Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances
Abstract
:1. Introduction
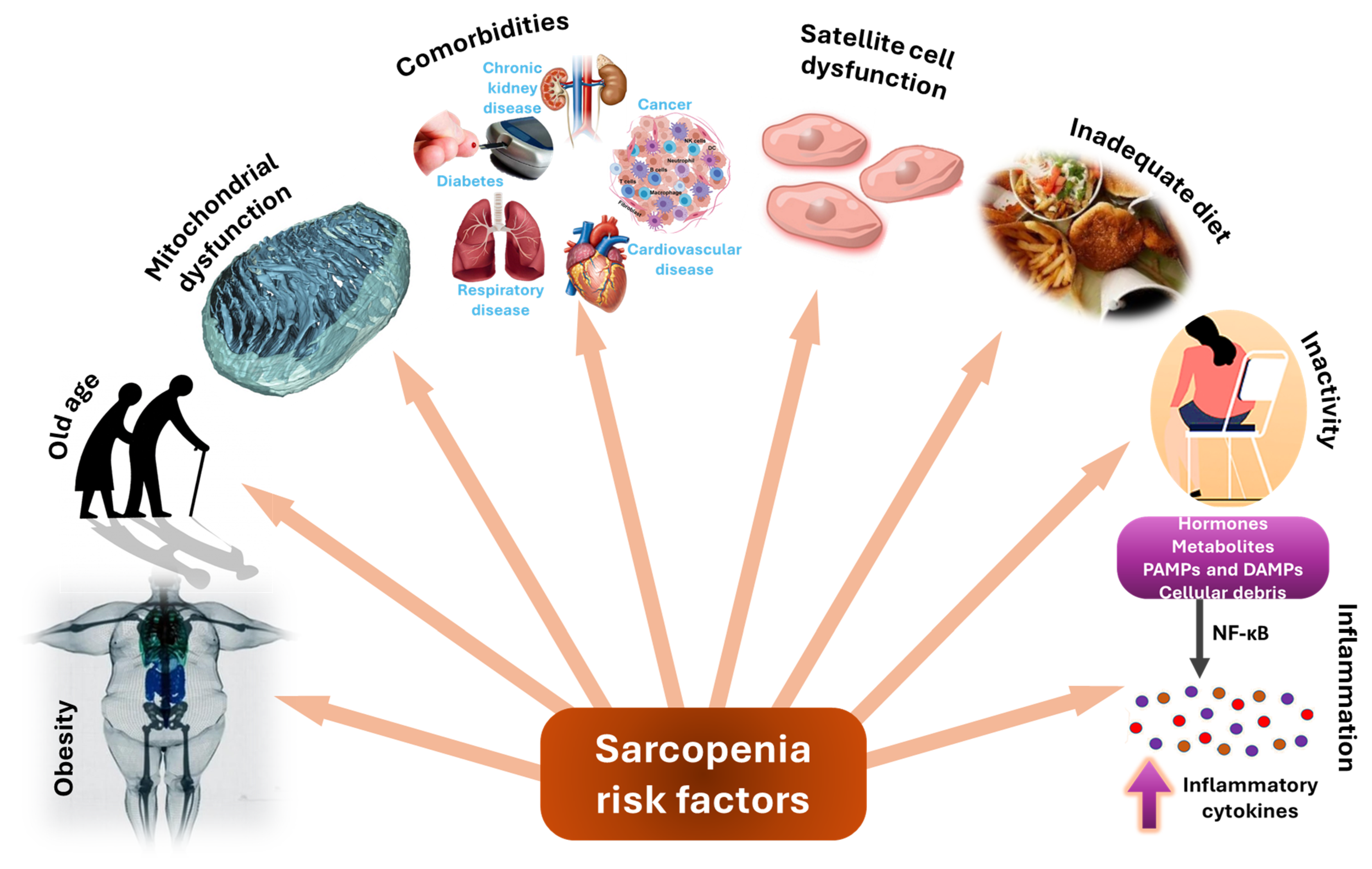
2. Sarcopenia—Etiology, Pathogenesis, and Risk Factors
3. Conventional Treatment Approaches
4. Emerging Treatment Approaches
4.1. New Therapeutic Formulations
4.2. Drug Delivery Systems
4.3. Stem Cell Therapies
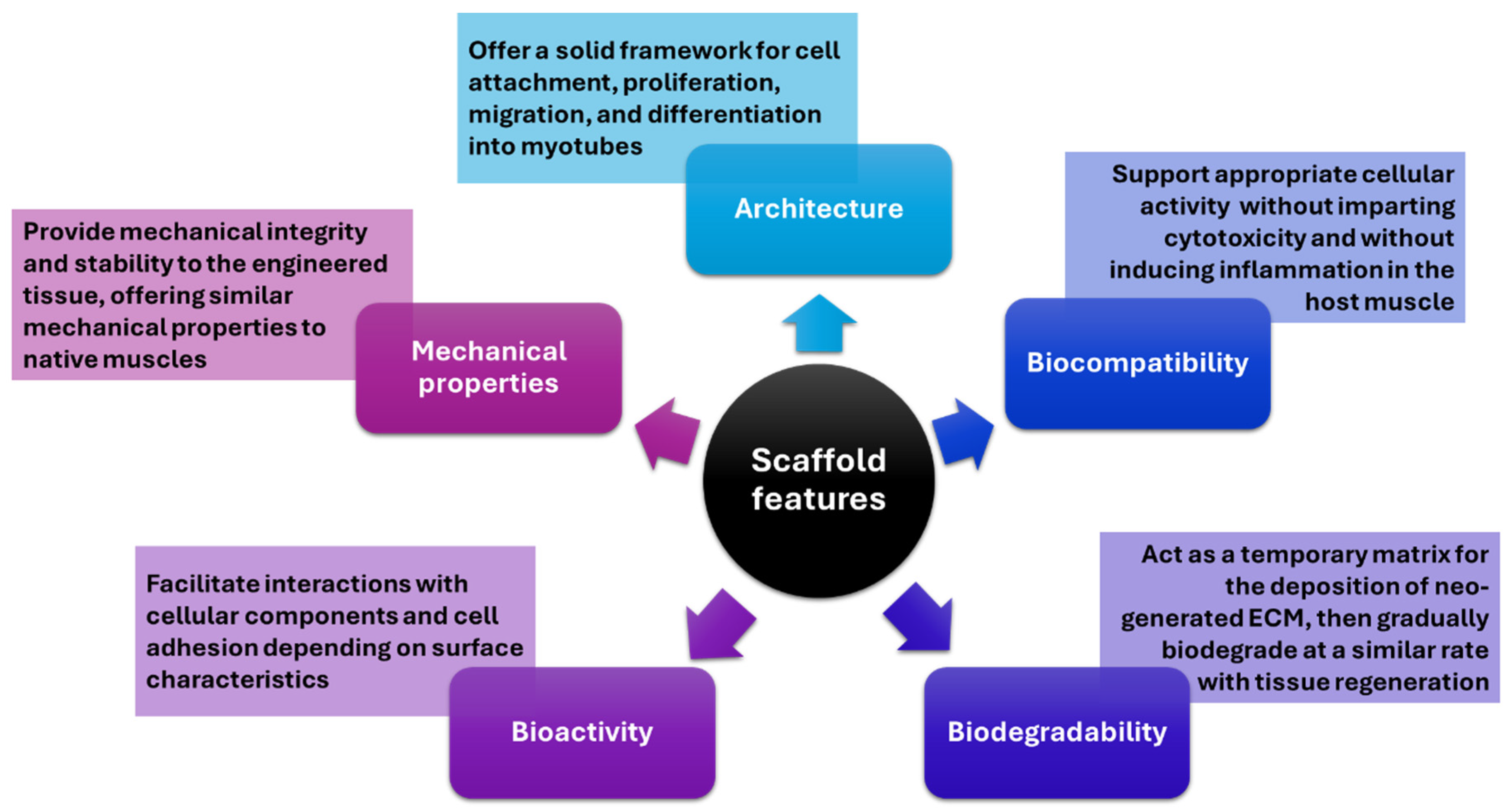
4.4. Tissue-Engineered Scaffolds
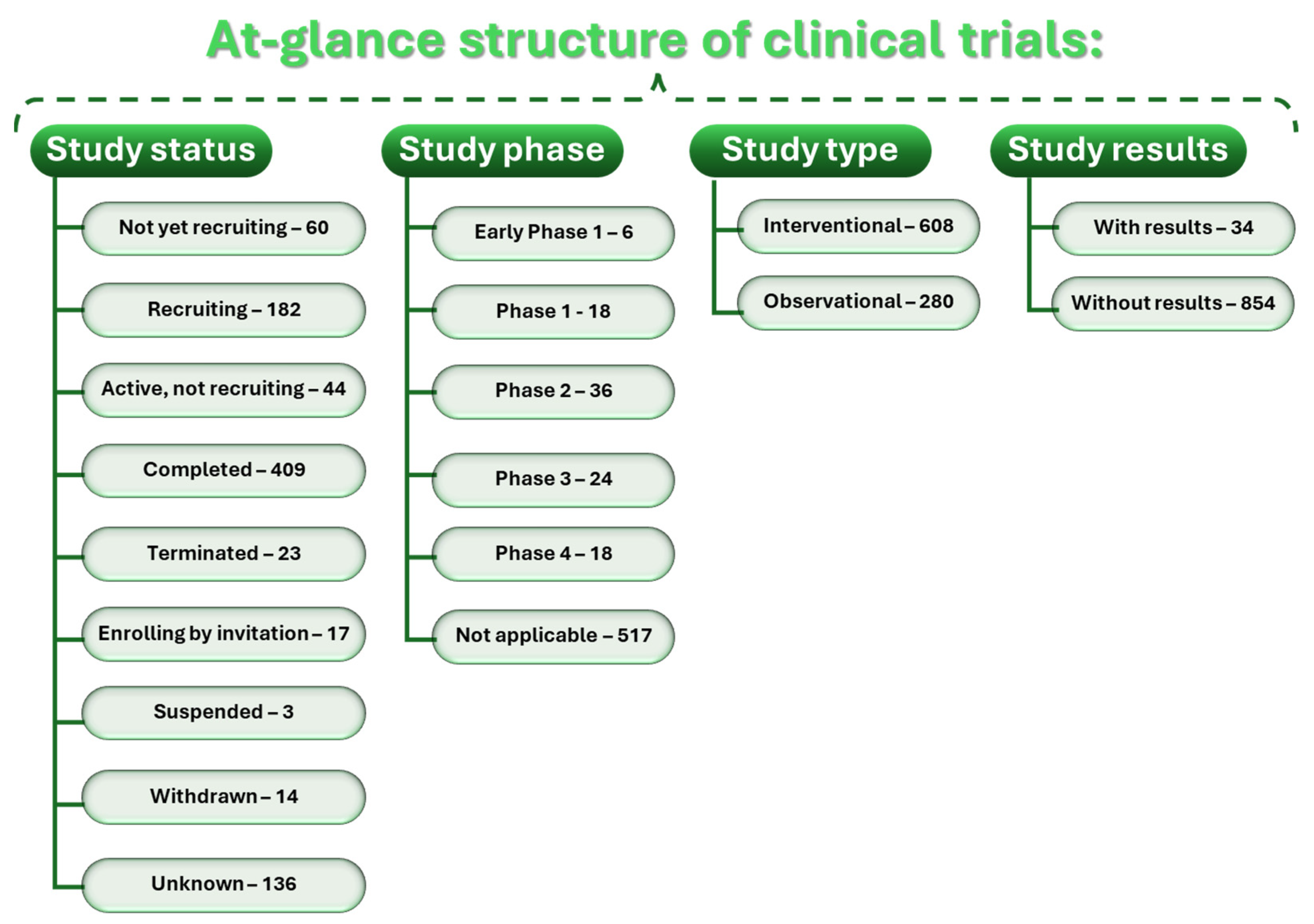
4.5. Clinical Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- del Carmen Ortuño-Costela, M.; García-López, M.; Cerrada, V.; Gallardo, M.E. iPSCs: A powerful tool for skeletal muscle tissue engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Terry, E.E.; Zhang, X.; Hoffmann, C.; Hughes, L.D.; Lewis, S.A.; Li, J.; Wallace, M.J.; Riley, L.A.; Douglas, C.M.; Gutierrez-Monreal, M.A.; et al. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. eLife 2018, 7, e34613. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, D.; Yang, Y.; Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Xiao, W.; Li, Y. The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia. Stem Cell Res. Ther. 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Becker, M.; Livshits, G. New Horizons in the Treatment of Age-Associated Obesity, Sarcopenia and Osteoporosis. Drugs Aging 2022, 39, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Muscle Delivery of Mitochondria-Targeted Drugs for the Treatment of Sarcopenia: Rationale and Perspectives. Pharmaceutics 2022, 14, 2588. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Muñoz-Cánoves, P. Regenerative decline of stem cells in sarcopenia. Mol. Asp. Med. 2016, 50, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, P.; Dou, Q.; Wang, C.; Zhang, W.; Yang, Y.; Wang, J.; Xie, X.; Zhou, J.; Zeng, Y. Falls among older adults with sarcopenia dwelling in nursing home or community: A meta-analysis. Clin. Nutr. 2020, 39, 33–39. [Google Scholar] [CrossRef]
- Zhang, F.-M.; Wu, H.-F.; Shi, H.-P.; Yu, Z.; Zhuang, C.-L. Sarcopenia and malignancies: Epidemiology, clinical classification and implications. Ageing Res. Rev. 2023, 91, 102057. [Google Scholar] [CrossRef]
- Feike, Y.; Zhijie, L.; Wei, C. Advances in research on pharmacotherapy of sarcopenia. Aging Med. 2021, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, S.; Wakabayashi, H.; Inuma, H.; Inose, T.; Shioya, M.; Aoyama, Y.; Hara, T.; Uchimura, K.; Tomita, K.; Okamoto, M. Rehabilitation nutrition and exercise therapy for sarcopenia. World J. Men’s Health 2022, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.; Anker, S.D.; von Haehling, S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: Highlights from the 12th Cachexia Conference. J. Cachexia Sarcopenia Muscle 2020, 11, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Zhao, Y.; Li, M.; Qin, Y.; Cheng, S.; Yang, Y.; Yin, P.; Zhang, L.; Tang, P. Advance in Drug Delivery for Ageing Skeletal Muscle. Front. Pharmacol. 2020, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Jang, Y.C.; García, A.J. Engineered matrices for skeletal muscle satellite cell engraftment and function. Matrix Biol. 2017, 60, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80. [Google Scholar] [CrossRef]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T.V. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015, 21, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, G.; Rouleau, A.-J.; Couture, V.; Söllrald, T.; Drouin, G.; Veillette, N.; Grandbois, M.; Grenier, G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS ONE 2015, 10, e0136217. [Google Scholar] [CrossRef]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.-K.; Lee, S.T.; Fiore, D.; Zhang, R.-H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M.V. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 2015, 21, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Jin, H.; Mikhail, H.; Pavel, V.; Yang, G.; Ji, B.; Lu, B.; Li, Y. Autophagy in sarcopenia: Possible mechanisms and novel therapies. Biomed. Pharmacother. 2023, 165, 115147. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. Expert Opin. Pharmacother. 2019, 20, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-Q.; Xie, W.-Q.; Luo, Y.-X.; Li, Y.-D.; Huang, W.-H.; Wu, Y.-X.; Li, Y.-S. High Intensity Interval Training: A Potential Method for Treating Sarcopenia. Clin. Interv. Aging 2023, 17, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Health consequences of sarcopenic obesity: A narrative review. Front. Endocrinol. 2020, 11, 530178. [Google Scholar] [CrossRef] [PubMed]
- Hajji, H.; Tabti, K.; En-nahli, F.; Bouamrane, S.; Lakhlifi, T.; Ajana, M.A.; Bouachrine, M. In silico investigation on the beneficial effects of medicinal plants on diabetes and obesity: Molecular docking, molecular dynamic simulations, and ADMET studies. Biointerface Res. Appl. Chem. 2021, 11, 6933–6949. [Google Scholar]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic Obesity: Prevalence and Association with Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef]
- Cauley, J.A. An Overview of Sarcopenic Obesity. J. Clin. Densitom. 2015, 18, 499–505. [Google Scholar] [CrossRef]
- Visser, M.; van Venrooij, L.M.W.; Vulperhorst, L.; de Vos, R.; Wisselink, W.; van Leeuwen, P.A.M.; de Mol, B.A.J.M. Sarcopenic obesity is associated with adverse clinical outcome after cardiac surgery. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 511–518. [Google Scholar] [CrossRef]
- Grillot, J.; D’Engremont, C.; Parmentier, A.-L.; Lakkis, Z.; Piton, G.; Cazaux, D.; Gay, C.; De Billy, M.; Koch, S.; Borot, S.; et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin. Nutr. 2020, 39, 3024–3030. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, I.P.; Mazurak, V.C.; Prado, C.M. Clinical Implications of Sarcopenic Obesity in Cancer. Curr. Oncol. Rep. 2016, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.J.; Mozer, M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr. Clin. Pract. 2017, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Thomas William, H.; Siddhartha, M.O.; Harnish, P.; Trevor, R.S. Getting to grips with sarcopenia: Recent advances and practical management for the gastroenterologist. Frontline Gastroenterol. 2021, 12, 53. [Google Scholar] [CrossRef]
- Chang, K.-V.; Hsu, T.-H.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Liang, C.K.; Chou, M.Y.; Liao, M.C.; Lin, Y.T.; Chen, L.K.; Lo, Y.K. Association of cognitive impairment, depressive symptoms and sarcopenia among healthy older men in the veterans retirement community in southern T aiwan: A cross-sectional study. Geriatr. Gerontol. Int. 2014, 14, 102–108. [Google Scholar] [CrossRef]
- Chen, X.; Han, P.; Yu, X.; Zhang, Y.; Song, P.; Liu, Y.; Jiang, Z.; Tao, Z.; Shen, S.; Wu, Y.; et al. Relationships between sarcopenia, depressive symptoms, and mild cognitive impairment in Chinese community-dwelling older adults. J. Affect. Disord. 2021, 286, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y.; Cheong, S.-K. Therapeutic potential of mesenchymal stem cells and their derivatives in sarcopenia. Malays. J. Pathol. 2022, 44, 429–442. [Google Scholar] [PubMed]
- Rooks, D.; Swan, T.; Goswami, B.; Filosa, L.A.; Bunte, O.; Panchaud, N.; Coleman, L.A.; Miller, R.R.; Garcia Garayoa, E.; Praestgaard, J.; et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2020836. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Bernetti, A.; Di Giacomo, G.; Viva, M.G.; Paoloni, M.; Mangone, M.; Santilli, V.; Masiero, S. Rehabilitative Good Practices in the Treatment of Sarcopenia: A Narrative Review. Am. J. Phys. Med. Rehabil. 2021, 100, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Hrisos, N.; Errington, L.; Rochester, L.; Rodgers, H.; Witham, M.; Sayer, A.A. Exercise as a treatment for sarcopenia: An umbrella review of systematic review evidence. Physiotherapy 2020, 107, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, S.H.; Kwak, H.-B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Moretti, A.; De Sire, A.; Liguori, S.; Toro, G.; Gimigliano, F. Pharmacological therapy of sarcopenia: Past, present and future. Clin. Cases Miner. Bone Metab. 2018, 15, 407–415. [Google Scholar]
- Sakuma, K.; Hamada, K.; Yamaguchi, A.; Aoi, W. Current Nutritional and Pharmacological Approaches for Attenuating Sarcopenia. Cells 2023, 12, 2422. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.-J.; Bae, G.-U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021, 44, 876–889. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.D.S. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023, 149, 155597. [Google Scholar] [CrossRef]
- Zazzara, M.B.; Penfold, R.S.; Onder, G. The Future of Drugs in Sarcopenia. In Sarcopenia: Research and Clinical Implications; Veronese, N., Beaudart, C., Sabico, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 181–208. [Google Scholar] [CrossRef]
- Cho, M.-R.; Lee, S.; Song, S.-K. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Liu, D.; Xiao, Z.; Greenbaum, J.; Shen, H.; Xiao, H.; Deng, H. Repurposing Approved Drugs for Sarcopenia Based on Transcriptomics Data in Humans. Pharmaceuticals 2023, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jung, D.-W.; Williams, D.R. Age Is Just a Number: Progress and Obstacles in the Discovery of New Candidate Drugs for Sarcopenia. Cells 2023, 12, 2608. [Google Scholar] [CrossRef]
- Bahat, G.; Ozkok, S. The Current Landscape of Pharmacotherapies for Sarcopenia. Drugs Aging 2024, 41, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Conte, E.; De Bellis, M.; Sanarica, F.; Camerino, G.M.; Pierno, S.; Cappellari, O.; Allegretti, M.; et al. BCAAs and Di-Alanine supplementation in the prevention of skeletal muscle atrophy: Preclinical evaluation in a murine model of hind limb unloading. Pharmacol. Res. 2021, 171, 105798. [Google Scholar] [CrossRef]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Cappellari, O.; Tulimiero, L.; Conte, E.; Cirmi, S.; Sanarica, F.; De Bellis, M.; Mele, A.; et al. Branched-Chain Amino Acids and Di-Alanine Supplementation in Aged Mice: A Translational Study on Sarcopenia. Nutrients 2023, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53. [Google Scholar] [CrossRef]
- Witham, M.D.; Granic, A.; Pearson, E.; Robinson, S.M.; Sayer, A.A. Repurposing Drugs for Diabetes Mellitus as Potential Pharmacological Treatments for Sarcopenia–A Narrative Review. Drugs Aging 2023, 40, 703–719. [Google Scholar] [CrossRef]
- Umbarkar, R.P.; Mittal, A.; Charde, M.S. Validated Stability-Indicating Assay UHPLC Method for Simultaneous Analysis of Saxagliptin and Metformin in Fixed-Dose Combinations. Biointerface Res. Appl. Chem. 2022, 12, 2729–2744. [Google Scholar]
- Ebert, S.M.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Murry, D.J.; Fox, D.K.; Bongers, K.S.; Lira, V.A.; Meyerholz, D.K.; Talley, J.J.; et al. Identification and small molecule inhibition of an activating transcription factor 4 (ATF4)-dependent pathway to age-related skeletal muscle weakness and atrophy. J. Biol. Chem. 2015, 290, 25497–25511. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Lee, S.Y.; Kim, C.M.; Kim, N.D.; Choe, S.; Lee, C.-H.; Shin, J.-H. Effect of loquat leaf extract on muscle strength, muscle mass, and muscle function in healthy adults: A randomized, double-blinded, and placebo-controlled trial. Evid.-Based Complement. Altern. Med. 2016, 2016, 4301621. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.S.; Seo, D.Y.; Chung, Y.M.; Oh, K.-M.; Park, J.J.; Arturo, F.; Jeong, S.-H.; Kim, N.; Han, J. Ursolic acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J. Physiol. Pharmacol. 2014, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of urolithin A supplementation on muscle endurance and mitochondrial health in older adults: A randomized clinical trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Nazemi, H. In silico interactions between curcumin derivatives and monoamine oxidase-a enzyme. Biointerface Res. Appl. Chem. 2022, 12, 3752–3761. [Google Scholar]
- Yusuf, M.; Sadiya, A.B.; Gulfishan, M. Modern perspectives of curcumin and its derivatives as promising bioactive and pharmaceutical agents. Biointerface Res. Appl. Chem. 2022, 12, 7177–7204. [Google Scholar]
- Saud Gany, S.L.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a Therapeutic Agent for Sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef] [PubMed]
- Ayubi, N.; Kusnanik, N.W.; Herawati, L.; Komaini, A.; Mutohir, T.C.; Gemaini, A.; Nugroho, A.S.; Pranoto, N.W. Effects of Curcumin on Inflammatory Response During Exercise-Induced Muscle Damage (Literature Review). Biointerface Res. Appl. Chem. 2023, 13, 146. [Google Scholar]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of prolonged dietary curcumin exposure on skeletal muscle biochemical and functional responses of aged male rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef] [PubMed]
- He, H.-J.; Wang, G.-Y.; Gao, Y.; Ling, W.-H.; Yu, Z.-W.; Jin, T.-R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N.; Juturu, V. Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar] [PubMed]
- Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water extract improves dexamethasone-induced sarcopenia by modulating the muscle-related gene and oxidative stress in mice. Antioxidants 2021, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-J.; Kim, S.; Park, J.; Kim, K.H.; Kim, K.; Jun, W. Antioxidant activities and protective effects of hot water extract from Curcuma longa L. on oxidative stress-induced C2C12 myoblasts. J. Korean Soc. Food Sci. Nutr. 2017, 46, 1408–1413. [Google Scholar]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3β activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef] [PubMed]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Mafi, F.; Biglari, S.; Afousi, A.G.; Gaeini, A.A. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Jativa, S.D.; Thapar, N.; Broyles, D.; Dikici, E.; Daftarian, P.; Jiménez, J.J.; Daunert, S.; Deo, S.K. Enhanced Delivery of Plasmid DNA to Skeletal Muscle Cells using a DLC8-Binding Peptide and ASSLNIA-Modified PAMAM Dendrimer. Mol. Pharm. 2019, 16, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ran, N.; Dong, X.; Zuo, B.; Yang, R.; Zhou, Q.; Moulton, H.M.; Seow, Y.; Yin, H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018, 10, eaat0195. [Google Scholar] [CrossRef] [PubMed]
- Poussard, S.; Decossas, M.; Le Bihan, O.; Mornet, S.; Naudin, G.; Lambert, O. Internalization and fate of silica nanoparticles in C2C12 skeletal muscle cells: Evidence of a beneficial effect on myoblast fusion. Int. J. Nanomed. 2015, 10, 1479–1492. [Google Scholar] [CrossRef]
- Ran, N.; Gao, X.; Dong, X.; Li, J.; Lin, C.; Geng, M.; Yin, H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 2020, 236, 119826. [Google Scholar] [CrossRef] [PubMed]
- Michiue, K.; Takayama, K.; Taniguchi, A.; Hayashi, Y.; Kogure, K. Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis. Pharmaceuticals 2023, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Kleppinger, A.; Annis, K.; Rathier, M.; Browner, B.; Judge, J.O.; McGee, D. Effects of Transdermal Testosterone on Bone and Muscle in Older Men with Low Bioavailable Testosterone Levels, Low Bone Mass, and Physical Frailty. J. Am. Geriatr. Soc. 2010, 58, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.W.; Otzel, D.M.; Bowser, A.; Nargi, D.; Agarwal, S.; Peterson, M.D.; Zou, B.; Borst, S.E.; Yarrow, J.F. Muscular responses to testosterone replacement vary by administration route: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xie, X.; Ling, H.; You, X.; Liang, S.; Lin, R.; Qiu, R.; Hou, H. Transdermal drug delivery via microneedles for musculoskeletal systems. J. Mater. Chem. B 2023, 11, 8327–8346. [Google Scholar] [CrossRef]
- Pin, F.; Huot, J.R.; Bonetto, A. The mitochondria-targeting agent MitoQ improves muscle atrophy, weakness and oxidative metabolism in C26 tumor-bearing mice. Front. Cell Dev. Biol. 2022, 10, 861622. [Google Scholar] [CrossRef]
- Campbell, M.D.; Duan, J.; Samuelson, A.T.; Gaffrey, M.J.; Merrihew, G.E.; Egertson, J.D.; Wang, L.; Bammler, T.K.; Moore, R.J.; White, C.C.; et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic. Biol. Med. 2019, 134, 268–281. [Google Scholar] [CrossRef]
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders. Life 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Munechika, R.; Kawamura, E.; Sakurai, Y.; Sato, Y.; Harashima, H. Mitochondrial delivery of doxorubicin using MITO-porter kills drug-resistant renal cancer cells via mitochondrial toxicity. J. Pharm. Sci. 2017, 106, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xiang, H.-J.; Wang, Y.; Zhang, Q.-L.; An, L.; Yang, S.-P.; Ma, Y.; Wang, Y.; Liu, J.-G. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem. Commun. 2017, 53, 3253–3256. [Google Scholar] [CrossRef] [PubMed]
- Kianamiri, S.; Dinari, A.; Sadeghizadeh, M.; Rezaei, M.; Daraei, B.; Bahsoun, N.E.-H.; Nomani, A. Mitochondria-targeted polyamidoamine dendrimer–curcumin construct for hepatocellular cancer treatment. Mol. Pharm. 2020, 17, 4483–4498. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.-H.; Zhang, L.; Yao, H.-J.; Zhang, Y.; Li, R.-J.; Ju, R.-J.; Wang, X.-X.; Zhou, J.; Li, N.; et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials 2012, 33, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Dhar, S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. USA 2012, 109, 16288–16293. [Google Scholar] [CrossRef]
- Van Giau, V.; An, S.S.A.; Hulme, J.P. Mitochondrial therapeutic interventions in Alzheimer’s disease. J. Neurol. Sci. 2018, 395, 62–70. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Pradhan, M.; Patel, R.J.; Singhvi, G.; Ajazuddin; Alexander, A. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed. Pharmacother. 2021, 141, 111919. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.S.; Flemming, N.B.; Gallo, L.A.; Fotheringham, A.K.; McCarthy, D.A.; Zhuang, A.; Tang, P.H.; Borg, D.J.; Shaw, H.; Harvie, B. Targeted mitochondrial therapy using MitoQ shows equivalent renoprotection to angiotensin converting enzyme inhibition but no combined synergy in diabetes. Sci. Rep. 2017, 7, 15190. [Google Scholar] [CrossRef]
- Karunanidhi, P.; Verma, N.; Kumar, D.N.; Agrawal, A.K.; Singh, S. Triphenylphosphonium functionalized Ficus religiosa L. extract loaded nanoparticles improve the mitochondrial function in oxidative stress induced diabetes. AAPS PharmSciTech 2021, 22, 158. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, D.-Z.; Zhang, C.-X.; Cui, H.; Liu, M.; Mei, Q.-B.; Lu, Z.-F.; Zhou, S.-Y. Mitochondria-targeted antioxidant delivery for precise treatment of myocardial ischemia–reperfusion injury through a multistage continuous targeted strategy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Matoba, T.; Ishikita, A.; Nagaoka, K.; Nakano, K.; Koga, J.i.; Tsutsui, H.; Egashira, K. Nanoparticle-Mediated Simultaneous Targeting of Mitochondrial Injury and Inflammation Attenuates Myocardial Ischemia-Reperfusion Injury. J. Am. Heart Assoc. 2021, 10, e019521. [Google Scholar] [CrossRef] [PubMed]
- Ishikita, A.; Matoba, T.; Ikeda, G.; Koga, J.i.; Mao, Y.; Nakano, K.; Takeuchi, O.; Sadoshima, J.; Egashira, K. Nanoparticle-mediated delivery of mitochondrial division inhibitor 1 to the myocardium protects the heart from ischemia-reperfusion injury through inhibition of mitochondria outer membrane permeabilization: A new therapeutic modality for acute myocardial infarction. J. Am. Heart Assoc. 2016, 5, e003872. [Google Scholar] [PubMed]
- Rong, S.; Wang, L.; Peng, Z.; Liao, Y.; Li, D.; Yang, X.; Nuessler, A.K.; Liu, L.; Bao, W.; Yang, W. The mechanisms and treatments for sarcopenia: Could exosomes be a perspective research strategy in the future? J. Cachexia Sarcopenia Muscle 2020, 11, 348–365. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Yoon, H.I.; Lee, K.S.; Choi, Y.C.; Yang, S.H.; Kim, I.-S.; Cho, Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control. Release 2016, 222, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.B.; Mumford, P.W.; McCarthy, J.J.; Miller, M.E.; Young, K.C.; Martin, J.S.; Beck, D.T.; Lockwood, C.M.; Roberts, M.D. Whey protein-derived exosomes increase protein synthesis and hypertrophy in C2C12 myotubes. J. Dairy Sci. 2017, 100, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Kahroba, H.; Hejazi, M.S.; Samadi, N. Exosomes: From carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell. Mol. Life Sci. 2019, 76, 1747–1758. [Google Scholar] [CrossRef]
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed remote ischemic preconditioning confersrenoprotection against septic acute kidney injury via exosomal miR-21. Theranostics 2019, 9, 405. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Pan, M.; Zhou, M.; Tang, Q.; Chen, M.; Hong, W.; Zhao, F.; Liu, K. Mitochondria Transplantation from Stem Cells for Mitigating Sarcopenia. Aging Dis. 2023, 14, 1700. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, W.; Li, H.; Jin, H.; Zhang, Y.; Li, Y. Cellular senescence in sarcopenia: Possible mechanisms and therapeutic potential. Front. Cell Dev. Biol. 2022, 9, 793088. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aty, H.E.; Zaazaa, A.M.; Mohamed, S.H.; Dayem, S.A.E.; Foda, F. Promising therapeutic efficacy of Trigonella-foenum graecum and bone marrow-derived mesenchymal stem cells on skeletal muscle atrophy in experimental rat model. Biointerface Res. Appl. Chem. 2023, 13, 133. [Google Scholar]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, B.; Zhai, J.; Wang, A.; Cao, N.; Liao, T.; Su, R.; He, L.; Li, Y.; Pei, X.; et al. Clinical-grade human umbilical cord-derived mesenchymal stem cells improved skeletal muscle dysfunction in age-associated sarcopenia mice. Cell Death Dis. 2023, 14, 321. [Google Scholar] [CrossRef]
- Sun, Y.; Duffy, R.; Lee, A.; Feinberg, A.W. Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater. 2013, 9, 7885–7894. [Google Scholar] [CrossRef]
- Zatti, S.; Zoso, A.; Serena, E.; Luni, C.; Cimetta, E.; Elvassore, N. Micropatterning topology on soft substrates affects myoblast proliferation and differentiation. Langmuir 2012, 28, 2718–2726. [Google Scholar] [CrossRef]
- Tsui, J.H.; Janebodin, K.; Ieronimakis, N.; Yama, D.M.P.; Yang, H.S.; Chavanachat, R.; Hays, A.L.; Lee, H.; Reyes, M.; Kim, D.-H. Harnessing sphingosine-1-phosphate signaling and nanotopographical cues to regulate skeletal muscle maturation and vascularization. ACS Nano 2017, 11, 11954–11968. [Google Scholar] [CrossRef] [PubMed]
- Maleiner, B.; Tomasch, J.; Heher, P.; Spadiut, O.; Rünzler, D.; Fuchs, C. The importance of biophysical and biochemical stimuli in dynamic skeletal muscle models. Front. Physiol. 2018, 9, 1130. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, N.; Shahini, A.; Asmani, M.; Vydiam, K.; Choudhury, D.; Nguyen, T.; Ikhapoh, I.; Zhao, R.; Lei, P.; Andreadis, S.T. Bioengineered skeletal muscle as a model of muscle aging and regeneration. Tissue Eng. Part A 2021, 27, 74–86. [Google Scholar] [CrossRef]
- Nambi, G. Integrated Physical Training with Protein Diet in Older Adults with Sarcopenia Symptoms. Available online: https://www.clinicaltrials.gov/study/NCT05224453 (accessed on 10 February 2024).
- Tuan, S.-H. VR-based Rehabilitation in the Treatment and Prevention of Sarcopenia of Older Residents. Available online: https://www.clinicaltrials.gov/study/NCT03809104 (accessed on 10 February 2024).
- Delmonico, M.J. Effects of a Resistance Training Program in Older Women with Sarcopenia (RESTORE-ME). Available online: https://www.clinicaltrials.gov/study/NCT02628145 (accessed on 10 February 2024).
- Novartis. Dose Range Finding Study of Bimagrumab in Sarcopenia. Available online: https://www.clinicaltrials.gov/study/NCT02333331 (accessed on 10 February 2024).
- Merck Sharp & Dohme LLC. A Study of the Safety and Efficacy of MK-0773 in Women with Sarcopenia (Loss of Muscle Mass) (MK-0773-005). Available online: https://www.clinicaltrials.gov/study/NCT00529659 (accessed on 10 February 2024).
- Novartis. A 24-Week Off-Drug Extension Study in Sarcopenic Elderly Who Completed Treatment in the 6-Month Core Study. Available online: https://www.clinicaltrials.gov/study/NCT02468674 (accessed on 10 February 2024).
- University of Arkansas. Maximizing the Dietary Pattern of Older Adults: The Effects of Protein Intake on Protein Kinetics. Available online: https://clinicaltrials.gov/study/NCT04830514 (accessed on 10 February 2024).
- Johns Hopkins University. A Study of Muscle Strength Maintenance in Older Adults. Available online: https://clinicaltrials.gov/study/NCT01989793 (accessed on 10 February 2024).
- University of Arkansas. Effect of an EAA/Whey Composition on Protein Metabolism. Available online: https://clinicaltrials.gov/study/NCT03502941 (accessed on 10 February 2024).
- Olek, R. Carnitine Supplementation and Skeletal Muscle Function. Available online: https://clinicaltrials.gov/study/NCT02692235 (accessed on 10 February 2024).
- The University of Texas Medical Branch, Galveston. Anabolic and Inflammatory Responses to Short-Term Testosterone Administration in Older Men. Available online: https://clinicaltrials.gov/study/NCT00957801 (accessed on 10 February 2024).
- University of Florida. Biological Effects of Weight Loss in Older, Obese Women (WL+E). Available online: https://clinicaltrials.gov/study/NCT01032733 (accessed on 10 February 2024).
- Dawson-Hughes, B. Effect of Potassium Bicarbonate Supplementation on Bone and Muscle in Older Adults. Available online: https://clinicaltrials.gov/study/NCT00357214 (accessed on 10 February 2024).
- University of Manitoba. Omega-3 Supplementation and Resistance Training. Available online: https://clinicaltrials.gov/study/NCT02617511 (accessed on 10 February 2024).
- University of Colorado, Denver. Acetaminophen and Impaired Musculoskeletal Adaptations to Exercise Training. Available online: https://clinicaltrials.gov/study/NCT01083901 (accessed on 10 February 2024).
- VA Office of Research and Development. Immune Function and Muscle Adaptations to Resistance Exercise in Older Adults (TDAP2). Available online: https://clinicaltrials.gov/study/NCT02261961 (accessed on 10 February 2024).
- Kestenbaum, B. Trial of Nicotinamide Riboside and Co-Enzyme Q10 in Chronic Kidney Disease (CoNR). Available online: https://clinicaltrials.gov/study/NCT03579693 (accessed on 10 February 2024).
- National Institutes of Health Clinical Center. Effects of Aromatase Inhibition versus Testosterone in Older Men with Low Testosterone: Randomized-Controlled Trial. Available online: https://clinicaltrials.gov/study/NCT00104572 (accessed on 10 February 2024).
- The University of Texas Medical Branch, Galveston. IMPACT: Inactivity Monitoring and Physical Activity Controlled Trial (IMPACT). Available online: https://clinicaltrials.gov/study/NCT01869348 (accessed on 10 February 2024).
- Espinoza, S. The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity (INOSO). Available online: https://clinicaltrials.gov/study/NCT03119610 (accessed on 10 February 2024).
- VA Office of Research and Development. 5-Alpha Reductase and Anabolic Effects of Testosterone. Available online: https://clinicaltrials.gov/study/NCT00475501 (accessed on 10 February 2024).
- Himmelfarb, J. Trial of Oral Glutamine on Mitochondrial Function in CKD. Available online: https://clinicaltrials.gov/study/NCT02838979 (accessed on 10 February 2024).
- University of Arkansas. A Physical Activity Program in End-State Liver Disease. Available online: https://clinicaltrials.gov/study/NCT02776553 (accessed on 10 February 2024).
- Nelson Joaquim Fortuna de Sousa. Study of the Long-Term Effects of Exercise on Heath Indicators in Older People. Available online: https://clinicaltrials.gov/study/NCT01874132 (accessed on 10 February 2024).
- Lee, J.L.; Zhang, C.; Westbrook, R.; Gabrawy, M.M.; Nidadavolu, L.; Yang, H.; Marx, R.; Wu, Y.; Anders, N.M.; Ma, L.; et al. Serum Concentrations of Losartan Metabolites Correlate with Improved Physical Function in a Pilot Study of Prefrail Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Church, D.D.; Azhar, G.; Schutzler, S.E.; Ferrando, A.A.; Wolfe, R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020, 17, 9. [Google Scholar] [CrossRef]
- Dennis, R.A.; Ponnappan, U.; Kodell, R.L.; Garner, K.K.; Parkes, C.M.; Bopp, M.M.; Padala, K.P.; Peterson, C.A.; Padala, P.R.; Sullivan, D.H. Immune Function and Muscle Adaptations to Resistance exercise in Older Adults: Study Protocol for a Randomized Controlled Trial of a Nutritional Supplement. Trials 2015, 16, 121. [Google Scholar] [CrossRef]
- Ahmadi, A.; Begue, G.; Valencia, A.P.; Norman, J.E.; Lidgard, B.; Bennett, B.J.; Van Doren, M.P.; Marcinek, D.J.; Fan, S.; Prince, D.K.; et al. Randomized crossover clinical trial of coenzyme Q10 and nicotinamide riboside in chronic kidney disease. JCI Insight 2023, 8, e167274. [Google Scholar] [CrossRef]
- Lewis, Z.H.; Swartz, M.C.; Martinez, E.; Lyons, E.J. Social Support Patterns of Middle-Aged and Older Adults within a Physical Activity App: Secondary Mixed Method Analysis. JMIR Aging 2019, 2, e12496. [Google Scholar] [CrossRef]
- Lyons, E.J.; Swartz, M.C.; Lewis, Z.H.; Martinez, E.; Jennings, K. Feasibility and Acceptability of a Wearable Technology Physical Activity Intervention with Telephone Counseling for Mid-Aged and Older Adults: A Randomized Controlled Pilot Trial. JMIR Mhealth Uhealth 2017, 5, e28. [Google Scholar] [CrossRef]
- Sousa, N.; Mendes, R.; Abrantes, C.; Sampaio, J.; Oliveira, J. A randomized study on lipids response to different exercise programs in overweight older men. Int. J. Sports Med. 2014, 35, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.; Mendes, R.; Silva, A.; Oliveira, J. Combined exercise is more effective than aerobic exercise in the improvement of fall risk factors: A randomized controlled trial in community-dwelling older men. Clin. Rehabil. 2017, 31, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Dawson-Hughes, B. No effect of bicarbonate treatment on insulin sensitivity and glucose control in non-diabetic older adults. Endocrine 2010, 38, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.P.; Melvin, D.; Shardell, M.; Ferrucci, L.; Chia, C.W.; Gharib, M.; Egan, J.M.; Basaria, S. Effects of Transdermal Testosterone Gel or an Aromatase Inhibitor on Prostate Volume in Older Men. J. Clin. Endocrinol. Metab. 2016, 101, 1865–1871. [Google Scholar] [CrossRef] [PubMed]


| ClinicalTrials.gov ID | Official Title | Intervention | Phase | (Estimated) Completion | Enrollment | Ref. |
|---|---|---|---|---|---|---|
| NCT05224453 | Comparative Effects of Integrated Physical Training With High Protein Diet Versus Low Protein Diet in COVID-19 Asymptomatic Older Adults With Sarcopenia Symptoms. | Other: Physical training and high protein diet Other: Physical training and low protein diet | N/A | 30 November 2021 | 76 | [126] |
| NCT03809104 | Virtual Reality-based Rehabilitation in the Treatment and Prevention of Sarcopenia of Older Residents in Caring Facilities—a Pilot Study in Rural Southern Taiwan | Other: Virtual reality-based rehabilitation programs | N/A | 8 October 2019 | 43 | [127] |
| NCT02628145 | Effects of a Resistance Training Program in Older Women With Sarcopenia | Behavioral: Resistance Training Intervention Behavioral: Active Control Group | N/A | July 2016 | 25 | [128] |
| NCT02333331 | A 28 Week, Randomized, Double-blind, Placebo-controlled, Two-part, Multi-center, Parallel Group Dose Range Finding Study to Assess the Effect of Monthly Doses of Bimagrumab 70, 210, and 700 mg on Skeletal Muscle Strength and Function in Older Adults With Sarcopenia (InvestiGAIT) | Drug: bimagrumab Other: placebo | 2 | 28 June 2018 | 217 | [129] |
| NCT00529659 | A Phase IIa Randomized, Placebo-Controlled Clinical Trial to Study the Efficacy and Safety of MK-0773 in Patients With Sarcopenia | Drug: Comparator: MK-0773 Drug: Comparator: Placebo | 2 | October 2009 | 170 | [130] |
| NCT02468674 | A 24 Week Off Drug Extension, Parallel Group, Study Assessing Durability of Effect on Skeletal Muscle Strength and Function Following a 6-month Double-blind, Placebo Controlled Study Evaluating Bimagrumab in Older Adults With Sarcopenia (InvestiGAIT Extension) | Drug: bimagrumab Drug: Placebo | 2 | 3 December 2018 | 160 | [131] |
| NCT04830514 | Maximizing the Dietary Pattern of Older Adults: the Effects of Protein Intake on Protein Kinetics | Other: Recommended Dietary Allowance of Protein Other: Habitual protein intake Other: Optimal Protein Intake | N/A | 15 March 2015 | 44 | [132] |
| NCT01989793 | A Study of Muscle Strength Maintenance in Older Adults | Drug: Losartan Drug: Placebo | 2 | October 2016 | 37 | [133] |
| NCT03502941 | Effect of an Essential Amino Acid/Protein Composition on Protein Metabolism | Dietary Supplement: 6.3 g of EAAs mixture and whey protein isolate Dietary Supplement: 12.6 g of EAAs mixture and whey protein isolate Dietary Supplement: 12.6 g of whey protein isolate | N/A | 17 July 2019 | 16 | [134] |
| NCT02692235 | Carnitine Supplementation and Skeletal Muscle Function in Aging | Dietary Supplement: carnitine Dietary Supplement: placebo | 3 | July 2017 | 28 | [135] |
| NCT00957801 | Anabolic and Inflammatory Responses to Short-Term Testosterone Administration in Older Men | Drug: Testosterone injection Drug: Testosterone gel Drug: Medrol | 4 | December 2015 | 29 | [136] |
| NCT01032733 | Biological Effects of Weight Loss Plus Exercise in Obese Older African-American Women: An Investigation of Aging-related Changes in Black and White Women | Behavioral: Lifestyle Counseling Other: Educational Control | 2 | October 2009 | 34 | [137] |
| NCT00357214 | Effect of Potassium Bicarbonate on Bone and Muscle | Dietary Supplement: Potassium Bicarbonate Dietary Supplement: Sodium Bicarbonate Dietary Supplement: Potassium Chloride Dietary Supplement: placebo (microcrystalline cellulose) | N/A | April 2008 | 171 | [138] |
| NCT02617511 | Omega-3 Fatty Acid Supplementation and Resistance Training on Inflammation and Body Composition in Older Men | Dietary Supplement: Omega-3 Supplementation Dietary Supplement: Placebo | N/A | November 2016 | 24 | [139] |
| NCT01083901 | Acetaminophen and Impaired Musculoskeletal Adaptations to Exercise Training | Behavioral: Resistance training | N/A | March 2011 | 34 | [140] |
| NCT02261961 | Immune Function and Muscle Adaptations to Resistance Exercise in Older Adults | Biological: TDAP Other: Acute Resistance Exercise Other: Resistance Exercise Training Other: Post-training Follow-up Dietary Supplement: Nutritional Supplement (Muscle Armor) Dietary Supplement: Placebo (Kool-Aid) | N/A | 30 September 2019 | 59 | [141] |
| NCT03579693 | Cross-over Randomized Controlled Trial of Coenzyme Q10 or Nicotinamide Riboside in Chronic Kidney Disease | Dietary Supplement: CoQ10 Dietary Supplement: Nicotinamide riboside Dietary Supplement: Placebo | 2 | 26 April 2021 | 26 | [142] |
| NCT00104572 | The Effects of Aromatase Inhibition and Testosterone Replacement in Sex Steroids, Pituitary Hormones, Markers of Bone Turnover, Muscle Strength, and Cognition in Older Men | Drug: Androgel (Testosterone Gel) Drug: Anastrozole (Aromatase Inhibitor) Drug: Placebo tablet Drug: Placebo gel Dietary Supplement: Calcium Cardone 500 mg with vitamin D 400 IU | 2 | January 2015 | 44 | [143] |
| NCT01869348 | IMPACT: Inactivity Monitoring and Physical Activity Controlled Trial | Behavioral: Monitor intervention | N/A | 15 April 2016 | 40 | [144] |
| NCT03119610 | The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity | Drug: Oxytocin nasal spray Drug: Placebo nasal spray | 1/2 | 17 December 2019 | 23 | [145] |
| NCT00475501 | 5-Alpha Reductase and Anabolic Effects of Testosterone | Drug: Testosterone Enanthate Drug: Finasteride Other: Placebo | 2 | October 2014 | 60 | [146] |
| NCT02838979 | Randomized Cross-over Trial of Oral L-Glutamine vs. Maltodextrin on Mitochondrial Function in Chronic Kidney Disease | Dietary Supplement: First Intervention (14 days) Other: Washout (3 weeks) Dietary Supplement: Second Intervention (14 days) | 2 | 31 January 2018 | 11 | [147] |
| NCT02776553 | A Physical Activity Program in End-stage Liver Disease: Pilot Study Assessing Changes in Physical Fitness, Sarcopenia, and the Metabolic Profile | Other: Nutritional consultation Behavioral: Physical training program Behavioral: Behavioral modification therapy | N/A | June 2020 | 20 | [148] |
| NCT01874132 | A Randomised Longitudinal Study of Exercise Prescription for Older Adults: Mode and Intensity to Induce the Highest Cardiovascular Health-related Benefits | Behavioral: Exercise training | N/A | September 2014 | 66 | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najm, A.; Niculescu, A.-G.; Grumezescu, A.M.; Beuran, M. Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances. Int. J. Mol. Sci. 2024, 25, 4300. https://doi.org/10.3390/ijms25084300
Najm A, Niculescu A-G, Grumezescu AM, Beuran M. Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances. International Journal of Molecular Sciences. 2024; 25(8):4300. https://doi.org/10.3390/ijms25084300
Chicago/Turabian StyleNajm, Alfred, Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu, and Mircea Beuran. 2024. "Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances" International Journal of Molecular Sciences 25, no. 8: 4300. https://doi.org/10.3390/ijms25084300








