Eldecalcitol Induces Minimodeling-Based Bone Formation and Inhibits Sclerostin Synthesis Preferentially in the Epiphyses Rather than the Metaphyses of the Long Bones in Rats
Abstract
1. Introduction
2. Results
2.1. Femoral BMD and Bone Histomorphometrical Analyses of the Tibial Metapsyses and Epiphyses in Sham, Vehicle, ELD30, and ELD90 Groups
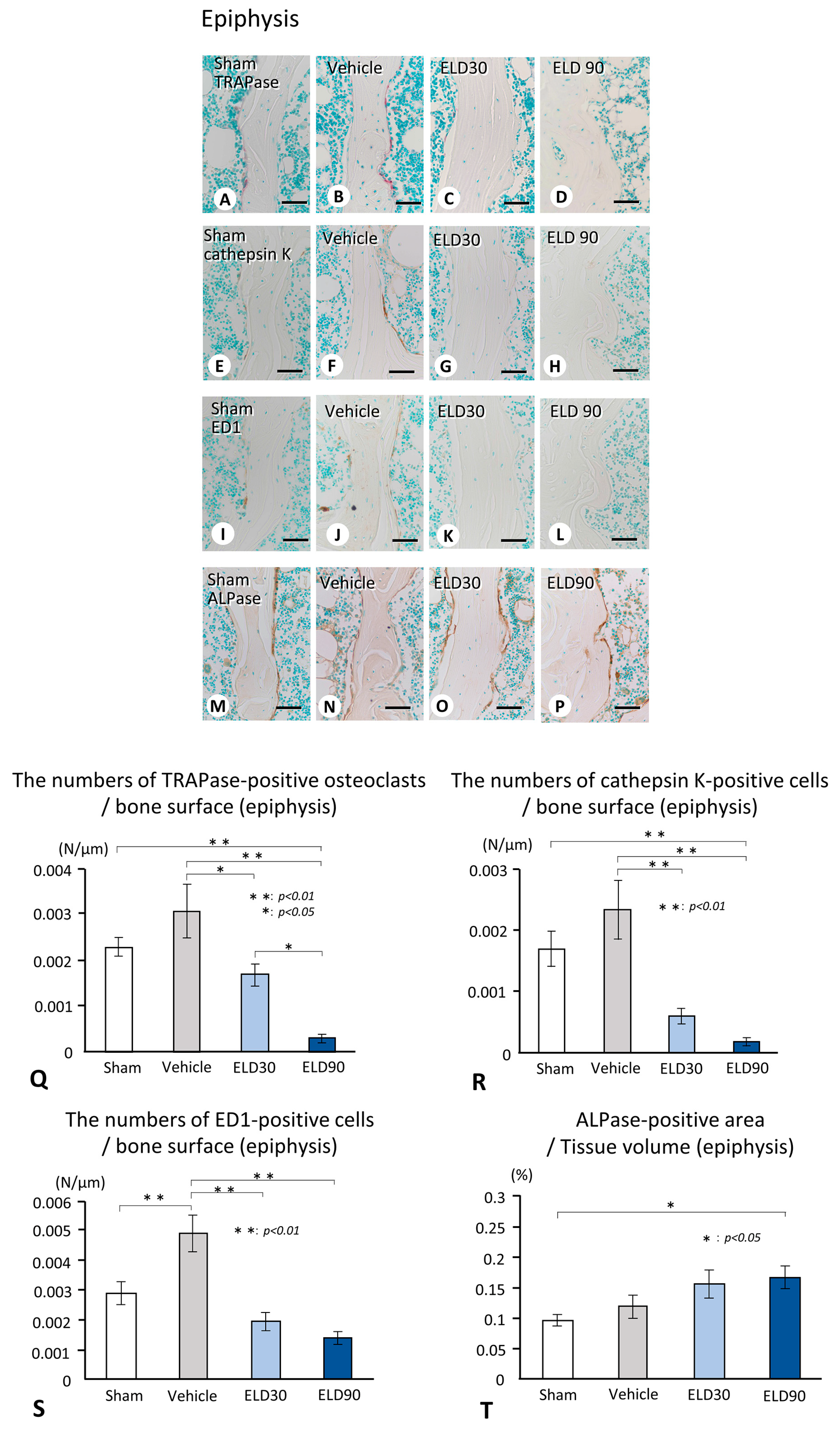
2.2. The Distribution of TRAPase-Reactive, Cathepsin K-Positive, and ED1-Immunoreactive Osteoclasts, and ALPase-Positive Osteoblasts in the ROI of Metaphysis and Epiphysis
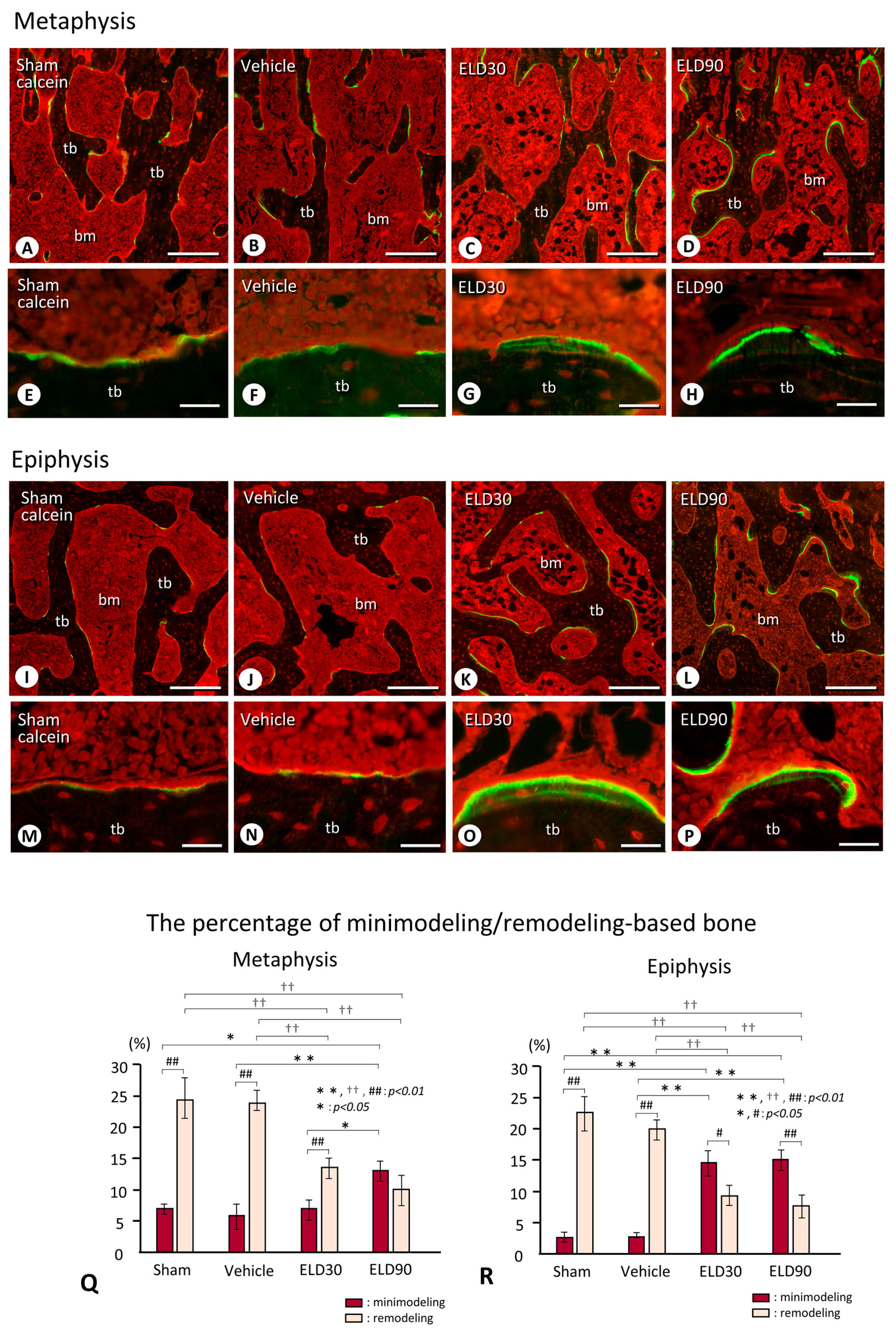
2.3. Minimodeling-Based Bone Formation and Remodeling-Based Bone Formation in Tibial Epiphyses and Metaphyses
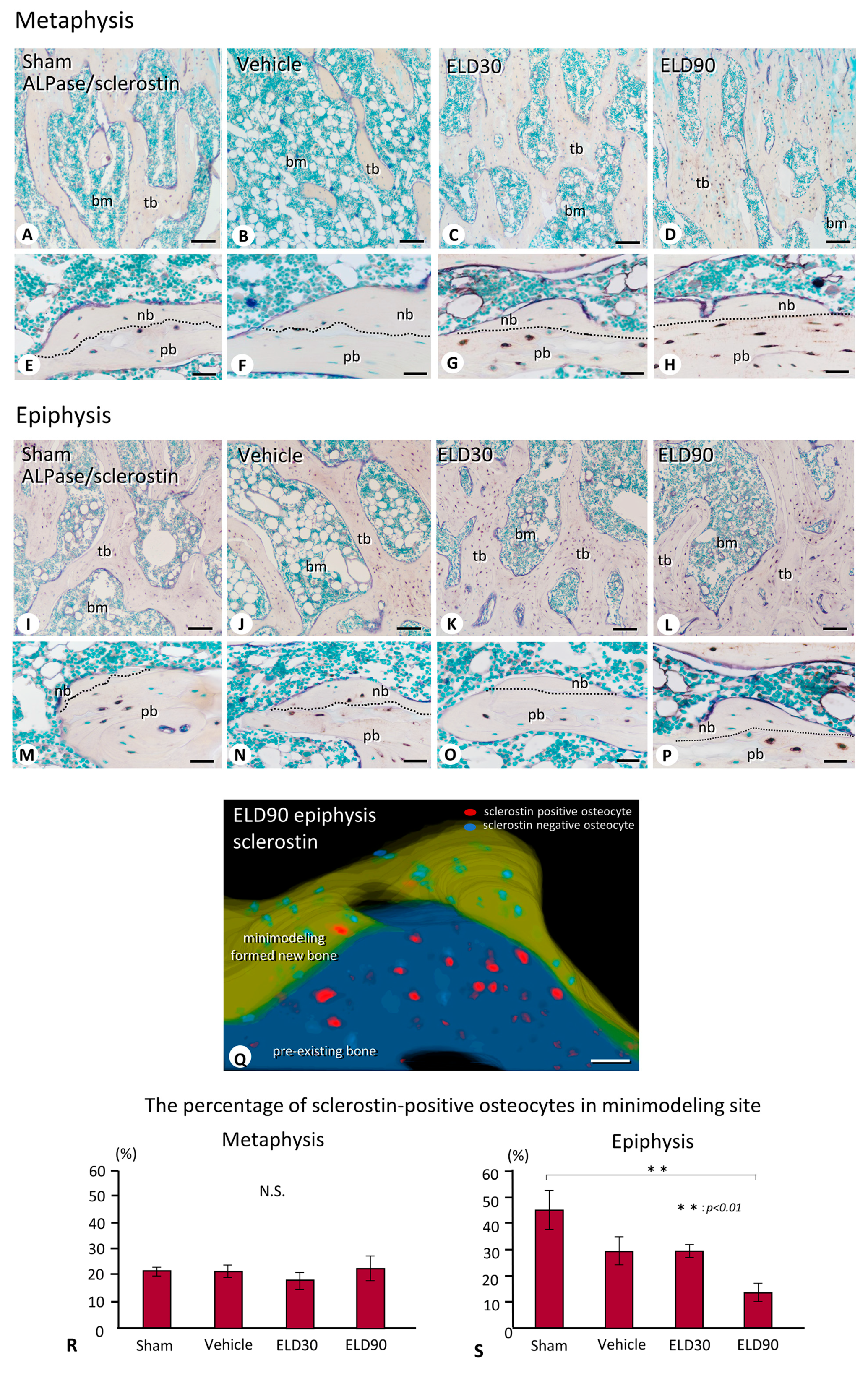
2.4. The Distribution of Sclerostin-Positive Osteocytes in Minimodeling-Induced New Bone
2.5. The Distribution of Osteocytic Lacunar Canalicular Network in Minimodeling-Induced New Bone
3. Discussion
4. Material and Methods
4.1. Animals and Tissue Preparation
4.2. Detection of BMD
4.3. Immunostaining for Tissue-Non-Specific Alkaline Phosphatase (ALPase), ED1, Cathepsin K, and Sclerostin
4.4. Enzyme Histochemistry for Tartrate-Resistant acid Phosphatase (TRAPase)
4.5. Bone Histomorphometry of BV/TV, Tb.N, Tb.Th, ALPase-Positive Osteoblastic Area, and the Analyses of TRAPase-Reactive, ED1-Positive, and Cathepsin K-Reactive Cells in Metaphyses and Epiphyses
4.6. Quantification of the Frequency of Minimodeling-Based/Remodeling-Based Bone in Metaphyses and Epiphyses
4.7. Quantification of the Percentage of Sclerostin-Positive Osteocytes in Minimodeling-Based and Remodeling-Based Bone in Metaphyses and Epiphyses
4.8. Statistical Analysis
4.9. Three-Dimensional Reconstruction of Minimodeling-Based Bone Formation including Sclerostin-Positive/Negative Osteocytes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frost, H.M. Dynamics of bone remodeling. In Bone Biodynamics; Frost, H.M., Ed.; Little and Brown Co.: Boston, MA, USA, 1964; pp. 315–333. [Google Scholar]
- Jee, W.S. The Skeletal tissues. In Histology, Cell and Tissue Biology, 5th ed.; Weiss, L., Ed.; The MacMillan Press: London, UK, 1983. [Google Scholar]
- Frost, H.M. Tetracycline-based histological analysis of bone remodeling. Calcif. Tissue. Res. 1969, 3, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff’s law: The bone modeling problem. Anat. Rec. 1990, 226, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Nordin, B.E.; Need, A.G.; Chatterton, B.E.; Horowitz, M.; Morris, H.A. The relative contributions of age and years since menopause to postmenopausal bone loss. J. Clin. Endocrinol. Metab. 1990, 70, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hattner, R.; Epker, B.N.; Frost, H.M. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 1965, 206, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takahashi, H.E.; Ito, A.; Saito, N.; Nawata, M.; Horiuchi, H.; Ohta, H.; Ito, A.; Iorio, R.; Yamamoto, N.; et al. Trabecular minimodeling in human iliac bone. Bone 2003, 32, 163–169. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakamura, T.; Nishida, S.; Suzuki, K.; Takeda, S.; Sato, K.; Nishii, Y. Effects of a synthetic vitamin D analog, ED-71, on bone dynamics and strength in cancellous and cortical bone in prednisolone-treated rats. J. Bone. Miner. Res. 1996, 11, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Higuchi, Y.; Takeda, S.; Masaki, T.; Shira-Ishi, A.; Sato, K.; Kubodera, N.; Ikeda, K.; Ogata, E. ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone 2002, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Miki, T.; Hagino, H.; Sugimoto, T.; Okamoto, S.; Hirota, T.; Tanigawara, Y.; Hayashi, Y.; Fukunaga, M.; Shiraki, M.; et al. A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: A randomized, double-blind, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2005, 90, 5031–5036. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takano, T.; Yamakido, S.; Takahashi, F.; Tsuji, N. Comparison of the effects of eldecalcitol and alfacalcidol on bone and calcium metabolism. J. Steroid. Biochem. Mol. Biol. 2010, 121, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ito, M.; Hayashi, Y.; Hirota, T.; Tanigawara, Y.; Sone, T.; Fukunaga, M.; Shiraki, M.; Nakamura, T. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—A randomized, active comparator, double-blind study. Bone 2011, 49, 605–612. [Google Scholar] [CrossRef]
- Miyamoto, K.; Murayama, E.; Ochi, K.; Watanabe, H.; Kubodera, N. Synthetic studies of vitamin D analogues XIV Synthesis and calcium regulating activity of vitamin D3 bearing a hydroxyalkoxy group at the 2β-position. Chem. Pharm. Bull. 1993, 41, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.H.L.; Hasegawa, T.; Takeda, S.; Sasaki, M.; Tabata, C.; Oda, K.; Li, M.; Saito, H.; Amizuka, N. Eldecalcitol, a second-generation vitamin D analog, drives bone minimodeling and reduces osteoclastic number in trabecular bone of ovariectomized rats. Bone 2011, 49, 335–342. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamamoto, T.; Sakai, S.; Miyamoto, Y.; Hongo, H.; Qiu, Z.; Abe, M.; Takeda, S.; Oda, K.; Freitas, P.H.L.; et al. Histological effects of the combined administration of eldecalcitol and a parathyroid hormone in the metaphyseal trabeculae of ovariectomized rats. J. Histochem. Cytochem. 2019, 67, 169–184. [Google Scholar] [CrossRef]
- Parfitt, A.M. The physiologic and clinical significance of bone histomorphometricaal data. In Bone Histomorphometry: Techniques and Interpretation; Recker, R.R., Ed.; CRC Press: Boca Raton, FL, USA, 1983; pp. 143–223. [Google Scholar]
- Jee, W.S.; Tian, X.Y.; Setterberg, R.B. Cancellous bone minimodeling-based formation: A Frost, Takahashi legacy. J. Musculoskelet. Neuronal. Interact. 2007, 7, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Birchman, R.; Xu, R.; Lindsay, R.; Shen, V. Temporal changes in cancellous bone structure of rats immediately after ovariectomy. Bone 1995, 16, 157–161. [Google Scholar] [CrossRef]
- Hilliam, R.A.; Skerry, T.M. Inhibition of bone resorption and stimulation of formation by mechanical loading of the modeling rat ulna in vivo. J. Bone. Miner. Res. 1995, 10, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Baldock, P.A.; Morris, H.A.; Need, A.G.; Moore, R.J.; Durbridge, T.C. Variation in the short-term changes in bone cell activity in three regions of the distal femur immediately following ovariectomy. J. Bone. Miner. Res. 1998, 13, 1451–1457. [Google Scholar] [CrossRef]
- Baldock, P.A.; Need, A.G.; Moore, R.J.; Durbridge, T.C.; Morris, H.A. Discordance between bone turnover and bone loss: Effects of aging and ovariectomy in the rat. J. Bone. Miner. Res. 1999, 14, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Bandeira, L.; Lewiecki, E.M.; Bilezikian, J.P. Romosozumab for the treatment of osteoporosis. Expert. Opin. Biol. Ther. 2017, 17, 255–263. [Google Scholar] [CrossRef]
- Ominsky, M.S.; Boyd, S.K.; Varela, A.; Jolette, J.; Felx, M.; Doyle, N.; Mellal, N.; Smith, S.Y.; Locher, K.; Buntich, S.; et al. Romosozumab improves bone mass and strength while maintaining bone quality in ovariectomized cynomolgus monkeys. J. Bone. Miner. Res. 2017, 32, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Chapurlat, R.; Portero-Muzy, N.; Roux, J.P.; Garcia, P.; Brown, J.P.; Libanati, C.; Boyce, R.W.; Wang, A.; Grauer, A. Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: Bone histomorphometry and microcomputed tomography analysis after 2 and 12 months of treatment. J. Bone. Miner. Res. 2019, 34, 1597–1608. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshino, H.; Haraguchi-Kitakamae, M.; Ishizu, H.; Shimizu, T.; Iwasaki, N.; Amizuka, N. Matrix vesicle-mediated mineralization and osteocytic regulation of bone mineralization. Int. J. Mol. Sci. 2022, 23, 9941. [Google Scholar] [CrossRef]
- Hikata, T.; Hasegawa, T.; Horiuchi, K.; Fujita, N.; Iwanami, A.; Watanabe, K.; Ishii, K.; Nakamura, M.; Amizuka, N.; Matsumoto, M. Histomorphometric analysis of minimodeling in the vertebrae in postmenopausal patients treated with anti-osteoporotic agents. Bone. Rep. 2016, 5, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; Roelen, B.A.; Visser, A.; van der Wee-Pals, L.; de Wilt, E.; Karperien, M.; Hamersma, H.; Papapoulos, S.E.; ten Dijke, P.; Löwik, C.W. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 2004, 199, 805–814. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef]
- Silvestrini, G.; Ballanti, P.; Leopizzi, M.; Sebastiani, M.; Berni, S.; Di Vito, M.; Bonucci, E. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J. Mol. Histol. 2007, 38, 261–269. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Tokunaga, S.; Yamamoto, T.; Sakai, M.; Hongo, H.; Kawata, T.; Amizuka, N. Evocalcet rescues secondary hyperparathyroidism-driven cortical porosity in chronic kidney disease male rats. Endocrinology 2023, 164, bqad022. [Google Scholar] [CrossRef]
- Oda, K.; Amaya, Y.; Fukushi-Irié, M.; Kinameri, Y.; Ohsuye, K.; Kubota, I.; Fujimura, S.; Kobayashi, J. A general method for rapid purification of soluble versions of glycosylphosphatidylinositol-anchored proteins expressed in insect cells: An application for human tissue-nonspecific alkaline phosphatase. J. Biochem. 1999, 126, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone. Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]



| Experimental Group | Operation | Treatment |
|---|---|---|
| Sham | Sham | Vehicle |
| Vehicle | OVX | Vehicle |
| ELD30 | OVX | ELD 30 ng/kgBW |
| ELD90 | OVX | ELD 90 ng/kgBW |
| Tissue | Target region | Analysis item |
| Left femur | Whole and distal | BMD |
| Right and left tibiae | Metaphysis | Histological assessment |
| Epiphysis | Bone histomorphometry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, T.; Yamamoto, T.; Hongo, H.; Yamamoto, T.; Haraguchi-Kitakamae, M.; Ishizu, H.; Shimizu, T.; Saito, H.; Sakai, S.; Yogo, K.; et al. Eldecalcitol Induces Minimodeling-Based Bone Formation and Inhibits Sclerostin Synthesis Preferentially in the Epiphyses Rather than the Metaphyses of the Long Bones in Rats. Int. J. Mol. Sci. 2024, 25, 4257. https://doi.org/10.3390/ijms25084257
Hasegawa T, Yamamoto T, Hongo H, Yamamoto T, Haraguchi-Kitakamae M, Ishizu H, Shimizu T, Saito H, Sakai S, Yogo K, et al. Eldecalcitol Induces Minimodeling-Based Bone Formation and Inhibits Sclerostin Synthesis Preferentially in the Epiphyses Rather than the Metaphyses of the Long Bones in Rats. International Journal of Molecular Sciences. 2024; 25(8):4257. https://doi.org/10.3390/ijms25084257
Chicago/Turabian StyleHasegawa, Tomoka, Tomomaya Yamamoto, Hiromi Hongo, Tsuneyuki Yamamoto, Mai Haraguchi-Kitakamae, Hotaka Ishizu, Tomohiro Shimizu, Hitoshi Saito, Sadaoki Sakai, Kenji Yogo, and et al. 2024. "Eldecalcitol Induces Minimodeling-Based Bone Formation and Inhibits Sclerostin Synthesis Preferentially in the Epiphyses Rather than the Metaphyses of the Long Bones in Rats" International Journal of Molecular Sciences 25, no. 8: 4257. https://doi.org/10.3390/ijms25084257
APA StyleHasegawa, T., Yamamoto, T., Hongo, H., Yamamoto, T., Haraguchi-Kitakamae, M., Ishizu, H., Shimizu, T., Saito, H., Sakai, S., Yogo, K., Matsumoto, Y., & Amizuka, N. (2024). Eldecalcitol Induces Minimodeling-Based Bone Formation and Inhibits Sclerostin Synthesis Preferentially in the Epiphyses Rather than the Metaphyses of the Long Bones in Rats. International Journal of Molecular Sciences, 25(8), 4257. https://doi.org/10.3390/ijms25084257





