Preclinical Evaluation of HER2-Targeting DARPin G3: Impact of Albumin-Binding Domain (ABD) Fusion
Abstract
:1. Introduction
2. Results
2.1. Production, Purification, and Characterisation of the ABD-Fused DARPin G3 Variants and G3 and Conjugation of the DOTA Chelator
2.2. Radiolabelling of the Probes with 177Lu and In Vitro Stability
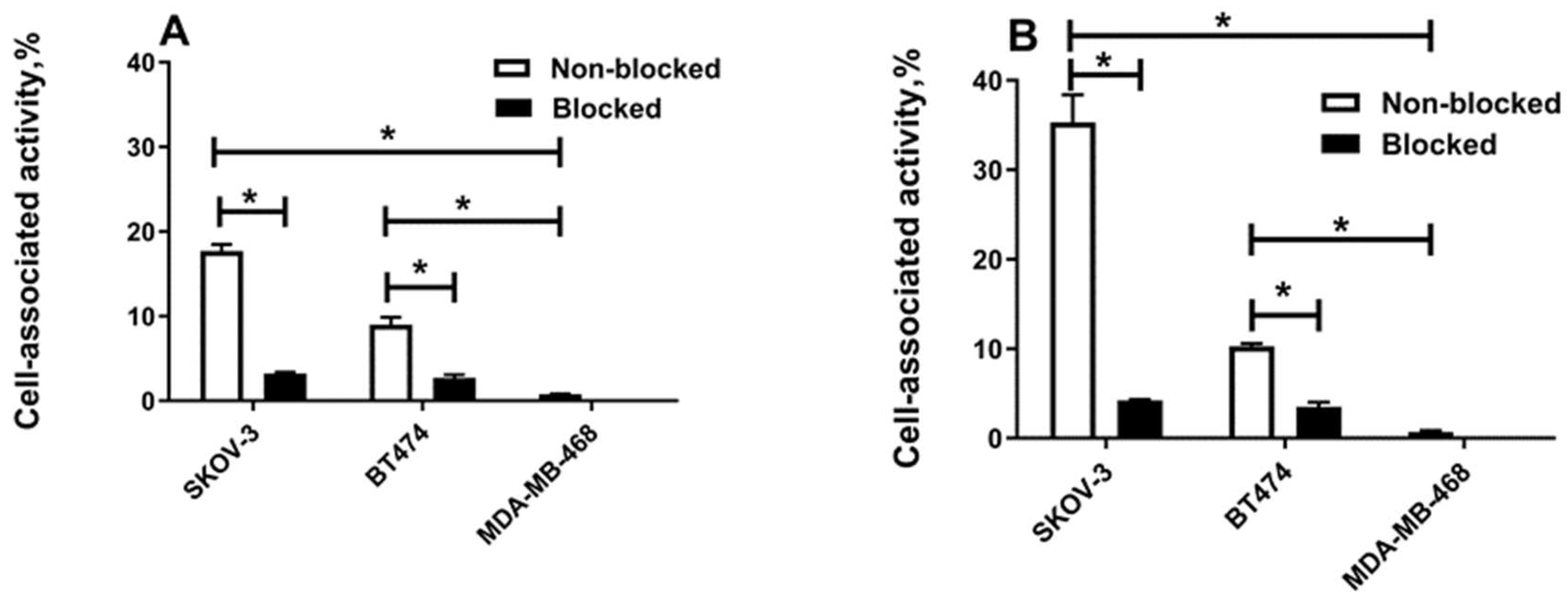
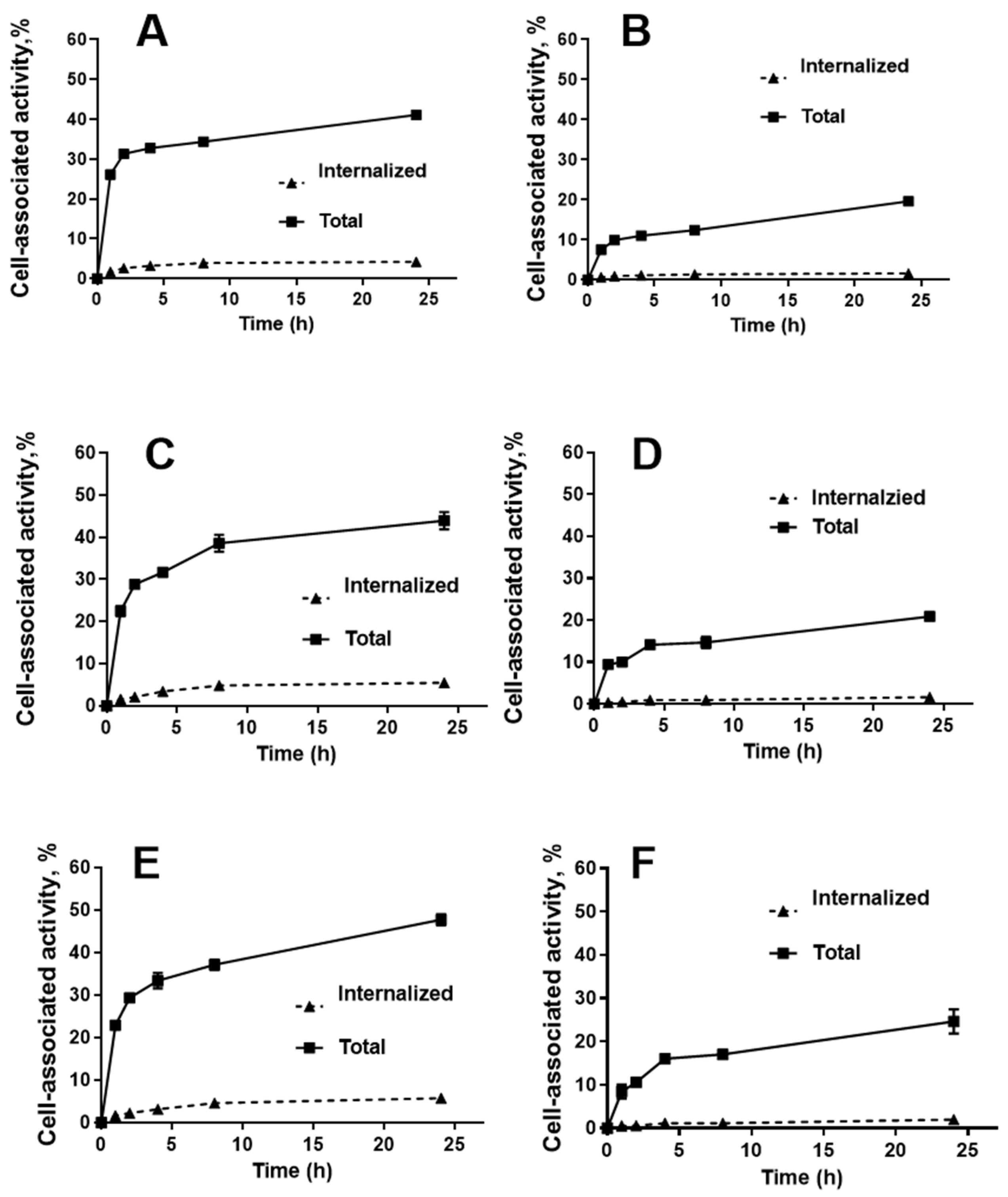
2.3. In Vitro Studies
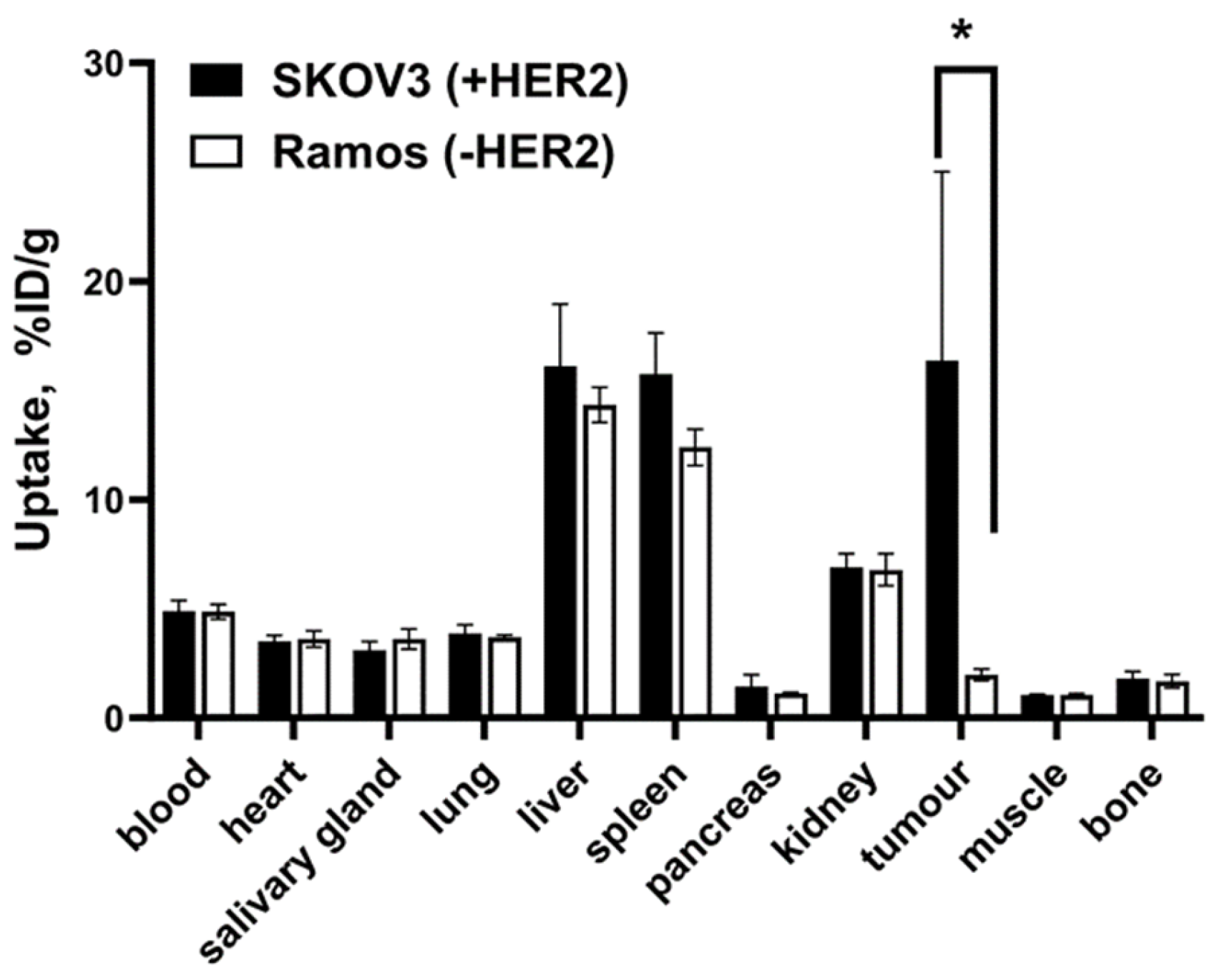
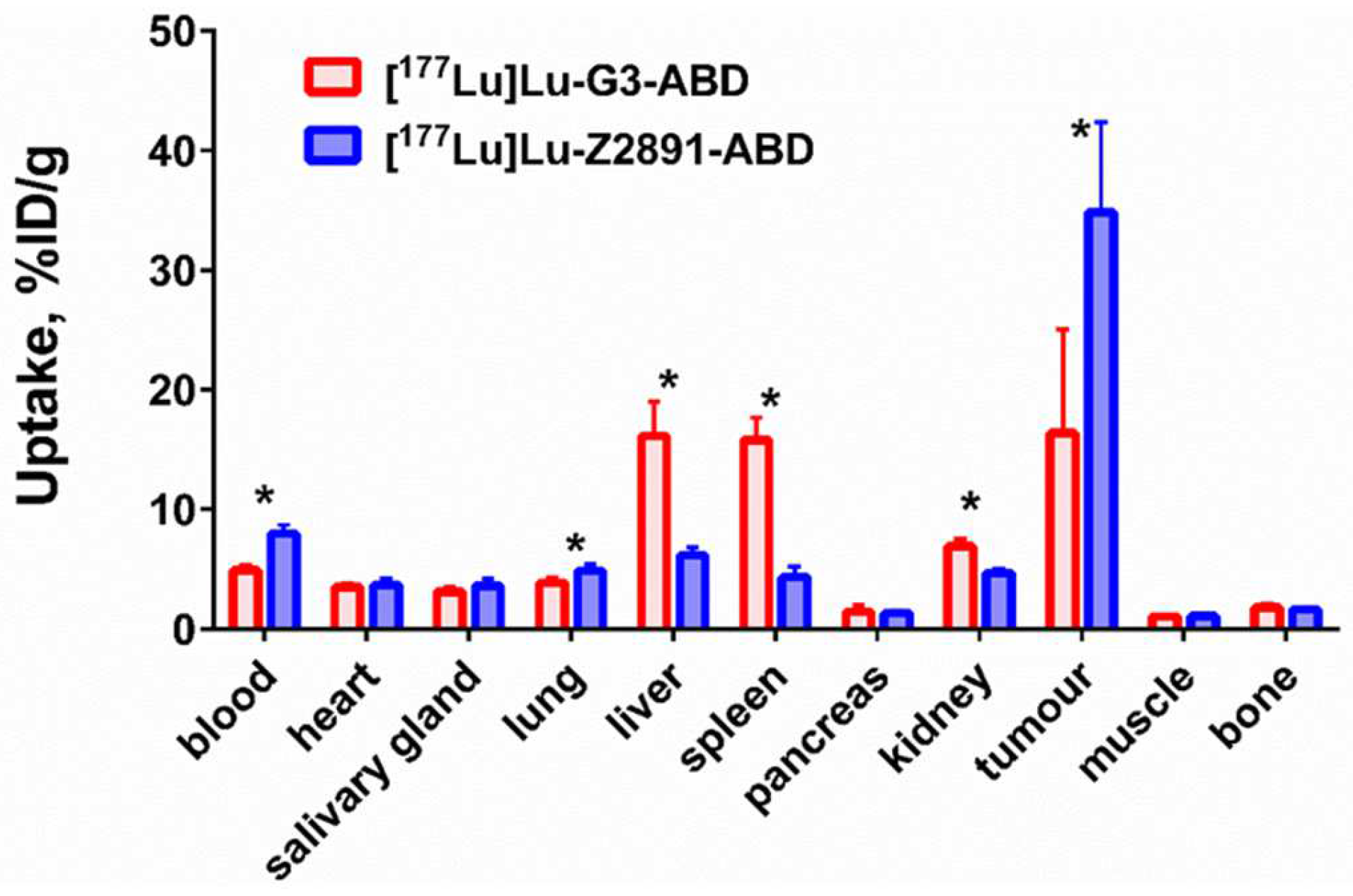
2.4. In Vivo Studies
3. Discussion
4. Materials and Methods
4.1. General
4.2. Production, Purification, and Characterisation of the ABD-Fused DARPin G3 Variants and G3 and Conjugation of the DOTA Chelator
4.3. Radiolabelling of DARPins with 177Lu and In Vitro Stability
4.4. In Vitro Studies
4.5. In Vivo Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Parker, B.A.; Schwab, R.; Kurzrock, R. HER2 aberrations in cancer: Implications for therapy. Cancer Treat. Rev. 2014, 40, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Chandarlapaty, S.; Modi, S. Management of patients with advanced-stage HER2-positive breast cancer: Current evidence and future perspectives. Nat. Rev. Clin. Oncol. 2024, 21, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; Goldenberg, D.M. Cancer radioimmunotherapy. Immunotherapy 2011, 3, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Kenanova, V.; Wu, A.M. Tailoring antibodies for radionuclide delivery. Expert Opin. Drug Deliv. 2006, 3, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Garousi, J.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Imaging using radio-labelled targeted proteins: Radioimmunodetection and beyond. EJNMMI Radiopharm. Chem. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, C.; Kawe, M.; Stumpp, M.T.; De Pasquale, C.; Tamaskovic, R.; Nagy-Davidescu, G.; Dreier, B.; Schibli, R.; Binz, H.K.; Waibel, R.; et al. Efficient Tumor Targeting with High-Affinity Designed Ankyrin Repeat Proteins: Effects of Affinity and Molecular Size. Cancer Res. 2010, 70, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Sosabowski, J.; Livanos, M.; Leyton, J.; Vigor, K.; Bhavsar, G.; Nagy-Davidescu, G.; Rashid, M.; Miranda, E.; Yeung, J.; et al. Development of the designed ankyrin repeat protein (DARPin) G3 for HER2 molecular imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu. Rev. Pharmacol. 2015, 55, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Deyev, S.; Vorobyeva, A.; Schulga, A.; Proshkina, G.; Güler, R.; Löfblom, J.; Mitran, B.; Garousi, J.; Altai, M.; Buijs, J.; et al. Comparative evaluation of two DARPin variants: Effect of affinity, size, and label on tumor targeting properties. Mol. Pharm. 2019, 16, 995–1008. [Google Scholar] [CrossRef]
- Bragina, O.; Chernov, V.; Schulga, A.; Konovalova, E.; Garbukov, E.; Vorobyeva, A.; Orlova, A.; Tashireva, L.; Sörensen, J.; Zelchan, R.; et al. Phase I trial of 99mTc-(HE)3-G3, a DARPin-based probe for imaging of HER2 expression in breast cancer. J. Nucl. Med. 2022, 63, 528–535. [Google Scholar] [CrossRef]
- Zahnd, C.; Wyler, E.; Schwenk, J.M.; Steiner, D.; Lawrence, M.C.; McKern, N.M.; Pecorari, F.; Ward, C.W.; Joos, T.O.; Plückthun, A. A Designed Ankyrin Repeat Protein Evolved to Picomolar Affinity to Her2. J. Mol. Biol. 2007, 369, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Theurillat, J.-P.; Dreier, B.; Nagy-Davidescu, G.; Seifert, B.; Behnke, S.; Zürrer-Härdi, U.; Ingold, F.; Plückthun, A.; Moch, H. Designed ankyrin repeat proteins: A novel tool for testing epidermal growth factor receptor 2 expression in breast cancer. Mod Pathol. 2010, 23, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Garousi, J.; Rinne, S.S.; Schulga, A.; Deyev, S.; Vorobyeva, A. On the prevention of kidney uptake of radiolabeled DARPins. EJNMMI Res. 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Half-life extended biotherapeutics. Expert Opin. Biol. Ther. 2016, 16, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Merz, F.W.; Sonderegger, I.; Gulotti-Georgieva, M.; Villemagne, D.; Phillips, D.J.; Forrer, P.; Stumpp, M.T.; Zitt, C.; Binz, H.K. Half-life extension using serum albumin-binding DARPin® domains. Protein Eng. Des. Sel. 2017, 30, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.; Merten, H.; Zimmermann, M.; Béhé, M.; Zangemeister-Wittke, U.; Plückthun, A. Influence of size and charge of unstructured polypeptides on pharmacokinetics and biodistribution of targeted fusion proteins. J. Control. Release 2019, 307, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.; Busslinger, S.; Zangemeister-Wittke, U.; Plückthun, A. Optimizing the anti-tumor efficacy of protein-drug conjugates by engineering the molecular size and half-life. J. Control. Release 2020, 327, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.; Bragina, O.; Altai, M.; Mitran, B.; Orlova, A.; Shulga, A.; Proshkina, G.; Chernov, V.; Tolmachev, V.; Deyev, S. Comparative Evaluation of Radioiodine and Technetium-Labeled DARPin 9_29 for Radionuclide Molecular Imaging of HER2 Expression in Malignant Tumors. Eur. J. Nucl. Med. Mol. Imaging 2018, 2018, 6930425. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A.; Pehrson, R.; Galli, J.; Baastrup, B.; Andersson, K.; Sandström, M.; Rosik, D.; Carlsson, J.; Lundqvist, H.; et al. Radionuclide Therapy of HER2-Positive Microxenografts Using a 177 Lu-Labeled HER2-Specific Affibody Molecule. Cancer Res. 2007, 67, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.T.; Sandlie, I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: Implications for development of new diagnostics and therapeutics. Drug Metab. Pharmacok. 2009, 24, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.T.; Pehrson, R.; Tolmachev, V.; Daba, M.B.; Abrahmsén, L.; Ekblad, C. Extending Half-life by Indirect Targeting of the Neonatal Fc Receptor (FcRn) Using a Minimal Albumin Binding Domain. J. Biol. Chem. 2011, 286, 5234–5241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vorobyeva, A.; Xu, T.; Orlova, A.; Loftenius, A.; Bengtsson, T.; Jonasson, P.; Tolmachev, V.; Frejd, F.Y. Comparative Preclinical Evaluation of HER2-Targeting ABD-Fused Affibody® Molecules 177Lu-ABY-271 and 177Lu-ABY-027: Impact of DOTA Position on ABD Domain. Pharmaceutics 2021, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Garousi, J.; Lindbo, S.; Borin, J.; Von Witting, E.; Vorobyeva, A.; Oroujeni, M.; Mitran, B.; Orlova, A.; Buijs, J.; Tolmachev, V.; et al. Comparative evaluation of dimeric and monomeric forms of ADAPT scaffold protein for targeting of HER2-expressing tumours. Eur. J. Pharm. Biopharm. 2019, 134, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Schulga, A.; Konovalova, E.; Güler, R.; Löfblom, J.; Sandström, M.; Garousi, J.; Chernov, V.; Bragina, O.; Orlova, A.; et al. Optimal composition and position of histidine-containing tags improves biodistribution of 99mTc-labeled DARPin G3. Sci. Rep. 2019, 9, 9405. [Google Scholar] [CrossRef]
- Watanabe, S.; Hashimoto, K.; Ishioka, N.S. Lutetium-177 complexation of DOTA and DTPA in the presence of competing metals. J. Radioanal. Nucl. Chem. 2015, 303, 1519–1521. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. (Russ). Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Yin, W.; Xu, T.; Altai, M.; Oroujeni, M.; Zhang, J.; Vorobyeva, A.; Vorontsova, O.; Vtorushin, S.V.; Tolmachev, V.; Gräslund, T.; et al. The Influence of Domain Permutations of an Albumin-Binding Domain-Fused HER2-Targeting Affibody-Based Drug Conjugate on Tumor Cell Proliferation and Therapy Efficacy. Pharmaceutics 2021, 13, 1974. [Google Scholar] [CrossRef] [PubMed]
- Alhuseinalkhudhur, A.; Lindman, H.; Liss, P.; Sundin, T.; Frejd, F.Y.; Hartman, J.; Iyer, V.; Feldwisch, J.; Lubberink, M.; Rönnlund, C.; et al. Human Epidermal Growth Factor Receptor 2-Targeting [68Ga]Ga-ABY-025 PET/CT Predicts Early Metabolic Response in Metastatic Breast Cancer. J. Nucl. Med. 2023, 64, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, S.; Staubach, P.; Dirschka, T.; Wetzel, D.; Weirich, O.; Niesmann, J.; Da Mota, R.; Rothhaar, A.; Ardabili, M.; Vlasitz, G.; et al. Izokibep for the treatment of moderate-to-severe plaque psoriasis: A phase II, randomized, placebo-controlled, double-blind, dose-finding multicentre study including long-term treatment. Br. J. Dermatol. 2023, 189, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrot, C.; Ultsch, M.; Dubnovitsky, A.; Abrahmsén, L.; Härd, T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA 2010, 107, 15039–15044. [Google Scholar] [CrossRef] [PubMed]
- Jost, C.; Schilling, J.; Tamaskovic, R.; Schwill, M.; Honegger, A.; Plückthun, A. Structural basis for eliciting a cytotoxic effect in HER2-overexpressing cancer cells via binding to the extracellular domain of HER2. Structure 2013, 21, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.; Dogan, J.; Herne, N.; Abrahmsén, L.; Nygren, P.A. Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng. Des. Sel. 2008, 21, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Barta, P.; Malmberg, J.; Melicharova, L.; Strandgård, J.; Orlova, A.; Tolmachev, V.; Laznicek, M.; Andersson, K. Protein interactions with HER-family receptors can have different characteristics depending on the hosting cell line. Int. J. Oncol. 2012, 40, 1677–1682. [Google Scholar] [PubMed]
- Leyton, J.V. Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics—The Tale of HER2. Antibodies 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Scagliola, R.; Brunelli, C. Venous Congestion and Systemic Hypoperfusion in Cardiorenal Syndrome: Two Sides of the Same Coin. Rev. Cardiovasc. Med. 2022, 23, 111. [Google Scholar] [CrossRef]
- Yin, W.; Xu, T.; Ding, H.; Zhang, J.; Bodenko, V.; Tretyakova, M.S.; Belousov, M.V.; Liu, Y.; Oroujeni, M.; Orlova, A.; et al. Comparison of HER2-targeted affibody conjugates loaded with auristatin- and maytansine-derived drugs. J. Control. Release 2023, 355, 515–527. [Google Scholar] [CrossRef]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007, 2, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Malakhov, M.P.; Mattern, M.R.; Malakhova, O.A.; Drinker, M.L.; Weeks, S.D.; Butt, T.R. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genom. 2004, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Tran, T.A.; Rosik, D.; Sjöberg, A.; Abrahmsén, L.; Orlova, A. Tumor targeting using affibody molecules: Interplay of affinity, target expression level, and binding site composition. J. Nucl. Med. 2012, 53, 953–960. [Google Scholar] [CrossRef] [PubMed]
- McLarty, K.; Cornelissen, B.; Scollard, D.A.; Done, S.J.; Chun, K.; Reilly, R.M. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 81–93. [Google Scholar] [CrossRef] [PubMed]
- DeFazio-Eli, L.; Strommen, K.; Dao-Pick, T.; Parry, G.; Goodman, L.; Winslow, J. Quantitative assays for the measurement of HER1-HER2 heterodimerization and phosphorylation in cell lines and breast tumors: Applications for diagnostics and targeted drug mechanism of action. Breast Cancer Res. 2011, 13, R44. [Google Scholar] [CrossRef] [PubMed]




| ka (M−1s−1) | kd (s−1) | KD (M) | |
|---|---|---|---|
| G3-ABD-DOTA | |||
| Human albumin | 1.31 × 105 | 2.38 × 10−4 | 1.82 × 10−9 |
| Murine albumin | 6.13 × 104 | 5.18 × 10−3 | 8.46 × 10−9 |
| ABD-G3-DOTA | |||
| Human albumin | 8.53 × 103 | 3.02 × 10−4 | 3.54 × 10−8 |
| Murine albumin | 2.35 × 104 | 1.91 × 10−3 | 8.13 × 10−8 |
| KD1 (pM) | Weight 1, % | KD2 (nM) | Weight 2, % | |
|---|---|---|---|---|
| [177Lu]Lu-G3-ABD | 901 ± 169 | 54 | 24 ± 0 | 45 |
| [177Lu]Lu-ABD-G3 | 1290 ± 920 | 38 | 67 ± 14 | 62 |
| [177Lu]Lu-G3 | 1200 ± 200 | 60 | 34 ± 12 | 40 |
| Uptake, %ID/g | ||||||
|---|---|---|---|---|---|---|
| Organ | [177Lu]Lu-G3-ABD | [177Lu]Lu-ABD-G3 | ||||
| 4 h | 48 h | 144 h | 4 h | 48 h | 144 h | |
| Blood | 23.1 ± 2.4 | 4.9 ± 0.5 b | 0.4 ± 0.1 c | 21.1 ± 3.4 | 1.3 ± 0.3 b | 0.047 ± 0.004 c |
| Heart | 6.7 ± 0.1 | 3.5 ± 0.3 b | 1.5 ± 0.1 c | 6.3 ± 0.8 | 1.66 ± 0.05 b | 0.66 ± 0.03 c |
| Salivary gland | 3.1 ± 0.7 | 3.1 ± 0.4 b | 2.0 ± 0.3 c | 2.9 ± 0.2 | 1.9 ± 0.3 b | 0.9 ± 0.1 c |
| Lung | 8.6 ± 1.1 | 3.9 ± 0.4 b | 1.1 ± 0.2 c | 8.5 ± 0.9 | 1.6 ± 0.3 b | 0.42 ± 0.02 c |
| Liver | 17.8 ± 1.8 a | 16.1 ± 2.9 b | 7.3 ± 0.8 c | 9.7 ± 0.9a | 8.6 ± 1.6 b | 4.4 ± 0.6 c |
| Spleen | 17.1 ± 3.8 a | 15.8 ± 1.9 b | 7.0 ± 1.5 c | 5.5 ± 1.4a | 3.7 ± 0.5 b | 2.1 ± 0.2 c |
| Pancreas | 1.3 ± 0.1 | 1.4 ± 0.5 | 0.6 ± 0.1 c | 1.6 ± 0.4 | 0.8 ± 0.5 | 0.3 ± 0.1 c |
| Kidney | 7.8 ± 0.6 a | 6.9 ± 0.6 b | 3.1 ± 0.5 c | 39.2 ± 6.0 a | 106.4 ± 9.3 b | 36.5 ± 6.9 c |
| Tumour | 7.5 ± 5.5 | 16.4 ± 8.7 | 7.9 ± 2.5 | 7.4 ± 5.4 | 11.3 ± 3.6 | 4.3 ± 1.7 |
| Muscle | 1.0 ± 0.2 | 1.05 ± 0.04 b | 0.48 ± 0.13 c | 1.2 ± 0.1 | 0.51 ± 0.0 b | 0.18 ± 0.02 c |
| Bone | 2.1 ± 0.2 | 1.8 ± 0.3 | 1.2 ± 0.2 | 2.4 ± 0.6 | 1.89 ± 0.04 | 1.5 ± 0.4 |
| Brain | 0.6 ± 0.2 | 0.17 ± 0.01 b | 0.04 ± 0.01 c | 0.52 ± 0.18 | 0.06 ± 0.02 b | 0.009 ± 0.003 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deyev, S.M.; Oroujeni, M.; Garousi, J.; Gräslund, T.; Li, R.; Rosly, A.H.B.; Orlova, A.; Konovalova, E.; Schulga, A.; Vorobyeva, A.; et al. Preclinical Evaluation of HER2-Targeting DARPin G3: Impact of Albumin-Binding Domain (ABD) Fusion. Int. J. Mol. Sci. 2024, 25, 4246. https://doi.org/10.3390/ijms25084246
Deyev SM, Oroujeni M, Garousi J, Gräslund T, Li R, Rosly AHB, Orlova A, Konovalova E, Schulga A, Vorobyeva A, et al. Preclinical Evaluation of HER2-Targeting DARPin G3: Impact of Albumin-Binding Domain (ABD) Fusion. International Journal of Molecular Sciences. 2024; 25(8):4246. https://doi.org/10.3390/ijms25084246
Chicago/Turabian StyleDeyev, Sergey M., Maryam Oroujeni, Javad Garousi, Torbjörn Gräslund, Ruonan Li, Alia Hani Binti Rosly, Anna Orlova, Elena Konovalova, Alexey Schulga, Anzhelika Vorobyeva, and et al. 2024. "Preclinical Evaluation of HER2-Targeting DARPin G3: Impact of Albumin-Binding Domain (ABD) Fusion" International Journal of Molecular Sciences 25, no. 8: 4246. https://doi.org/10.3390/ijms25084246







