathogenesis of Acute Appendicitis. International Journal of Molecular Sciences, 25(8), 4216. https://doi.org/10.3390/ijms25084216






