MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis
Abstract
:1. Introduction
2. Results
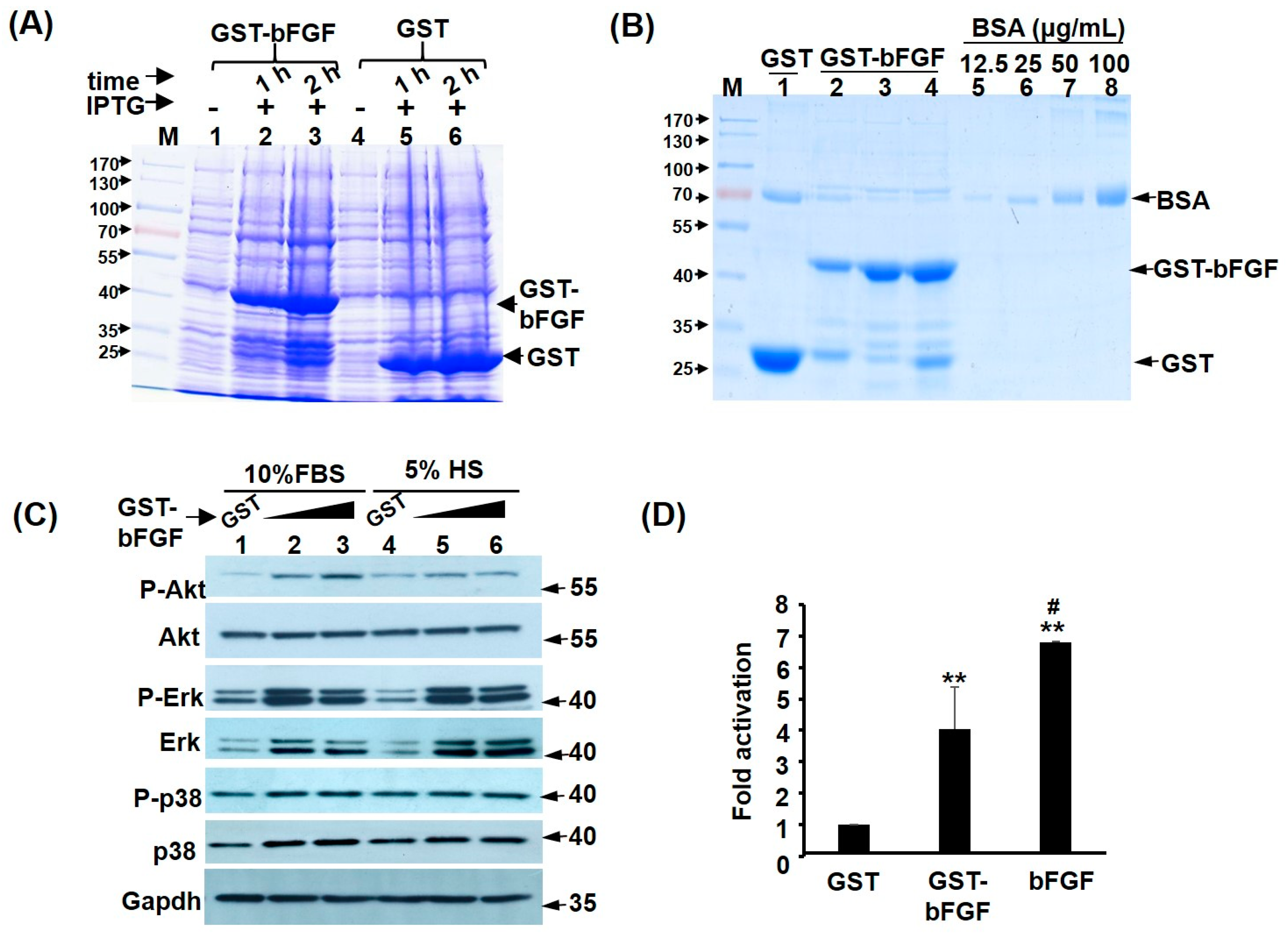
2.1. Recombinant GST-FGF Induces Akt and MAPK Signalling in Myoblasts
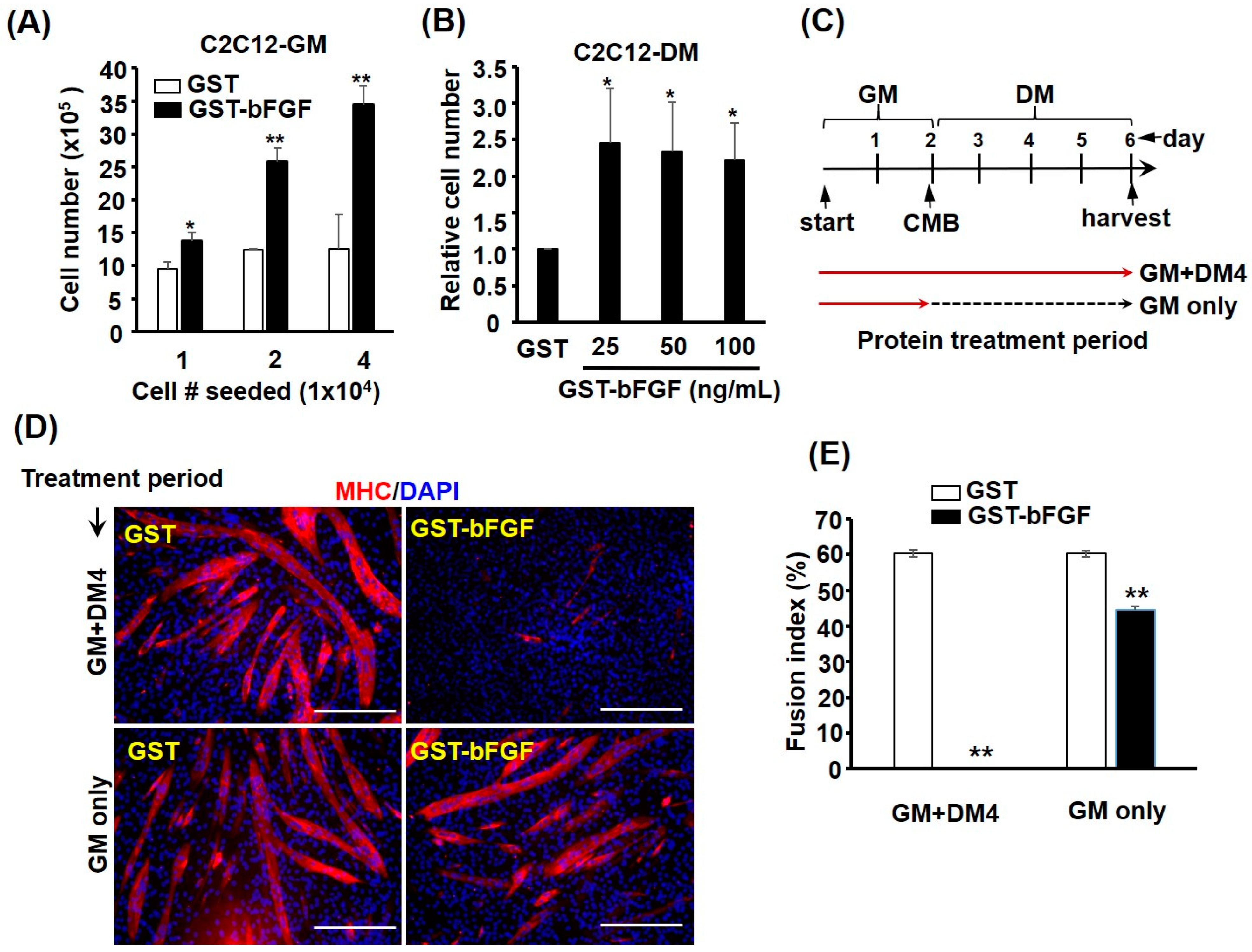
2.2. GST-bFGF Is a Strong Proliferation Inducer of Myoblasts
2.3. GST-bFGF Reversibly Repressed Myogenic Differentiation
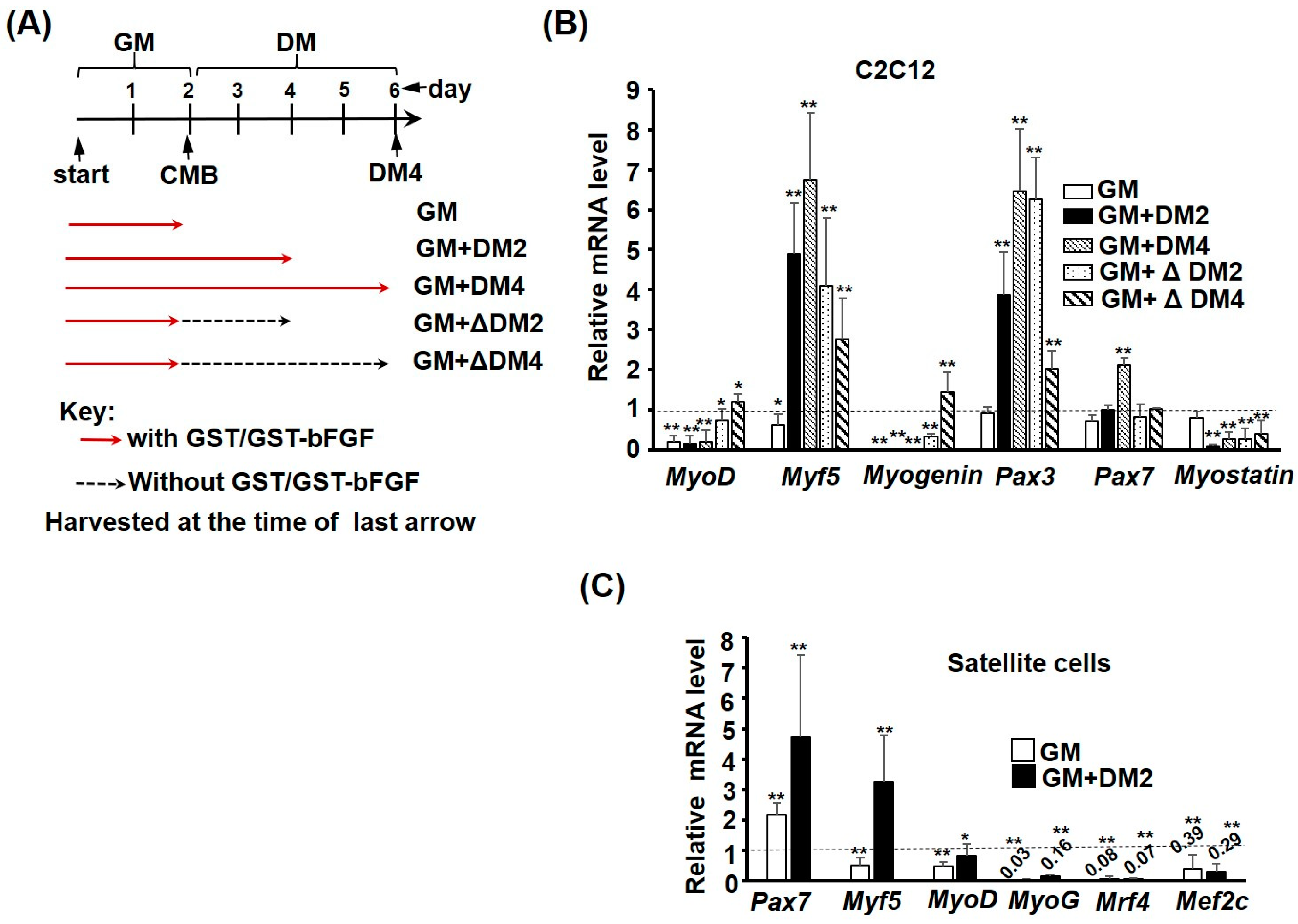
2.4. GST-bFGF Induces Cell Cycle Genes but Selectively Represses Myogenic Genes
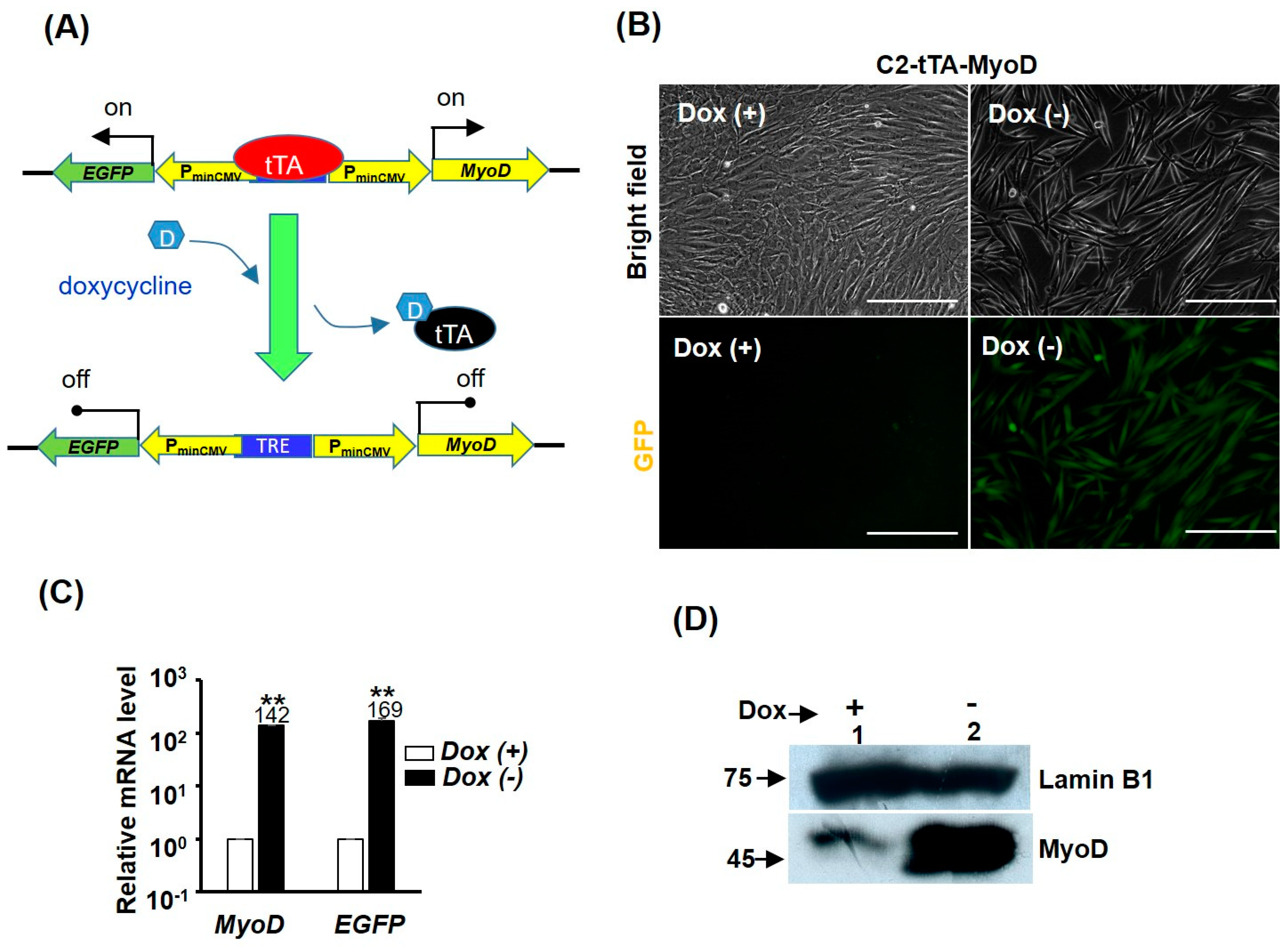
2.5. MyoD Repression Is Dispensable for GST-bFGF Induced Cell Proliferation
2.6. GST-bFGF Signaling Enhances the Stemness of Satellite Cells
3. Discussion
3.1. GST-bFGF Conserves the Property of Native bFGF
3.2. Differential Effects on Free and Myofiber-Associated Satellite Cells
3.3. MyoD Is the Major Target of bFGF in Myogenic Repression
4. Materials and Methods
4.1. Plasmids
4.2. Cell Culture and Promoter Assay
4.3. Recombinant protein Expression and Purification
4.4. Quantitative RT-PCR (qRT-PCR)
4.5. Western Blot
4.6. Satellite Cell Isolation and Culture
4.7. Flowcytometry
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckingham, M.; Rigby, P.W. Gene Regulatory Networks and Transcriptional Mechanisms that Control Myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Asahara, H. The myogenic transcriptional network. Cell. Mol. Life Sci. 2011, 68, 1843–1849. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Chang, N.C.; Bentzinger, C.F.; Rudnicki, M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012, 22, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Froeschlé, A.; Alric, S.; Kitzmann, M.; Carnac, G.; Auradé, F.; Rochette-Egly, C.; Bonnieu, A. Retinoic acid receptors and muscle b-HLH proteins: Partners in retinoid-induced myogenesis. Oncogene 1998, 16, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Stern, H.M.; Lin-Jones, J.; Hauschka, S.D. Synergistic interactions between bFGF and a TGF-beta family member may mediate myogenic signals from the neural tube. Development 1997, 124, 3511–3523. [Google Scholar] [CrossRef] [PubMed]
- Muscat, G.E.; Mynett-Johnson, L.; Dowhan, D.; Downes, M.; Griggs, R. Activation ofmyoDgene transcription by 3,5,3′-triiodo-L-thyronine: A direct role for the thyroid hormone and retinoid X receptors. Nucleic Acids Res. 1994, 22, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.E.; Boxhorn, L.K. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 1989, 138, 311–315. [Google Scholar] [CrossRef]
- Joseph-Silverstein, J.; Consigli, S.A.; Lyser, K.M.; Pault, C.V. Basic fibroblast growth factor in the chick embryo: Immunolocalization to striated muscle cells and their precursors. J. Cell Biol. 1989, 108, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- DiMario, J.; Buffinger, N.; Yamada, S.; Strohman, R.C. Fibroblast Growth Factor in the Extracellular Matrix of Dystrophic (mdx) Mouse Muscle. Science 1989, 244, 688–690. [Google Scholar] [CrossRef]
- La Venuta, G.; Zeitler, M.; Steringer, J.P.; Müller, H.-M.; Nickel, W. The Startling Properties of Fibroblast Growth Factor 2: How to Exit Mammalian Cells without a Signal Peptide at Hand. J. Biol. Chem. 2015, 290, 27015–27020. [Google Scholar] [CrossRef]
- Rabouille, C.; Malhotra, V.; Nickel, W. Diversity in unconventional protein secretion. J. Cell Sci. 2012, 125, 5251–5255. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Laußmann, M.; Wegehingel, S.; Kaderali, L.; Erfle, H.; Reichert, J.; Lechner, J.; Beer, H.-D.; Pepperkok, R.; Nickel, W. Tec-Kinase-Mediated Phosphorylation of Fibroblast Growth Factor 2 is Essential for Unconventional Secretion. Traffic 2010, 11, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, B.; Vogler, T.O.; Gadek, K.; Olwin, B.B. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev. Dyn. 2017, 246, 359–367. [Google Scholar] [CrossRef]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar] [CrossRef]
- Bizen, N.; Inoue, T.; Shimizu, T.; Tabu, K.; Kagawa, T.; Taga, T. A Growth-Promoting Signaling Component Cyclin D1 in Neural Stem Cells Has Antiastrogliogenic Function to Execute Self-Renewal. Stem Cells 2013, 32, 1602–1615. [Google Scholar] [CrossRef] [PubMed]
- Galimov, A.; Merry, T.L.; Luca, E.; Rushing, E.J.; Mizbani, A.; Turcekova, K.; Hartung, A.; Croce, C.M.; Ristow, M.; Krützfeldt, J. MicroRNA-29a in Adult Muscle Stem Cells Controls Skeletal Muscle Regeneration During Injury and Exercise Downstream of Fibroblast Growth Factor-2. Stem Cells 2016, 34, 768–780. [Google Scholar] [CrossRef]
- Weintraub, H.; Tapscott, S.J.; Davis, R.L.; Thayer, M.J.; Adam, M.A.; Lassar, A.B.; Miller, A.D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc. Natl. Acad. Sci. USA 1989, 86, 5434–5438. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.C.; Wang, X.W.; Teng, H.F.; Wu, Y.J.; Chang, H.C.; Chen, S.L. Wnt3a signal pathways activate MyoD expression by targeting cis-elements inside and outside its distal enhancer. Biosci. Rep. 2015, 35, e00180. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-F.; Tsao, K.-C.; Lee, S.-J.; Yang, R.-B. SCUBE3 (Signal Peptide-CUB-EGF Domain-containing Protein 3) Modulates Fibroblast Growth Factor Signaling during Fast Muscle Development. J. Biol. Chem. 2014, 289, 18928–18942. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Weseman, J.; Moran, J.S.; Lindstrom, J. Effect of fibroblast growth factor on the division and fusion of bovine myoblasts. J. Cell Biol. 1976, 70, 395–405. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Purification of a fibroblast growth factor from bovine pituitary. J. Biol. Chem. 1975, 250, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, B.; Olson, E.; Glaser, L. Control by fibroblast growth factor of differentiation in the BC3H1 muscle cell line. J. Cell Biol. 1985, 100, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.E.; Dodson, M.V.; Luiten, L.S. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp. Cell Res. 1984, 152, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rando, T.A.; Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994, 125, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Barlowe, C.K.; Miller, E.A. Secretory Protein Biogenesis and Traffic in the Early Secretory Pathway. Genetics 2013, 193, 383–410. [Google Scholar] [CrossRef] [PubMed]
- Impens, F.; Radoshevich, L.; Cossart, P.; Ribet, D. Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli. Proc. Natl. Acad. Sci. USA 2014, 111, 12432–12437. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, M.; Ohmae, H.; Tsuji, S.; Tanaka, Y.; Koyama, N.; Nishimura, O. epsilon-N-acetylation in the production of recombinant human basic fibroblast growth factor mutein. Prep. Biochem. Biotechnol. 1996, 26, 259–270. [Google Scholar] [CrossRef]
- Feige, J.J.; Baird, A. Glycosylation of the basic fibroblast growth factor receptor. The contribution of carbohydrate to receptor function. J. Biol. Chem. 1988, 263, 14023–14029. [Google Scholar] [CrossRef]
- Wegehingel, S.; Zehe, C.; Nickel, W. Rerouting of fibroblast growth factor 2 to the classical secretory pathway results in post-translational modifications that block binding to heparan sulfate proteoglycans. FEBS Lett. 2008, 582, 2387–2392. [Google Scholar] [CrossRef]
- Bailly, K.; Soulet, F.; Leroy, D.; Amalric, F.; Bouche, G. Uncoupling of cell proliferation and differentiation activities of basic fibroblast growth factor. FASEB J. 2000, 14, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gysin, R.; Kapur, S.; Baylink, D.J.; Lau, K.W. Modifications of the fibroblast growth factor-2 gene led to a marked enhancement in secretion and stability of the recombinant fibroblast growth factor-2 protein. J. Cell. Biochem. 2007, 100, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Evans, S.; Stewart, D.; Spencer, K.H.; Sheikh, F.; Hui, E.E.; Christman, K.L. Fibroblasts influence muscle progenitor differentiation and alignment in contact independent and dependent manners in organized co-culture devices. Biomed. Microdevices 2012, 15, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hannon, K.; Kudla, A.J.; McAvoy, M.J.; Clase, K.L.; Olwin, B.B. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 1996, 132, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Mitchell, C.; McGeachie, J.; Grounds, M.D. The Time Course of Basic Fibroblast Growth Factor Expression in Crush-Injured Skeletal Muscles of SJL/J and BALB/c Mice. Exp. Cell Res. 1995, 216, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z.; Seger, R.; Rivera, A.J. Fibroblast Growth Factor Promotes Recruitment of Skeletal Muscle Satellite Cells in Young and Old Rats. J. Histochem. Cytochem. 1999, 47, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z.; Anderson, J.E. Satellite cells from dystrophic (Mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev. Dyn. 2005, 235, 203–212. [Google Scholar] [CrossRef]
- Tajbakhsh, S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J. Intern. Med. 2009, 266, 372–389. [Google Scholar] [CrossRef]
- Vaidya, T.B.; Rhodes, S.J.; Taparowsky, E.J.; Konieczny, S.F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol. Cell. Biol. 1989, 9, 3576–3579. [Google Scholar] [CrossRef]
- Brunetti, A.; Goldfine, I.D. Differential Effects of Fibroblast Growth Factor on Insulin Receptor and Muscle Specific Protein Gene Expression in BC3H-1 Myocytes. Mol. Endocrinol. 1990, 4, 880–885. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kao, C.H.; Villarroya, F.; Chang, H.Y.; Chang, H.C.; Hsiao, S.P.; Liou, G.-G.; Chen, S.L. Bhlhe40 Represses PGC-1α Activity on Metabolic Gene Promoters in Myogenic Cells. Mol. Cell. Biol. 2015, 35, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Kao, C.H.; Chung, S.Y.; Chen, W.C.; Aninda, L.P.; Chen, Y.H.; Juan, Y.A.; Chen, S.L. Bhlhe40 differentially regulates the function and number of peroxisomes and mitochondria in myogenic cells. Redox Biol. 2018, 20, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, R.J.; Deasy, B.M.; Cao, B.; Gates, C.; Huard, J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J. Cell Sci. 2002, 115, 4361–4374. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, W.-C.; Hsiao, W.-P.; Huang, K.-F.; Liao, Y.-S.; Lin, H.-B.; Wu, Y.-J.; Kao, C.-H.; Chen, S.-L. Reciprocal Regulation of Peroxisome Biogenesis and Myogenic Factors Is Critical for Myogenesis. Int. J. Mol. Sci. 2023, 24, 12262. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.-H.; Li, N.; Huang, K.-F.; Chang, Y.-T.; Wu, C.-C.; Chen, S.-L. MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis. Int. J. Mol. Sci. 2024, 25, 4308. https://doi.org/10.3390/ijms25084308
Fan S-H, Li N, Huang K-F, Chang Y-T, Wu C-C, Chen S-L. MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis. International Journal of Molecular Sciences. 2024; 25(8):4308. https://doi.org/10.3390/ijms25084308
Chicago/Turabian StyleFan, Shu-Hsin, Ning Li, Kai-Fan Huang, Yun-Ting Chang, Chuan-Che Wu, and Shen-Liang Chen. 2024. "MyoD Over-Expression Rescues GST-bFGF Repressed Myogenesis" International Journal of Molecular Sciences 25, no. 8: 4308. https://doi.org/10.3390/ijms25084308




