Apoptotic Effect of Isoimpertorin via Inhibition of c-Myc and SIRT1 Signaling Axis
Abstract
1. Introduction
2. Results
2.1. Isoimperatorin Inhibits the Viability of Huh7 and Hep3B Cells
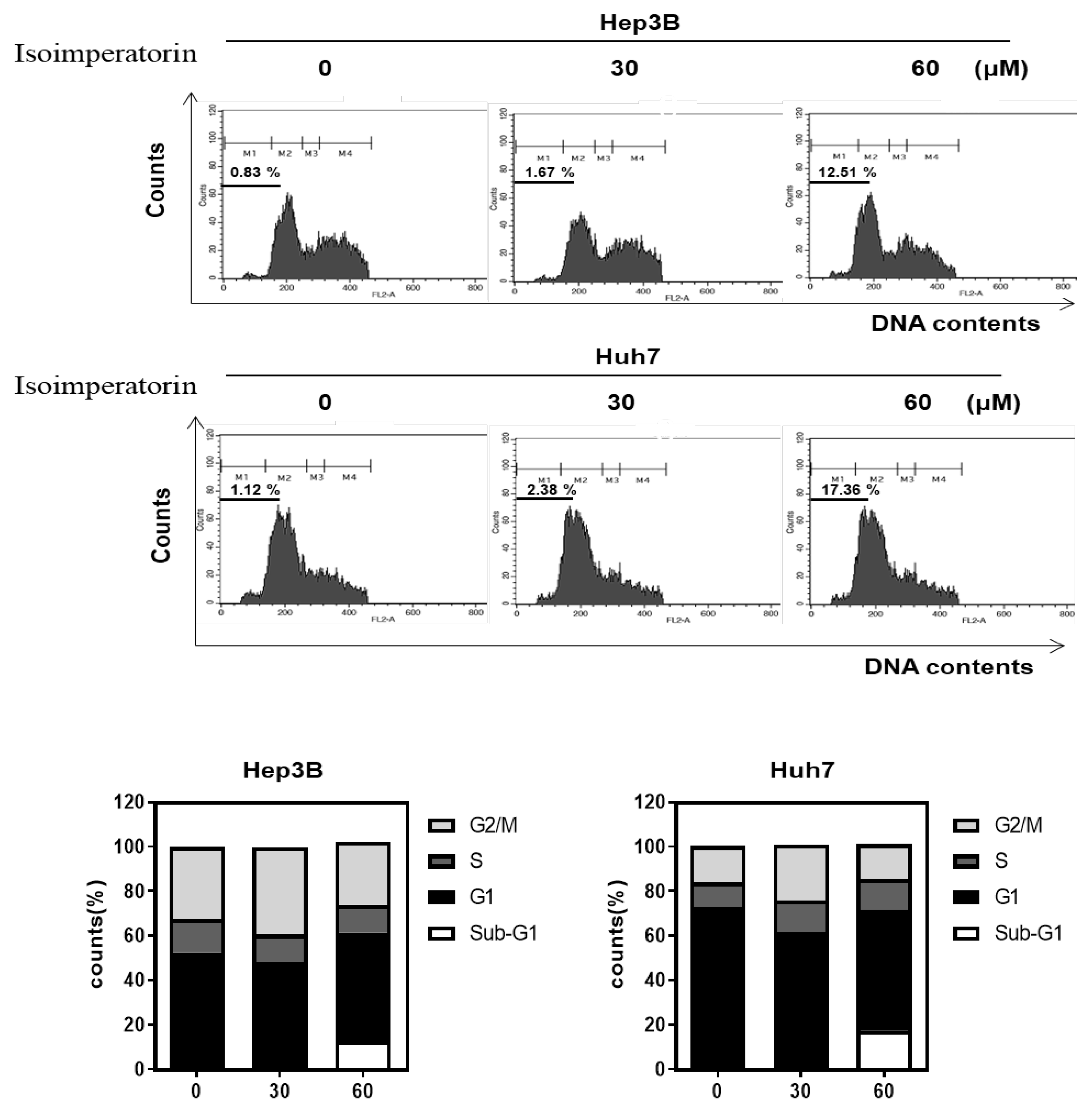
2.2. Isoimperatorin Increases Sub G1 Population in Huh7 and Hep3B Cells
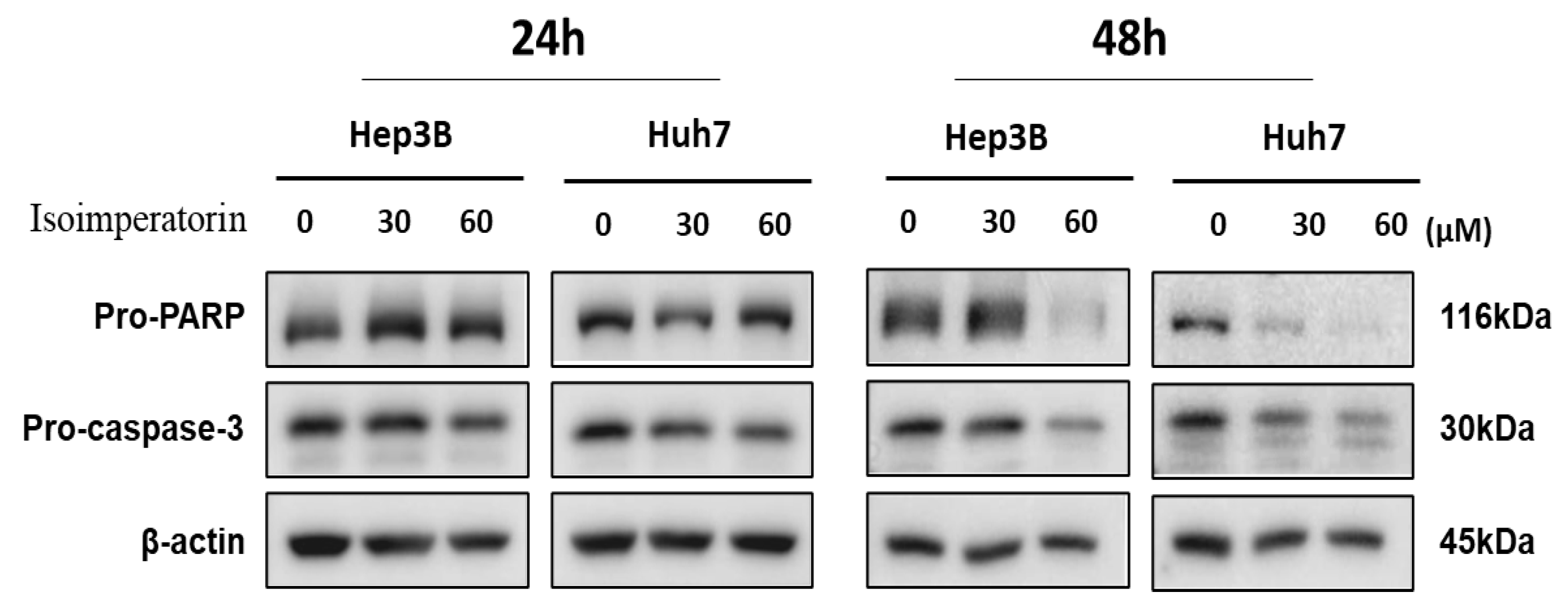
2.3. Isoimperatorin Attenuates the Expression of Pro-PARP and Pro-Caspase 3 in Huh7 and Hep3B Cells
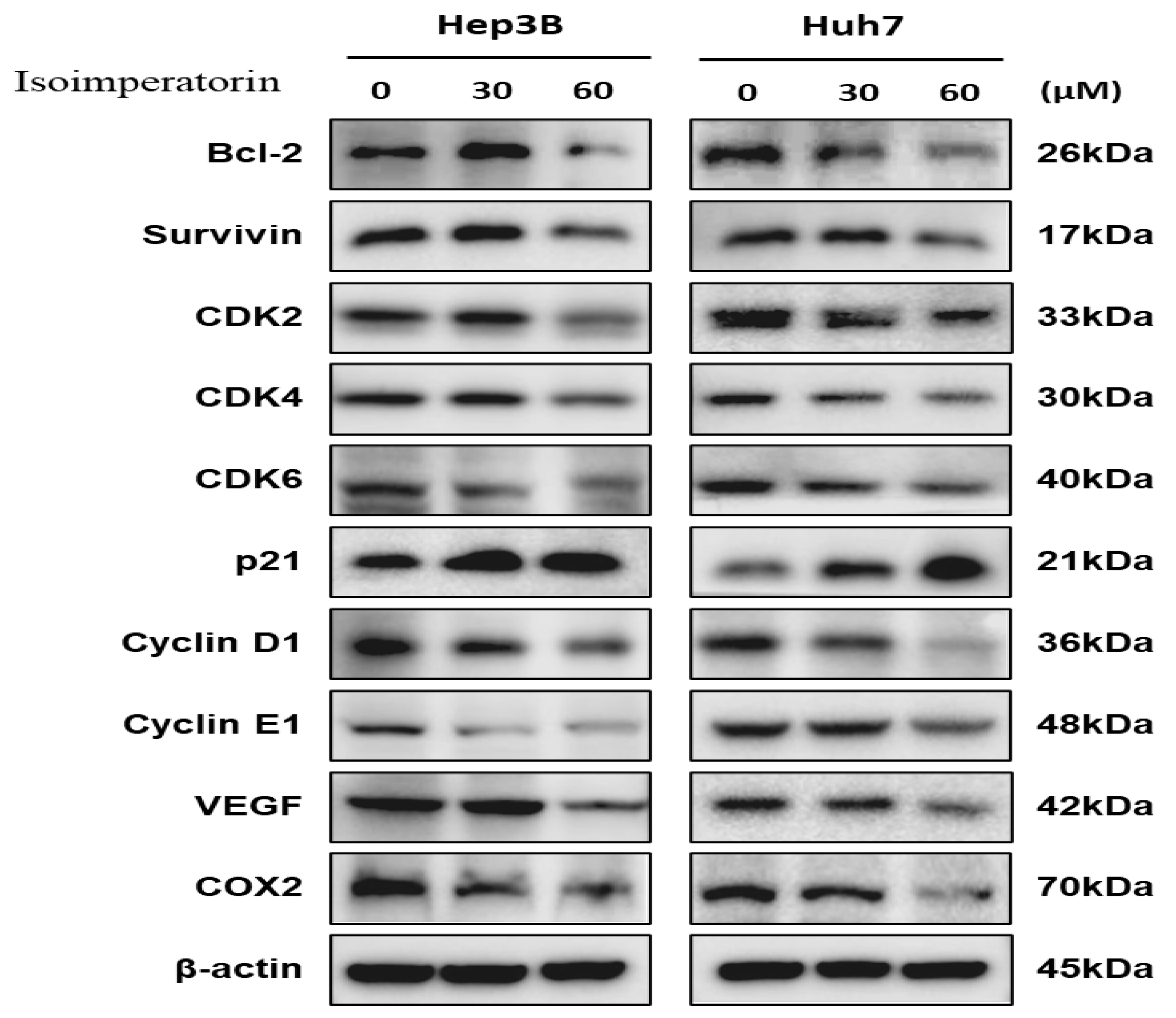
2.4. Isoimperatorin Modulates Cell Cycle- and Survival-Related Proteins in Huh7 and Hep3B Cells
2.5. Isoimperatorin Inhibits c-Myc Stability in Huh7 and Hep3B Cells
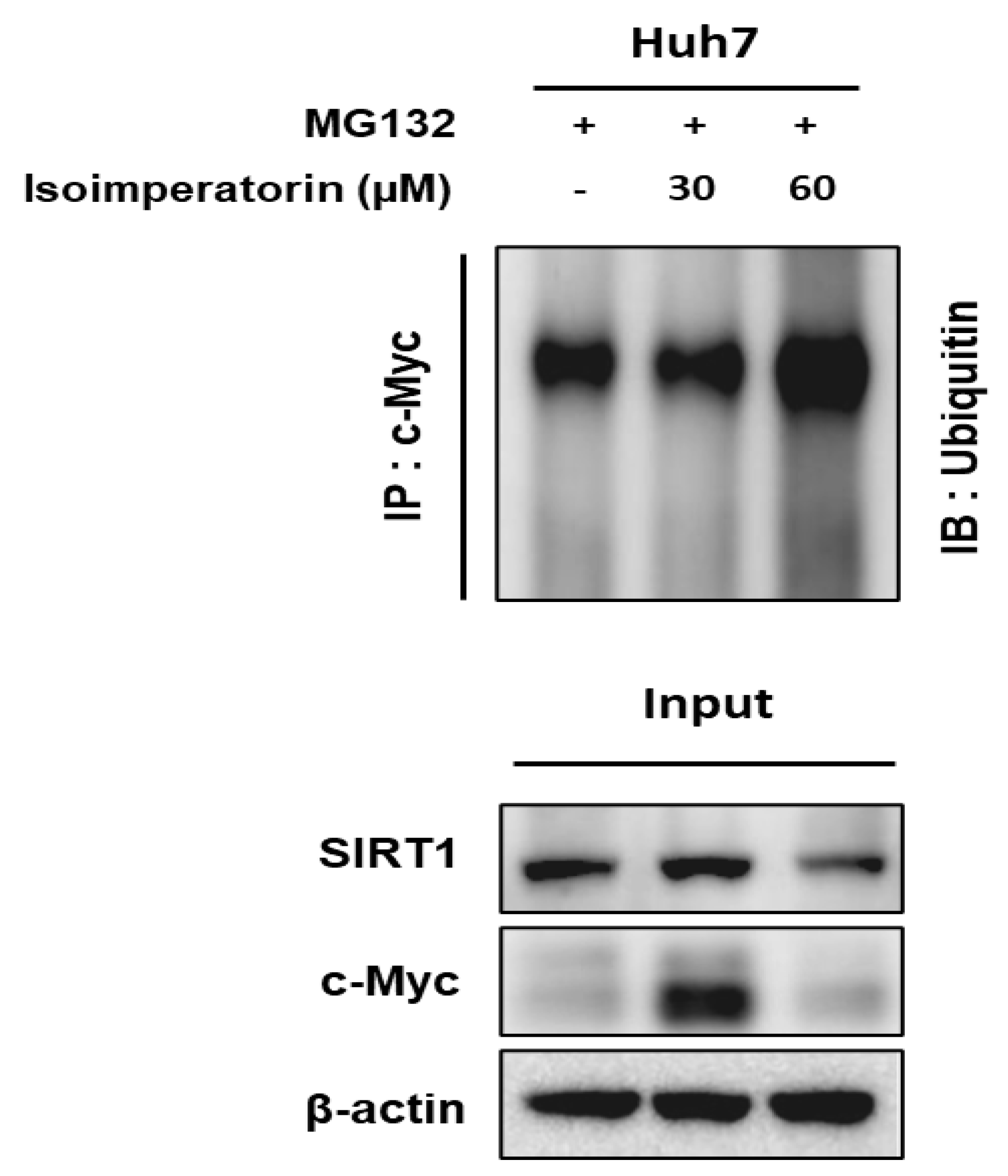
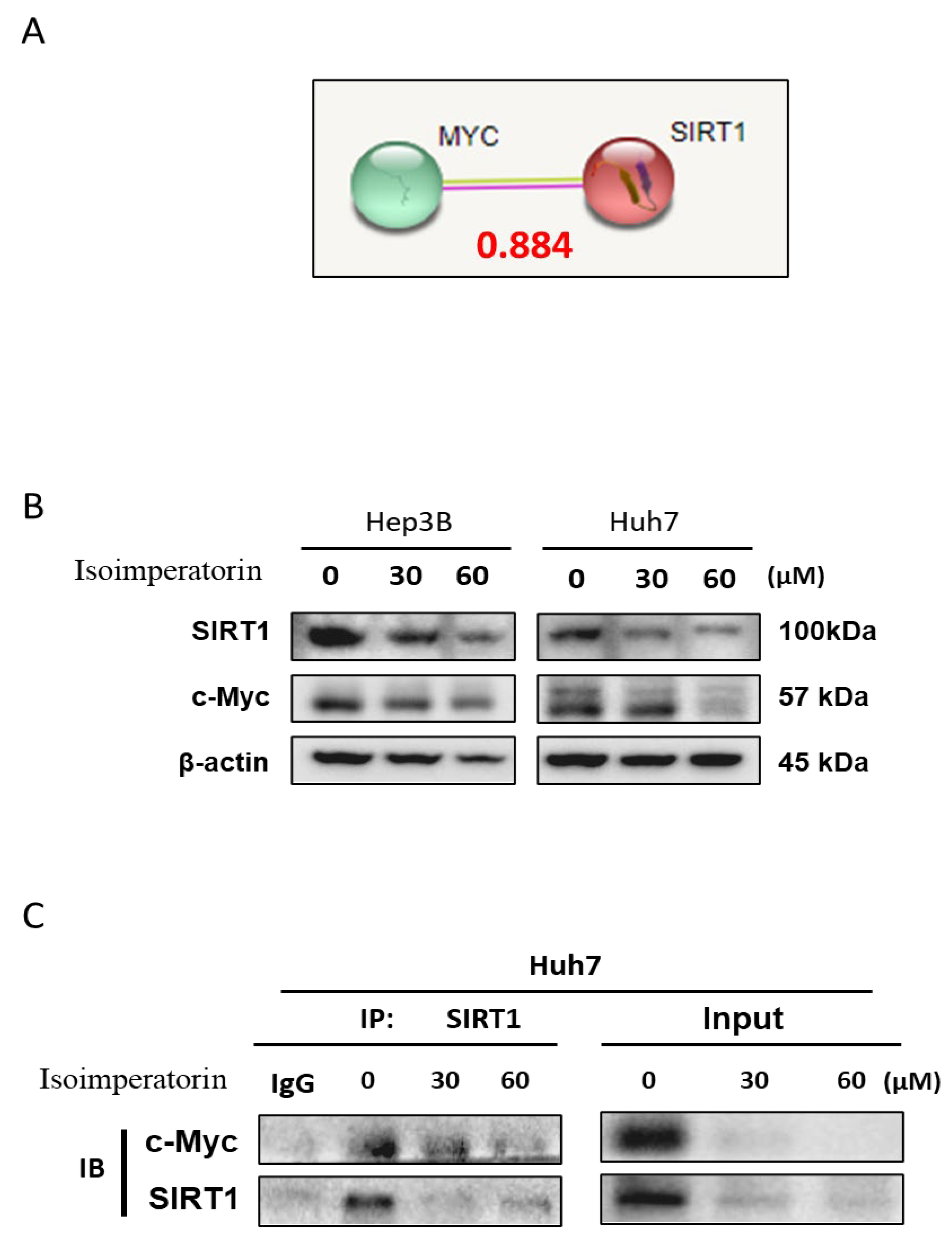
2.6. Isoimperatorin Inhibits the Expression of c-Myc and SIRT1 via c-Myc Degradation in Huh7 Cells
2.7. Isoimperatorin Disturbs the Binding between c-Myc and SIRT1 in Huh7 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cytotoxicity Assay
4.3. Wound-Healing Assay
4.4. Cell Cycle Analysis
4.5. RNA Interference
4.6. Western Blotting
4.7. Cycloheximide Assay
4.8. Ubiquitination Assay
4.9. Co-Immunoprecipitation Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Satriano, L.; Lewinska, M.; Rodrigues, P.M.; Banales, J.M.; Andersen, J.B. Metabolic rearrangements in primary liver cancers: Cause and consequences. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Mejia, J.C.; Pasko, J. Primary liver cancers: Intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Surg. Clin. N. Am. 2020, 100, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Park, H.; Ro, S.W. C-myc-driven hepatocarcinogenesis. Anticancer. Res. 2021, 41, 4937–4946. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. Myc as a target for cancer treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Yin, J.; Gan, Y.; Xu, S.; Gu, Y.; Huang, W. Alternative approaches to target myc for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of sirt1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Dong, L.; Li, Y.; Liu, G. Sirt1 and hif1α signaling in metabolism and immune responses. Cancer Lett. 2018, 418, 20–26. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Huang, S.; Deng, C.; Zhou, S.; Yang, J.; Cao, Y.; Xu, L.; Yuan, Y.; Yang, J.; et al. Sirt1 inhibits gastric cancer proliferation and metastasis via stat3/mmp-13 signaling. J. Cell Physiol. 2019, 234, 15395–15406. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. Sirt1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Zhou, L.; Wang, Y.; Shang, P. Sirt1: A potential tumour biomarker and therapeutic target. J. Drug Target. 2019, 27, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Deng, X.; Chen, Z.; Ba, X.; Qin, K.; Huang, Y.; Huang, Y.; Li, T.; Yan, J.; Tu, S. Sirt1: A potential therapeutic target in autoimmune diseases. Front. Immunol. 2021, 12, 779177. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Q.; He, B. Sirt1 as a potential therapeutic target for chronic obstructive pulmonary disease. Lung 2023, 201, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Jin, M.; Son, J.K.; Chang, H.W. The effects of isoimperatorin isolated from angelicae dahuricae on cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2008, 31, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Han, T.; Zhan, S.; Jiang, Y.; Liu, X.; Li, G. Antiviral activity of isoimperatorin against influenza a virus in vitro and its inhibition of neuraminidase. Front. Pharmacol. 2021, 12, 657826. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, X.; Yan, Z.; Wang, X.; Gun, S. Isoimperatorin enhances 3t3-l1 preadipocyte differentiation by regulating pparγ and c/ebpα through the akt signaling pathway. Exp. Ther. Med. 2019, 18, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Kausar, M.A.; Shahid, S.; Anwar, S.; Kuddus, M.; Khan, M.K.A.; Khalifa, A.M.; Khatoon, F.; Alotaibi, A.D.; Alkhodairy, S.F.; Snoussi, M.; et al. Identifying the alpha-glucosidase inhibitory potential of dietary phytochemicals against diabetes mellitus type 2 via molecular interactions and dynamics simulation. Cell Mol. Biol. 2022, 67, 16–26. [Google Scholar] [CrossRef]
- Fan, L.; Li, Z.; Gao, L.; Zhang, N.; Chang, W. Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating erk1/2 and nf-κb pathways. Open Life Sci. 2023, 18, 20220541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.M.; Shen, J.Z.; Wang, Y.; Lu, A.X.; Ho, W.S. Anti-oxidant and anti-cancer activities of angelica dahurica extract via induction of apoptosis in colon cancer cells. Phytomedicine 2016, 23, 1267–1274. [Google Scholar] [CrossRef]
- Tong, K.; Xin, C.; Chen, W. Isoimperatorin induces apoptosis of the sgc-7901 human gastric cancer cell line via the mitochondria-mediated pathway. Oncol. Lett. 2017, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Gao, H.R.; Ren, Y.J.; Fang, F.X.; Tian, H.T.; Gao, Z.J.; Song, W.; Huang, S.M.; Zhao, A.F. Effects of isoimperatorin on proliferation and apoptosis of human gastric carcinoma cells. Oncol. Lett. 2018, 15, 7993–7998. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.S.; Sears, R.C. Myc degradation. Cold Spring Harb. Perspect. Med. 2014, 4, a014365. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Peng, Z. Mg132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac. J. Clin. Oncol. 2013, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Moon, H.J.; You, B.R.; Park, W.H. The attenuation of mg132, a proteasome inhibitor, induced a549 lung cancer cell death by p38 inhibitor in ros-independent manner. Oncol. Res. 2010, 18, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.A.; Hann, S.R. C-myc proteolysis by the ubiquitin-proteasome pathway: Stabilization of c-myc in burkitt’s lymphoma cells. Mol. Cell Biol. 2000, 20, 2423–2435. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Kim, J.Y.; Li, G.; Lee, K.S.; Seo, C.S.; Yan, J.J.; Jung, J.S.; Kim, H.J.; Chang, H.W.; Son, J.K. Agents protecting against sepsis from the roots of angelica dahurica. Biol. Pharm. Bull. 2005, 28, 380–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, N.Y.; Jung, Y.Y.; Yang, M.H.; Um, J.Y.; Sethi, G.; Ahn, K.S. Isoimperatorin down-regulates epithelial mesenchymal transition through modulating nf-κb signaling and cxcr4 expression in colorectal and hepatocellular carcinoma cells. Cell Signal 2022, 99, 110433. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.D. Caspases in cell death, inflammation, and pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. Cdk4/6 inhibition in cancer: Beyond cell cycle arrest. Trends Cell Biol 2018, 28, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.; Teixeira, L.K. Cyclin e/cdk2: DNA replication, replication stress and genomic instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: P53-p21-rb signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Pope, E.D., 3rd; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A., 3rd; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert. Opin. Ther. Targets 2019, 23, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Feng, F.; Wu, J.; Fan, S.; Han, J.; Wang, S.; Yang, L.; Liu, W.; Wang, C.; Xu, K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022, 181, 106270. [Google Scholar] [CrossRef]
- Sridhar, S.; Sharma, I.; Sankpal, U.T.; Ghabach, B.; Narra, K.; Neerukonda, L.; Basha, R. Targeted molecular therapeutic options for hepatocellular carcinoma. Crit. Rev. Oncog. 2020, 25, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yin, S.; Zhang, E.; Fan, L.; Ye, M.; Zhang, Y.; Hu, H. Glycycoumarin exerts anti-liver cancer activity by directly targeting t-lak cell-originated protein kinase. Oncotarget 2016, 7, 65732–65743. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of sirt1 and its roles in inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.R.; Park, W.Y.; Lee, H.J.; Sim, D.Y.; Im, E.; Park, J.E.; Ahn, C.H.; Shim, B.S.; Kim, S.H. Antitumor effect of morusin via g1 arrest and antiglycolysis by ampk activation in hepatocellular cancer. Int. J. Mol. Sci. 2021, 22, 619. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, H.; Kim, J.H.; Sim, D.Y.; Ahn, H.; Kim, B.; Chang, S.; Kim, S.H. P53-dependent apoptotic effect of puromycin via binding of ribosomal protein l5 and l11 to mdm2 and its combination effect with rita or doxorubicin. Cancers 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Aprelikova, O.; Chen, K.; El Touny, L.H.; Brignatz-Guittard, C.; Han, J.; Qiu, T.; Yang, H.H.; Lee, M.P.; Zhu, M.; Green, J.E. The epigenetic modifier jmjd6 is amplified in mammary tumors and cooperates with c-myc to enhance cellular transformation, tumor progression, and metastasis. Clin. Epigenetics 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, H.-J.; Park, S.-Y.; Sim, D.Y.; Kim, S.-H.; Hur, S.; Lee, J.-H.; Kim, Y. Apoptotic Effect of Isoimpertorin via Inhibition of c-Myc and SIRT1 Signaling Axis. Int. J. Mol. Sci. 2024, 25, 4248. https://doi.org/10.3390/ijms25084248
Ko H-J, Park S-Y, Sim DY, Kim S-H, Hur S, Lee J-H, Kim Y. Apoptotic Effect of Isoimpertorin via Inhibition of c-Myc and SIRT1 Signaling Axis. International Journal of Molecular Sciences. 2024; 25(8):4248. https://doi.org/10.3390/ijms25084248
Chicago/Turabian StyleKo, Hwan-Joo, Su-Yeon Park, Deok Yong Sim, Sung-Hoon Kim, Soyoung Hur, Jang-Hoon Lee, and Youngchul Kim. 2024. "Apoptotic Effect of Isoimpertorin via Inhibition of c-Myc and SIRT1 Signaling Axis" International Journal of Molecular Sciences 25, no. 8: 4248. https://doi.org/10.3390/ijms25084248
APA StyleKo, H.-J., Park, S.-Y., Sim, D. Y., Kim, S.-H., Hur, S., Lee, J.-H., & Kim, Y. (2024). Apoptotic Effect of Isoimpertorin via Inhibition of c-Myc and SIRT1 Signaling Axis. International Journal of Molecular Sciences, 25(8), 4248. https://doi.org/10.3390/ijms25084248





