Geniposide and Harpagoside Functionalized Cerium Oxide Nanoparticles as a Potential Neuroprotective
Abstract
1. Introduction
2. Results
2.1. Characterization of Encapsulation of GH into Cerium Nanoparticles
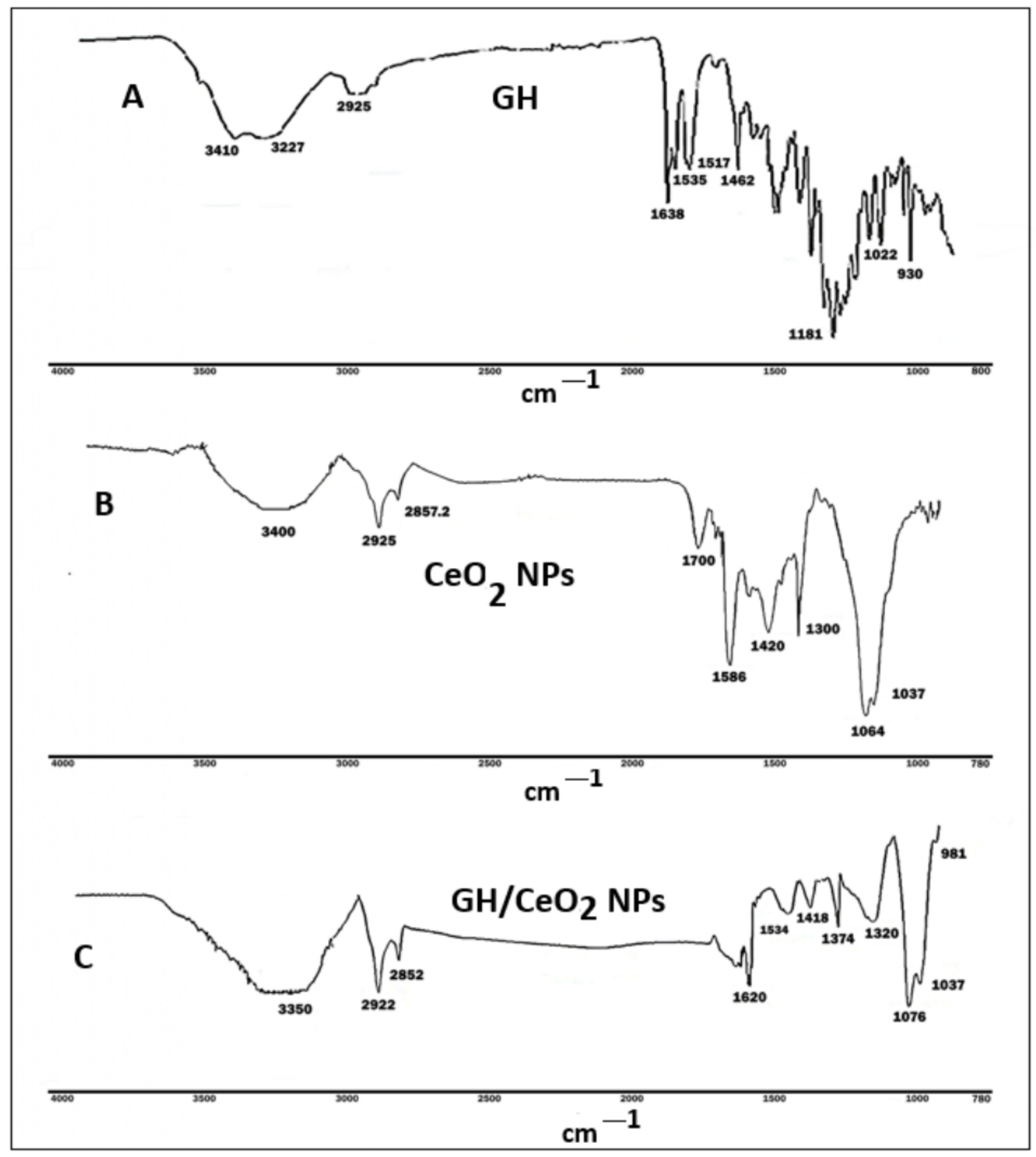
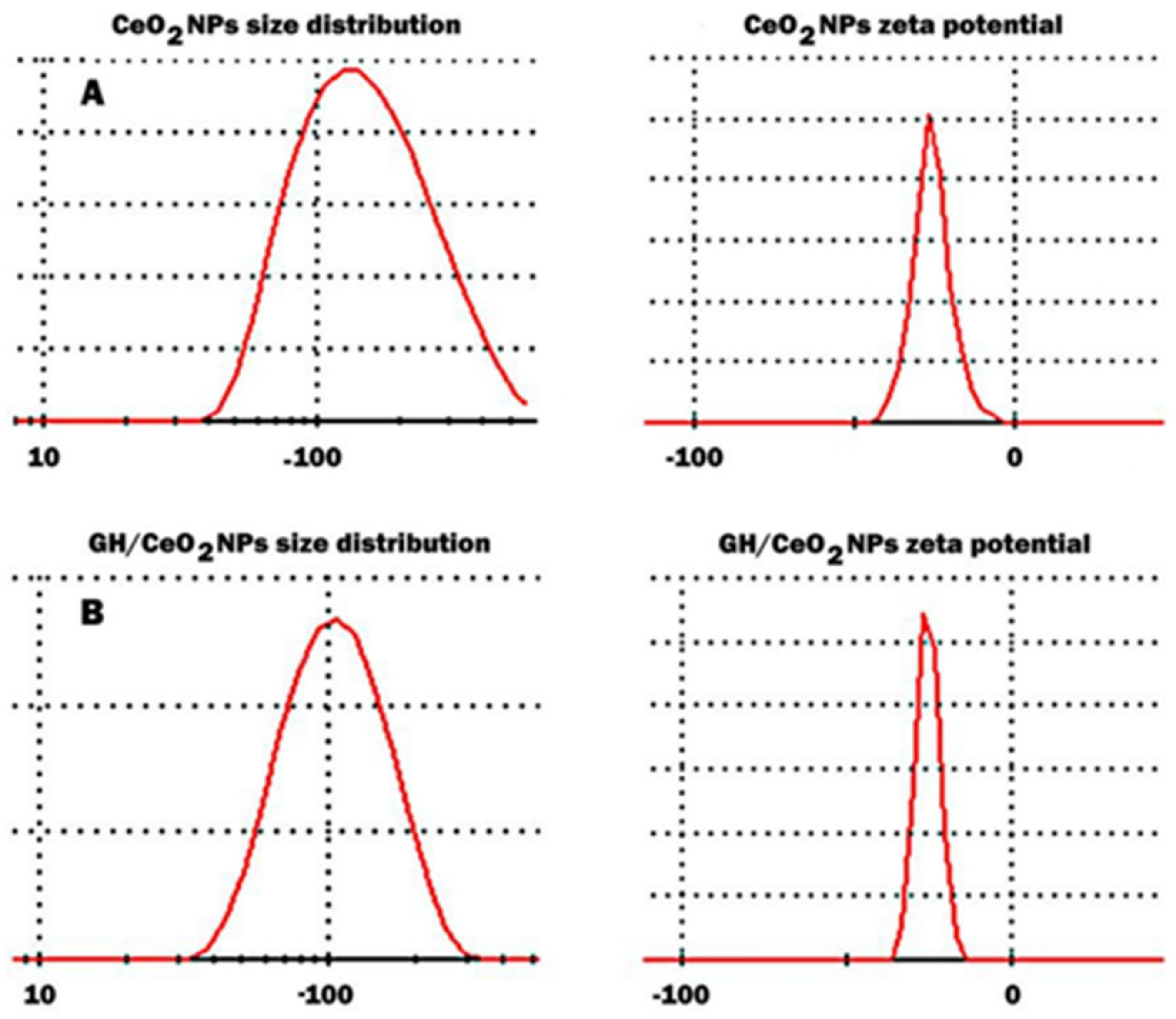
2.1.1. Particle Size Distribution
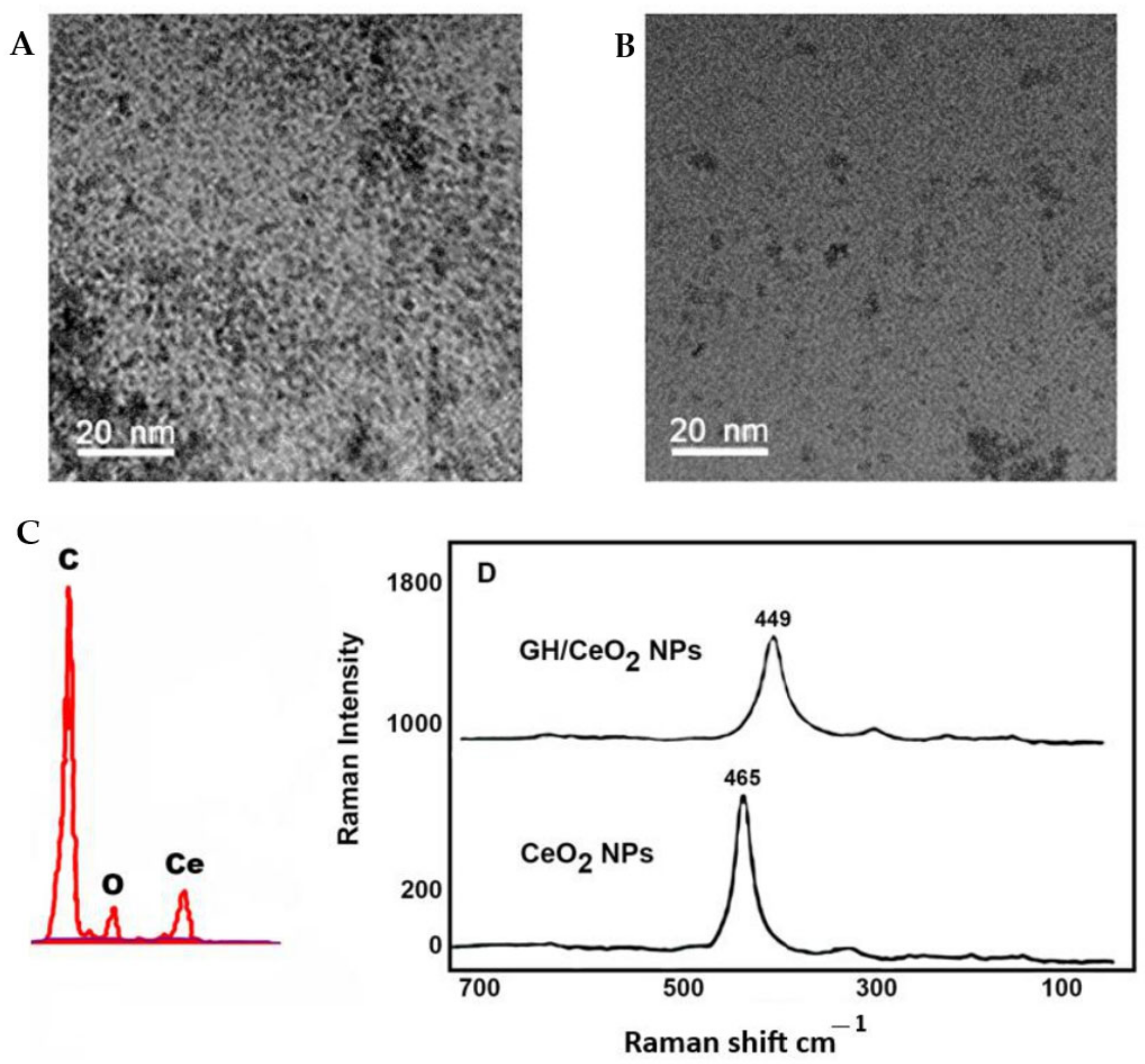
2.1.2. Transmission Electron Microscopy, Scanning Electron Microscopy
2.1.3. X-ray Diffraction (XRD) Analysis of GH/Cerium Nanoparticles
2.1.4. Raman Spectroscopy Imaging
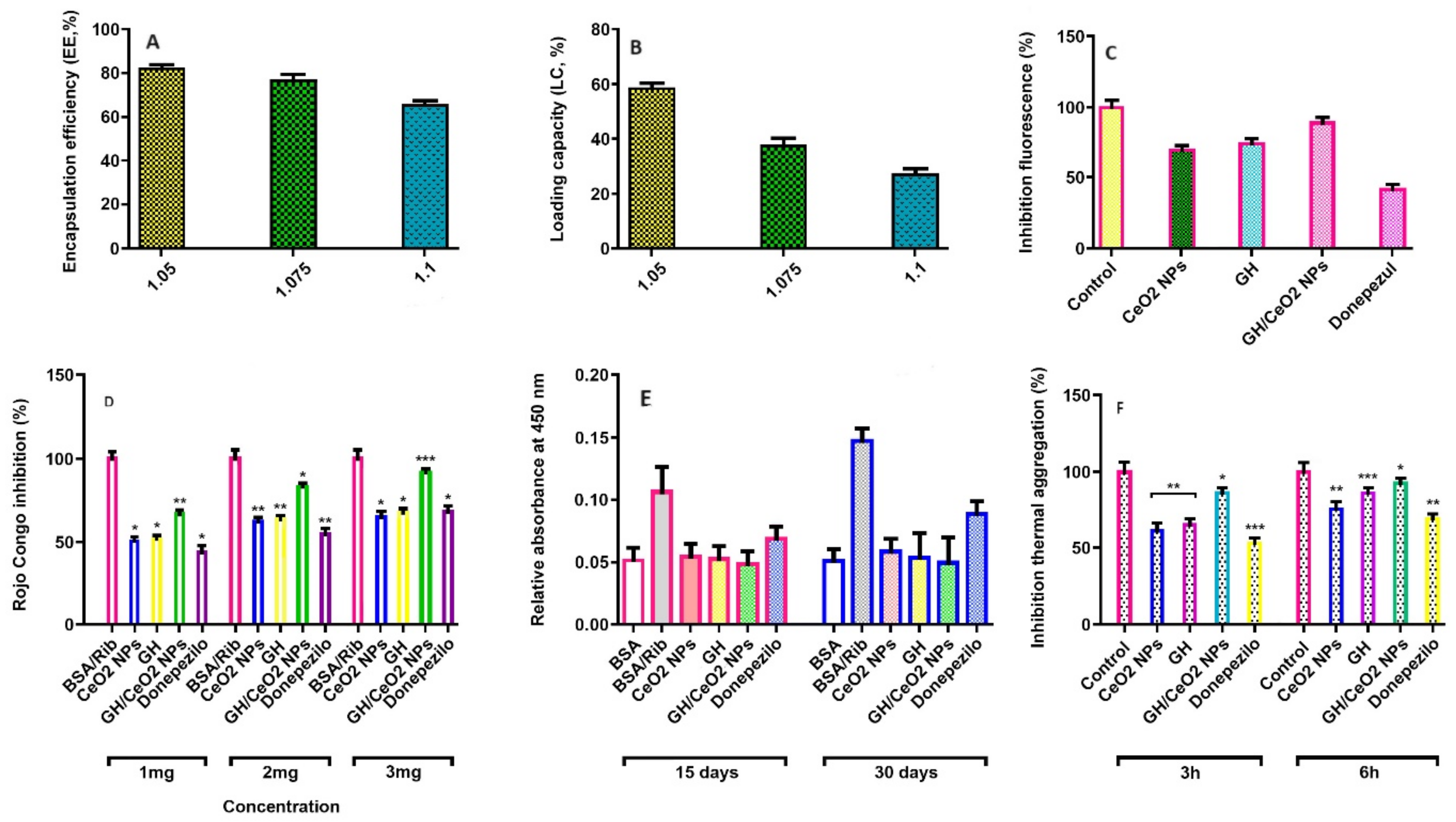
2.1.5. Encapsulation Efficiency (EE, %) and Loading Capacity (LC, %) of GH in CeO2-NPs
2.2. GH and GH/CeO2-NPs Inhibited Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE) Activities
2.3. Evaluation of Anti-Aggregatory Activity
2.3.1. Total AGEs-Fluorescence
2.3.2. Fluorescence Intensity Studies of BSA-Ribose with Nanoparticles
2.3.3. Dynamic Light Scattering
2.3.4. Determination of Amyloid Aggregation by Thioflavin T
2.3.5. Determination of Amyloid-Beta Aggregation by Congo Red Assay (CR)
2.3.6. Measure of Aggregation of BSA by Turbidity and Thermal Assays
2.3.7. Amyloid-β1–42 Aggregation
2.3.8. Tau Protein Aggregation
2.4. Effect of CeO2-NPs, GH, and GH/CeO2-NPs on Behavioral Condition in Treated AlCl3 Mice
2.4.1. Nanoparticles Enhanced Cognitive Abilities of AD Mice in an Open-Field Test
2.4.2. Effect of CeO2-NPs, GH, and GH/CeO2-NPs on Object Recognition Ability
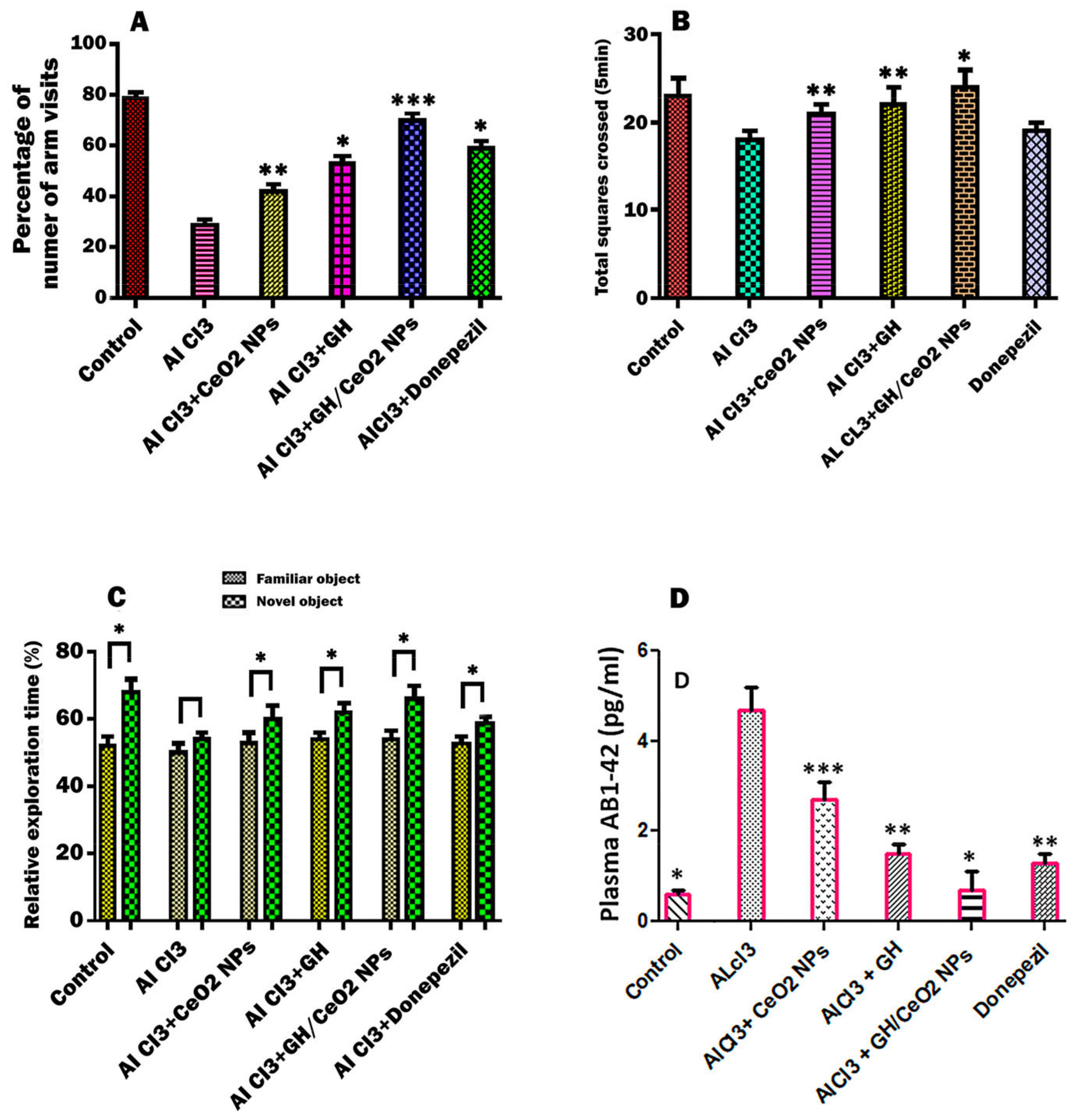
2.4.3. Nanoparticles Treatment Reduces Plasma Aβ Deposition in C57BL6/J Mice
2.4.4. Cytotoxicity of CeO2 NPs and GH/CeO2 NPs
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.1.1. Synthesis of CeO2-NPs
4.1.2. Encapsulation of GH into Cerium
4.1.3. Characterization of CeO2 and GG/CeO2 Nanoparticles
4.1.4. Encapsulation Efficiency (EE, %) and Loading Capacity (LC, %) of GH in CeO2 NPs
4.1.5. Preparation of In Vitro Glycated Albumin (BSA)
4.1.6. Dynamic Light Scattering Used to Determine the Aggregation of BSA
4.1.7. Acetylcholinesterase (AChE) and Butyryl Thiocholine (BuChE) Inhibition
4.2. Aggregation Assays
4.2.1. Determination of Amyloid-β Aggregation by Thioflavin T (Th. T)
4.2.2. Binding of Congo Red
4.2.3. Turbidimetric Aggregation Analysis
4.2.4. Thermal Aggregation
4.2.5. β1–42 Fibrils Formation by ThT Assay
4.2.6. Aggregation of Tau Proteins in Vitro
4.3. Behavioral Studies
4.3.1. Animals of Experimentation
4.3.2. Experimental Protocol
4.3.3. T-Maze Test, Novel Objection Recognition (NOR) Test
4.3.4. Novel Objection Recognition (NOR) Test
4.3.5. Exploratory Behavior in Open-field Test
4.3.6. ELISA Detection of Amyloid Aggregates in Plasma
4.3.7. Cytotoxicity of GH/CeO2-NPs
4.3.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, P.; Siddiqi, K.; Chturvedi, S.K.; Khan, R.H. Protein aggregation: From background to inhibition strategies. Int. J. Biol. Macromol. 2017, 103, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Russo, A.; Ficarra, S. Protective effects of the caffeine against neurodegenerative diseases. Curr. Med. Chem. 2019, 25, 5137–5151. [Google Scholar] [CrossRef]
- Thal, L.J. Physostigmine in Alzheimer’s disease. In Cholinergic Basis for Alzheimer Therapy Boston; Becker, R., Giacobini, E., Eds.; Birkhauser: Basel, Switzerland, 1991; pp. 107–115. [Google Scholar]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Neuropharmacology 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.; Martins, R.; Rowe, T.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Kaniakova, M.; Nepovimova, E.; Kleteckova, L.; Skrenkova, K.; Holubova, K.; Chrienova, Z.; Hepnarova, V.; Kucera, T.; Kobrlova, T.; Vales, K.; et al. Combination of memantine and 6-chlorotacrine as novel multi-target compound against Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, K.; Hosseini, Z.; Farshadian, S.; Naderi-Manesh, H. Synthesis, characterization, oxidative degradation, antibacterial activity and acetylcholinesterase/butyrylcholinesterase inhibitory effects of some new phosphorus(V) hydrazides. Eur. J. Med. Chem. 2010, 45, 5130–5139. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.; Ma, B.; Nussinov, R. Polymorphism in Alzheimer Abeta amyloid organization reflects conformational selection in a rugged energy landscape. Chem. Rev. 2010, 110, 4820–4838. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.A.; Feany, M.B. The unfolded protein response protects from tau neurotoxicity in vivo. PLoS ONE 2010, 5, e13084. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Melnikova, A.K.; Seferbekova, Z.N.; Barinova, K.V.; Schmalhausen, E.V. Glycation, Glycolysis, and Neurodegenerative Diseases: Is There Any Connection? Biochem. (Mosc.) 2017, 82, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Piccininni, C.; Dibello, V.; Stallone, R.; Giannelli, G.; Bellomo, A.; Greco, A.; et al. BACE inhibitors in clinical development for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2018, 18, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, M.; Sheng, R.; Ma, Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ1–42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 174–186. [Google Scholar] [CrossRef]
- Lacosta, A.M.; Pascual-Lucas, M.; Pesini, P.; Casabona, D.; Pérez-Grijalba, V.; Marcos-Campos, I.; Sarasa, L.; Canudas, J.; Badi, H.; Monleón, I.; et al. Safety, tolerability and immunogenicity of an active anti-Aβ40 vaccine (ABvac40) in patients with Alzheimer’s disease: A randomised, double-blind, placebo-controlled, phase I trial. Alzheimer’s Res. Ther. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Kim, H.G.; Oh, M.S. Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 57–75. [Google Scholar] [PubMed]
- Lu, Y.; Du, S.; Bai, J.; Li, P.; Wen, R.; Zhao, X. Bioavailability and brain-targeting in gardenia- borneol co-compound by different administration routes in mice. Int. J. Mol. Sci. 2012, 13, 14127–14135. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Yang, X.; Lou, J. Geniposide reduces a-synuclein by blocking microRNA-21/lysosome- associated membrane protein 2A interaction in Parkinson disease models. Brain Res. 2016, 1644, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, X.; Zhang, R.; Jiang, W.; Sun, X.; Xia, Z.; Wang, X.; Wu, E.; Zhang, Y.; Hu, Y. Harpagoside ameliorates the amyloid-β-induced cognitive impairment in rats via up-regulating BDNF expression and MAPK/PI3K pathways. Neuroscience 2015, 303, 103–114. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef] [PubMed]
- Danish, S.M.; Gupta, A.; Khan, U.A.; Hasan, N.; Ahmad, F.J.; Warsi, M.H.; Ali, A.M.A.; Zafar, A.; Jain, G.K. Intranasal cerium oxide nanoparticles ameliorate cognitive function in rats with Alzheimer’s via anti-oxidative pathway. Pharmaceutics 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Hanzha, V.V.; Rozumna, N.M.; Kravenska, Y.V.; Spivak, M.Y.; Lukyanetz, E.A. The effect of cerium dioxide nanoparticles on the viability of hippocampal neurons in Alzheimer’s disease modeling. Front. Cell. Neurosci. 2023, 17, 1131168. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, G.; Venkatasubbu, D.; Mohidee, S.S. Investigation of therapeutic potential of cerium oxide nanoparticles in Alzheimer’s disease using transgenic Drosophila. Biotech 2021, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Weisheng, L.; Yue-wern, Y.; Xiao-Dong, Z.; Yinfa, M. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Rodea-Palomares, I.; Das, S.; Sakthivel, T.S.; Leganes, F.; Rosal, R.; Seal, S. Untangling the biological effects of cerium oxide nanoparticles: The role of surface states. Sci. Rep. 2015, 5, 15613. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Pandey, A.K. Cerium oxide nanoparticles induced toxicity in human lung cells: Role of ROS mediated DNA damage and apoptosis. Biomed Res. Int. 2014, 2014, 891934. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Patil, S.; Seal, J.; McGinnis, F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; da Ana, R.; Fonseca, J.; Szalata, M.; Wielgus, K.; Fathi, F.; Oliveira, M.B.P.P.; Staszewski, R.; Karczewski, J.; Souto, E.B. A phytocannabinoids: Chromatographic screening of cannabinoids and loading into lipid nanoparticles. Molecules 2023, 28, 2875. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.P.; Sharma, R.; Kumar, S.K. Mehta, nanoscale surface designing of cerium oxide nanoparticles for controlling growth, stability, optical and thermal properties. Ceram. Int. 2015, 41, 10995–11003. [Google Scholar] [CrossRef]
- Rane, A.S.S.; Choi, P. Polydispersity index: How accurately does it measure the breadth of the molecular weight distribution? Chem. Mater. 2005, 17, 926. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Doroftei, F.; Ardeleanu, T.S.; Dobromir, M.; Tîmpu, D.; Ursu, E.-L. Phytomediated-assisted preparation of cerium oxide nanoparticles using plant extracts and assessment of their structural and optical properties. Int. J. Mol. Sci. 2023, 24, 8917. [Google Scholar] [CrossRef]
- Tiseanu, C.V.; Parvulescu, D.; Avram, B.; Cojocaru, N.; Apostol, A.V.; Vela-Gonzalez, M. Structural, down- and phase selective up-conversion emission properties of mixed valent Pr doped into oxides with tetravalent cations. Phys. Chem. Chem. Phys. 2014, 16, 5793–5802. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nikitchenko, Y.V.; Klochkov, V.K.; Kavok, N.S. CeO2 nanoparticles improve rooxidant/antioxidant balance, life quality and survival of old male rats. Biogerontology 2023, 24, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, K.; Shi, M.; Xie, L.; Deng, M.; Chen, H.; Li, X. Therapeutic potential of catalpol and geniposide in Alzheimer’s and Parkinson’s diseases: A snapshot of their underlying mechanisms. Brain Res. Bull. 2021, 174, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Tonnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Penumala, M.; Zinka, R.B.; Shaik, J.B.; Mallepalli, S.K.R.; Vadde, R.; Amooru, D.G. Phytochemical profiling and in vitro screening for anticholinesterase, antioxidant, antiglucosidase and neuroprotective effect of three traditional medicinal plants for Alzheimer’s disease and diabetes mellitus dual therapy. BMC Comp. Alt. Med. 2018, 18, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S. Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M.; Rigacci, S. Protein folding and aggregation into amyloid: The interference by natural phenolic compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef] [PubMed]
- Pallo, S.P.; Johnson, G.V. Tau facilitates Aβ-Induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons. Neurosci. Lett. 2015, 597, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zatta, P. Aluminium (III) as a promoter of cellular oxidation. Coord. Chem. Rev. 2002, 228, 271–284. [Google Scholar] [CrossRef]
- Zannou, O.; Oussou, K.F.; Chabi, I.B.; Awad, N.M.H.; Aïssi, M.V.; Goksen, G.; Mortas, M.; Oz, F.; Proestos, C.; Kayodé, A.P.P. Nanoencapsulation of cyanidin 3-o-glucoside: Purpose, technique, bioavailability, and stability. Nanomaterials 2023, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Markowicz-Piasecka, M.; Broncel, M.; Mikiciuk-Olasik, E. Extract of Aronia melanocarpa-modified hemostasis: In vitro studies. Eur. J. Nutr. 2014, 53, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Sunyer, B.; Höger, H.; Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the multiple T-maze and in the Morris water maze. Behav. Brain Res. 2000, 198, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.Q.; Kim, J.; Choi, J.M.; Song, J.; Lee, S.; Cho, F.J. Cirsium japonicum var. Maackii improves cognitive impairment under amyloid Beta25-35-Induced Alzheimer’s disease model. BioMed Res. Int. 2022, 2022, 4513998. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.L. Bohacek exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. J. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Shenqiang, Z.; Xiaofang, Z.; Lirong, Z.; Fan, G.; Youwen, T.; Aihua, G.; Zhengzou, F.; Huixiang, J.; Chaoyang, W.; Fengyi, D. Biomineralization-inspired synthesis of cerium-doped carbonaceous nanoparticles for highly hydroxyl radical scavenging activity. Nanoscale Res. Lett. 2018, 13, 76. [Google Scholar] [CrossRef]

| Sample | Inhibition (%) | |
|---|---|---|
| ACHE | BuChE | |
| CeO2NPs | ||
| 10 µg | 33.9 | 37.4 |
| 20 µg | 36.9 | 39.2 |
| 30 µg | 41.7 | 44.5 |
| GH | ||
| 10 µg | 43.6 | 43.4 |
| 20 µg | 47.4 | 46.1 |
| 30 µg | 49.7 | 49.7 |
| GH/CeO2NPs | ||
| 10 µg | 49.4 | 57.2 |
| 20 µg | 52.3 | 61.3 |
| 30 µg | 67.0 | 63.1 |
| Donepezil | ||
| 10 µg | 58.8 | 55.4 |
| 20 µg | 65.8 | 67.2 |
| 30 µg | 88.7 | 84.0 |
| Sample | Zeta Potential (mV) | Average Diameter (nm) | Polydispersity Index | Fluorescence (A.U) |
|---|---|---|---|---|
| BSA | −8.41 | 9.2 | 0.13 | 8.6 |
| BSA/ribose | −34.6 | 4355 | 1.0 | 74.9 |
| BSA/ribose + GH | −22.8 | 2078 | 0.397 | 42.6 |
| BSA/ribose + CeO2 NPs | −27.2 | 2482 | 0.412 | 50.2 |
| BSA/ribose + GH/CeO2 NPs | −19.1 | 1765 | 0.365 | 35.1 |
| BSA/ribose + Donepezil | −29.3 | 3223 | 0.638 | 61.5 |
| Th Assay β1–42 Aggregation Fluorescence in RFU/Inhibition (%) | |||||
|---|---|---|---|---|---|
| Sample | 4 h | 6 h | 8 h | 12 h | 24 h |
| Amyloid β1–42 | 6200 | 7800 | 9865 | 11,750 | 12,120 |
| CeO2 NPs | 5743 (7.4) | 6821 (12.5) | 6832 (30.7) | 5131 (56.3) | 2253 (81) |
| GH | 5282 (14.8) | 6120 (21.5) | 6432 (34.7) | 4765 (59) | 1876 (84) |
| GH/CeO2 NPs | 5181 (16.4) | 5623 (27.9) | 6138 (37.7) | 4322 (63.2) | 1087 (91) |
| Th Assay Tau Aggregation Fluorescence in RFU/Inhibition (%) | ||||||
|---|---|---|---|---|---|---|
| Sample | 20 h | 40 h | 60 h | 80 h | 100 h | 120 h |
| Tau protein | 2500 | 4100 | 6341 | 8641 | 9851 | 11,500 |
| CeO2 NPs | 2000 (20) | 2300 (43.9) | 2650 (58.2) | 3284 (62) | 3648 (63) | 3401 (70.4) |
| GH | 1832 (26.7) | 2125 (48.1) | 2360 (62.8) | 2460 (71) | 2619 (73.4) | 2671 (77) |
| GH/CeO2 NPs | 1654 (33.8) | 1720 (58) | 1964 (69) | 2000 (76.8) | 2003 (79.5) | 2004 (83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Gutiérrez, R.M.; Rodríguez-Serrano, L.M.; Laguna-Chimal, J.F.; de la Luz Corea, M.; Paredes Carrera, S.P.; Téllez Gomez, J. Geniposide and Harpagoside Functionalized Cerium Oxide Nanoparticles as a Potential Neuroprotective. Int. J. Mol. Sci. 2024, 25, 4262. https://doi.org/10.3390/ijms25084262
Pérez Gutiérrez RM, Rodríguez-Serrano LM, Laguna-Chimal JF, de la Luz Corea M, Paredes Carrera SP, Téllez Gomez J. Geniposide and Harpagoside Functionalized Cerium Oxide Nanoparticles as a Potential Neuroprotective. International Journal of Molecular Sciences. 2024; 25(8):4262. https://doi.org/10.3390/ijms25084262
Chicago/Turabian StylePérez Gutiérrez, Rosa Martha, Luis Miguel Rodríguez-Serrano, José Fidel Laguna-Chimal, Mónica de la Luz Corea, Silvia Patricia Paredes Carrera, and Julio Téllez Gomez. 2024. "Geniposide and Harpagoside Functionalized Cerium Oxide Nanoparticles as a Potential Neuroprotective" International Journal of Molecular Sciences 25, no. 8: 4262. https://doi.org/10.3390/ijms25084262
APA StylePérez Gutiérrez, R. M., Rodríguez-Serrano, L. M., Laguna-Chimal, J. F., de la Luz Corea, M., Paredes Carrera, S. P., & Téllez Gomez, J. (2024). Geniposide and Harpagoside Functionalized Cerium Oxide Nanoparticles as a Potential Neuroprotective. International Journal of Molecular Sciences, 25(8), 4262. https://doi.org/10.3390/ijms25084262










