Analgesic, Anti-Inflammatory, and Chondroprotective Activities of Siraitia grosvenorii Residual Extract
Abstract
1. Introduction
2. Results
2.1. NHGRE Down-Regulates COX and 5-LOX Activities
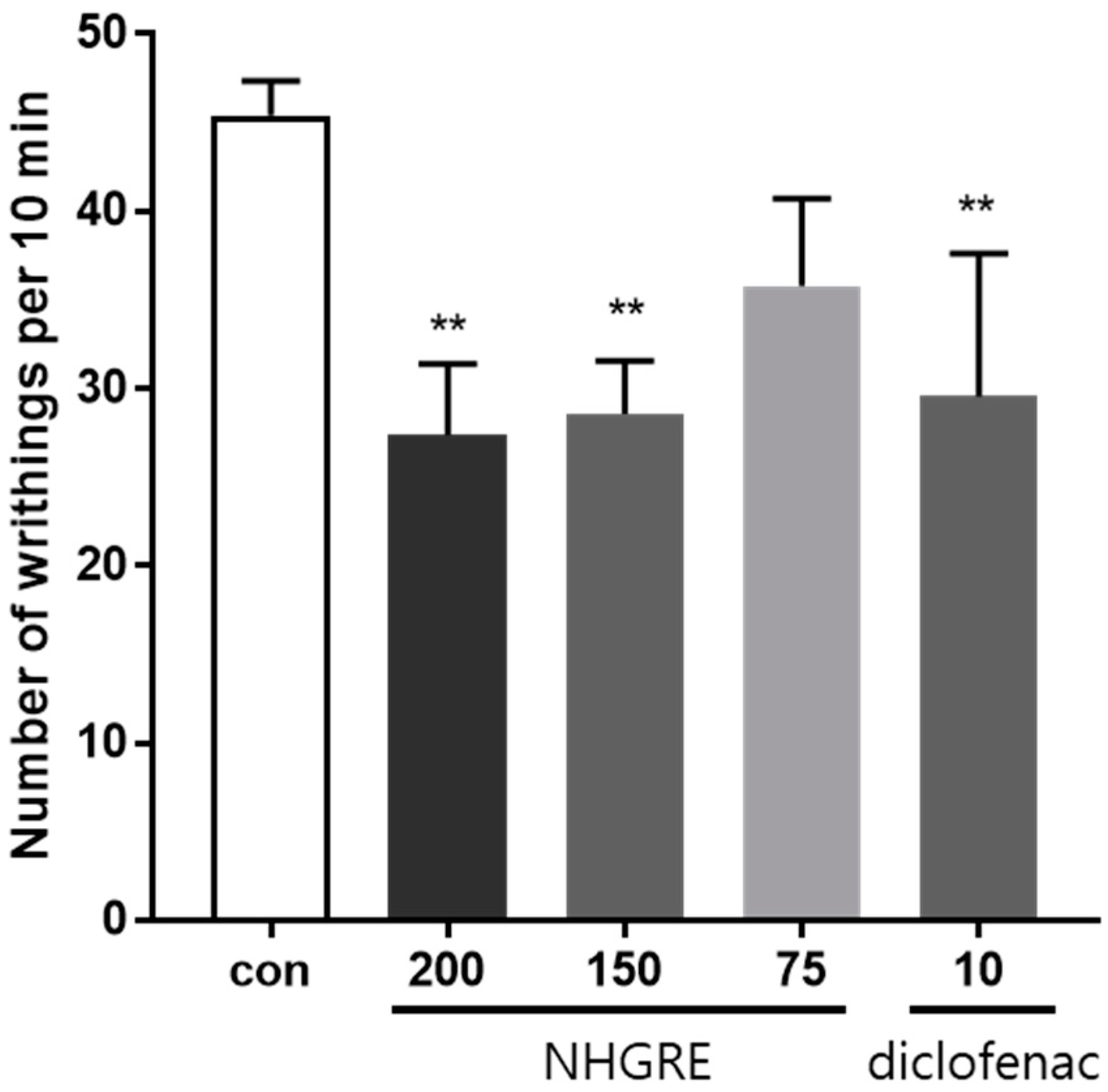
2.2. Effect of NHGRE on Acetic Acid-Induced Writhing Response
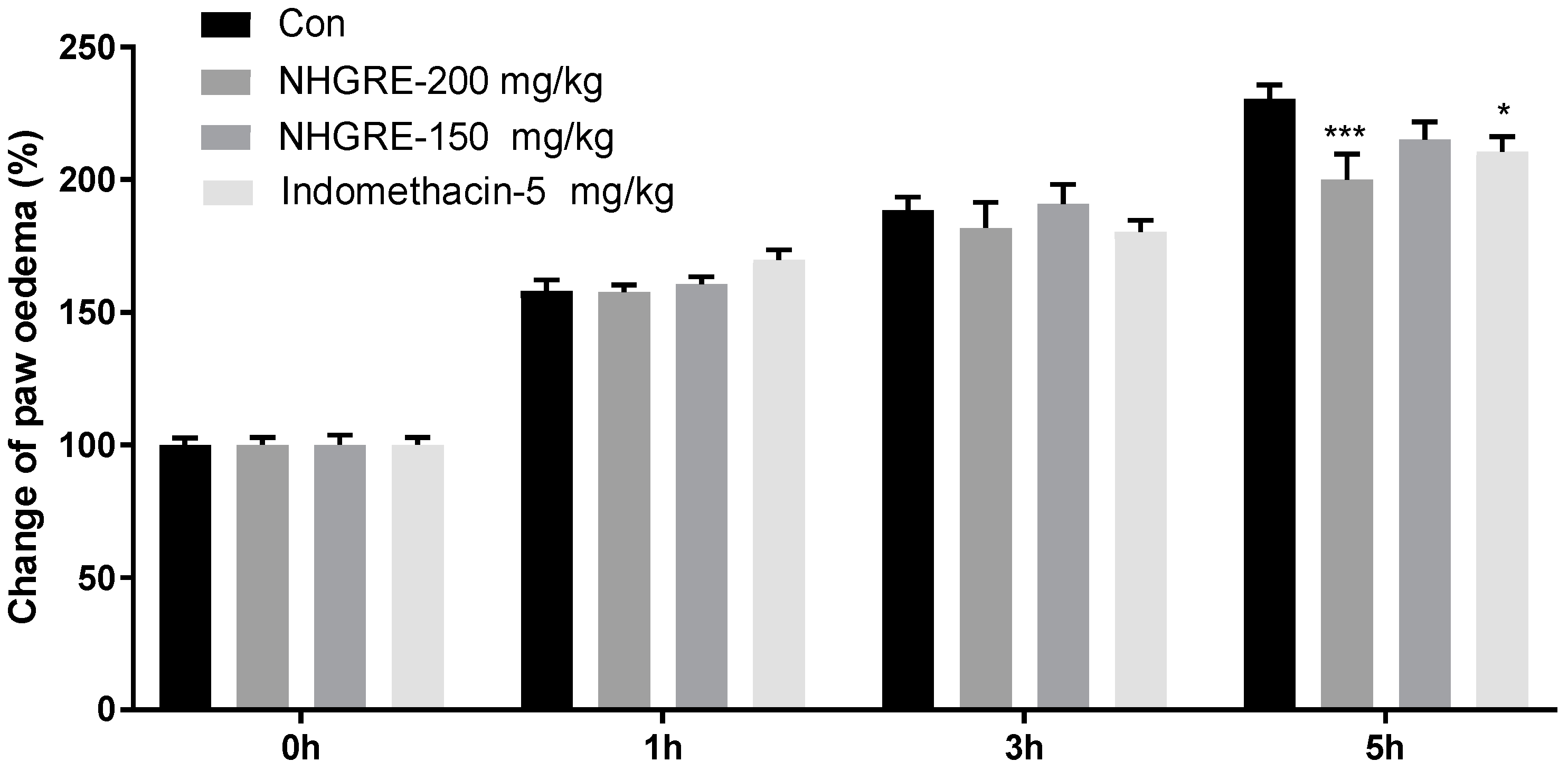
2.3. Effect of NHGRE on Carrageenan-Induced Paw Oedema
2.4. Effect of NHGRE on TNF-α, IL-6, and PGE2 Production in IL-1β-Treated SW1353 Cells
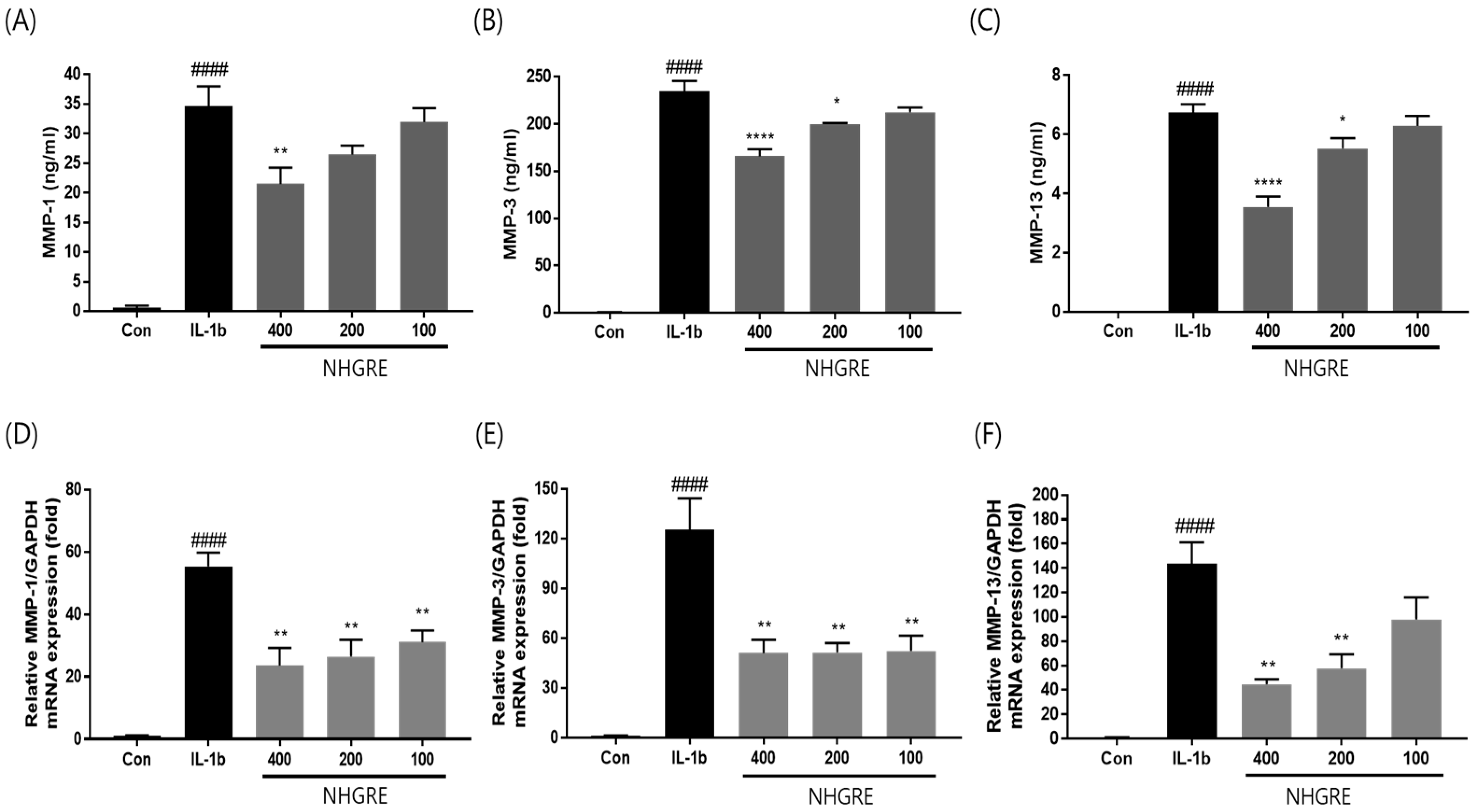
2.5. Effects of NHGRE on MMP Expression in IL-1β-Stimulated SW1353 Cells
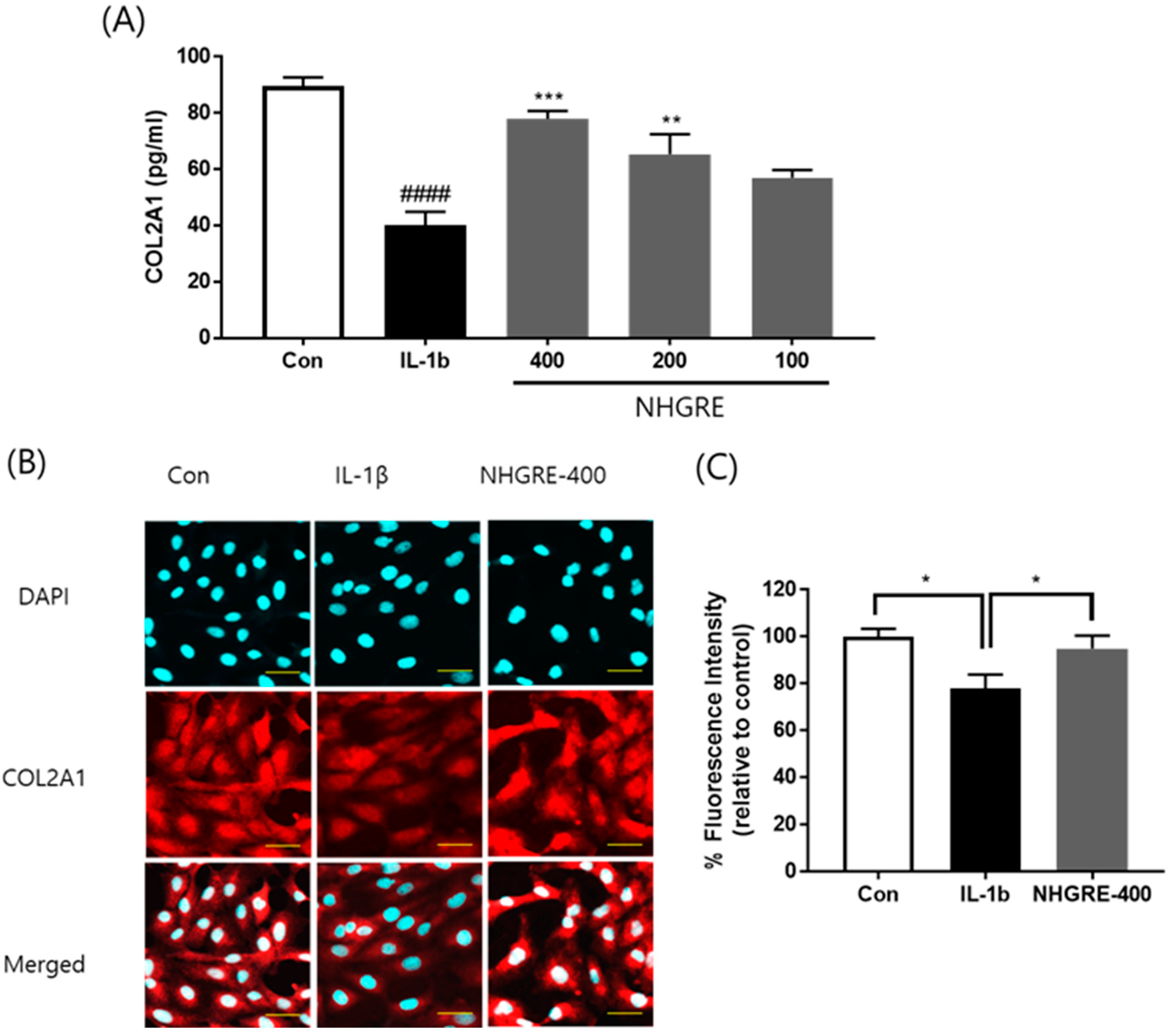
2.6. Effects of NHGRE on Type II Collagen Degradation in IL-1β-Stimulated SW1353 Cells
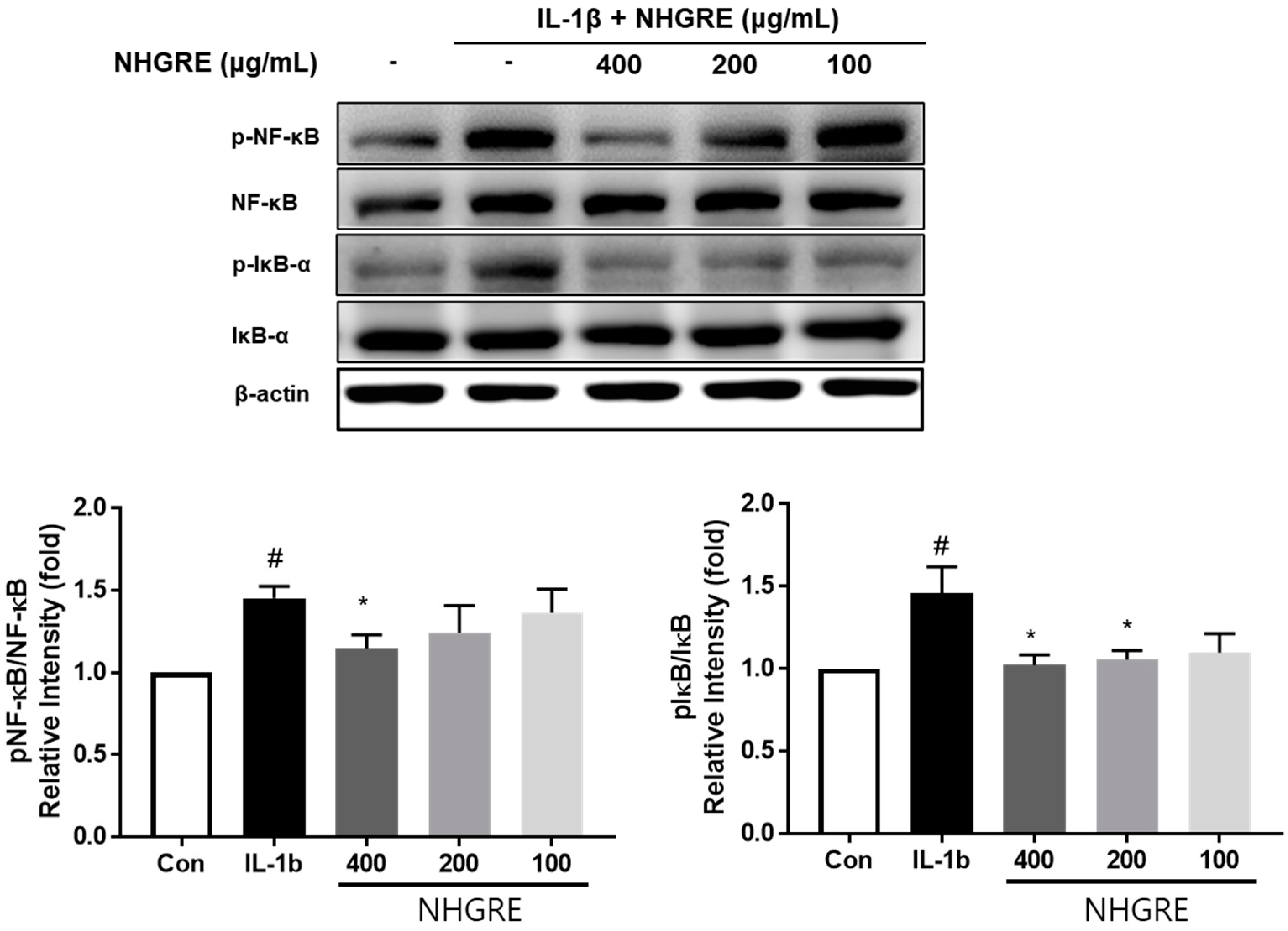
2.7. Effects of NHGRE on IL-1β-Stimulated NF-κB Activation
3. Discussion
4. Materials and Methods
4.1. Preparation of NHGRE
4.2. COX and 5-LOX Activity Assay
4.3. Acetic Acid-Induced Writhing Response
4.4. Carrageenan-Induced Paw Oedema
4.5. Cell Culture and Cytotoxicity Measurement
4.6. ELISA
4.7. RNA Isolation and RT-PCR
4.8. Western Blotting
4.9. Immunofluorescence Staining
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karim, N.; Khan, I.; Khan, W.; Khan, I.; Khan, A.; Halim, S.A.; Khan, H.; Hussain, J.; Al-Harrasi, A. Anti-nociceptive and anti-inflammatory activities of asparacosin A involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: An in-vitro, in-vivo, and in-silico approach. Front. Immunol. 2019, 10, 581. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Fu, X.; Lin, Z.; Yu, K. Geraniol-mediated osteoarthritis improvement by down-regulating PI3K/Akt/NF-kappaB and MAPK signals: In vivo and in vitro studies. Int. Immunopharmacol. 2020, 86, 106713. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Deng, B.; Yan, L. Syringaresinol attenuates osteoarthritis via regulating the NF-kappaB pathway. Int. Immunopharmacol. 2023, 118, 109982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, C.; Lou, B.; Pang, Q.; Su, Y.; Song, Y.; Lei, C.; Li, Y.; Wen, Y. First report of Watermelon silver mottle orthotospovirus infecting Siraitia grosvenorii in China. Plant Dis. 2023, 107, 3323. [Google Scholar] [CrossRef]
- Wu, J.; Jian, Y.; Wang, H.; Huang, H.; Gong, L.; Liu, G.; Yang, Y.; Wang, W. A review of the phytochemistry and pharmacology of the fruit of Siraitia grosvenorii (Swingle): A traditional Chinese medicinal food. Molecules 2022, 27, 6618. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Peng, Y.; Wang, M.; Li, X. Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of Luohanguo extract on obese T2DM rats. J. Ethnopharmacol. 2020, 247, 112273. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Meng, G.; Zhang, C.; Chen, F.; Tang, S.; Hong, H.; Zhang, C. Neuroprotective effect of mogrol against Abeta(1-42) -induced memory impairment neuroinflammation and apoptosis in mice. J. Pharm. Pharmacol. 2019, 71, 869–877. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Lu, R.; Hu, J.; Liu, X.; Yu, L.; Hu, J.; Jiang, H.; Liu, S.; Li, M.; He, J.; Yang, X.; et al. Mogroside-rich extract from Siraitia grosvenorii fruits protects against heat stress-induced intestinal damage by ameliorating oxidative stress and inflammation in mice. Food Funct. 2023, 14, 1238–1247. [Google Scholar] [CrossRef]
- Tao, L.; Cao, F.; Xu, G.; Xie, H.; Zhang, M.; Zhang, C. Mogroside IIIE attenuates LPS-induced acute lung injury in mice partly through regulation of the TLR4/MAPK/NF-kappaB axis via AMPK activation. Phytother. Res. 2017, 31, 1097–1106. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, M.; Yuk, H.J.; Kim, S.H.; Kim, D.S. Siraitia grosvenorii residual extract inhibits inflammation in RAW264.7 macrophages and attenuates osteoarthritis progression in a rat model. Nutrients 2023, 15, 1417. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Jacob, J.P.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Sanghi, S.; MacLaughlin, E.J.; Jewell, C.W.; Chaffer, S.; Naus, P.J.; Watson, L.E.; Dostal, D.E. Cyclooxygenase-2 inhibitors: A painful lesson. Cardiovasc. Hematol. Disord. Drug Targets 2006, 6, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Eltayeb, W.A.; Rakshit, G.; El-Arabey, A.A.; Khan, J.; Aldosari, S.M.; Alshehri, B.; Abdalla, M. Dual synergistic inhibition of COX and LOX by potential chemicals from Indian daily spices investigated through detailed computational studies. Sci. Rep. 2023, 13, 8656. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Non-steroidal anti-inflammatory drugs: Recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem. 2022, 13, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Subhawa, S.; Arpornchayanon, W.; Jaijoy, K.; Chansakaow, S.; Soonthornchareonnon, N.; Sireeratawong, S. Anti-inflammatory, antinociceptive, antipyretic, and gastroprotective effects of Eurycoma longifolia Jack ethanolic extract. Life 2023, 13, 1465. [Google Scholar] [CrossRef] [PubMed]
- Bighetti, E.J.; Hiruma-Lima, C.A.; Gracioso, J.S.; Brito, A.R. Anti-inflammatory and antinociceptive effects in rodents of the essential oil of Croton cajucara Benth. J. Pharm. Pharmacol. 1999, 51, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Cecen, B.; Keles, D.; Oktay, G.; Kozaci, L.D. Effects of simvastatin on matrix metalloproteinase regulation in IL-1beta-induced SW1353 cells. Chem. Biol. Interact. 2019, 310, 108730. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Huang, X.; Xi, Y.; Pan, Q.; Mao, Z.; Zhang, R.; Ma, X.; You, H. Caffeic acid protects against IL-1beta-induced inflammatory responses and cartilage degradation in articular chondrocytes. Biomed. Pharmacother. 2018, 107, 433–439. [Google Scholar] [CrossRef]
- Hardingham, T. Extracellular matrix and pathogenic mechanisms in osteoarthritis. Curr. Rheumatol. Rep. 2008, 10, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Li, M.; Yuan, X.; Yu, X.; Chen, A.; Shao, M.; Kang, H.; Cheng, P. Rutaecarpine ameliorates osteoarthritis by inhibiting PI3K/AKT/NF-kappaB and MAPK signalling transduction through integrin alphaVbeta3. Int. J. Mol. Med. 2023, 52, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, J.; Gu, J.H.; Wang, F.Y.; Shang, X.S.; Tao, H.R.; Wang, X. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of NF-kappaB signaling in rat chondrocytes. Mol. Med. Rep. 2017, 16, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-kappaB signaling pathways in osteoarthritic cartilage destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-kappaB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, S.H.; Yuk, H.J.; Yang, W.K.; Lee, Y.M.; Son, E.; Kim, D.S. Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice. Phytomedicine 2019, 61, 152835. [Google Scholar] [CrossRef]
- Yimam, M.; Brownell, L.; Hodges, M.; Jia, Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet Suppl. 2012, 9, 155–165. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.C.; Moore, B.; Jiao, P.; Hong, M.; Nam, J.B.; Kim, M.R.; Kim, T.W.; Kim, H.J.; Hyun, E.J.; et al. UP1304, a Botanical Composition Containing Two Standardized Extracts of Curcuma longa and Morus alba, Mitigates Pain and Inflammation in Adjuvant-induced Arthritic Rats. Pharmacogn. Res. 2016, 8, 112–117. [Google Scholar] [CrossRef]
- Lee, Y.M.; Son, E.; Kim, S.H.; Kim, D.S. Protective Effects of Glycine soja Leaf and Stem Extract against Chondrocyte Inflammation and Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 4829. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | NHGRE Dose (µg/mL) | Inhibition Rates (%) | IC50 (µg/mL) |
|---|---|---|---|
| COX-1 | 25 | 29.29 ± 5.21 | 62.00 ± 3.59 |
| 50 | 44.63 ± 12.03 | ||
| 100 | 70.16 ± 2.96 | ||
| COX-2 | 25 | 14.02 ± 9.79 | 82.33 ± 9.90 |
| 50 | 38.00 ± 5.52 | ||
| 100 | 58.56 ± 10.40 | ||
| 5-LOX | 100 | 41.37 ± 0.15 | 270.95 ± 6.63 |
| 250 | 48.89 ± 0.73 | ||
| 500 | 61.62 ± 0.44 |
| Gene | Direction | Primer Sequence |
|---|---|---|
| MMP-1 | Forward | 5′-GACAGAGATGAAGTCCGGTTT-3′ |
| Reverse | 5′-GCCAAAGGAGCTGTAGATGTC-3′ | |
| MMP-3 | Forward | 5′-ATTCCATGGAGCCAGGCTTTC-3′ |
| Reverse | 5′-CATTTGGGTCAAACTCCAACTGT-3′ | |
| MMP-13 | Forward | 5′-AGCCACTTTATGCTTCCTGA-3′ |
| Reverse | 5′-TGGCATCAAGGGATAAGGAAG-3′ | |
| GAPDH | Forward | 5′-CACCCACTCCTCCACCTTTG-3′ |
| Reverse | 5′-CCACCACCCTGTGCTGTAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-M.; Kim, D.-S. Analgesic, Anti-Inflammatory, and Chondroprotective Activities of Siraitia grosvenorii Residual Extract. Int. J. Mol. Sci. 2024, 25, 4268. https://doi.org/10.3390/ijms25084268
Lee Y-M, Kim D-S. Analgesic, Anti-Inflammatory, and Chondroprotective Activities of Siraitia grosvenorii Residual Extract. International Journal of Molecular Sciences. 2024; 25(8):4268. https://doi.org/10.3390/ijms25084268
Chicago/Turabian StyleLee, Yun-Mi, and Dong-Seon Kim. 2024. "Analgesic, Anti-Inflammatory, and Chondroprotective Activities of Siraitia grosvenorii Residual Extract" International Journal of Molecular Sciences 25, no. 8: 4268. https://doi.org/10.3390/ijms25084268
APA StyleLee, Y.-M., & Kim, D.-S. (2024). Analgesic, Anti-Inflammatory, and Chondroprotective Activities of Siraitia grosvenorii Residual Extract. International Journal of Molecular Sciences, 25(8), 4268. https://doi.org/10.3390/ijms25084268






