Abstract
Hearing loss represents a multifaceted and pervasive challenge that deeply impacts various aspects of an individual’s life, spanning psychological, emotional, social, and economic realms. Understanding the molecular underpinnings that orchestrate hearing loss remains paramount in the quest for effective therapeutic strategies. This review aims to expound upon the physiological, biochemical, and molecular aspects of hearing loss, with a specific focus on its correlation with diabetes. Within this context, phytochemicals have surfaced as prospective contenders in the pursuit of potential adjuvant therapies. These compounds exhibit noteworthy antioxidant and anti-inflammatory properties, which hold the potential to counteract the detrimental effects induced by oxidative stress and inflammation—prominent contributors to hearing impairment. Furthermore, this review offers an up-to-date exploration of the diverse molecular pathways modulated by these compounds. However, the dynamic landscape of their efficacy warrants recognition as an ongoing investigative topic, inherently contingent upon specific experimental models. Ultimately, to ascertain the genuine potential of phytochemicals as agents in hearing loss treatment, a comprehensive grasp of the molecular mechanisms at play, coupled with rigorous clinical investigations, stands as an imperative quest.
1. Introduction
1.1. Hearing Loss
Hearing loss (HL) has far-reaching consequences on a person’s life, impacting psychological, emotional, societal, and economic aspects. The costs of hearing aids and specialized services further contribute to the economic burden faced by individuals with HL. Addressing the impacts of hearing loss requires comprehensive support systems, awareness campaigns, and accessible resources to enrich the quality of life for individuals with hearing impairment. In this review, we explore the physiological, biochemical, and molecular aspects of HL, alongside the current and emerging therapeutic strategies, outlining their potential benefits.
By 2050, approximately 2.5 billion individuals are expected to experience some level of hearing impairment, and at the minimum hearing rehabilitation will be warranted for 700 million individuals. Notably, the prevalence of hearing loss surpasses that of diabetes or cancer, being twice as frequent [1]. The incidence of hearing loss rises with age and is most pronounced among males, individuals of White ethnicity, those with lower income, and individuals with lower educational attainment [1]. The elderly represent the most vulnerable population for hearing impairment; however, unhealthy listening practices put over 1.1 billion young adults at risk of permanent yet preventable hearing loss [2]. At speech frequencies that commonly present in older adults, hearing loss is correlated with emotions of loneliness and social isolation stemming from its impact on communication and social interactions [3,4]. This isolation can lead to negative psychiatric consequences, such as depression, distress, and anxiety, which are often reported alongside more severe hearing loss [5,6]. Moreover, there is a well-established relationship between age-related hearing loss (ARHL) and cognitive decline, with ongoing research exploring the causal mechanism. Among modifiable risk factors for dementia, including diabetes mellitus, hypertension, and smoking, hearing loss has the highest relative risk for the development of dementia throughout a person’s life [7]. Two hypotheses have been investigated to understand the connection between hearing loss and cognitive decline: one suggests that cognitive decline precedes hearing loss, and the other attributes hearing loss as the precursor to cognitive decline [8]. In addition to the implications for psychiatric disorders and cognitive decline, a study investigating findings reported by the National Health and Nutrition Examination Survey conducted from 1999 to 2007 revealed that speech-frequency hearing loss is significantly associated with increased heart disease mortality and overall death rates [9]. Hearing loss should not be viewed solely as an impairment of the sensory system but as a condition with wide-ranging consequences for an individual’s overall health. Unfortunately, as the global population ages in the coming decades, hearing loss and its related health issues pose a growing concern.
1.2. Statistics and Classifications of Hearing Loss
In the U.S., self-reported incidences of hearing difficulties can reach as high as 16.8% of the adult population, equivalent to 43.4 million adults [10]. Although self-reporting may inflate the frequency of hearing impairment, low rates of hearing screening and referrals to audiology specialists might underestimate the prevalence in the U.S. According to one study, only 14.6% of primary care physicians regularly perform hearing screenings, potentially leading to missed diagnoses [10]. Moreover, out of the 48.8 million individuals who sought medical attention for hearing complaints in a 5-year period, only 60% were referred to otolaryngologists or audiologists for continued treatment. According to the American Academy of Otolaryngology–Head and Neck Surgery, the shortage of otolaryngologists and audiologists, especially in rural areas, is a significant concern. The current distribution of medical professionals tends to favor urban areas, leaving rural patients at a disadvantage in accessing hearing specialists. This scarcity may hinder access to specialized care, and it does not meet forecasted demand as the US population ages, thus leading to delayed treatment and worsening of hearing impairment [11]. Consequently, many patients remain undiagnosed and untreated despite the increasing prevalence of hearing loss, primarily due to the low rates of screening, referrals, and limited access to specialized care.
In low-income countries, the accessibility to hearing services is severely limited, with 78% and 93% having fewer than one otolaryngologist and audiologist per million people, respectively [12]. Without a large initiative to improve healthcare infrastructure in these regions, this disparity will continue to worsen, as the World Health Organization (WHO) estimates that over the next three decades the greatest increase in hearing loss will be seen in the African and eastern Mediterranean regions [13]. To best address the frequency and extent of hearing loss across the globe, the Global Burden of Disease Expert Group in Hearing Loss recommends an improved classification system. This new system defines seven distinct grades of bilateral hearing impairment (Table 1), each with functional descriptions, and includes a category for unilateral hearing impairment [14]. However, without a substantial initiative to enhance healthcare infrastructure in these regions, this disparity is likely to persist.

Table 1.
Global Burden of Disease Expert Group in Hearing Loss recommended classification of hearing loss types.
The World Report on Hearing observed that individuals with moderate hearing impairment (≥35 dB threshold shift in the better ear) face the highest risk of adverse effects and stand to benefit significantly from medical interventions like hearing aids and cochlear implants if their hearing loss progresses further [13,15]. Moderate hearing loss can be considered the threshold for disabling hearing loss, which affects approximately 27% (430 million) of individuals with hearing impairments [12]. Since age is the most significant risk factor for hearing loss, regions with an aging population are projected to have greater increases in individuals experiencing disabling hearing loss [13]. An increase in disabling hearing loss along with the disparity in hearing specialists and severely low rates of hearing aid use do not confer hopeful predictions about the future state of hearing health.
Unfortunately, the usage of hearing aids in the U.S. is dismally low (28.8 million), with only 15% of individuals who meet the criteria for hearing aids actually using them [15,16]. Notably, the U.S. rate of hearing aid usage is even lower than the global estimates provided by the World Report on Hearing, which indicate that only 17–23% of eligible individuals worldwide use hearing aids [2]. The reasons for such low usage are not solely related to healthcare access but also involve factors like cost, usability challenges, and the unfortunate social stigma associated with wearing hearing aids [15]. To combat the increasing number of people with hearing impairments, there is an urgent need for improved management that addresses disparities in access to hearing specialists, the utilization of hearing aids and cochlear implants, and the availability of therapies to protect hearing. Advancements in prevention and treatment of hearing loss require a comprehensive understanding of different types of hearing loss and the underlying mechanisms of auditory dysfunction.
1.3. Types of Hearing Loss
Hearing loss is categorized into three groups, based on which part of the auditory system is affected. These categories are known as conductive, sensorineural, and mixed hearing loss. When considering years lost to disability, hearing loss ranks as the third most significant contributor, and among sensory impairments, it causes the highest number of years lost [13].
1.3.1. Conductive Hearing Loss (CHL)
CHL occurs when the outer and middle ear fail to convert sound waves into mechanical vibrations that are transmitted to the inner ear [17]. The process of conducting sound involves sound waves entering the auditory canal, causing the tympanic membrane to vibrate, which, in turn, transmits the vibrations through the ossicular chain to the oval window and into the cochlea. Damage or blockage in any of these structures can result in CHL. For example, the auditory canal may be blocked by an accumulation of cerumen, exostosis, or Otitis externa [15]. The tympanic membrane can develop perforations due to acoustic trauma, leading to sclerosis, which reduces the membrane’s flexibility and sensitivity to sound waves [15]. Additionally, the ossicular chain can be disrupted by conditions such as cholesteatoma, Otitis media with effusions, and otosclerosis, thereby interrupting the conduction of sound waves from the auditory canal to the cochlea [15]. Given that the inner ear remains generally unaffected in CHL cases, once this conductive barrier is surpassed, individuals often experience clear hearing.
1.3.2. Sensorineural Hearing Loss (SNHL)
SNHL occurs when the inner ear is unable to convert fluid waves into auditory nerve signals due to damage in either the cochlear epithelium or auditory nerve and may range from a mild loss to profound hearing loss. The causes of SNHL are varied, with congenital factors including cochlear malformations, auditory nerve deficiencies, and abnormalities in the internal auditory canal [18]. Acquired causes comprise Meniere’s disease, age, noise exposure, and ototoxic medications [15,19,20].
ARHL, commonly known as presbycusis, represents the most prevalent form of SNHL. It typically develops gradually and can be worsened by factors such as exposure to loud noise, usage of ototoxic medications, and other ear-related disorders [21,22]. In presbycusis, higher frequency sounds are affected first with progression to the mid and lower frequencies, leading to a decline in the overall quality of life [5]. In addition to worsening outcomes in ARHL, noise- and ototoxic medication-induced hearing loss are discussed as primary causes of SNHL. Noise-induced hearing loss (NIHL) presents as bilateral loss of hearing and is of chief concern in occupational settings [21,23]. Ototoxicity from medications is not limited to but largely investigated with the antineoplastic cisplatin and aminoglycoside antibiotics [24].
On the other hand, sudden SNHL is a distinct condition characterized by an acute loss of at least 30 dB in hearing within three separate instances over a period of 72 h [15]. Unlike presbycusis, this sudden form of SNHL can be treated according to current clinical guidelines, which recommend a combination of steroid therapy and hyperbaric oxygen therapy [25]. In pediatric patients, progressive SNHL can be associated with genetic conditions or chronic infections, while fluctuating symptoms can also occur due to Meniere’s disease or endolymphatic hydrops [21].
1.3.3. Mixed Hearing Loss
Mixed hearing loss manifests with aspects of both CHL and SNHL, either separately or interconnected. Various conditions can affect both the middle and inner ears, such as otosclerosis, chronic infections, and trauma to the temporal bone [21]. However, for the rest of this review, the primary focus will be on SNHL, as it is the most prevalent type of hearing loss.
2. Cellular Mechanisms of Cochlear Hair Cell Degeneration
Over the years, the cellular mechanisms of SNHL have been slowly elucidated to identify major pathways contributing to the pathology. A pervasive commonality among the three major categories is the loss of cochlear hair cells and spiral ganglion neurons; however, the deterioration of these cell groups is attributed to separate and overlapping mechanisms.
Traditionally, it was believed that outer hair cells (OHCs) are the most vulnerable to insults. However, emerging research suggests that in ARHL models, cochlear synaptopathy and spiral ganglion cell degeneration may occur before actual hair cell loss [26]. Noise exposure, even without causing permanent threshold shifts (i.e., noticeable hearing loss) and hair cell death, may exacerbate this process [26,27,28]. Neuronal losses alone are associated with decreased sound discrimination in noisy environments, a condition termed “hidden hearing loss” [29,30]. Over time, accumulated neuronal losses due to aging and exposure to environmental noise can lead to threshold shifts, but it is estimated that this occurs only after losing 80–90% of connections with inner hair cells (IHCs) [30]. Another possible mechanism involves noise-induced glutamate excitotoxicity, which may lead to progressive spiral ganglion cell degeneration by disrupting essential neurotrophin signaling from hair cells [29].
While the concept of hidden hearing loss is intriguing, a full conformation in humans remains challenging due to difficulties in measuring synaptic degeneration [31]. Currently, supporting data for this hypothesis, apart from animal models, are limited to archival temporal bone studies [32,33]. The mechanisms behind cochlear hair cell degeneration are also not completely understood but provide a foundation for comprehending the pathogenesis of SNHL. Studies suggest that early permanent threshold shifts are associated with dysfunction and cell death of OHCs, most commonly in the basal cochlea [34,35]. Recent investigations have revealed that both basal and apical OHCs are lost in the cochleae of aged humans and rats [33,36]. However, even though OHCs deteriorate in both tonotopic regions, the loss of high-frequency hearing precedes the progression into lower frequencies. This phenomenon might be linked to the OHCs’ capability to amplify high-frequency sounds by 50–70 dB, compared to only 20 dB in low frequencies. As a result, threshold shifts would be most significant in the higher frequencies [37].
To protect itself from permanent threshold shifts caused by noise exposure, the cochlea induces temporary threshold shifts, akin to physiological habituation. After noise exposure, temporary threshold shifts occur through the activation of the purinergic receptor P2X 2 ion channel [38]. This channel opens, leading to a reduction in the endocochlear potential around the hair cells, which, in turn, decreases signal transduction and lowers noise sensitivity [39,40]. Additionally, in guinea pigs, noise-induced temporary threshold shifts have been shown to upregulate Bcl-xL, an anti-apoptotic Bcl-2 family member [41].
Prolonged or high dB noise exposure overwhelms this adaptive mechanism and induces permanent threshold shifts by the upregulation of pro-apoptotic Bcl-2 family member, Bak, that lead to hair cell loss [41]. Likewise, noise exposure upregulates endogenous proinflammatory cytokine and chemokine production through activation of nuclear factor kappa B (NF-kB) signaling and recruits immune cells to the cochlea [42,43,44]. Tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1-beta (IL-1β) expressed in response to excessive noise can overcome anti-apoptotic signals to induce hair cell death (Figure 1) [45,46]. In addition to apoptosis, necroptosis has been implicated in outer hair cell death through an upregulation of receptor-interacting serine/threonine protein kinases in chinchilla and mice cochleae [47,48]. Necroptosis varies from apoptosis by permeabilizing plasma and intracellular membranes releasing cellular components into the surrounding environment [48]. Upon release, the cellular components act as damage associated molecular patterns which can be recognized by Toll-like receptors and receptors for advanced glycation products potentiating the pro-apoptotic signaling [49,50]. Moreover, hair cell death is also regulated through the induction of intrinsic, pro-apoptotic caspase cascades.
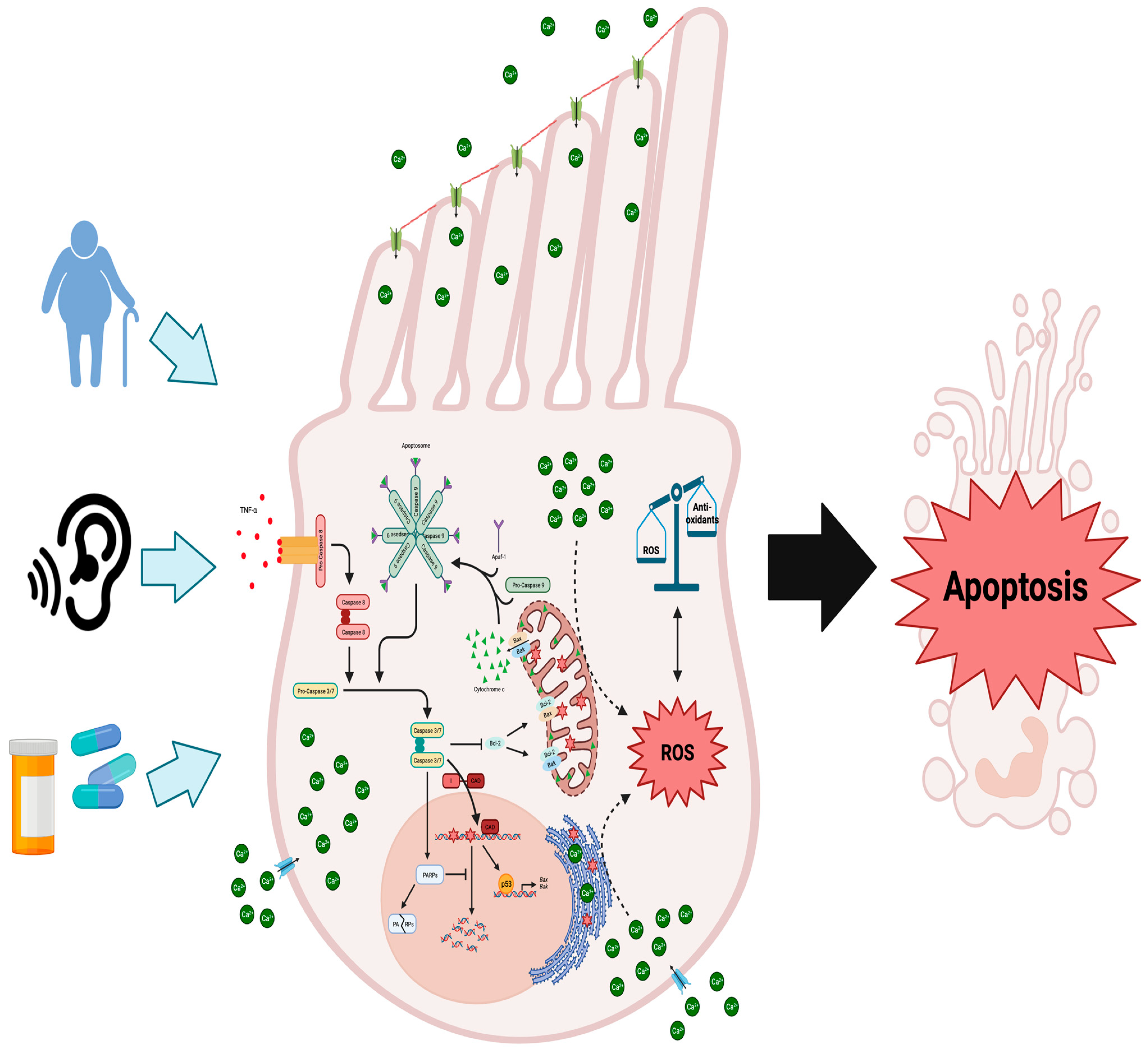
Figure 1.
Molecular mechanisms underlying cochlear hair cell demise due to aging, noise exposure, or ototoxic medications: When cochlear hair cells are injured, the intrinsic and extrinsic apoptotic pathways are initiated, alongside the generation of ROS and disruptions to calcium balance. Damage to these cells triggers external inflammatory molecules like TNF-α to bind to their receptors on the cell membrane, initiating the cleavage and activation of procaspase 8. This sets off the extrinsic apoptotic cascade, activating effector caspases 3 and 7. Caspase 3 further triggers the degradation of anti-apoptotic Bcl-2 protein, releasing cytochrome c from mitochondria. It also activates CAD, leading to DNA fragmentation by cleaving its inhibitor ICAD. Typically, DNA repair is facilitated by PARPs, but caspases 3/7 inhibit this process, contributing to cellular degradation. Excessive DNA damage boosts the pro-apoptotic role of p53, upregulating genes like Bax and Bak. Furthermore, p53 directly interacts with Bak at the outer mitochondrial membrane, promoting cytochrome c release. Through the recruitment of procaspase 9 and Apaf-1, cytosolic cytochrome c assembles the apoptosome, which further activates caspases 3 and 7. Excessive ROS production surpassing antioxidant systems induces oxidative stress and DNA damage. Figure created with BioRender.com.
The activation of intrinsic caspase cascades is initiated by the permeabilization of the mitochondria membrane and release of cytochrome c, which is crucial for the subsequent activation of downstream effector caspases 3 and 7 (Figure 1) [51]. This process involves the formation of the apoptosome, which consists of cytochrome c, Apaf-1, and procaspase 9 (Figure 1). The apoptosome then cleaves and activates the zymogens, procaspases 3 and 7, thereby initiating the apoptotic program [51]. Caspases 3 and 7 act as proteases that target essential cell survival proteins like Bcl-2 [52]. Bcl-2 plays a crucial role in preventing the oligomerization of the pro-apoptotic Bcl-2 family member, Bax, on the outer mitochondrial membrane (Figure 1). Bax recruits the smaller pro-apoptotic Bcl-2 family member, Bak, to the mitochondrial membrane, where both oligomerizes in conjunction [53].The oligomerization of both Bax and Bak forms pores that permeabilize the outer mitochondrial membrane, resulting in the release of cytochrome c, which is necessary for the activation of the intrinsic caspase cascade leading to apoptosis (Figure 1) [53,54,55]. Therefore, this mechanism of action creates a positive feedback loop, as the activation of the intrinsic caspase cascade leads to the degradation of Bcl-2, facilitating Bax-dependent cytochrome c release and further reinforcing the initial apoptotic signal.
In an auditory hair cell line, cisplatin treatment enhanced activation of both the intrinsic and extrinsic pathways as judged by the activation of caspase 8 and 9 (Figure 1) [56]. Further evidence for the role of the extrinsic and intrinsic pathways in hair cell loss comes from a study that exposed chinchillas to loud noise and found caspase 8 is activated alongside caspase 9 in hair cells in response to noise [57]. However, in cisplatin-treated guinea pigs, only caspase 9 was shown to be activated in cochlear hair cells [58]. Despite these results that show the extrinsic caspase cascade to be involved in NIHL, caspase 3 activation, downstream of both pathways, is evident and essential for caspase-dependent apoptosis in cochlear hair cells (Figure 1). Both in vitro and in vivo models display caspase 3 activation in response to cisplatin [58,59], aminoglycosides [60,61], and noise exposure [57,62]. Furthermore, selective inhibition of caspase 3 protects cochlear hair cells from cisplatin-induced apoptosis in vivo [58]. Supporting the role of the intrinsic pathway, in response to the same cochlear insults, Bax activation increases in cochlear hair cells of gerbils and guinea pigs treated with cisplatin and aged gerbils [58,63,64]. Cytochrome c release into the cytosol connects Bax activation and the intrinsic caspase cascade, which has been shown to occur in vivo in response to cisplatin [58], aminoglycoside [61], or noise exposure [57] (Figure 1). Concomitant with the release of cytochrome c, Bax oligomerization also alters the mitochondrial transmembrane potential and causes an increase in cytosolic reactive oxygen species (ROS) [49,55]. Increased generation of ROS overwhelms the antioxidant systems of the inner ear and offers another potential mechanism for cochlear hair cell death.
Accumulation of cytosolic ROS induces oxidative stress when endogenous antioxidant systems are downregulated or overwhelmed shifting the cell towards apoptosis. Aging, ototoxic drugs, and noise are all demonstrated to cause cochlear hair cell death and hearing loss through oxidative stress [65]. Additionally, genetic polymorphisms in antioxidant systems, namely, glutathione S-transferase [66], N-acetyltransferase 2 [67,68], and superoxide dismutase (SOD) 2 [69], predispose individuals to develop ARHL. In the CBA/J experimental model of ARHL, ROS increased alongside the decrease in SOD2 at 18 months of age [70]. This suggests that SNHL may be driven by oxidative stress-related DNA and mitochondrial damage. For instance, in the SAMP8 mouse model of ARHL, 8-oxoguanine, a marker of both nuclear and mitochondrial oxidized DNA, was detected in hair, supporting, and spiral ganglion cells of the cochlea [71]. In a follow-up study from the same laboratory, hydrogen peroxide was shown to induce DNA damage leading to increased auditory brainstem response (ABR) threshold shifts as compared to control mice of the same age [72]. Administration of EUK-207, a SOD/catalase mimetic, prevented threshold shift in SAMP8 mice, suggesting that exogenous antioxidants provide an effective avenue for SNHL prevention. In vitro, HEI-OC1 cells, a heterogenous cell line isolated from the mouse organ of Corti, exposed to hydrogen peroxide became senescent through alterations to the morphology and functionality of the mitochondria [73]. While oxidative stress-induced models of ARHL proceed through promoting senescence, NIHL may be caused through calcium-dependent oxidative stress-induced apoptosis (Figure 1). Exposure to noise increasing ROS in the inner ear was established in guinea pigs and corroborated after in C57BL/6J mice, which suggests that NIHL can be mediated through oxidative stress [74,75].
As indicated above, OHCs appear to be affected first, as opposed to IHCs, in the early progression of hearing loss, and this susceptibility may be attributed to lower antioxidant content [76]. Also, it is plausible that ROS are elevated in response to increased levels of cytosolic calcium increases, leading to OHC death, as has been shown in neurons [77]. Cytosolic calcium increase in neurons was shown to cause a loss of the mitochondrial transmembrane potential and undergo caspase-dependent apoptosis [78,79]. Similarly, in guinea pigs, it was found that noise-induced temporary threshold shifts led to an increase in cytosolic calcium levels in OHCs [80], leading to a rise in ROS production, potentially creating a positive feedback loop that accelerates the progression of NIHL (Figure 1). In the cochlea of guinea pigs, noise exposure was also shown to result in the generation of isoprostanes, vasoactive lipid peroxidation products, which cause ROS accumulation and induce ischemia in the inner ear, restricting blood flow to hair cells and further promoting ROS generation [81].
Unmitigated oxidative stress can have detrimental effects on the cellular environment, leading to damage in cellular membranes and DNA. Notably, it has been observed that 67% of patients with mitochondrial DNA (mtDNA) disorders will develop sensorineural hearing loss, supporting the idea that the accumulation of oxidative stress-induced mtDNA damage may contribute to this condition [82]. Given that oxidative stress is a fundamental factor in multiple pathologies, including type 2 diabetes mellitus (T2DM), emerging evidence supports a strong association between T2DM and SNHL. For instance, mothers that develop T2DM are at risk for mtDNA mutations that are then maternally inherited by their children. The presence of either a 10.4kb mtDNA deletion or point mutation in the mitochondrial tRNA (Leu) passed on from the mother caused all children to develop both T2DM and sensorineural deafness [83,84]. In the third generation born to mothers with mtDNA mutations, eight of the thirteen children developed sensorineural deafness and only two had both conditions [84]. These lineages suggest that oxidative stress in T2DM patients may be related to the development of SNHL.
3. Anti-Hearing Loss Therapies
Numerous therapeutic strategies have been identified to tackle hearing loss at the cellular and molecular levels. While research in pharmacological treatments for hearing loss is still in its infancy, several drugs have shown promise in clinical trials or preclinical studies targeting SNHL as well as various other hearing loss models (Table 2).

Table 2.
Exploring hearing loss therapies: clinical trials and key findings.
3.1. D-Methionine
As an antioxidant, D-methionine has been acknowledged for its potential therapeutic value in the prevention of certain types of hearing loss. For instance, in a phase II exploratory clinical trial, oral administration of D-methionine was examined for its safety and efficacy against cisplatin-induced ototoxicity in cancer patients. Patients receiving D-methionine prior to cisplatin treatment did not show significant threshold shifts at various testing frequencies in comparison to placebo. All documented adverse effects were found to be already common to cisplatin therapy. These results indicate that supplementation of D-methionine protects cancer patients against chemotherapeutic-induced hearing loss without added adverse effects [88], although the molecular mechanisms are yet to be fully elucidated. Pre-clinical investigations have substantiated the protective properties of D-methionine against NIHL. In chinchillas, select windows of D-methionine preloading effectively reduced ABR threshold shifts and increased certain antioxidant levels in both steady state and impulse noise exposures, thus revealing the otoprotective properties of D-methionine against NIHL [98]. These protective effects were corroborated in a randomized controlled trial, where D-methionine preloading demonstrated efficacy against NIH [89]. Similarly, post-administration of D-methionine was also able to protect against noise insults to the cochlea. Using a mouse model of NIHL, Wang et al. demonstrated that both pre- and post-administration of D-methionine treatment reduced cochlear hair cell loss, suppressed apoptosis, and preserved the expression of gap junction proteins, such as connexin 26 and 30 [99]. Additionally, several studies have highlighted the ability of D-methionine to modulate the activities of antioxidant enzymes, including SOD and catalase, thereby attenuating noise-induced oxidative stress [100,101]. However, it is important to note that while oxidative stress is a prominent factor in the pathology of hearing loss, recent ex vivo research has cautioned against high-dose antioxidant treatment. Cochlear explants subjected to prolonged exposure to high concentrations of antioxidants, such as D-methionine at 50 μM, exhibited adverse effects including severe damage to hair cell structure and complete loss of spiral ganglion neurons and supporting fibers [102].
3.2. Ebselen/SPI-1005
Ebselen (SPI-1005) stands out as a pharmaceutical candidate for treating hearing loss due to its unique mimicry of the antioxidant enzyme, glutathione peroxidase (GPx) 1 [103]. During phase II clinical testing in healthy adults, 400 mg of ebselen given orally twice daily was found effective and safe to reduce acute noise-induced hearing loss [90]. Other completed, yet unpublished, clinical trials have focused on investigating ebselen’s potential for treating Meniere’s disease, a rare disorder of the inner ear that can cause a range of hearing-related disturbances [104]. Phase Ib and IIb trials conducted in Meniere’s or Meniere’s disease-probable patients found that subjects treated with SPI-1005, in comparison to placebo, showed clinically relevant improvements of several symptoms that characterize Meniere’s disease [91]. Moreover, some preclinical data confer that ebselen may be beneficial for patients diagnosed with cystic fibrosis [105]. Tobramycin, an aminoglycoside, is commonly used long-term to help treat cystic fibrosis-associated pulmonary bacterial infections [106]. Unfortunately, tobramycin is known to induce ototoxicity [107,108]. Gu and colleagues, however, demonstrated that mice co-treated with ebselen are able to alleviate tobramycin-induced ototoxicity of the cochlea and co-treated cochlear explants were observed to have reduced tobramycin-induced hair cell loss [105]. Likewise, in a model of NIHL, ebselen protected rats from OHC loss and damage to the stria vascularis [103]. Kil and colleagues provided evidence suggesting that ebselen directly scavenges ROS/RNS and possibly upregulates or preserves endogenous GPx1. This hypothesis is supported by previous research demonstrating that ebselen preserves GPx1 levels in spiral ganglion cells exposed to peroxynitrite [109]. Although the precise molecular mechanisms remain unclear, it is plausible that ebselen’s protective effects against hearing loss primarily result from its ability to mitigate oxidative stress. Notably, ebselen has been shown to alleviate inflammation in db/db mice by inhibiting SHIP2 [110] and to attenuate renal damage through the upregulation of autophagy and sequential initiation of the nuclear factor erythroid 2-related factor-2 (Nrf2) pathway [111]. However, further research is needed to confirm whether these mechanisms contribute to its protective effects in hearing loss models.
3.3. Glucocorticoids
In 1980, a double-blind clinical trial revealed the efficacy of oral steroids in treating idiopathic sudden sensorineural hearing loss (ISSNHL) [112], and since, a wealth of clinical trials has investigated the most commonly prescribed steroid medicine for hearing loss, dexamethasone [113]. Various studies have indicated that dexamethasone protects against chemotherapeutic-induced hearing loss [85], mild-to-severe ISSNHL [86], and Meniere’s disease [87,114], among others. However, collective evidence on dexamethasone and its genuine impact on hearing disorders, or as preventative care, appears to be less conclusive. For instance, the benefits of intratympanic dexamethasone remain a controversial subject. Some evidence suggests intratympanic (IT) injection of dexamethasone is a resourceful route of treatment, as IT dexamethasone was reported as being easy to administer, even possibly safer than, and just as effective as IV administration [115,116,117]. Supportively, Ni and colleagues reported no significant differences in hearing improvements between routes of corticoid administration—specifically systemic, IT, or combination therapy—in patients with sudden deafness [118]. In contrast, according to others, IT administration of dexamethasone may be the riskier therapy. In another clinical trial of sudden hearing loss, two patients from the IT cohort reported having tears to the tympanic membrane [119]. Additionally, the true efficacy of dexamethasone has been under review, as Salt and Plontke brought forward that the pharmacokinetic properties of dexamethasone-phosphate, the pro-drug commonly used in clinical practice, may not be compatible with local drug delivery and thus would fail to be clinically relevant [120]. Other discrepancies such as higher incidence of postoperative retching [121] and null outcomes [122] have also been reported with dexamethasone therapy.
While direct human evidence remains scarce, preclinical investigations suggest that aldosterone might have the potential to decelerate or even halt the advancement of presbycusis. Halonen et al. conducted experiments on middle-aged CBA/CaJ mice to elucidate the otoprotective properties of this mineralocorticoid hormone. Their findings indicated that animals receiving long-term aldosterone treatment exhibited stabilized ABR thresholds. In contrast, the untreated group experienced a notable shift in noise threshold, increasing from 39 dB to 49 dB [123]. Further in vivo models have demonstrated that aldosterone [124], synthetic aldosterone, or a combination of aldosterone and prednisone at low doses [125,126] are also effective at restoring autoimmune mediated hearing loss.
3.4. Sodium Thiosulfate
Sodium thiosulfate, an FDA-approved drug, has emerged as a potential intervention to reduce the likelihood and severity of cisplatin-induced hearing loss in pediatric patients. Despite common side effects such as vomiting and nausea, sodium thiosulfate maintains an overall safety profile, making it a pertinent consideration for improving the quality of life in pediatric cancer patients undergoing cisplatin-based chemotherapy. Such perspective is supported by results from few animal studies [127,128] and human clinical trials. For example, in a study by Brock et al. [96], sodium thiosulfate administered six hours post-cisplatin chemotherapy in children with standard-risk hepatoblastoma demonstrated a lower incidence of hearing loss without compromising overall or event-free survival. Similarly, Rischin et al. highlighted the potential of DB-020 to reduce cisplatin-induced ototoxicity [97]. DB-020 is an intratympanic formulation of sodium thiosulfate [129]. Additionally, the role of sodium thiosulfate in preventing cisplatin-induced hearing loss is supported by a multicenter, randomized, phase 3 trial (ACCL0431), which found a significant decrease in the incidence of hearing loss among participants treated with sodium thiosulfate compared to the control group [95]. These findings, along with results from a prospective analysis comparing hearing loss incidence in a phase III randomized trial versus IV high-dose cisplatin chemoradiation without sodium thiosulfate, provide evidence supporting the potential of sodium thiosulfate to mitigate cisplatin-induced hearing loss in pediatric patients. The collective results emphasize the promising role of sodium thiosulfate in addressing this side effect and its potential impact on the well-being of pediatric cancer patients [94]. It is worth noting that while the molecular mechanisms mediating sodium thiosulfate autoprotective effects are yet to be determined, studies by Hochman and colleagues were unable to demonstrate that sodium thiosulfate can attenuate gentamicin-induced ototoxicity in mice [130]. This underscores the need for additional evidence and a more comprehensive understanding of sodium thiosulfate’s modes of action.
3.5. Additional Therapies
A phase 1 study tested the safety and audiometric effects of FX-322 for the treatment of SNHL. The authors reported that patients with chronic SNHL treated with an intratympanic injection of FX-322 exhibited clinically meaningful improvements in speech recognition and hearing function without drug intolerances [92]. Notably, clinical phase 2a trials are up and coming.
Moreover, IGF-1 has recently shown some early therapeutic promise for treating SNHL, yet symptoms such as pain, dizziness, and headache are all adverse effects reported by treated patients [93].
Tinnitus or phantom auditory perception is a condition that is frequently reported among patients with hearing loss [131,132]. A noncompetitive NMDA receptor antagonist, AM-101, was specifically evaluated for its efficacy and safety to treat several hearing loss pathologies. Some clinical outcomes have shown that intratympanic injections of AM-101 are well tolerated and safe with some patient-reported improvements in tinnitus-related symptoms [133,134,135]. However, in a placebo-controlled phase II trial, the primary end point to assess the efficacy of AM-101 for acute inner ear tinnitus was unsuccessful. Moreover, the benefit of AM-101 treatment failed to transfer to ISSNHL-related tinnitus patients, a subcategory of the total study population [133].
Because SNHL results from permanent damage to and loss of cochlear hair cells, a fair portion of therapeutic research has set out to explore the potential of hair cell regeneration [136]. Presently, three companies have advanced preclinical data into clinical works, and these have been outlined and well described in a recent review by Hinton et al. Briefly, clinical hair cell regeneration therapies primarily embrace gene therapy and small molecule inhibitor or combination approaches [137].
4. Phytochemicals as Medicine
Phytochemicals have begun to emerge as efficacious treatment options and adjuvants in Western medicine since biomedical research seeks alternatives to pharmaceuticals. Phytochemicals—non-nutrient bioactive compounds originating from natural sources, most commonly plants, but also including some fungi and algae compounds [138]—have been shown to be beneficial in combatting a plethora of chronic and non-chronic diseases across the board including cancer, aging, diabetes, osteoporosis, and cardiovascular disease. Phytochemicals exhibit potent antioxidative, anti-inflammatory, and neuroprotective characteristics attributed to their structural diversity and abundance [139]. Phytochemicals encompass various classes, including polyphenols, triterpenoids, steroids, saponins, and alkaloids, each containing distinct subgroups [138]. Among these, polyphenols are particularly noteworthy, as they are the most abundant antioxidants found in foods. They can be further categorized into flavonoids, phenolic acids, stilbenes, tannins, and coumarins. The clinical community has shown support for phytochemicals due to their demonstrated effectiveness with fewer side effects and lower costs compared to pharmaceuticals [139]. In the subsequent section, we will elucidate the protective properties of several phytochemicals against disruptions in cochlear hair cell homeostasis and functionality, along with their potential in mitigating hearing loss.
4.1. Phytochemicals in Sensorineural Hearing Loss
The activation of antioxidant and anti-inflammatory pathways, such as Nrf2 and inhibition of NF-κB, is crucial for mitigating the harmful effects that lead to cochlear hair cell death (Figure 2). Additionally, maintaining proper blood flow to the cochlea is essential for nutrient circulation and waste removal, as any disruption in this process can result in damage to the hair and supporting cells. Phytochemicals have been found to influence these pathways, offering potential benefits for chronic diseases that share similar etiology with SNHL, such as cardiovascular disease, nephropathy, and T2DM. In this section, we will explore the body of research on specific phytochemicals and their effects on SNHL, including those that have demonstrated efficacy in treating T2DM. Given the association between T2DM and SNHL, it is possible that phytochemicals could be used to address both conditions and potentially improve outcomes for individuals with these health concerns.
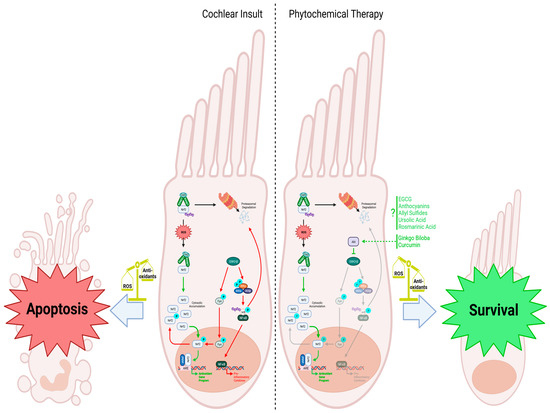
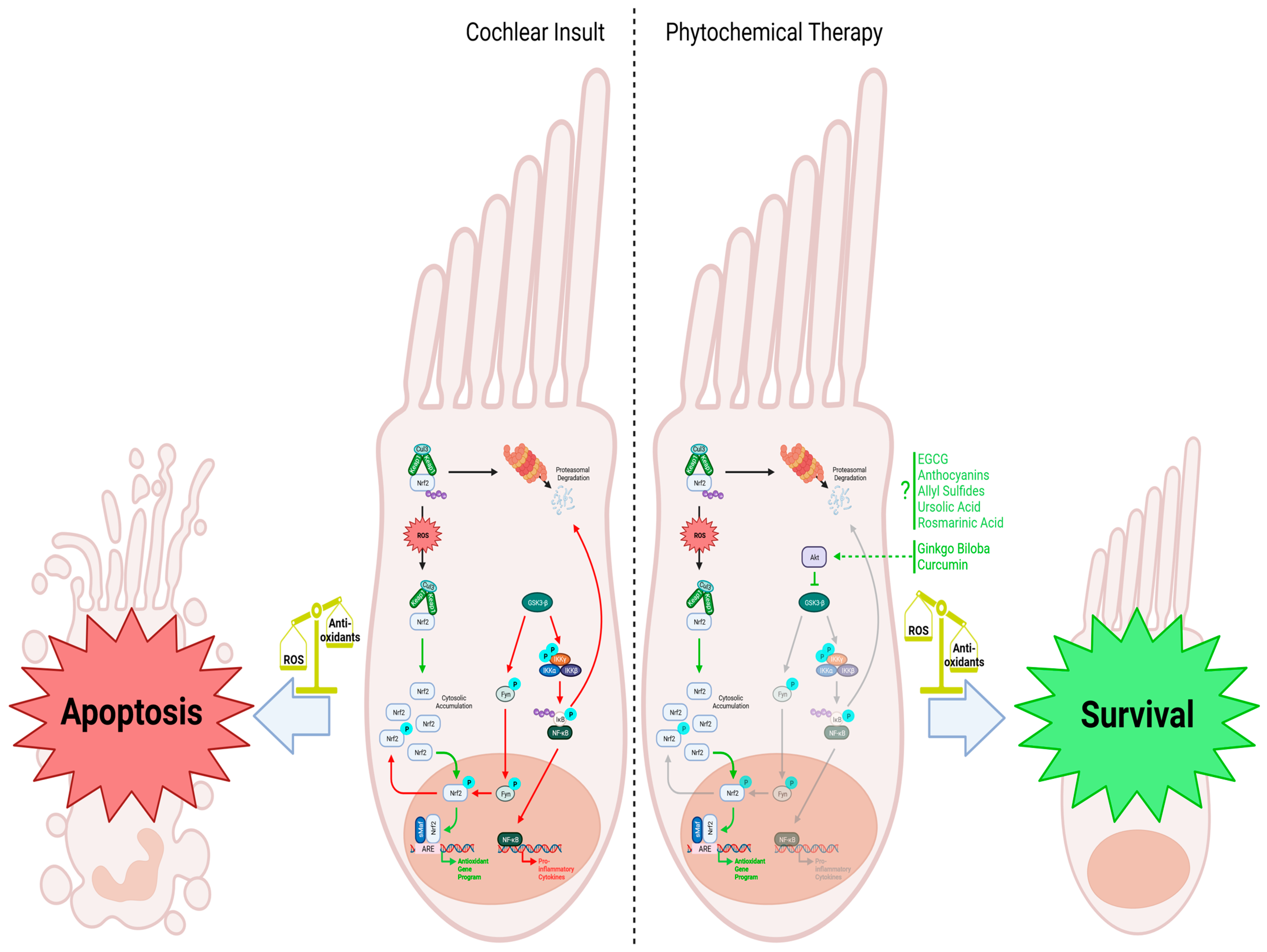
Figure 2.
Cochlear insult: When cellular reactive oxygen species (ROS) are low, Nrf2 is degraded via proteasomal degradation facilitated by the Keap1 homodimer, which recruits the ubiquitin ligase Cul3 for subsequent ubiquitination. However, when cochlear hair cell injury occurs and ROS levels rise, Keap1 undergoes oxidation, leading to the accumulation of Nrf2 within the cytosol. This prompts Nrf2 to translocate into the nucleus, where it forms a complex with the leucine zipper-type transcription factor sMaf and binds to the antioxidant response element (ARE). This activation leads to the upregulation of antioxidant genes, counteracting the increased ROS levels in the cell. GSK3-β negatively regulates the Nrf2-dependent gene expression mechanism by phosphorylating Fyn, leading to its translocation into the nucleus, where it phosphorylates Nrf2. Phosphorylated Nrf2 is then exported from the nucleus back into the cytosol, inhibiting the antioxidant response. Additionally, GSK3-β activates the IKK complex and leads to the phosphorylation of IκB and its subsequent ubiquitination and degradation. The release of NF-κB and translocation to the nucleus promotes the transcription of proinflammatory cytokines that further worsens cell injury. The reduced antioxidant response, combined with the upregulation of proinflammatory cytokines, makes the cell more susceptible to apoptosis. Phytochemical therapy involving substances like rosmarinic acid, Ginkgo biloba, and curcumin enhances the cellular response to increased ROS levels. Ginkgo biloba and curcumin activate Akt, inhibiting the GSK3-β-induced phosphorylation cascade. Without Fyn stimulating Nrf2 nuclear exclusion, Nrf2 can activate the antioxidant program, helping to scavenge ROS. Although various phytochemicals are known to stimulate Nrf2 and the transcription of its downstream antioxidant genes, specific mechanisms are still being investigated. Nevertheless, administering these phytochemicals effectively combats oxidative stress, promoting the survival and function of cochlear hair cells. Figure created with BioRender.com.
4.1.1. Ginkgo biloba
Ginkgo biloba stands out as one of the most thoroughly investigated extracts for the prevention and treatment of hearing loss, yet its clinical effectiveness continues to be a subject of debate. Multiple clinical trials and several animal models have shown beneficial results for its use in ARHL, NIHL, and hearing loss induced by cisplatin. In human studies, supplementation with Ginkgo biloba extract EGb 761 during chemotherapy with cisplatin showed significant hearing protection, as measured by distortion product otoacoustic emission (DPOAE) tests, compared to the control group [140]. Similarly, in another trial using Ginkocer, a Ginkgo biloba supplement, ARHL patients experienced an average of 15.56% improvement in hearing [141]. Moreover, coal miners exposed to blast noise damage and treated with Ginkgo biloba through a tympanic injection, in combination with hyperbaric oxygen therapy, exhibited significant improvement in acoustic response rates compared to those who only received traditional therapy [142].
These clinical findings are supported by in vivo evidence from various animal models. For instance, Ginkgo biloba supplementation to cisplatin-exposed rats showed significantly improved DPOAE tests compared to the cisplatin group and resulted in fewer apoptotic cells in the organ of Corti and stria vascularis [143]. These results corroborate previous investigations employing rats and guinea pigs as experimental models of cisplatin-induced ototoxicity [144,145], further substantiating the effectiveness of Ginkgo biloba in ameliorating cochlear dysfunction induced by cisplatin. Notably, Ginkgo biloba’s antioxidant properties also protected guinea pigs from ototoxicity caused by pesticides and antibiotic-induced ototoxicity [146,147]. In models of NIHL, the prophylactic use of EGb 761 in Mongolian gerbils reduced NIHL and prevented permanent threshold shift [148,149]. Additionally, in C57BL/6 mice, a combination of cilostazol and EGb 761, called Renexin®, protected the organ of Corti from degeneration and NIHL, suggesting potential for reversing permanent NIHL [150]. Furthermore, in studies investigating ARHL in young and aged rats, EGb 761 correlated with reduced caspase 3/7 activity and threshold shifts [151].
Mechanistically, Ginkgo biloba exhibits a diverse range of actions that contribute to its effectiveness in various models of SNHL. In vitro studies exploring its effects on neural stem cells (NSCs) demonstrated Ginkgo biloba’s ability to promote NSC proliferation and differentiation into functional neurons [152]. When exposed to oxidative stress induced by hydrogen peroxide, Ginkgo biloba’s antioxidant effects appeared to protect NSCs by preventing mitochondrial depolarization and apoptosis through modulation of the intrinsic apoptotic pathway, as evidenced by the upregulation of Bcl-2 and downregulation of Bax and Caspase-3 [153]. Similarly, in HEI-OC1 cells treated with cisplatin, Ginkgolide B exhibited comparable results by inhibiting the intrinsic pathway of apoptosis. These effects were attributed to reduced ROS levels via downregulation of NADPH oxidase 2 expression and upregulation of Akt/Nrf2/HO-1 signaling (Figure 2) [154]. Moreover, EGb 761 was proposed to operate through the upregulation of sirtuin 1 (SIRT1), which was found to alleviate SNHL induced by 3-nitropropionic acid in rats, potentially protecting fibrocytes and spiral ganglion cells through SIRT1 expression [155]. Additionally, EGb 761 preserved the organ of Corti from gap junction intercellular communication disturbance by inhibiting cisplatin-induced downregulation of connexins [156]. The observed anti-inflammatory effects of Ginkgo biloba were evident in a rat model of NIHL, where it inhibited IL-1β, IL-6, TNF-α, and cyclooxygenase (COX) 2 while increasing heat shock protein 70 and heat shock factor 1, ultimately reducing NIHL [157]. EGb 761 was also found to lower total oxidant status and DNA damage in NIHL while reducing threshold shifts. Consistent with these findings, when Renexin®, a pharmaceutical formulation comprising cilostazol and Ginkgo biloba leaf extract, known for its decongestant and appetite suppressant properties, as well as its potential in alleviating ischemic symptoms associated with peripheral arterial disease [158], was administrated to a rat model of cisplatin-induced hearing loss, a significant decline in ROS levels and apoptosis was observed [159]. Nevertheless, it is imperative to underscore that there exists conflicting evidence regarding the efficacy of Ginkgo biloba. Indeed, investigations involving guinea pigs and zebrafish have documented either a lack of therapeutic response or even adverse effects when Ginkgo biloba is administered concurrently with the antibiotic amikacin [160,161,162]. Furthermore, a clinical trial focusing on the treatment of ARHL using Ginkgo biloba and other phytochemicals failed to demonstrate statistically significant alterations in hearing thresholds [163]. These contradictory outcomes suggest that the efficacy of Ginkgo biloba in managing SNHL may be contingent upon the specific experimental model and context in which it is employed. Further research is warranted to elucidate the determinants influencing its efficacy and to delineate the optimal conditions for its therapeutic application.
4.1.2. Resveratrol
Resveratrol, a naturally occurring polyphenol classified as a stilbenoid and found in over 70 plant species [164], has been subject to extensive research aiming at unveiling its therapeutic potential against SNHL. Various mechanisms have been proposed to elucidate its efficacy across diverse experimental models. For example, in a rat model of cisplatin-induced hearing impairment, the co-administration of resveratrol with cisplatin resulted in a significant reduction in ABR thresholds concomitant with improvement in DPOAEs compared to cisplatin treatment alone [165,166]. Resveratrol has also shown protective effects on the inner ear, reducing damage and lowering ROS levels in rats and guinea pigs, respectively [167,168]. Furthermore, in a model of diabetes-induced ototoxicity in rats, resveratrol at multiple dosages decreased apoptosis in the inner ear, as evidenced by reduced caspase-3, Bax, and TUNEL staining [169]. Notably, resveratrol was effective against amikacin-induced ototoxicity, in contrast to Ginkgo biloba [170]. In other studies, resveratrol’s protection against cisplatin-induced hearing loss was suggested to involve modulation of the PTEN/PI3K/Akt pathway, resulting in the upregulation of miRNA-455-5p [171]. Furthermore, resveratrol decreased proinflammatory gene expression after cisplatin treatment [172]. However, at high doses, resveratrol may potentiate cisplatin-induced ototoxicity [173]. It is worth noting that the protective effects of resveratrol are not limited to its antioxidant properties, as it has been also shown to regulate gene expression to ameliorate the effects of ototoxic drugs. For example, in aminoglycoside-induced ototoxicity, resveratrol upregulates antioxidant enzymes to relieve oxidative stress while downregulating proinflammatory genes [174]. Additionally, resveratrol has been identified as a potent SIRT1 agonist, and its induction of SIRT1/PGC1-α signaling pathways has been shown to ameliorate the effects of oxidative stress in guinea pig models of NIHL and C57BL/6 mice exposed to noise [175,176]. Furthermore, resveratrol has been proposed to protect against NIHL by inhibiting serotonin transporters to increase serotonin levels [177]. The attenuation of COX2 expression may also play a role in reducing threshold shifts after noise exposure [178].
In models of NIHL, resveratrol has been shown to lower ABR threshold shifts and protect the cochlea from acoustic damage [179,180]. Resveratrol has been observed to decrease the expression of pro-apoptotic genes, such as Bax and Bak, while concurrently upregulating anti-apoptotic genes, including Bcl-2 and Bcl-xL [181]. Furthermore, in the same study, the treatment with resveratrol resulted in the downregulation of inflammatory and apoptotic genes, such as NF-κB, COX2; inducible nitric oxide synthase (iNOS); as well as caspases 3 and 9. These effects seem to be mediated by SIRT1, an NAD+-dependent deacetylase that was shown to exhibit a protective role in aging cochlea [182]. Notably, resveratrol protected mice from hair cell loss through the induction of the miRNA-34-a/SIRT1/p53 signaling pathway [183]. These observations were substantiated in a recent investigation, where resveratrol was demonstrated to elicit the same signaling cascade to preserve aging cochlear hair cells, spiral ganglion cells, and the stria vascularis [184]. Furthermore, resveratrol modulates the involvement of autophagy in aging by regulating SIRT1 expression [185].
4.1.3. Curcumin
Due to curcumin’s limited bioavailability, researchers have explored nanoencapsulation as a targeted delivery method to the cochlea. Studies in guinea pigs have demonstrated that nanoparticle-encapsulated curcumin can be more effective than dexamethasone in preventing hearing loss, indicating its potential as a promising therapeutic option [186]. Nanoparticles have also shown safety and efficacy in mitigating cochlear damage induced by cisplatin [187]. Furthermore, curcumin has proven effective in a model of gentamicin-induced ototoxicity and has demonstrated potential as a prophylactic agent, with similar protective effects observed in the gentamicin-induced ototoxicity model [188]. In rat models of ototoxicity induced by the chemotherapeutic agent paclitaxel or cisplatin, curcumin administered prior to or in combination with the drugs alleviated cochlear damage [189,190]. Rat models of NIHL have also shown that curcumin induces Nrf2 signaling, offering protection against DPOAE shifts and loss of inner and outer hair cells (Figure 2) [189,191].
Although the specific molecular mechanisms of curcumin’s action are not fully elucidated, several pathways have been identified. Following noise exposure, curcumin treatment protected the cochlea by reducing NF-κB activation [192]. Curcumin has also been found to protect supporting tissue in the cochlea by decreasing the expression of calcineurin, NFATc1, and overall lowering the apoptotic index [193]. Another possible mechanism involves the inhibition of IKKβ phosphorylation that suppresses CXCL5 and the induction of MKP-1, which negatively regulates p38-MAPK signaling [194]. Interestingly, curcumin has been shown to preserve cisplatin’s action against tumors while protecting against ototoxicity by activating STAT3 and Nrf2 in vivo (Figure 2) [195]. Additionally, curcumin may regulate HO-1 expression and block the JNK-mediated cell death pathway to preserve cochlear cells [196,197]. Overall, while the precise mechanisms of curcumin’s action require further investigation, the accumulating evidence suggests its potential as a therapeutic agent for various forms of hearing loss.
4.1.4. Salvia miltiorrhiza (Danshen)
Salvia miltiorrhiza, also known as Danshen in China, is commonly used therapeutically in an injectable form. It has shown promise in protecting against cisplatin-induced ototoxicity in guinea pigs by reducing damage to the cochlea and spiral ganglion through the attenuation of iNOS expression [198]. Salvia miltiorrhiza has also been found to safeguard against ABR threshold shifts when kanamycin is used to induce ototoxicity, without compromising the antibiotic’s efficacy [199]. The effectiveness of Salvia miltiorrhiza in combatting hearing loss appears to be linked to its regulation of iNOS expression.
For instance, in a guinea pig model of aminoglycoside-induced hearing loss, Salvia miltiorrhiza was found to attenuate the expression of iNOS and caspase 3, resulting in a decrease in the number of apoptotic cells [200]. Similarly, Salvia miltiorrhiza was shown to exhibit protective effects against gentamicin-induced damage to the stria vascularis through reducing iNOS activity [201]. Additionally, it seems that Salvia miltiorrhiza’s ability to reduce acetylcholinesterase may contribute to protecting the cochlea and spiral ganglion cells after streptomycin administration [202]. Corroborating these findings, another study demonstrated a reduction in iNOS activity in response to streptomycin when cotreated with Salvia miltiorrhiza [203]. An interesting alternative mechanism of Salvia miltiorrhiza’s action was observed in guinea pigs, where it increased superoxide dismutase activity and reduced lipid peroxidation, leading to improvements in auditory function [204].
4.1.5. Epigallocatechin Gallate (EGCG)
EGCG has been touted as an effective treatment to a wide variety of conditions. As with curcumin, EGCG has been packaged into nanoparticles, providing enhanced protection against damage to the cochlea in C57/BL6 mice [205]. It is worth noting, however, that EGCG demonstrated protection of spiral ganglion cells appears to deviate from the mechanisms employed by other phytochemicals. Unlike many, EGCG does not predominantly rely on the upregulation of antioxidant systems, as evidenced by its ability to suppress SOD2 expression [206]. Instead, EGCG may exert its effectiveness through the regulation of the STAT1/STAT3 axis. Studies using mice and rats with cisplatin-induced ototoxicity revealed that EGCG’s protective effects involve the suppression of STAT1 activity while preserving the function of STAT3 and Bcl-xL to reduce outer hair cell death [207,208,209]. EGCG also appears to act through other pathways to reduce oxidative stress and inflammation. Investigations in aminoglycoside ototoxicity using the network pharmacology approach have shown that EGCG induces a cellular response involving Akt1, dihydrofolate reductase, and the hepatocyte growth factor receptor product of the proto-oncogene MET, which helps preserve outer hair cells [210]. Moreover, EGCG has been demonstrated to prevent hair cell loss in the organ of Corti by inhibiting NF-κB and caspase-3 activity in a study of NO ototoxicity [211]. Additionally, EGCG has shown promise in promoting hearing recovery by increasing neural stem cell proliferation and differentiation [212]. Notably, it has also been identified as a novel inhibitor of Notch signaling, which may contribute to hair cell proliferation [213]. In summary, EGCG exhibits a multifaceted approach in protecting against hearing loss, involving the regulation of various molecular pathways and cellular responses, making it a promising candidate for potential therapeutic applications in hearing-related conditions.
4.1.6. Panax ginseng
Panax ginseng exerts protection against medication, palmitate, ischemia, and noise-induced cochlear hair cell damage through a class of saponins referred to as ginsenosides. Largely, Panax ginseng is studied as a whole-food supplement and research into isolated ginsenosides has offered some insight into the molecular mechanisms of action. Sprague–Dawley rats exposed to gentamicin had increased hearing thresholds and significant stereocilia damage [214]. When rats were supplemented with Korean red ginseng (KRG) for one week prior and during exposure to gentamicin, researchers observed a significant improvement in hearing thresholds and reduction in cochlear hair cell damage [214]. In rats exposed to prolonged high-decibel noise, supplementation with KRG protected against cell death in both the organ of Corti and spiral ganglion [215]. In an effort to understand the effects of isolated ginsenosides, Fujita and colleagues demonstrated ginsenoside-Rb1 reduces the loss of spiral ganglion cells after the induction of cochlear ischemia by occlusion [216].
In vitro studies conducted in HEI-OC1 and UB/OC-2 cochlear cell lines better characterized the molecular effects of the cochlear insults and the protection offered by P. ginseng and its bioactive components. Exposure to cisplatin shifted HEI-OC1 cells to undergo apoptosis due to the generation of ROS and proinflammatory protein expression. Solubilized KRG prevented these pro-apoptotic processes by attenuating ROS generation and the activation of NF-κB inflammatory signaling [217,218]. Kim and co-authors proposed that the mitigation of inflammation occurs through the attenuation of phosphorylation and activation of IKK, leading to a reduction in the transcriptional activity of NF-κB [218]. Additionally, cochlear hair cell death can be induced through the activation of the unfolded protein response and ER stress. Palmitate induces apoptosis in UB/OC-2 cells through the activation of ER stress and inflammation in addition to increasing the generation of ROS [219]. The inhibition of only one of these stress pathways is insufficient to block cell death; however, ginsenoside-Rc protected against palmitate-induced cell death through the amelioration of all three pathways [219]. An alternative mechanism of protection also includes a reduction of PARP cleavage after gentamicin exposure in HEI-OC1 cells treated with ginsenoside-Rb1 and -Rb2 [214]. While clinical human data validating the potential role of ginsenosides in mitigating hearing loss are limited, the existing evidence aligns with the concept that Panax ginseng provides protection against cochlear damage. Textile workers are exposed to continuous, high-decibel occupational noise, which makes them an interesting population to study the efficacy of P. ginseng. In a study spanning 14 days, textile workers were administered oral doses of ginseng, with hearing thresholds assessed both prior to and following the trial [220]. The results indicated that ginseng provided defense against noise-induced temporary threshold shifts, although its efficacy was surpassed by supplementation with N-acetyl-cysteine [220]. Additional long-term studies are needed to ascertain whether Panax ginseng confers protection against permanent threshold shifts, which arise following cochlear hair cell and spiral ganglion cell damage induced by factors such as noise exposure, medication, aging, or diabetes.
4.1.7. Astragalosides
Astragalosides have primarily been investigated in the context of NIHL, specifically in acute impulse studies. A clinical trial investigating the effectiveness of astragalosides in recovering from acute acoustic trauma revealed that the group supplemented with astragalosides experienced significantly shorter recovery times [221]. In comparison to other antioxidants, namely, α-lipoic acid and vitamin E, astragalosides and α-lipoic acid were notably more effective in reducing ABR thresholds and protecting hair cells, outperforming vitamin E [222]. In guinea pigs subjected to impulse noise exposure, administration of astragalosides led to a significant reduction in ABR thresholds, hair cell damage, and the expression of iNOS and reactive nitrogen species (RNS) production [223]. These observations are corroborated by another investigation, which indicated that astragalosides protect cochlear hair cells by mitigating ROS levels and suppressing caspase-3 activity [224]. Furthermore, astragalosides were demonstrated to maintain the integrity of connexin 26 and KCNQ1, thereby safeguarding the stria vascularis in guinea pigs [225]. Additionally, astragalosides effectively mitigated cisplatin-induced ototoxicity by preserving outer hair cells through the reduction of malondialdehyde and nitric oxide levels [226].
4.1.8. Garlic
Several primary bioactive components present in garlic, including S-allylmercaptocysteine (SAMC), diallyl disulfide (DD), and S-allylcysteine, have demonstrated effectiveness in treating SNHL, as well as other forms of HL [227,228,229,230]. In studies involving rats with gentamicin-induced ototoxicity, all three components effectively mitigated the condition, as evidenced by reductions in ABR thresholds [228]. However, SAMC and DD exhibited greater potency than S-allylcysteine. Additionally, rats supplemented with garlic in their diet were protected from gentamicin-induced BERA threshold shifts [229]. Moreover, in a rat model of noise-induced hearing loss, SAMC and DD were found to be more effective than vitamin B12 in protecting the cochlea from noise-induced damage [230]. Likewise, the use of MEKRITEN, a garlic extract liquid formulated by the Tashkent Pharmaceutical Institute and promoted for its therapeutic benefits, has shown efficacy in healing suppurative otitis with fewer side effects compared to commonly used treatments in patients [227]. Although the exact mechanism of action for garlic and its derivatives is not fully understood, the current proposed mechanism seems to involve the attenuation of caspase-dependent and PARP-1/AIF-dependent cell death [231]. For instance, allicin, a garlic product, was observed to reduce caspase-3 cleavage, PARP-1, and AIF nuclear translocation in the stria vascularis [231]. Similarly, in mice with cisplatin-induced ototoxicity, allicin protected spiral ganglion neurons by reducing cytochrome c release and the expression of Bax, caspases 3 and 9, and p53 [232]. Allicin also upregulated SOD, thereby preserving the outer hair and supporting cells of the cochlea [232].
4.1.9. Additional Phytochemicals
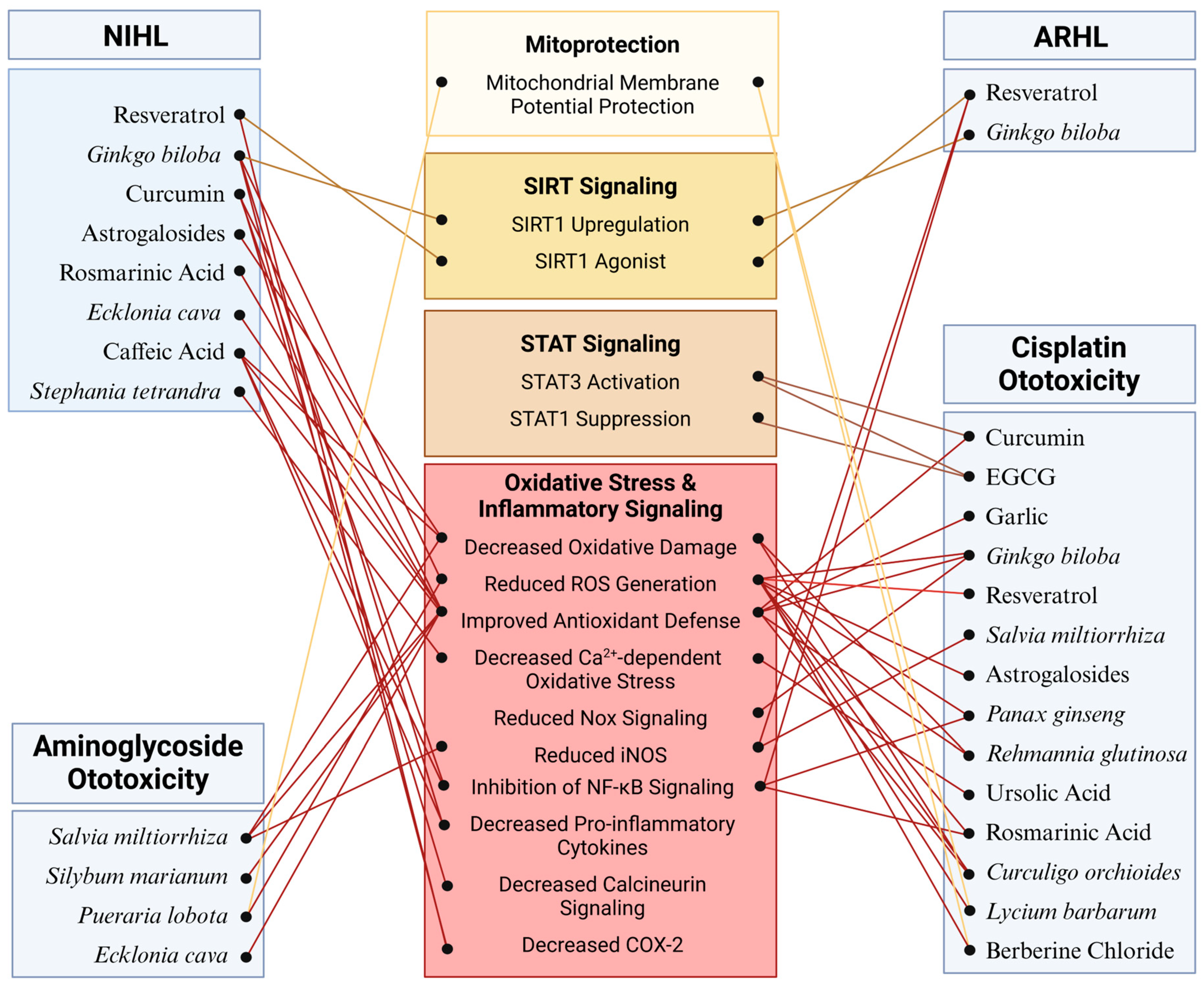
Many additional phytochemicals (Table 3) have been investigated for their potential uses as primary strategies to preserve auditory function or combat the ototoxicity of medications while maintaining drug efficacy. Largely, these phytochemicals act through similar mechanisms to the ones above by attenuating ROS generation either by upregulation of endogenous antioxidant enzymes in cochlear hair cells or by sequestration of free radicals by the phytochemical itself (Figure 3). Many have anti-inflammatory effects as well, thereby reducing NF-κB activation and expression of interleukin cytokine species (Figure 3) [233,234]. Ameliorating oxidative stress and inflammatory signaling combats apoptotic signaling that can be directly observed through the inhibition of caspase 3 and PARP cleavage as well as mitochondrial apoptotic signaling [233,235,236]. Novel mechanisms of action for hearing preservation have also been elucidated in these investigations. Ursolic acid was shown to reduce oxidative stress through the inhibition of the TRPV1/Ca2+/calpain pathway that is activated during cisplatin-induced ototoxicity [237]. Silymarin, the bioactive component in milk thistle (Silybum marianum), protected against gentamicin damage by restoring NGF production and upregulating its receptor, Trk-A [238]. Puerarin, isolated from Pueraria lobota, reduced threshold shift after noise exposure in mice through its action to decrease PKCγ expression and increase GABAB receptor 1 and 2 expression [239]. Berberine chloride acts on spiral ganglion cells to reduce cytomegalovirus apoptosis by reducing Nox3-dependent ROS generation (Figure 3) [240]. The wealth of phytochemical species and mechanisms of action constitute an arsenal for the preservation of hearing across all classes of SNHL.

Table 3.
Exploring the effects of select phytochemicals in the prevention and treatment of hearing loss.
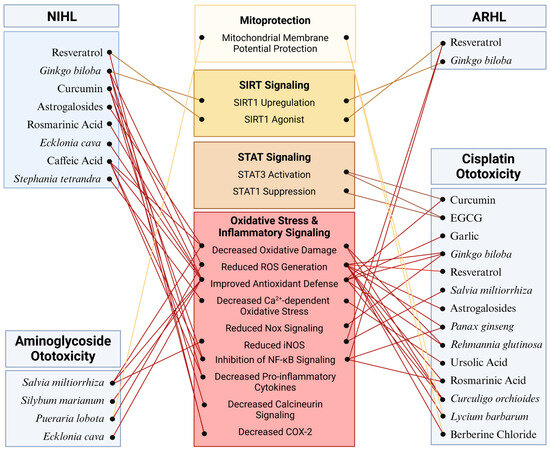
Figure 3.
Molecular mechanisms mediating the potential protective effects of phytochemicals against SNHL: The effectiveness of phytochemicals has been evaluated across various models of SNHL with particular emphasis on cisplatin ototoxicity. Across these models, phytochemicals demonstrate a protective effect against cochlear damage primarily by regulating oxidative stress and inflammatory pathways. Additionally, phytochemicals such as resveratrol and curcumin provide protection by activating SIRT and STAT signaling pathways, respectively. Further research is warranted to elucidate additional specific targets mediating the effects of phytochemicals in the cochlea. Figure created with BioRender.com.
5. Conclusions
In summary, hearing loss poses a complex and widespread challenge affecting 20% of the global population. In this review, we delineated the extent, clinical variations, and current understanding of the molecular foundations of SNHL. We elucidated how the molecular pathophysiology of SNHL, particularly involving the induction of oxidative stress and inflammation, parallels that of other pathological conditions. Within this context, phytochemicals have emerged as promising candidates for addressing hearing loss, leveraging their antioxidant and anti-inflammatory properties, potentially offering a safer avenue for mitigating hearing loss compared to strategies involving hair cell regeneration or gene therapy. Furthermore, we provided an up-to-date exploration of the diverse molecular pathways influenced by these compounds. Because phytochemicals do modulate more than one molecular pathway, and can also act epigenetically, comprehensive studies of their effects in different biochemical contexts should be combined with clinical investigations to elucidate their potential in mitigating hearing loss. As discussed above, a wealth of preliminary studies suggests that investigation of phytochemicals for hearing loss treatment holds significant promise. Notably, further exploration, and efforts to enhance their bioavailability, delivery, efficacy, and safety, should also be pursued.
Author Contributions
Writing—original draft preparation, all authors; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reed, N.S.; Garcia-Morales, E.E.; Myers, C.; Huang, A.R.; Ehrlich, J.R.; Killeen, O.J.; Hoover-Fong, J.E.; Lin, F.R.; Arnold, M.L.; Oh, E.S.; et al. Prevalence of Hearing Loss and Hearing Aid Use Among US Medicare Beneficiaries Aged 71 Years and Older. JAMA Netw. Open 2023, 6, e2326320. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021; p. 252. [Google Scholar]
- Agrawal, Y.; Platz, E.A.; Niparko, J.K. Prevalence of hearing loss and differences by demographic characteristics among US adults: Data from the National Health and Nutrition Examination Survey, 1999–2004. Arch. Intern. Med. 2008, 168, 1522–1530. [Google Scholar] [CrossRef]
- Shukla, A.; Harper, M.; Pedersen, E.; Goman, A.; Suen, J.J.; Price, C.; Applebaum, J.; Hoyer, M.; Lin, F.R.; Reed, N.S. Hearing Loss, Loneliness, and Social Isolation: A Systematic Review. Otolaryngol.-Head Neck Surg. 2020, 162, 622–633. [Google Scholar] [CrossRef]
- Cosh, S.; Helmer, C.; Delcourt, C.; Robins, T.G.; Tully, P.J. Depression in elderly patients with hearing loss: Current perspectives. Clin. Interv. Aging 2019, 14, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Nordvik, O.; Laugen Heggdal, P.O.; Brannstrom, J.; Vassbotn, F.; Aarstad, A.K.; Aarstad, H.J. Generic quality of life in persons with hearing loss: A systematic literature review. BMC Ear Nose Throat Disord. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Age-related hearing loss and cognitive decline: MRI and cellular evidence. Ann. N. Y. Acad. Sci. 2021, 1500, 17–33. [Google Scholar] [CrossRef]
- Feng, X.; Li, W.; Cheng, M.; Qiu, W.; Liang, R.; Li, M.; Chen, W.; Wang, D. Association of hearing loss with total and cause-specific mortality in US adults. Environ. Sci. Pollut. Res. Int. 2022, 29, 5032–5042. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, H.; Lin, H.W.; Bhattacharyya, N. Prevalence, Characteristics, and Treatment Patterns of Hearing Difficulty in the United States. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 65–70. [Google Scholar] [CrossRef]
- Hughes, C.A.; McMenamin, P.; Mehta, V.; Pillsbury, H.; Kennedy, D. Otolaryngology workforce analysis. Laryngoscope 2016, 126 (Suppl. S9), S5–S11. [Google Scholar] [CrossRef]
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 2021, 99, 242–242A. [Google Scholar] [CrossRef]
- GBD 2019 Hearing Loss Collaborators. Hearing loss prevalence and years lived with disability, 1990-2019: Findings from the Global Burden of Disease Study 2019. Lancet 2021, 397, 996–1009. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Davis, A.C.; Hoffman, H.J. Hearing loss grades and the International classification of functioning, disability and health. Bull. World Health Organ. 2019, 97, 725–728. [Google Scholar] [CrossRef]
- Michels, T.C.; Duffy, M.T.; Rogers, D.J. Hearing Loss in Adults: Differential Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 98–108. [Google Scholar]
- Berenbrok, L.A.; Mormer, E. Over-the-counter Hearing Aids. JAMA J. Am. Med. Assoc. 2023, 329, 1225–1226. [Google Scholar] [CrossRef] [PubMed]
- Dallos, P. The role of outer hair cells in cochlear function. Prog. Clin. Biol. Res. 1985, 176, 207–230. [Google Scholar]
- Shekdar, K.V.; Bilaniuk, L.T. Imaging of Pediatric Hearing Loss. Neuroimaging Clin. N. Am. 2019, 29, 103–115. [Google Scholar] [CrossRef]
- Wang, D.T.; Ramakrishnaiah, R.; Kanfi, A. Sensorineural Hearing Loss through the Ages. Semin. Roentgenol. 2019, 54, 207–214. [Google Scholar] [CrossRef]
- Lowe, L.H.; Vezina, L.G. Sensorineural hearing loss in children. Radiographics 1997, 17, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Newsted, D.; Rosen, E.; Cooke, B.; Beyea, M.M.; Simpson, M.T.W.; Beyea, J.A. Approach to hearing loss. Can. Fam. Physician 2020, 66, 803–809. [Google Scholar] [PubMed]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Su, S.B.; Chen, K.T. An overview of occupational noise-induced hearing loss among workers: Epidemiology, pathogenesis, and preventive measures. Environ. Health Prev. Med. 2020, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.G.; Lee, J.A.; Liu, Y.F.; Endriukaitis, L.; Isaac, J.L.; Bullington, W.M. Drug-Induced Ototoxicity: A Comprehensive Review and Reference Guide. Pharmacotherapy 2020, 40, 1265–1275. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol.-Head Neck Surg. 2019, 161 (Suppl. S1), S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Acceleration of age-related hearing loss by early noise exposure: Evidence of a misspent youth. J. Neurosci. 2006, 26, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, K.A.; Jeffers, P.W.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. J. Neurosci. 2015, 35, 7509–7520. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Bramhall, N.F.; McMillan, G.P.; Kujawa, S.G.; Konrad-Martin, D. Use of non-invasive measures to predict cochlear synapse counts. Hear. Res. 2018, 370, 113–119. [Google Scholar] [CrossRef]
- Viana, L.M.; O’Malley, J.T.; Burgess, B.J.; Jones, D.D.; Oliveira, C.A.; Santos, F.; Merchant, S.N.; Liberman, L.D.; Liberman, M.C. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res. 2015, 327, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Z.; Liberman, L.D.; Bennett, K.; de Gruttola, V.; O’Malley, J.T.; Liberman, M.C. Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience 2019, 407, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, L.G.; Hawkins, J.E., Jr. Sensory and neural degeneration with aging, as seen in microdissections of the human inner ear. Ann. Otol. Rhinol. Laryngol. 1972, 81, 179–193. [Google Scholar] [CrossRef]
- Nadol, J.B., Jr. Application of electron microscopy to human otopathology. Ultrastructural findings in neural presbycusis, Meniere’s disease and Usher’s syndrome. Acta Otolaryngol. 1988, 105, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Keithley, E.M.; Feldman, M.L. Hair cell counts in an age-graded series of rat cochleas. Hear. Res. 1982, 8, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Keithley, E.M. Pathology and mechanisms of cochlear aging. J. Neurosci. Res. 2020, 98, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Morton-Jones, R.; Vlajkovic, S.M.; Telang, R.S.; Paramananthasivam, V.; Tadros, S.F.; Wong, A.C.; Froud, K.E.; Cederholm, J.M.; Sivakumaran, Y.; et al. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc. Natl. Acad. Sci. USA 2013, 110, 7494–7499. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.R.; Munoz, D.J.; Housley, G.D. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the Guinea pig. J. Assoc. Res. Otolaryngol. 2004, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Morton-Jones, R.T.; Vlajkovic, S.M.; Thorne, P.R.; Cockayne, D.A.; Ryan, A.F.; Housley, G.D. Properties of ATP-gated ion channels assembled from P2X2 subunits in mouse cochlear Reissner’s membrane epithelial cells. Purinergic Signal. 2015, 11, 551–560. [Google Scholar] [CrossRef]
- Yamashita, D.; Minami, S.B.; Kanzaki, S.; Ogawa, K.; Miller, J.M. Bcl-2 genes regulate noise-induced hearing loss. J. Neurosci. Res. 2008, 86, 920–928. [Google Scholar] [CrossRef]
- Yamamoto, H.; Omelchenko, I.; Shi, X.; Nuttall, A.L. The influence of NF-kappaB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J. Neurosci. Res. 2009, 87, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef]
- Tornabene, S.V.; Sato, K.; Pham, L.; Billings, P.; Keithley, E.M. Immune cell recruitment following acoustic trauma. Hear. Res. 2006, 222, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Infante, E.B.; Channer, G.A.; Telischi, F.F.; Gupta, C.; Dinh, J.T.; Vu, L.; Eshraghi, A.A.; Van De Water, T.R. Mannitol protects hair cells against tumor necrosis factor alpha-induced loss. Otol. Neurotol. 2012, 33, 1656–1663. [Google Scholar] [CrossRef]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.P.; Henderson, D.; Hu, B.H.; Nicotera, T.M. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear. Res. 2004, 196, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.W.; Chen, J.; Sha, S.H. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014, 5, e1262. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Vethanayagam, R.R.; Yang, W.; Dong, Y.; Hu, B.H. Toll-like receptor 4 modulates the cochlear immune response to acoustic injury. Cell Death Dis. 2016, 7, e2245. [Google Scholar] [CrossRef]
- Stennicke, H.R.; Salvesen, G.S. Caspases—Controlling intracellular signals by protease zymogen activation. Biochim. Biophys. Acta 2000, 1477, 299–306. [Google Scholar] [CrossRef]
- Kirsch, D.G.; Doseff, A.; Chau, B.N.; Lim, D.S.; de Souza-Pinto, N.C.; Hansford, R.; Kastan, M.B.; Lazebnik, Y.A.; Hardwick, J.M. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 1999, 274, 21155–21161. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Pena-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol. Cell 2022, 82, 933–949.E9. [Google Scholar] [CrossRef] [PubMed]
- Borner, C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003, 39, 615–647. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Jockel, J.; Wei, M.C.; Korsmeyer, S.J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998, 17, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P.; Savoca, M.; Castaneda, M.P.; Park, M.S.; Esteban-Cruciani, N.; Kalinec, G.; Kalinec, F. Cisplatin-induced apoptosis in auditory cells: Role of death receptor and mitochondrial pathways. Hear. Res. 2002, 174, 45–54. [Google Scholar] [CrossRef]
- Nicotera, T.M.; Hu, B.H.; Henderson, D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 466–477. [Google Scholar] [CrossRef]
- Wang, J.; Ladrech, S.; Pujol, R.; Brabet, P.; Van De Water, T.R.; Puel, J.L. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004, 64, 9217–9224. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Ding, D.; Salvi, R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience 2003, 120, 191–205. [Google Scholar] [CrossRef]
- Cheng, A.G.; Cunningham, L.L.; Rubel, E.W. Hair cell death in the avian basilar papilla: Characterization of the in vitro model and caspase activation. J. Assoc. Res. Otolaryngol. 2003, 4, 91–105. [Google Scholar] [CrossRef]
- Mangiardi, D.A.; McLaughlin-Williamson, K.; May, K.E.; Messana, E.P.; Mountain, D.C.; Cotanche, D.A. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J. Comp. Neurol. 2004, 475, 1–18. [Google Scholar] [CrossRef]
- Hu, B.H.; Henderson, D.; Nicotera, T.M. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear. Res. 2002, 166, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.A.; Oshima, T.; Suzuki, M.; Kawase, T.; Takasaka, T.; Ikeda, K. The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope 2001, 111, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.A.; Ikeda, K.; Oshima, T.; Suzuki, M.; Kawase, T.; Kikuchi, T.; Takasaka, T. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear. Res. 2000, 141, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Bottger, E.C.; Schacht, J. The mitochondrion: A perpetrator of acquired hearing loss. Hear. Res. 2013, 303, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bared, A.; Ouyang, X.; Angeli, S.; Du, L.L.; Hoang, K.; Yan, D.; Liu, X.Z. Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol.-Head Neck Surg. 2010, 143, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Tamer, L.; Dogruer, Z.N.; Yildirim, H.; Vayisoglu, Y.; Camdeviren, H. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope 2005, 115, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- Van Eyken, E.; Van Camp, G.; Fransen, E.; Topsakal, V.; Hendrickx, J.J.; Demeester, K.; Van de Heyning, P.; Maki-Torkko, E.; Hannula, S.; Sorri, M.; et al. Contribution of the N-acetyltransferase 2 polymorphism NAT2*6A to age-related hearing impairment. J. Med. Genet. 2007, 44, 570–578. [Google Scholar] [CrossRef]
- Nolan, L.S.; Cadge, B.A.; Gomez-Dorado, M.; Dawson, S.J. A functional and genetic analysis of SOD2 promoter variants and their contribution to age-related hearing loss. Mech. Ageing Dev. 2013, 134, 298–306. [Google Scholar] [CrossRef]
- Jiang, H.; Talaska, A.E.; Schacht, J.; Sha, S.H. Oxidative imbalance in the aging inner ear. Neurobiol. Aging 2007, 28, 1605–1612. [Google Scholar] [CrossRef]
- Menardo, J.; Tang, Y.; Ladrech, S.; Lenoir, M.; Casas, F.; Michel, C.; Bourien, J.; Ruel, J.; Rebillard, G.; Maurice, T.; et al. Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid. Redox Signal. 2012, 16, 263–274. [Google Scholar] [CrossRef]
- Benkafadar, N.; Francois, F.; Affortit, C.; Casas, F.; Ceccato, J.C.; Menardo, J.; Venail, F.; Malfroy-Camine, B.; Puel, J.L.; Wang, J. ROS-Induced Activation of DNA Damage Responses Drives Senescence-Like State in Postmitotic Cochlear Cells: Implication for Hearing Preservation. Mol. Neurobiol. 2019, 56, 5950–5969. [Google Scholar] [CrossRef]
- Kamogashira, T.; Hayashi, K.; Fujimoto, C.; Iwasaki, S.; Yamasoba, T. Functionally and morphologically damaged mitochondria observed in auditory cells under senescence-inducing stress. NPJ Aging Mech. Dis. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999, 4, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Taylor, R.; Forge, A.; Schacht, J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Hsu, L.F.; Jou, S.B.; Reiter, R.J.; Yang, C.M.; Chiao, C.C.; Lin, Y.F.; Chen, C.C. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. J. Pineal Res. 2010, 48, 20–38. [Google Scholar] [CrossRef]
- Petersen, A.; Castilho, R.F.; Hansson, O.; Wieloch, T.; Brundin, P. Oxidative stress, mitochondrial permeability transition and activation of caspases in calcium ionophore A23187-induced death of cultured striatal neurons. Brain Res. 2000, 857, 20–29. [Google Scholar] [CrossRef]
- Fridberger, A.; Flock, A.; Ulfendahl, M.; Flock, B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc. Natl. Acad. Sci. USA 1998, 95, 7127–7132. [Google Scholar] [CrossRef]
- Ohinata, Y.; Miller, J.M.; Altschuler, R.A.; Schacht, J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000, 878, 163–173. [Google Scholar] [CrossRef]
- Gold, M.; Rapin, I. Non-Mendelian mitochondrial inheritance as a cause of progressive genetic sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 1994, 30, 91–104. [Google Scholar] [CrossRef]
- Ballinger, S.W.; Shoffner, J.M.; Hedaya, E.V.; Trounce, I.; Polak, M.A.; Koontz, D.A.; Wallace, D.C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat. Genet. 1992, 1, 11–15. [Google Scholar] [CrossRef] [PubMed]
- van den Ouweland, J.M.; Lemkes, H.H.; Ruitenbeek, W.; Sandkuijl, L.A.; de Vijlder, M.F.; Struyvenberg, P.A.; van de Kamp, J.J.; Maassen, J.A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat. Genet. 1992, 1, 368–371. [Google Scholar] [CrossRef]
- Marshak, T.; Steiner, M.; Kaminer, M.; Levy, L.; Shupak, A. Prevention of Cisplatin-Induced Hearing Loss by Intratympanic Dexamethasone: A Randomized Controlled Study. Otolaryngol.-Head Neck Surg. 2014, 150, 983–990. [Google Scholar] [CrossRef]
- Koltsidopoulos, P.; Bibas, A.; Sismanis, A.; Tzonou, A.; Seggas, I. Intratympanic and systemic steroids for sudden hearing loss. Otol. Neurotol. 2013, 34, 771–776. [Google Scholar] [CrossRef]
- Molnar, A.; Maihoub, S.; Tamas, L.; Szirmai, A. Intratympanically administered steroid for progressive sensorineural hearing loss in Meniere’s disease. Acta Otolaryngol. 2019, 139, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.C.; Rehemtulla, A.; Sunkara, P.; Hamstra, D.; Buhnerkempe, M.; Ross, B. Oral D-methionine protects against cisplatin-induced hearing loss in humans: Phase 2 randomized clinical trial in India. Int. J. Audiol. 2022, 61, 621–631. [Google Scholar] [CrossRef]
- Ge, Z.; Ma, S.; Jia, X.; Zhang, L.; Song, L. Study of protective effects on noise-induced hearing impairment by D-methionine tablets pre-loading. Lin. Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2014, 28, 1232–1234. [Google Scholar]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Kil, J.; Harruff, E.E.; Longenecker, R.J. Development of ebselen for the treatment of sensorineural hearing loss and tinnitus. Hear. Res. 2022, 413, 108209. [Google Scholar] [CrossRef] [PubMed]
- McLean, W.J.; Hinton, A.S.; Herby, J.T.J.; Salt, A.N.; Hartsock, J.J.; Wilson, S.; Lucchino, D.L.; Lenarz, T.; Warnecke, A.; Prenzler, N.; et al. Improved Speech Intelligibility in Subjects With Stable Sensorineural Hearing Loss Following Intratympanic Dosing of FX-322 in a Phase 1b Study. Otol. Neurotol. 2021, 42, e849–e857. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.J.; Joshi, A.; Bradoo, R.; Prajapati, M.; Shah, K. Effects of Insulin-Like Growth Factor (IGF-1) in Patients with Sensorineural Hearing Loss. J. Int. Adv. Otol. 2021, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zuur, C.L.; Simis, Y.J.; Lansdaal, P.E.; Hart, A.A.; Schornagel, J.H.; Dreschler, W.A.; Rasch, C.R.; Balm, A.J. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J. Clin. Oncol. 2007, 25, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Chen, L.; Krailo, M.D.; Knight, K.; Villaluna, D.; Bliss, B.; Pollock, B.H.; Ramdas, J.; Lange, B.; Van Hoff, D.; et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 63–74. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; O’Leary, S.J.; Hart, C.D.; Diwakarla, C.S.; Seetharamu, N.; Lee, J.; Raines, S.; Quigley, T.; Wolff, H.M.; Lapuerta, P.; et al. A phase 1 clinical trial of DB-020 intratympanic injections administered prior to high dose cisplatin chemotherapy to reduce ototoxicity. J. Clin. Oncol. 2023, 41 (Suppl. S16), 6043. [Google Scholar] [CrossRef]
- Campbell, K.; Cosenza, N.; Meech, R.; Buhnerkempe, M.; Qin, J.; Rybak, L.; Fox, D. Preloaded D-methionine protects from steady state and impulse noise-induced hearing loss and induces long-term cochlear and endogenous antioxidant effects. PLoS ONE 2021, 16, e0261049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Y.; Chen, X.; Zhang, P.; Su, D.; Wang, L.; Yang, F.; Yang, J. Effects of D-methionine in mice with noise-induced hearing loss mice. J. Int. Med. Res. 2019, 47, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Rewerska, A.; Pawelczyk, M.; Rajkowska, E.; Politanski, P.; Sliwinska-Kowalska, M. Evaluating D-methionine dose to attenuate oxidative stress-mediated hearing loss following overexposure to noise. Eur. Arch. Otorhinolaryngol. 2013, 270, 1513–1520. [Google Scholar] [CrossRef]
- Samson, J.; Wiktorek-Smagur, A.; Politanski, P.; Rajkowska, E.; Pawlaczyk-Luszczynska, M.; Dudarewicz, A.; Sha, S.H.; Schacht, J.; Sliwinska-Kowalska, M. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by D-methionine. Neuroscience 2008, 152, 146–150. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, J.; Liu, F.; Li, P.; Qi, W.; Xing, Y.; Shi, H.; Jiang, H.; Sun, H.; Yin, S.; et al. Antioxidative stress-induced damage in cochlear explants. J. Otol. 2020, 15, 36–40. [Google Scholar] [CrossRef]
- Kil, J.; Pierce, C.; Tran, H.; Gu, R.; Lynch, E.D. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 2007, 226, 44–51. [Google Scholar] [CrossRef]
- Nakashima, T.; Pyykko, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.H. Meniere’s disease. Nat. Rev. Dis. Primers 2016, 2, 16028. [Google Scholar] [CrossRef]
- Gu, R.; Longenecker, R.J.; Homan, J.; Kil, J. Ebselen attenuates tobramycin-induced ototoxicity in mice. J. Cyst. Fibros. 2021, 20, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Shteinberg, M.; Elborn, J.S. Use of inhaled tobramycin in cystic fibrosis. Adv. Ther. 2015, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harruff, E.E.; Kil, J.; Ortiz, M.G.T.; Dorgan, D.; Jain, R.; Poth, E.A.; Fifer, R.C.; Kim, Y.J.M.; Shoup, A.G.; Flume, P.A. Ototoxicity in cystic fibrosis patients receiving intravenous tobramycin for acute pulmonary exacerbation: Ototoxicity following tobramycin treatment. J. Cyst. Fibros. 2021, 20, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, R.J.; Gu, R.; Homan, J.; Kil, J. Development of Tinnitus and Hyperacusis in a Mouse Model of Tobramycin Cochleotoxicity. Front. Mol. Neurosci. 2021, 14, 715952. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Chen, F.; Wang, M.; Xiao, Y.; Wang, H.; Xu, L.; Liu, W. Glutathione Peroxidase 1 Protects Against Peroxynitrite-Induced Spiral Ganglion Neuron Damage Through Attenuating NF-κB Pathway Activation. Front. Cell. Neurosci. 2022, 16, 841731. [Google Scholar] [CrossRef]
- Polianskyte-Prause, Z.; Tolvanen, T.A.; Lindfors, S.; Kon, K.; Hautala, L.C.; Wang, H.; Wada, T.; Tsuneki, H.; Sasaoka, T.; Lehtonen, S. Ebselen enhances insulin sensitivity and decreases oxidative stress by inhibiting SHIP2 and protects from inflammation in diabetic mice. Int. J. Biol. Sci. 2022, 18, 1852–1864. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, H.; Xu, Y.; Pei, J.; Song, S.; Chen, W.; Xu, S. Ebselen ameliorates renal ischemia-reperfusion injury via enhancing autophagy in rats. Mol. Cell. Biochem. 2022, 477, 1873–1885. [Google Scholar] [CrossRef]
- Wilson, W.R.; Byl, F.M.; Laird, N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch. Otolaryngol. 1980, 106, 772–776. [Google Scholar] [CrossRef]
- Mao, H.; Chen, Y. Noise-Induced Hearing Loss: Updates on Molecular Targets and Potential Interventions. Neural Plast. 2021, 2021, 4784385. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Zhang, D.; Li, X.; Han, Y.; Li, Y.; Wang, J.; Song, Y.; Kong, L.; Jian, H.; Fan, Z.; et al. Dexamethasone protects the hearing of Meniere’s disease patients after triple semicircular canal plugging. Acta Otolaryngol. 2020, 140, 803–807. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, K.; Chen, W.; Zhang, Q.; Han, Y.; Weng, J.; Pan, H. Effect of tympanic dexamethasone injection in the treatment of different types of sudden deafness. Lin. Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 34, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Cao, X.; Wang, W. Differences in hearing recovery following intratympanic alone or intravenous dexamethasone with rescue intratympanic steroids for sudden sensorineural hearing loss: A randomised trial. Clin. Otolaryngol. 2021, 46, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M.B.; Yoo, S.Y.; Park, S.N.; Nam, E.C.; Moon, I.S.; Lee, H.K. Clinical effect of intratympanic dexamethasone injection in acute unilateral tinnitus: A prospective, placebo-controlled, multicenter study. Laryngoscope 2018, 128, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.M.; Liu, L.; Zhao, R.; Nie, M.; Yang, X.H. Analysis of related factors of coins foreign bodies crossing the esophagus in 204 cases of children. Lin. Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2018, 32, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B.; Zhou, S.; Xu, W.J. Intratympanic dexamethasone vesus post-auricular subperiosteal injection of methylprednisolone treatment for sudden hearing loss. Lin. Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017, 31, 1265–1268. [Google Scholar] [CrossRef]
- Salt, A.N.; Plontke, S.K. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear. Res. 2018, 368, 28–40. [Google Scholar] [CrossRef]
- Safaeian, R.; Hassani, V.; Asghari, A.; Mohseni, M.; Ashraf, H.; Koleini, Z.S. The effects of ondansetron versus dexamethasone on electrocardiographic markers of ventricular repolarization in children undergoing cochlear implant. Int. J. Pediatr. Otorhinolaryngol. 2020, 132, 109896. [Google Scholar] [CrossRef]
- Moreno, I.; Belinchon, A. Evaluating the Efficacy of Intratympanic Dexamethasone in Protecting Against Irreversible Hearing Loss in Patients on Cisplatin-Based Cancer Treatment: A Randomized Controlled Phase IIIB Clinical Trial. Ear Hear. 2022, 43, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.; Hinton, A.S.; Frisina, R.D.; Ding, B.; Zhu, X.; Walton, J.P. Long-term treatment with aldosterone slows the progression of age-related hearing loss. Hear. Res. 2016, 336, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Trune, D.R.; Kempton, J.B.; Kessi, M. Aldosterone (mineralocorticoid) equivalent to prednisolone (glucocorticoid) in reversing hearing loss in MRL/MpJ-Fas1pr autoimmune mice. Laryngoscope 2000, 110, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Trune, D.R.; Kempton, J.B. Low dose combination steroids control autoimmune mouse hearing loss. J. Neuroimmunol. 2010, 229, 140–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacArthur, C.J.; Kempton, J.B.; DeGagne, J.; Trune, D.R. Control of chronic otitis media and sensorineural hearing loss in C3H/HeJ mice: Glucocorticoids vs mineralocorticoids. Otolaryngol.-Head Neck Surg. 2008, 139, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Church, M.W.; Kaltenbach, J.A.; Blakley, B.W.; Burgio, D.L. The comparative effects of sodium thiosulfate, diethyldithiocarbamate, fosfomycin and WR-2721 on ameliorating cisplatin-induced ototoxicity. Hear. Res. 1995, 86, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, J.A.; Church, M.W.; Blakley, B.W.; McCaslin, D.L.; Burgio, D.L. Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol.-Head Neck Surg. 1997, 117, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Viglietta, V.; Shi, F.; Hu, Q.Y.; Ren, Y.; Keilty, J.; Wolff, H.; McCarthy, R.; Kropp, J.; Weber, P.; Soglia, J. Phase 1 study to evaluate safety, tolerability and pharmacokinetics of a novel intra-tympanic administered thiosulfate to prevent cisplatin-induced hearing loss in cancer patients. Investig. New Drugs 2020, 38, 1463–1471. [Google Scholar] [CrossRef]
- Hochman, J.; Blakley, B.W.; Wellman, M.; Blakley, L. Prevention of aminoglycoside-induced sensorineural hearing loss. J. Otolaryngol. 2006, 35, 153–156. [Google Scholar]
- Jastreboff, P.J. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci. Res. 1990, 8, 221–254. [Google Scholar] [CrossRef]
- Martines, F.; Bentivegna, D.; Martines, E.; Sciacca, V.; Martinciglio, G. Characteristics of tinnitus with or without hearing loss: Clinical observations in Sicilian tinnitus patients. Auris Nasus Larynx 2010, 37, 685–693. [Google Scholar] [CrossRef] [PubMed]
- van de Heyning, P.; Muehlmeier, G.; Cox, T.; Lisowska, G.; Maier, H.; Morawski, K.; Meyer, T. Efficacy and safety of AM-101 in the treatment of acute inner ear tinnitus—A double-blind, randomized, placebo-controlled phase II study. Otol. Neurotol. 2014, 35, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Staecker, H.; Maxwell, K.S.; Morris, J.R.; van de Heyning, P.; Morawski, K.; Reintjes, F.; Meyer, T. Selecting appropriate dose regimens for AM-101 in the intratympanic treatment of acute inner ear tinnitus. Audiol. Neurootol. 2015, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Staecker, H.; Morelock, M.; Kramer, T.; Chrbolka, P.; Ahn, J.H.; Meyer, T. Safety of Repeated-Dose Intratympanic Injections with AM-101 in Acute Inner Ear Tinnitus. Otolaryngol.-Head Neck Surg. 2017, 157, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cao, W.; Niu, Y.; He, S.; Chai, R.; Yang, J. Autophagy Regulates the Survival of Hair Cells and Spiral Ganglion Neurons in Cases of Noise, Ototoxic Drug, and Age-Induced Sensorineural Hearing Loss. Front. Cell. Neurosci. 2021, 15, 760422. [Google Scholar] [CrossRef]
- Hinton, A.S.; Yang-Hood, A.; Schrader, A.D.; Loose, C.; Ohlemiller, K.K.; McLean, W.J. Approaches to Treat Sensorineural Hearing Loss by Hair-Cell Regeneration: The Current State of Therapeutic Developments and Their Potential Impact on Audiological Clinical Practice. J. Am. Acad. Audiol. 2021, 32, 661–669. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef]
- Dias, M.A.; Sampaio, A.L.; Venosa, A.R.; Meneses Ede, A.; Oliveira, C.A. The chemopreventive effect of Ginkgo biloba extract 761 against cisplatin ototoxicity: A pilot study. Int. Tinnitus J. 2015, 19, 12–19. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, R.M.; Chaturvedi, V.N. Role of ginkgo biloba extract in acquired sensorineural hearing loss. Indian. J. Otolaryngol. Head Neck Surg. 2000, 52, 212–219. [Google Scholar] [CrossRef]
- Tan, J.; Peng, H. Clinical analysis of Ginkgo biloba injection combined with traditional therapy in treatment of explosive deafness. Chin. J. Ind. Hyg. Occup. Dis. 2015, 33, 279–281. [Google Scholar]
- Esen, E.; Ozdogan, F.; Gurgen, S.G.; Ozel, H.E.; Baser, S.; Genc, S.; Selcuk, A. Ginkgo biloba and Lycopene are Effective on Cisplatin Induced Ototoxicity? J. Int. Adv. Otol. 2018, 14, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Cakil, B.; Basar, F.S.; Atmaca, S.; Cengel, S.K.; Tekat, A.; Tanyeri, Y. The protective effect of Ginkgo biloba extract against experimental cisplatin ototoxicity: Animal research using distortion product otoacoustic emissions. J. Laryngol. Otol. 2012, 126, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Xu, O.; Lu, H.; Li, P.Q.; Zhang, X.; Lu, Z. Effect of combination of Ginkgo leaf extract and deferoxamine in preventing and treating ototoxicity of cisplatin. Chin. J. Integr. Tradit. West. Med. 2004, 24, 915–918. [Google Scholar]
- Finkler, A.D.; Silveira, A.F.; Munaro, G.; Zanrosso, C.D. Otoprotection in guinea pigs exposed to pesticides and ginkgo biloba. Braz. J. Otorhinolaryngol. 2012, 78, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Cho, Y.B.; Kim, J.S.; Cho, S.W.; Yang, H.C.; Jung, K.H.; Kim, J.Y.; Choi, C.H.; Lim, Y.; Park, H.; et al. Effect of Ginkgo biloba extract on endotoxin-induced labyrinthitis. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Krauss, P.; Tziridis, K.; Buerbank, S.; Schilling, A.; Schulze, H. Therapeutic Value of Ginkgo biloba Extract EGb 761(R) in an Animal Model (Meriones unguiculatus) for Noise Trauma Induced Hearing Loss and Tinnitus. PLoS ONE 2016, 11, e0157574. [Google Scholar] [CrossRef]
- Tziridis, K.; Korn, S.; Ahlf, S.; Schulze, H. Protective effects of Ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development. Neural Plast. 2014, 2014, 427298. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Back, S.A.; Kim, H.L.; Kim, D.K.; Yeo, S.W.; Park, S.N. Renexin as a rescue regimen for noise-induced hearing loss. Noise Health 2014, 16, 257–264. [Google Scholar] [CrossRef]
- Nevado, J.; Sanz, R.; Sanchez-Rodriguez, C.; Garcia-Berrocal, J.R.; Martin-Sanz, E.; Gonzalez-Garcia, J.A.; Esteban-Sanchez, J.; Ramirez-Camacho, R. Ginkgo biloba extract (EGb761) protects against aging-related caspase-mediated apoptosis in rat cochlea. Acta Otolaryngol. 2010, 130, 1101–1112. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z. Ginkgo Biloba Extract Enhances Differentiation and Performance of Neural Stem Cells in Mouse Cochlea. Cell. Mol. Neurobiol. 2015, 35, 861–869. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B. Ginkgo Biloba Extract Attenuates Oxidative Stress and Apoptosis in Mouse Cochlear Neural Stem Cells. Phytother. Res. 2016, 30, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hu, J.; Cheng, Y.; Wang, J.; Zhang, X.; Xu, M. Ginkgolide B protects against cisplatin-induced ototoxicity: Enhancement of Akt-Nrf2-HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother. Pharmacol. 2015, 75, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Rhee, J.; Kim, S.H.; Kim, Y.H. The Protective Effect of Egb 761 Against 3-Nitropropionic Acid-Induced Hearing Loss: The Role of Sirtuin 1. Clin. Exp. Otorhinolaryngol. 2018, 11, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, S.W.; Lee, J.B.; Lim, H.J.; Kim, Y.J.; Tian, C.; So, H.S.; Park, R.; Choung, Y.H. Gingko biloba extracts protect auditory hair cells from cisplatin-induced ototoxicity by inhibiting perturbation of gap junctional intercellular communication. Neuroscience 2013, 244, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Dogan, R.; Sjostrand, A.P.; Yenigun, A.; Karatas, E.; Kocyigit, A.; Ozturan, O. Influence of Ginkgo Biloba extract (EGb 761) on expression of IL-1 Beta, IL-6, TNF-alfa, HSP-70, HSF-1 and COX-2 after noise exposure in the rat cochlea. Auris Nasus Larynx 2018, 45, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.; Yu, C.W.; Choi, D.; Ahn, T.H.; Chang, K.; Cho, J.-M.; Kim, S.-J.; Park, C.G.; Cho, D.-K.; Kim, S.-H.; et al. Efficacy and Safety of SID142 in Patients with Peripheral Arterial Disease: A Multicenter, Randomized, Double-Blind, Active-Controlled, Parallel-Group, Phase III Clinical Trial. Clin. Ther. 2022, 44, 508–528. [Google Scholar] [CrossRef]
- Tian, C.J.; Kim, Y.J.; Kim, S.W.; Lim, H.J.; Kim, Y.S.; Choung, Y.H. A combination of cilostazol and Ginkgo biloba extract protects against cisplatin-induced Cochleo-vestibular dysfunction by inhibiting the mitochondrial apoptotic and ERK pathways. Cell Death Dis. 2013, 4, e509. [Google Scholar] [CrossRef] [PubMed]
- Lamm, K.; Arnold, W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO2 and auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear. Res. 2000, 141, 199–219. [Google Scholar] [CrossRef]
- Miman, M.C.; Ozturan, O.; Iraz, M.; Erdem, T.; Olmez, E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear. Res. 2002, 169, 121–129. [Google Scholar] [CrossRef]
- Neveux, S.; Smith, N.K.; Roche, A.; Blough, B.E.; Pathmasiri, W.; Coffin, A.B. Natural Compounds as Occult Ototoxins? Ginkgo biloba Flavonoids Moderately Damage Lateral Line Hair Cells. J. Assoc. Res. Otolaryngol. 2017, 18, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Polanski, J.F.; Cruz, O.L. Evaluation of antioxidant treatment in presbyacusis: Prospective, placebo-controlled, double-blind, randomised trial. J. Laryngol. Otol. 2013, 127, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Simsek, G.; Tas, B.M.; Muluk, N.B.; Azman, M.; Kilic, R. Comparison of the protective efficacy between intratympanic dexamethasone and resveratrol treatments against cisplatin-induced ototoxicity: An experimental study. Eur. Arch. Otorhinolaryngol. 2019, 276, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Erdem, T.; Bayindir, T.; Filiz, A.; Iraz, M.; Selimoglu, E. The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur. Arch. Otorhinolaryngol. 2012, 269, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Simsek, G.; Tokgoz, S.A.; Vuralkan, E.; Caliskan, M.; Besalti, O.; Akin, I. Protective effects of resveratrol on cisplatin-dependent inner-ear damage in rats. Eur. Arch. Otorhinolaryngol. 2013, 270, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Yumusakhuylu, A.C.; Yazici, M.; Sari, M.; Binnetoglu, A.; Kosemihal, E.; Akdas, F.; Sirvanci, S.; Yuksel, M.; Uneri, C.; Tutkun, A. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Erkan, S.O.; Tuhanioglu, B.; Gurgen, S.G.; Ozdas, T.; Tastekin, B.; Pelit, A.; Gorgulu, O. The effect of resveratrol on the histologic characteristics of the cochlea in diabetic rats. Laryngoscope 2019, 129, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Avci, D.; Erkan, M.; Sonmez, M.F.; Kokoglu, K.; Gunes, M.S.; Gundogdu, R.; Gulec, S.; Karabulut, D. A Prospective Experimental Study on the Protective Effect of Resveratrol against Amikacin-Induced Ototoxicity in Rats. J. Int. Adv. Otol. 2016, 12, 290–297. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Zhang, F.; Yang, J.; He, J. Resveratrol upregulates miR-455-5p to antagonize cisplatin ototoxicity via modulating the PTEN-PI3K-AKT axis. Biochem. Cell Biol. 2021, 99, 385–395. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, K.W.; Lee, S.M.; Kim, S.Y. Dose-Dependent Effects of Resveratrol on Cisplatin-Induced Hearing Loss. Int. J. Mol. Sci. 2020, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Olgun, Y.; Kirkim, G.; Kolatan, E.; Kiray, M.; Bagriyanik, A.; Olgun, A.; Kizmazoglu, D.C.; Ellidokuz, H.; Serbetcioglu, B.; Altun, Z.; et al. Friend or foe? Effect of oral resveratrol on cisplatin ototoxicity. Laryngoscope 2014, 124, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alcantara, F.; Murillo-Cuesta, S.; Pulido, S.; Bermudez-Munoz, J.M.; Martinez-Vega, R.; Milo, M.; Varela-Nieto, I.; Rivera, T. The expression of oxidative stress response genes is modulated by a combination of resveratrol and N-acetylcysteine to ameliorate ototoxicity in the rat cochlea. Hear. Res. 2018, 358, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Jiang, Y.H.; Li, C.C.; Chen, X.M.; Huang, L.G.; Zhang, M.; Ruan, B.; Wang, X.C. Involvement of the SIRT1/PGC-1alpha Signaling Pathway in Noise-Induced Hidden Hearing Loss. Front. Physiol. 2022, 13, 798395. [Google Scholar] [CrossRef]
- Xiong, H.; Ou, Y.; Xu, Y.; Huang, Q.; Pang, J.; Lai, L.; Zheng, Y. Resveratrol Promotes Recovery of Hearing following Intense Noise Exposure by Enhancing Cochlear SIRT1 Activity. Audiol. Neurootol. 2017, 22, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Li, I.H.; Shih, J.H.; Jhao, Y.T.; Chen, H.C.; Chiu, C.H.; Chen, C.F.; Huang, Y.S.; Shiue, C.Y.; Ma, K.H. Regulation of Noise-Induced Loss of Serotonin Transporters with Resveratrol in a Rat Model Using 4-[18F]-ADAM/Small-Animal Positron Emission Tomography. Molecules 2019, 24, 1344. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Tang, W.; Bai, V.U.; Ahmad, N.; Jiang, H.; Media, J.; Patel, N.; Rubin, C.J.; Standring, R.T. Resveratrol decreases noise-induced cyclooxygenase-2 expression in the rat cochlea. Otolaryngol.-Head Neck Surg. 2013, 148, 827–833. [Google Scholar] [CrossRef]
- Seidman, M.; Babu, S.; Tang, W.; Naem, E.; Quirk, W.S. Effects of resveratrol on acoustic trauma. Otolaryngol.-Head Neck Surg. 2003, 129, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hanci, D.; Ulusoy, S.; Acar, M.; Burukoglu, D.; Kutlu, H.M.; Bayar Muluk, N.; Cingi, C. Potential protective effect of resveratrol on acoustic trauma: Electron microscopy study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3469–3475. [Google Scholar]
- Muderris, T.; Yar Saglam, A.S.; Unsal, D.; Mulazimoglu, S.; Sevil, E.; Kayhan, H. Efficiency of resveratrol in the prevention and treatment of age-related hearing loss. Exp. Ther. Med. 2022, 23, 40. [Google Scholar] [CrossRef]
- Zhao, T.; Tian, G. Potential therapeutic role of SIRT1 in age- related hearing loss. Front. Mol. Neurosci. 2022, 15, 984292. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Pang, J.; Yang, H.; Dai, M.; Liu, Y.; Ou, Y.; Huang, Q.; Chen, S.; Zhang, Z.; Xu, Y.; et al. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: Implications for age-related hearing loss. Neurobiol. Aging 2015, 36, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Chen, S.; Lai, L.; Yang, H.; Xu, Y.; Pang, J.; Su, Z.; Lin, H.; Zheng, Y. Modulation of miR-34a/SIRT1 signaling protects cochlear hair cells against oxidative stress and delays age-related hearing loss through coordinated regulation of mitophagy and mitochondrial biogenesis. Neurobiol. Aging 2019, 79, 30–42. [Google Scholar] [CrossRef]
- Pang, J.; Xiong, H.; Ou, Y.; Yang, H.; Xu, Y.; Chen, S.; Lai, L.; Ye, Y.; Su, Z.; Lin, H.; et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol. Aging 2019, 80, 127–137. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Wang, C.; Su, Y.; Xiong, M.; Feng, X.; Chen, D.; Ke, Z.; Wen, L.; Chen, G. Curcumin-Encapsulated Chitosan-Coated Nanoformulation as an Improved Otoprotective Strategy for Ototoxic Hearing Loss. Mol. Pharm. 2022, 19, 2217–2230. [Google Scholar] [CrossRef]
- Salehi, P.; Akinpelu, O.V.; Waissbluth, S.; Peleva, E.; Meehan, B.; Rak, J.; Daniel, S.J. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol. Neurotol. 2014, 35, 1131–1139. [Google Scholar] [CrossRef]
- Haryuna, T.S.H.; Fauziah, D.; Anggraini, S.; Harahap, M.P.H.; Harahap, J. Antioxidant Effect of Curcumin on the Prevention of Oxidative Damage to the Cochlea in an Ototoxic Rat Model Based on Malondialdehyde Expression. Int. Arch. Otorhinolaryngol. 2022, 26, e119–e124. [Google Scholar] [CrossRef]
- Haryuna, T.S.H.; Amellya, D.; Munir, D.; Zubaidah, T.S.H. The Benefits of Curcuminoid to Expression Nuclear Factor Erythroid 2 Related Factor 2 (NRF2) and Signal to Noise Ratio (SNR) Value in the Noise Exposed Organ of Corti of Rattus Norvegicus. Rep. Biochem. Mol. Biol. 2021, 10, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Soyalic, H.; Gevrek, F.; Koc, S.; Avcu, M.; Metin, M.; Aladag, I. Intraperitoneal curcumin and vitamin E combination for the treatment of cisplatin-induced ototoxicity in rats. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 173–178. [Google Scholar] [CrossRef]
- Soyalic, H.; Gevrek, F.; Karaman, S. Curcumin protects against acoustic trauma in the rat cochlea. Int. J. Pediatr. Otorhinolaryngol. 2017, 99, 100–106. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yoneyama, M.; Onaka, Y.; Imaizumi, A.; Ogita, K. Preventive effect of curcumin and its highly bioavailable preparation on hearing loss induced by single or repeated exposure to noise: A comparative and mechanistic study. J. Pharmacol. Sci. 2017, 134, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Haryuna, T.S.; Riawan, W.; Nasution, A.; Ma’at, S.; Harahap, J.; Adriztina, I. Curcumin Reduces the Noise-Exposed Cochlear Fibroblasts Apoptosis. Int. Arch. Otorhinolaryngol. 2016, 20, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Konduru, A.S.; Lee, B.C.; Li, J.D. Curcumin suppresses NTHi-induced CXCL5 expression via inhibition of positive IKKbeta pathway and up-regulation of negative MKP-1 pathway. Sci. Rep. 2016, 6, 31695. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Mezzogori, D.; Rolesi, R.; Eramo, S.L.; Paludetti, G.; Troiani, D. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: The role of curcumin on pSTAT3 and Nrf-2 signalling. Br. J. Cancer 2015, 113, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Eramo, S.L.; Paciello, F.; Rolesi, R.; Podda, M.V.; Troiani, D.; Paludetti, G. Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol. Neurotol. 2014, 35, e169–e177. [Google Scholar] [CrossRef] [PubMed]
- Scarpidis, U.; Madnani, D.; Shoemaker, C.; Fletcher, C.H.; Kojima, K.; Eshraghi, A.A.; Staecker, H.; Lefebvre, P.; Malgrange, B.; Balkany, T.J.; et al. Arrest of apoptosis in auditory neurons: Implications for sensorineural preservation in cochlear implantation. Otol. Neurotol. 2003, 24, 409–417. [Google Scholar] [CrossRef]
- Xu, O.; Liu, Y.; Li, X.; Yang, Y.; Zhang, Z.; Wang, N.; Zhang, Y.; Lu, H. Protective effects of Salvia miltiorrhiza against cisplatin-induced ototoxicity in guinea pigs. Am. J. Otolaryngol. 2011, 32, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Sha, S.H.; Lesniak, W.; Schacht, J. Tanshinone (Salviae miltiorrhizae extract) preparations attenuate aminoglycoside-induced free radical formation in vitro and ototoxicity in vivo. Antimicrob. Agents Chemother. 2003, 47, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; An, Y.; Wang, A.; Gao, Q.; Yang, Y. The protective effect of Salvia miltiorrhiza on gentamicin-induced ototoxicity. Am. J. Otolaryngol. 2014, 35, 171–179. [Google Scholar] [CrossRef]
- Wang, A.M.; Tang, H.; Shen, J.; Cui, G.Y.; Cui, C. Effect of injectio Salvia Miltiorrhiza on gentamicin ototoxicity-induced activity of nitric oxide synthase in cochlear stria vascularis of guinea pig. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2001, 17, 89–92. [Google Scholar]
- Shi, L.J.; Guan, J.; Tang, H. iNOS and AChE expression on guinea pigs cochlea spiral ganglion induced by streptomycin and attenuation by Salvia miltiorrhiza injection. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2009, 25, 264–267. [Google Scholar] [PubMed]
- Shi, L.J.; Tang, H. Effect of injection salvia miltiorrhiza on the expression of inducible nitric oxide synthase in the cochlea of guinea pig damaged by streptomycin. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2005, 21, 96–99. [Google Scholar] [PubMed]
- Wang, A.M.; Tang, H.; Shen, J.; Cui, C. Effects of injectio Salvia miltiorrhiza on gentamicin-induced free radical formation in guinea pig cochlea. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2004, 20, 406–409. [Google Scholar] [PubMed]
- Chen, Y.; Gu, J.; Liu, Y.; Xu, K.; Song, J.; Wang, X.; Yu, D.; Wu, H. Epigallocatechin gallate-loaded tetrahedral DNA nanostructures as a novel inner ear drug delivery system. Nanoscale 2022, 14, 8000–8011. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Liu, G.; Zhu, G.; Wu, W.; Ge, S. (−)-Epigallocatechin-3-gallate protects cultured spiral ganglion cells from H2O2-induced oxidizing damage. Acta Otolaryngol. 2004, 124, 464–470. [Google Scholar] [CrossRef]
- Schmitt, N.C.; Rubel, E.W.; Nathanson, N.M. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. J. Neurosci. 2009, 29, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ray, A.; Rybak, L.P.; Brenner, M.J. Role of STAT1 and Oxidative Stress in Gentamicin-Induced Hair Cell Death in Organ of Corti. Otol. Neurotol. 2016, 37, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Borse, V.; Al Aameri, R.F.H.; Sheehan, K.; Sheth, S.; Kaur, T.; Mukherjea, D.; Tupal, S.; Lowy, M.; Ghosh, S.; Dhukhwa, A.; et al. Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis. 2017, 8, e2921. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Chen, F.; Li, S.; Zhang, H. (−)-Epigallocatechin-3-gallate (EGCG) prevents aminoglycosides-induced ototoxicity via anti-oxidative and anti-apoptotic pathways. Int. J. Pediatr. Otorhinolaryngol. 2021, 150, 110920. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.H.; Kim, B.S.; So, H.S.; Park, R.; Myung, N.Y.; Um, J.Y.; Hong, S.H. (−)-Epigallocatechin-3-gallate protects against NO-induced ototoxicity through the regulation of caspase-1, caspase-3, and NF-kappaB activation. PLoS ONE 2012, 7, e43967. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Dong, J.; Jia, Z.; Hao, F.; Shan, C. Effects of epigallocatechin-3-gallate on proliferation and differentiation of mouse cochlear neural stem cells: Involvement of PI3K/Akt signaling pathway. Eur. J. Pharm. Sci. 2016, 88, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.T.; Yang, J.; Su, S.Z.; Liu, W.W.; Shi, Z.G.; Wang, Q.R. Green Tea Polyphenols Protects Cochlear Hair Cells from Ototoxicity by Inhibiting Notch Signalling. Neurochem. Res. 2015, 40, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Choung, Y.H.; Kim, S.W.; Tian, C.; Min, J.Y.; Lee, H.K.; Park, S.N.; Lee, J.B.; Park, K. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope 2011, 121, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Mungan Durankaya, S.; Olgun, Y.; Aktas, S.; Eskicioglu, H.E.; Gurkan, S.; Altun, Z.; Mutlu, B.; Kolatan, E.; Dogan, E.; Yilmaz, O.; et al. Effect of Korean Red Ginseng on Noise-Induced Hearing Loss. Turk. Arch. Otorhinolaryngol. 2021, 59, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hakuba, N.; Hata, R.; Morizane, I.; Yoshida, T.; Shudou, M.; Sakanaka, M.; Gyo, K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci. Lett. 2007, 415, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Im, G.J.; Chang, J.W.; Choi, J.; Chae, S.W.; Ko, E.J.; Jung, H.H. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother. Res. 2010, 24, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwak, H.J.; Kim, D.S.; Choi, H.M.; Sim, J.E.; Kim, S.H.; Um, J.Y.; Hong, S.H. Protective mechanism of Korean Red Ginseng in cisplatin-induced ototoxicity through attenuation of nuclear factor-kappaB and caspase-1 activation. Mol. Med. Rep. 2015, 12, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.B.; Dowker-Key, P.D.; Hubbard, K.; Voy, B.H.; Whelan, J.; Hedrick, M.; Bettaieb, A. Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury. Int. J. Mol. Sci. 2023, 24, 7345. [Google Scholar] [CrossRef] [PubMed]
- Doosti, A.; Lotfi, Y.; Moossavi, A.; Bakhshi, E.; Talasaz, A.H.; Hoorzad, A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health 2014, 16, 223–227. [Google Scholar] [CrossRef]
- Xiong, M.; He, Q.; Lai, H.; Huang, W.; Wang, L.; Yang, C.; Wang, J. Radix Astragali injection enhances recovery from acute acoustic trauma. Acta Otolaryngol. 2011, 131, 1069–1073. [Google Scholar] [CrossRef]
- Xiong, M.; Lai, H.; Yang, C.; Huang, W.; Wang, J.; Fu, X.; He, Q. Comparison of the Protective Effects of Radix Astragali, alpha-Lipoic Acid, and Vitamin E on Acute Acoustic Trauma. Clin. Med. Insights Ear Nose Throat 2012, 5, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Lai, H.; He, Q.; Wang, J. Astragaloside IV attenuates impulse noise-induced trauma in guinea pig. Acta Otolaryngol. 2011, 131, 809–816. [Google Scholar] [CrossRef]
- Xiong, M.; He, Q.; Lai, H.; Wang, J. Astragaloside IV inhibits apoptotic cell death in the guinea pig cochlea exposed to impulse noise. Acta Otolaryngol. 2012, 132, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhu, Y.; Lai, H.; Fu, X.; Deng, W.; Yang, C.; He, Q.; Zheng, G. Radix astragali inhibits the down-regulation of connexin 26 in the stria vascularis of the guinea pig cochlea after acoustic trauma. Eur. Arch. Otorhinolaryngol. 2015, 272, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; He, Q.; Wang, J.; Lai, H. Astragalosides reduce cisplatin ototoxicity in guinea pigs. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mukhitdinov, A.; Olimov, N.; Olimova, S. Comparative Clinical Study of the Effectiveness of MEKRITEN in Patients with Chronic Suppurative Otitis. Turk. J. Pharm. Sci. 2018, 15, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Kokten, N.; Cam, O.H.; Kalcioglu, M.T.; Ugur, M.B.; Tekin, M.; Acar, G.O. The Effect of Garlic Derivatives (S-Allylmercaptocysteine, Diallyl Disulfide, and S-Allylcysteine) on Gentamicin Induced Ototoxicity: An Experimental Study. Clin. Exp. Otorhinolaryngol. 2016, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Balbaloglu, E.; Akinci, H. Garlic-supplemented diet attenuates gentamicin-induced ototoxicity: An experimental study. Ann. Otol. Rhinol. Laryngol. 2012, 121, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.M.; Ugur, M.B.; Karamert, R.; Aytekin, S.; Kabis, B.; Duzlu, M.; Seymen, C.; Elmas, C.; Gokdogan, C.; Unlu, S. Evaluation of Effect of Garlic Aged Extracts and Vitamin B12 on Noise-Induced Hearing Loss. Noise Health 2018, 20, 232–239. [Google Scholar] [CrossRef]
- Cai, J.; Wu, X.; Li, X.; Ma, C.; Xu, L.; Guo, X.; Li, J.; Wang, H.; Han, Y. Allicin Protects against Cisplatin-Induced Stria Vascularis Damage: Possible Relation to Inhibition of Caspase-3 and PARP-1-AIF-Mediated Apoptotic Pathways. ORL J. Otorhinolaryngol. Relat. Spec. 2019, 81, 202–214. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Song, Y.; Li, H.; Bai, X.; Liu, W.; Han, Y.; Xu, L.; Li, J.; Zhang, D.; et al. Allicin protects auditory hair cells and spiral ganglion neurons from cisplatin—Induced apoptosis. Neuropharmacology 2017, 116, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Choi, Y.; Kim, M.H.; Kang, I.C.; Lee, J.H.; Park, C.; Park, R.; Kim, H.M. Rosmarinic acid, active component of Dansam-Eum attenuates ototoxicity of cochlear hair cells through blockage of caspase-1 activity. PLoS ONE 2011, 6, e18815. [Google Scholar] [CrossRef] [PubMed]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Lee, J.E.; Do, N.Y. Protective effect of silymarin against cisplatin-induced ototoxicity. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Sun, Y.; Wang, S.; Li, G.; Tang, X.; Sun, J.; Pan, C.; Sun, J. Puerarin alleviates the ototoxicity of gentamicin by inhibiting the mitochondria-dependent apoptosis pathway. Mol. Med. Rep. 2021, 24, 851. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Xu, T.; Tian, Y.; Ma, T.; Qu, D.; Wang, Y.; Lin, Y.; Bao, D.; Yu, L.; Liu, S.; et al. Ursolic acid protects against cisplatin-induced ototoxicity by inhibiting oxidative stress and TRPV1-mediated Ca2+-signaling. Int. J. Mol. Med. 2020, 46, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Draz, E.I.; Abdin, A.A.; Sarhan, N.I.; Gabr, T.A. Neurotrophic and antioxidant effects of silymarin comparable to 4-methylcatechol in protection against gentamicin-induced ototoxicity in guinea pigs. Pharmacol. Rep. 2015, 67, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liao, Y.H.; Kou, Z.Z.; Wei, Y.Y.; Huang, J.; Chen, J.; Yanagawa, Y.; Wu, S.X.; Shi, M.; Li, Y.Q. Puerarin alleviates noise-induced hearing loss via affecting PKCgamma and GABAB receptor expression. J. Neurol. Sci. 2015, 349, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Li, T.; Wang, C.; Shi, X.; Li, Y.; Zhang, S.; Zhao, Z.; Dong, H.; Qiao, Y. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic. Biol. Med. 2018, 121, 127–135. [Google Scholar] [CrossRef]
- Long, M.; Smouha, E.E.; Qiu, D.; Li, F.; Johnson, F.; Luft, B. Flavanoid of Drynaria fortunei protects against gentamicin ototoxicity. Phytother. Res. 2004, 18, 609–614. [Google Scholar] [CrossRef]
- Yu, H.H.; Seo, S.J.; Kim, Y.H.; Lee, H.Y.; Park, R.K.; So, H.S.; Jang, S.L.; You, Y.O. Protective effect of Rehmannia glutinosa on the cisplatin-induced damage of HEI-OC1 auditory cells through scavenging free radicals. J. Ethnopharmacol. 2006, 107, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Hur, J.M.; Seo, S.J.; Moon, H.D.; Kim, H.J.; Park, R.K.; You, Y.O. Protective effect of ursolic acid from Cornus officinalis on the hydrogen peroxide-induced damage of HEI-OC1 auditory cells. Am. J. Chin. Med. 2009, 37, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Eramo, S.L.; Mancuso, C.; Troiani, D.; Paludetti, G. Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radic. Biol. Med. 2015, 85, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Eramo, S.L.M.; Di Pino, A.; Rolesi, R.; Paciello, F.; Grassi, C.; Troiani, D.; Paludetti, G. The Antioxidant Effect of Rosmarinic Acid by Different Delivery Routes in the Animal Model of Noise-Induced Hearing Loss. Otol. Neurotol. 2018, 39, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.N.; You, Y.O.; Kang, T.H. Curculigo orchioides, natural compounds for the treatment of noise-induced hearing loss in mice. Arch. Pharm. Res. 2011, 34, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Hong, B.N.; Jung, S.Y.; Lee, J.H.; So, H.S.; Park, R.; You, Y.O. Curculigo orchioides protects cisplatin-induced cell damage. Am. J. Chin. Med. 2013, 41, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Hu, L.; Wang, D. Lycium barbarum polysaccharides attenuate cisplatin-induced hair cell loss in rat cochlear organotypic cultures. Int. J. Mol. Sci. 2011, 12, 8982–8992. [Google Scholar] [CrossRef] [PubMed]
- Tavanai, E.; Mohammadkhani, G.; Farahani, S.; Jalaie, S. Protective Effects of Silymarin Against Age-Related Hearing Loss in an Aging Rat Model. Indian. J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S2), 1248–1257. [Google Scholar] [CrossRef]
- Mohammadkhani, G.; Pourbakht, A.; Khanavi, M.; Faghihzadeh, S. Protective effect of silymarin on noise-induced hearing loss in Guinea pigs. Iran. Red Crescent Med. J. 2013, 15, e8890. [Google Scholar] [CrossRef]
- Woo, H.; Kim, M.K.; Park, S.; Han, S.H.; Shin, H.C.; Kim, B.G.; Oh, S.H.; Suh, M.W.; Lee, J.H.; Park, M.K. Effect of Phlorofucofuroeckol A and Dieckol Extracted from Ecklonia cava on Noise-induced Hearing Loss in a Mouse Model. Mar. Drugs 2021, 19, 443. [Google Scholar] [CrossRef]
- Chang, M.Y.; Han, S.Y.; Shin, H.C.; Byun, J.Y.; Rah, Y.C.; Park, M.K. Protective effect of a purified polyphenolic extract from Ecklonia cava against noise-induced hearing loss: Prevention of temporary threshold shift. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Byon, S.H.; Shin, H.C.; Han, S.E.; Kim, J.Y.; Byun, J.Y.; Lee, J.D.; Park, M.K. Protective effects of the seaweed phlorotannin polyphenolic compound dieckol on gentamicin-induced damage in auditory hair cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 31–36. [Google Scholar] [CrossRef]
- Aydemir, F.; Ulku, C.H.; Elmas, C.; Seymen, C.M. Analysis of potential protective effects of caffeic acid phenethyl ester against gentamicin ototoxicity: An experimental study. Iran. J. Basic Med. Sci. 2022, 25, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, M.; Sengul, E.; Kinis, V.; Alabalik, U.; Yilmaz, B.; Topcu, I. Effects of caffeic acid phenethyl ester on cisplatin ototoxicity. B-ENT 2016, 12, 211–218. [Google Scholar]
- Bakir, S.; Ozbay, M.; Gun, R.; Yorgancilar, E.; Kinis, V.; Keles, A.; Abakay, A.; Gokalp, O.; Topcu, I. The protective role of caffeic acid phenethyl ester against streptomycin ototoxicity. Am. J. Otolaryngol. 2013, 34, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, A.; Kalcioglu, M.T.; Ozerol, E.; Iraz, M.; Gulec, M.; Akyol, O.; Ozturan, O. Caffeic acid phenethyl ester ameliorated ototoxicity induced by cisplatin in rats. J. Chemother. 2004, 16, 381–387. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, B.; Bao, J.; Mulvany, J.; Bielefeld, E.; Harrison, R.T.; Neton, S.A.; Thirumala, P.; Chen, Y.; Lei, D.; et al. Otoprotective Effects of Stephania tetrandra S. Moore Herb Isolate against Acoustic Trauma. J. Assoc. Res. Otolaryngol. 2018, 19, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, G.; Qin, C.; Chen, W.; Chen, G.; Wen, L. Structure Characterization and Otoprotective Effects of a New Endophytic Exopolysaccharide from Saffron. Molecules 2019, 24, 749. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, Z.; Naveena, K.; Lei, G.; Qiu, S.; Li, X.; Li, T.; Shi, X.; Zhuang, W.; Li, Y.; et al. ROS-Responsive Nanoparticle as a Berberine Carrier for OHC-Targeted Therapy of Noise-Induced Hearing Loss. ACS Appl. Mater. Interfaces 2021, 13, 7102–7114. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, J.I.; Lee, I.K.; Kim, U.K.; Kim, Y.R.; Lee, K.Y. Protective effect of berberine chloride against cisplatin-induced ototoxicity. Genes Genom. 2022, 44, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).