Loose Coupling between the Voltage Sensor and the Activation Gate in Mammalian HCN Channels Suggests a Gating Mechanism
Abstract
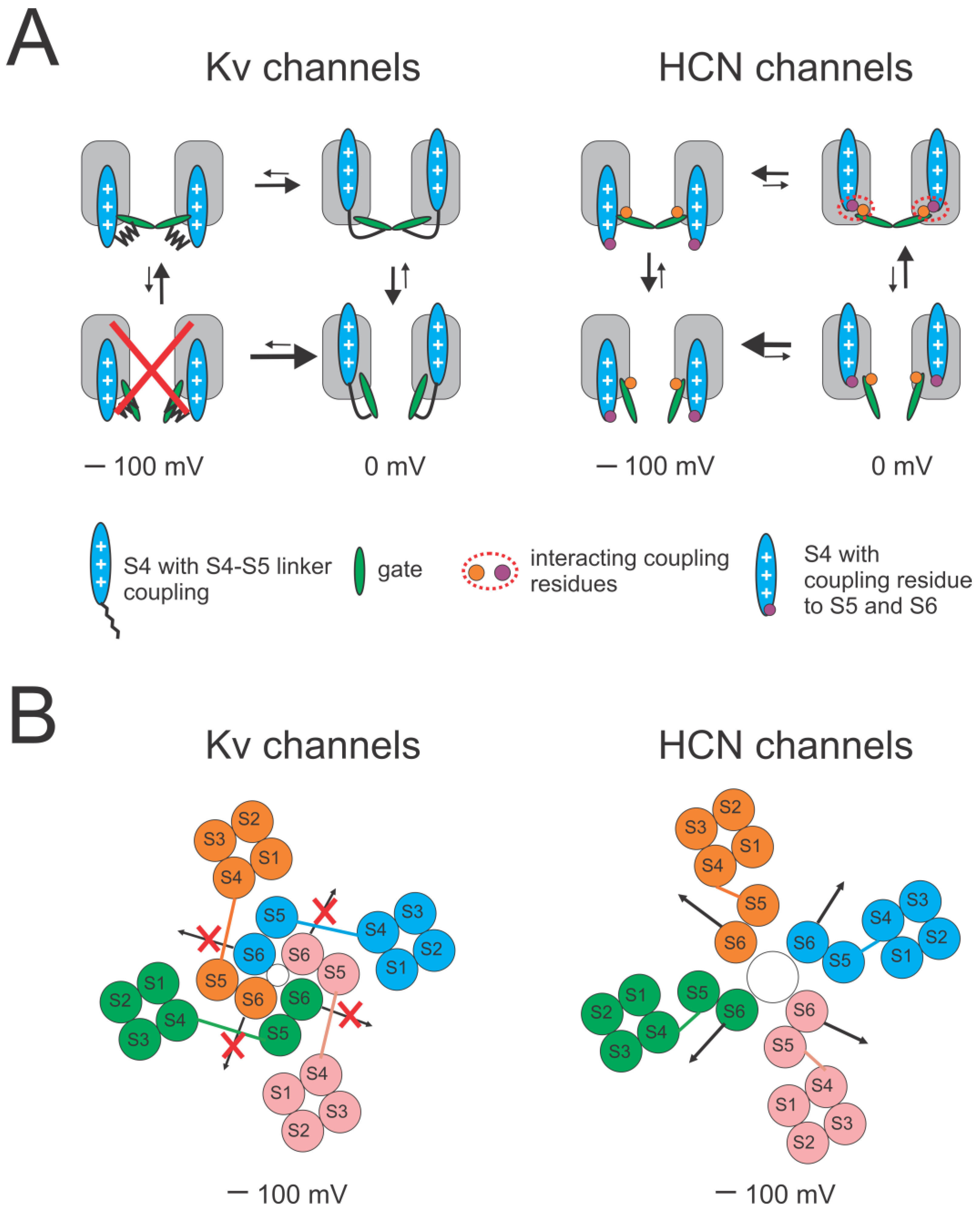
1. Introduction
2. Results
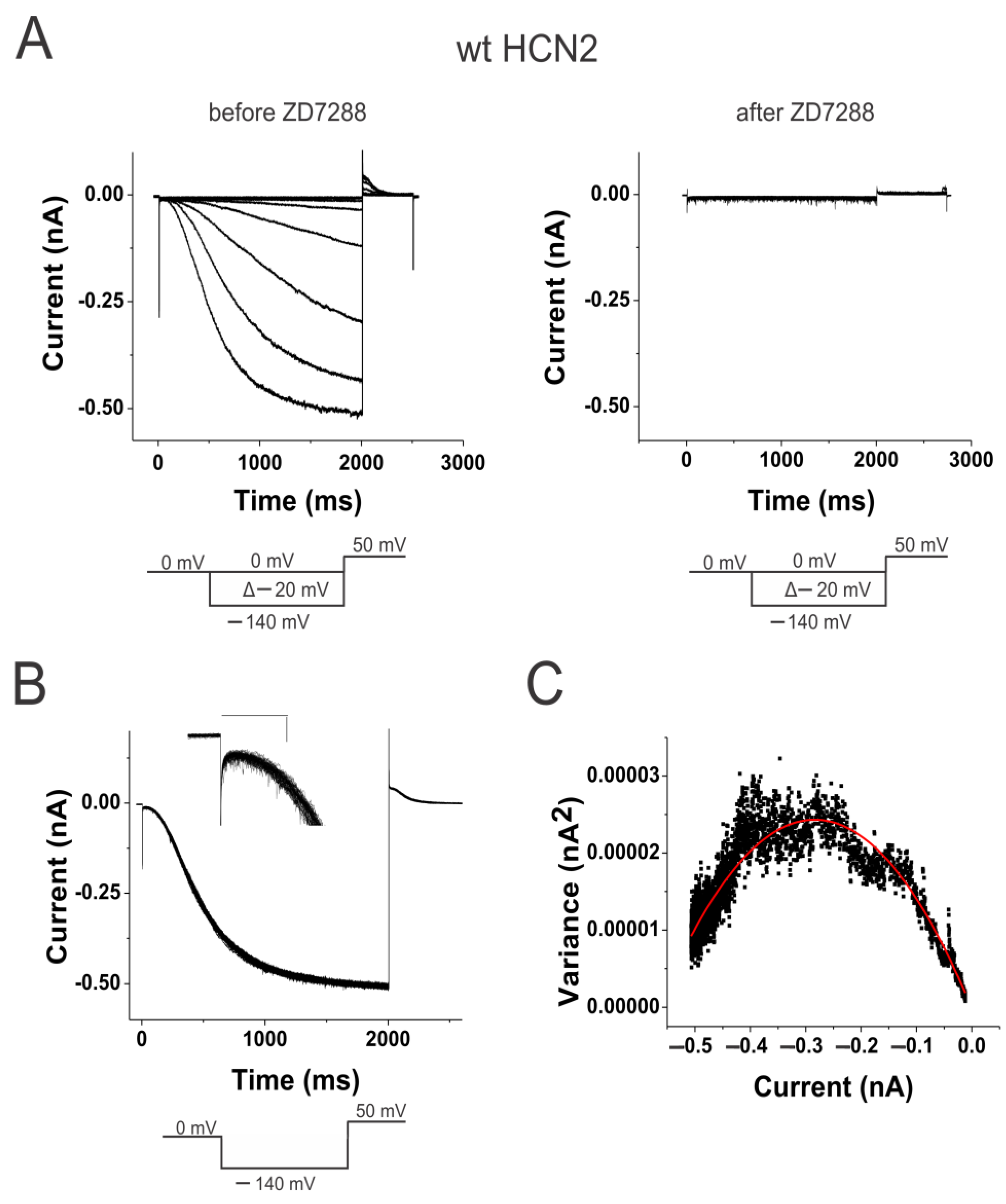
2.1. Weak Coupling in HCN Channels
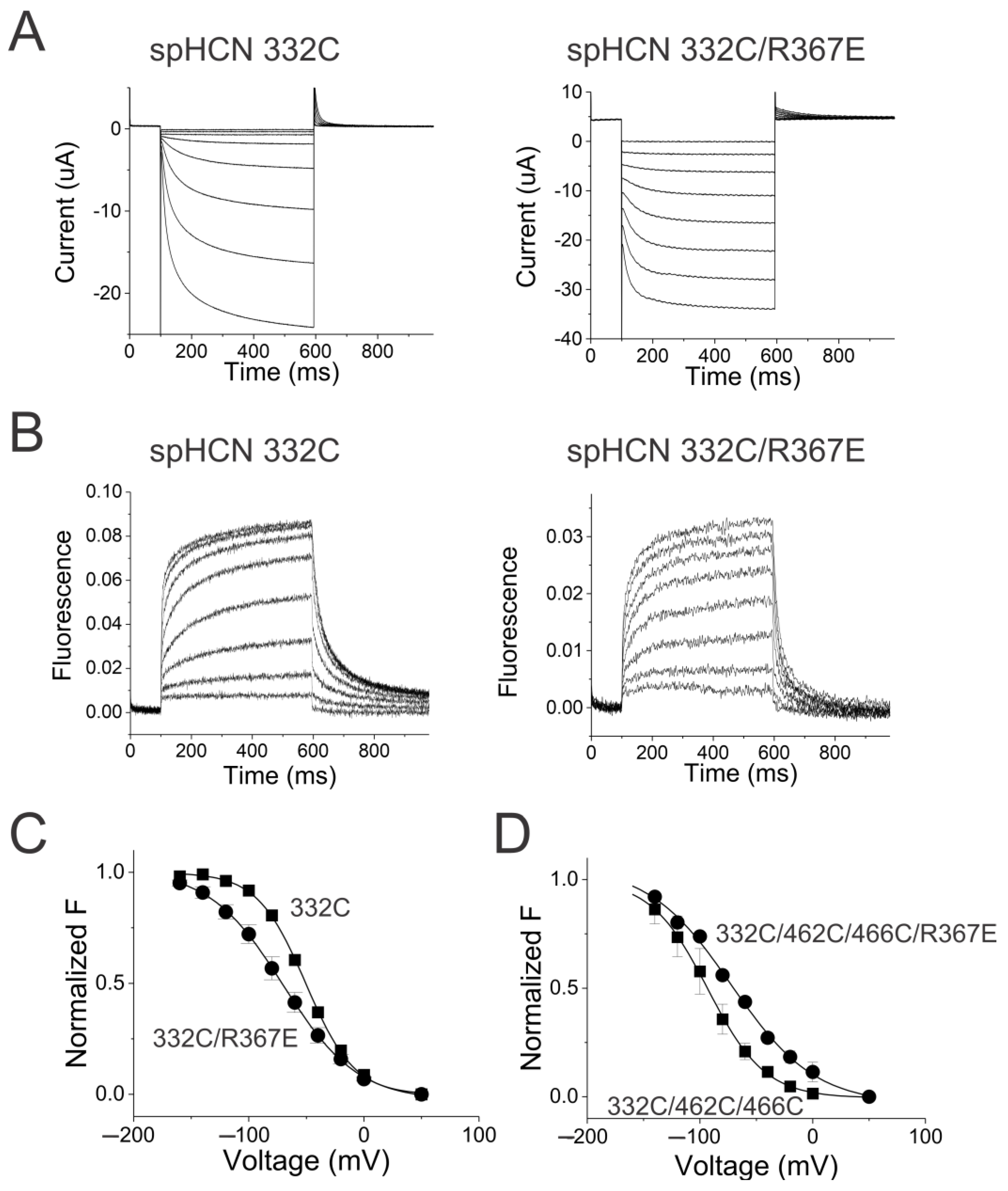
2.2. S4–S5 Mutation Further Weakens the Coupling

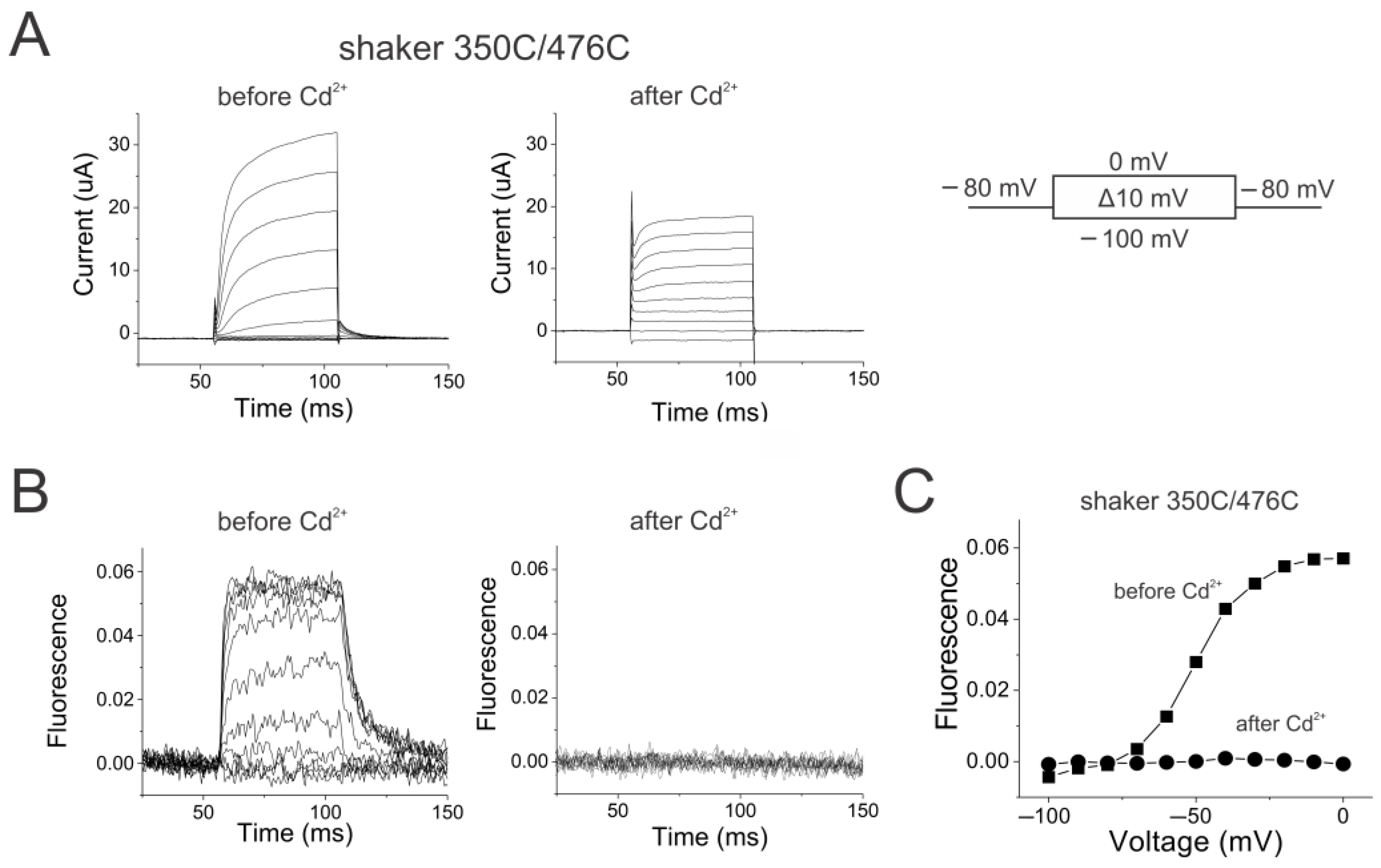
2.3. Reciprocal Effects of Gate and Voltage Sensor in Strongly Coupled Ion Channels
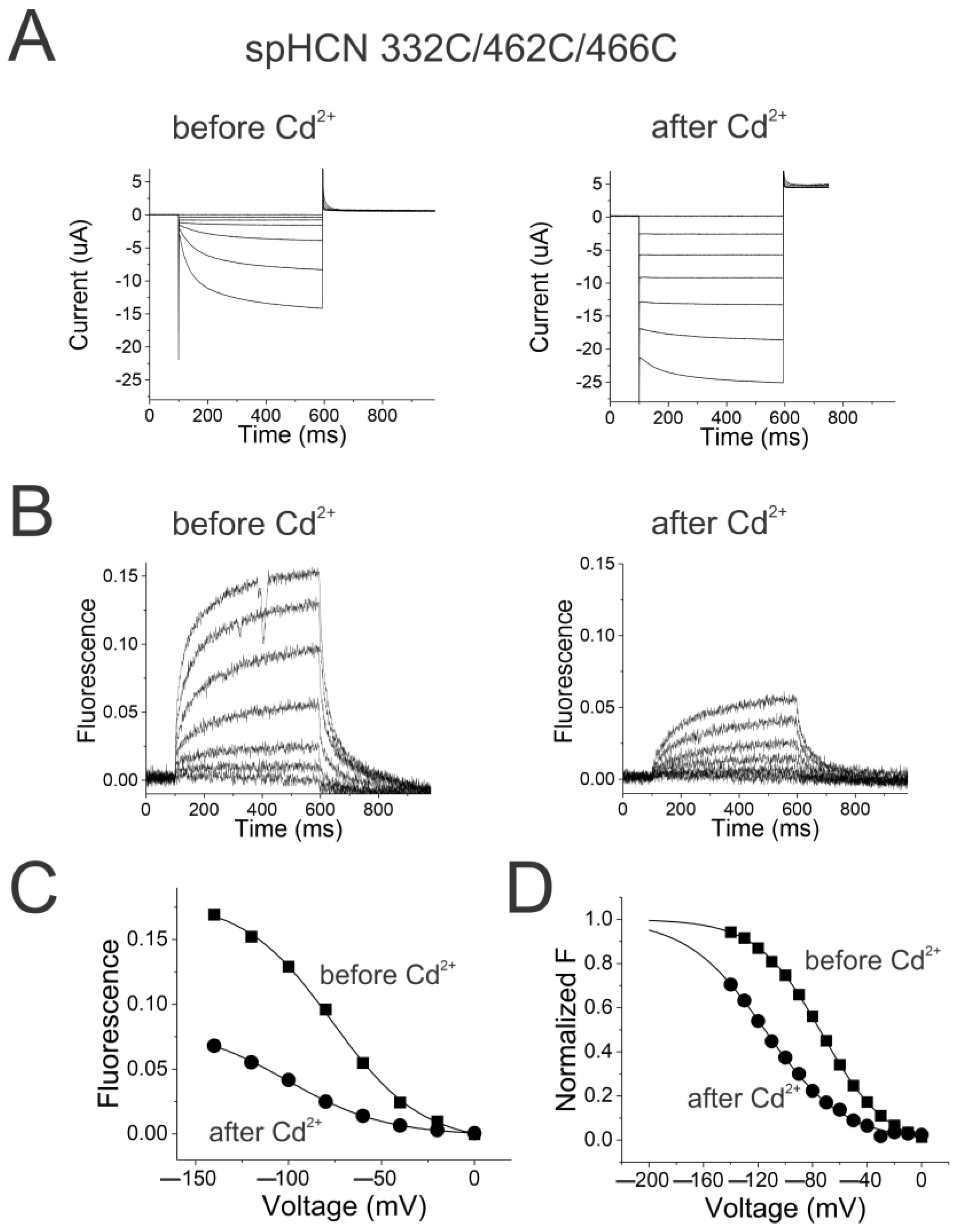
2.4. Reciprocal Effects of Gate and Voltage Sensor in Weakly Coupled Ion Channels
2.5. Coupling Mutation Affects Both Open Probability and S4 Movement
3. Discussion
4. Materials and Methods
4.1. Expression System
4.2. Two-Electrode Voltage Clamp
4.3. Voltage Clamp Fluorometry
4.4. Noise Analysis
4.5. Coupling Constants
4.6. Molecular Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benzoni, P.; Bertoli, G.; Giannetti, F.; Piantoni, C.; Milanesi, R.; Pecchiari, M.; Barbuti, A.; Baruscotti, M.; Bucchi, A. The funny current: Even funnier than 40 years ago. Uncanonical expression and roles of HCN/f channels all over the body. Prog. Biophys. Mol. Biol. 2021, 166, 189–204. [Google Scholar] [CrossRef]
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflugers Arch. Eur. J. Physiol. 2020, 472, 931–951. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, A.-S.S.; Tytgat, J.; Peigneur, S. Review: HCN Channels in the Heart. Curr. Cardiol. Rev. 2022, 18, e040222200836. [Google Scholar] [PubMed]
- Gauss, R.; Seifert, R.; Kaupp, U.B. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 1998, 393, 583–587. [Google Scholar] [CrossRef]
- Ludwig, A.; Zong, X.; Jeglitsch, M.; Hofmann, F.; Biel, M. A family of hyperpolarization-activated mammalian cation channels. Nature 1998, 393, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; MacKinnon, R. Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 2017, 168, 111–120.e11. [Google Scholar] [CrossRef]
- Tan, X.-F.; Bae, C.; Stix, R.; Fernández-Mariño, A.I.; Huffer, K.; Chang, T.-H.; Jiang, J.; Faraldo-Gómez, J.D.; Swartz, K.J. Structure of the Shaker Kv channel and mechanism of slow C-type inactivation. Sci. Adv. 2022, 8, eabm7814. [Google Scholar] [CrossRef]
- Whicher, J.R.; MacKinnon, R. Structure of the voltage-gated K + channel Eag1 reveals an alternative voltage sensing mechanism. Science 2016, 353, 664–669. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. The 70-year search for the voltage-sensing mechanism of ion channels. J. Physiol. 2022, 600, 3227–3247. [Google Scholar] [CrossRef]
- Larsson, H.; Baker, O.S.; Dhillon, D.S.; Isacoff, E.Y. Transmembrane Movement of the Shaker K+ Channel S4. Neuron 1996, 16, 387–397. [Google Scholar] [CrossRef]
- Männikkö, R.; Elinder, F.; Larsson, H.P. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature 2002, 419, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Mandala, V.S.; MacKinnon, R. Voltage-sensor movements in the Eag Kv channel under an applied electric field. Proc. Natl. Acad. Sci. USA 2022, 119, e2214151119. [Google Scholar] [CrossRef] [PubMed]
- Long, S.B.; Campbell, E.B.; MacKinnon, R. Voltage Sensor of Kv1.2: Structural Basis of Electromechanical Coupling. Science 2005, 309, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Klem, A.M.; Ramu, Y. Coupling between Voltage Sensors and Activation Gate in Voltage-gated K+ Channels. J. Gen. Physiol. 2002, 120, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Aman, T.K.; DiMaio, F.; Zagotta, W.N. The HCN channel voltage sensor undergoes a large downward motion during hyperpolarization. Nat. Struct. Mol. Biol. 2019, 26, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; MacKinnon, R. Voltage Sensor Movements during Hyperpolarization in the HCN Channel. Cell 2019, 179, 1582–1589.e7. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ramentol, R.; Perez, M.E.; Noskov, S.Y.; Larsson, H.P. A second S4 movement opens hyperpolarization-activated HCN channels. Proc. Natl. Acad. Sci. USA 2021, 118, e2102036118. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Aman, T.K.; DiMaio, F.; Zagotta, W.N. Electromechanical coupling mechanism for activation and inactivation of an HCN channel. Nat. Commun. 2021, 12, 2802. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, A.; Bauer, D.; Giese, M.H.; Swuec, P.; Porro, A.; Gasparri, F.; Sharifzadeh, A.S.; Chaves-Sanjuan, A.; Alberio, L.; Parisi, G.; et al. Gating movements and ion permeation in HCN4 pacemaker channels. Mol. Cell 2021, 81, 2929–2943.e6. [Google Scholar] [CrossRef]
- Long, S.B.; Campbell, E.B.; Mackinnon, R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science 2005, 7, 7. [Google Scholar] [CrossRef]
- Lu, Z.; Klem, A.M.; Ramu, Y. Ion conduction pore is conserved among potassium channels. Nature 2001, 413, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tristani-Firouzi, M.; Chen, J.; Sanguinetti, M.C. Interactions between S4–S5 Linker and S6 Transmembrane Domain Modulate Gating of HERG K+ Channels. J. Biol. Chem. 2002, 277, 18994–19000. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.E.; Zagotta, W.N. Insights into the molecular mechanism for hyperpolarization-dependent activation of HCN channels. Proc. Natl. Acad. Sci. USA 2018, 115, E8086–E8095. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; MacKinnon, R. Cryo-EM Structure of the Open Human Ether-à-go-go -Related K + Channel hERG. Cell 2017, 169, 422–430.e10. [Google Scholar] [CrossRef] [PubMed]
- Islas, L.D.; Sigworth, F.J. Voltage Sensitivity and Gating Charge in Shaker and Shab Family Potassium Channels. J. Gen. Physiol. 1999, 114, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F.; Perozo, E.; Stefani, E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys. J. 1994, 66, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F.; White, M.M.; Taylor, R.E. Gating currents associated with potassium channel activation. Nature 1982, 296, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Yellen, G. Charge movement in gating-locked HCN channels reveals weak coupling of voltage sensors and gate. J. Gen. Physiol. 2012, 140, 469–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.; Mitcheson, J.S.; Tristani-Firouzi, M.; Lin, M.; Sanguinetti, M.C. The S4–S5 linker couples voltage sensing and activation of pacemaker channels. Proc. Natl. Acad. Sci. USA 2001, 98, 11277–11282. [Google Scholar] [CrossRef]
- Bruening-Wright, A.; Elinder, F.; Larsson, H.P. Kinetic Relationship between the Voltage Sensor and the Activation Gate in spHCN Channels. J. Gen. Physiol. 2007, 130, 71–81. [Google Scholar] [CrossRef]
- Gandhi, C.S.; Loots, E.; Isacoff, E.Y. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron 2000, 27, 585–595. [Google Scholar] [CrossRef]
- Mannuzzu, L.M.; Isacoff, E.Y. Independence and Cooperativity in Rearrangements of a Potassium Channel Voltage Sensor Revealed by Single Subunit Fluorescence. J. Gen. Physiol. 2000, 115, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, M.; Shin, K.S.; Yellen, G. The Activation Gate of a Voltage-Gated K+ Channel Can Be Trapped in the Open State by an Intersubunit Metal Bridge. Neuron 1998, 21, 617–621. [Google Scholar] [CrossRef]
- Rothberg, B.S.; Shin, K.S.; Yellen, G. Movements near the Gate of a Hyperpolarization-activated Cation Channel. J. Gen. Physiol. 2003, 122, 501–510. [Google Scholar] [CrossRef]
- Altomare, C.; Bucchi, A.; Camatini, E.; Baruscotti, M.; Viscomi, C.; Moroni, A.; DiFrancesco, D. Integrated Allosteric Model of Voltage Gating of Hcn Channels. J. Gen. Physiol. 2001, 117, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Larsson, H.P. The Search Is on for the Voltage Sensor-to-gate Coupling. J. Gen. Physiol. 2002, 120, 475–481. [Google Scholar] [CrossRef]
- Jiang, Y.; Ruta, V.; Chen, J.; Lee, A.; MacKinnon, R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature 2003, 423, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Holmgren, M.; Jurman, M.E.; Yellen, G. Gated Access to the Pore of a Voltage-Dependent K+ Channel. Neuron 1997, 19, 175–184. [Google Scholar] [CrossRef]
- Rothberg, B.S.; Shin, K.S.; Phale, P.S.; Yellen, G. Voltage-Controlled Gating at the Intracellular Entrance to a Hyperpolarization-Activated Cation Channel. J. Gen. Physiol. 2002, 119, 83–91. [Google Scholar] [CrossRef]
- Shin, K.S.; Rothberg, B.S.; Yellen, G. Blocker State Dependence and Trapping in Hyperpolarization-activated Cation Channels. Evidence for an intracellular activation gate. J. Gen. Physiol. 2001, 117, 91–102. [Google Scholar] [CrossRef]
- Decher, N.; Chen, J.; Sanguinetti, M.C. Voltage-dependent gating of hyperpolarization-activated, cyclic nucleotide-gated pacemaker channels: Molecular coupling between the S4–S5 and C-linkers. J. Biol. Chem. 2004, 279, 13859–13865. [Google Scholar] [CrossRef]
- Ramentol, R.; Perez, M.E.; Larsson, H.P. Gating mechanism of hyperpolarization-activated HCN pacemaker channels. Nat. Commun. 2020, 11, 1419. [Google Scholar] [CrossRef]
- Wu, X.; Cunningham, K.P.; Ramentol, R.; Perez, M.E.; Larsson, H.P. Similar voltage-sensor movement in spHCN channels can cause closing, opening, or inactivation. J. Gen. Physiol. 2023, 155, e202213170. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Zhou, L.; George, M.S.; Siegelbaum, S.A. Voltage Sensor Movement and cAMP Binding Allosterically Regulate an Inherently Voltage-independent Closed−Open Transition in HCN Channels. J. Gen. Physiol. 2007, 129, 175–188. [Google Scholar] [CrossRef]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. Characterization of the pace-maker current kinetics in calf Purkinje fibres. J. Physiol. 1984, 348, 341–367. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D.; Ferroni, A. Delayed activation of the cardiac pacemaker current and its dependence on conditioning pre-hyperpolarizations. Pflugers Arch. Eur. J. Physiol. 1983, 396, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, J.; Klenchin, V.A.; Alvarez-Baron, C.; Tewari, D.; Blair, A.; Chanda, B. Bipolar switching by HCN voltage sensor underlies hyperpolarization activation. Proc. Natl. Acad. Sci. USA 2019, 116, 670–678. [Google Scholar] [CrossRef]
- Bruening-Wright, A.; Larsson, H.P. Slow conformational changes of the voltage sensor during the mode shift in hyperpolarization-activated cyclic-nucleotide-gated channels. J. Neurosci. 2007, 27, 270–278. [Google Scholar] [CrossRef]
- Cha, A.; Bezanilla, F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron 1997, 19, 1127–1140. [Google Scholar] [CrossRef]
- Mannuzzu, L.M.; Moronne, M.M.; Isacoff, E.Y. Direct Physical Measure of Conformational Rearrangement Underlying Potassium Channel Gating. Science 1996, 271, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, O.; Gonzalez, C.; Latorre, R. Counting channels: A tutorial guide on ion channel fluctuation analysis. Adv. Physiol. Educ. 2002, 26, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Neumcke, B.; Nonner, W.; Stämpfli, R. Conductance fluctuations from the inactivation process of sodium channels in myelinated nerve fibres. J. Physiol. 1980, 308, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.H.; Conti, F. Nonstationary noise analysis and application to patch clamp recordings. Methods Enzym. 1992, 207, 131–148. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Cunningham, K.P.; Bruening-Wright, A.; Pandey, S.; Larsson, H.P. Loose Coupling between the Voltage Sensor and the Activation Gate in Mammalian HCN Channels Suggests a Gating Mechanism. Int. J. Mol. Sci. 2024, 25, 4309. https://doi.org/10.3390/ijms25084309
Wu X, Cunningham KP, Bruening-Wright A, Pandey S, Larsson HP. Loose Coupling between the Voltage Sensor and the Activation Gate in Mammalian HCN Channels Suggests a Gating Mechanism. International Journal of Molecular Sciences. 2024; 25(8):4309. https://doi.org/10.3390/ijms25084309
Chicago/Turabian StyleWu, Xiaoan, Kevin P. Cunningham, Andrew Bruening-Wright, Shilpi Pandey, and H. Peter Larsson. 2024. "Loose Coupling between the Voltage Sensor and the Activation Gate in Mammalian HCN Channels Suggests a Gating Mechanism" International Journal of Molecular Sciences 25, no. 8: 4309. https://doi.org/10.3390/ijms25084309
APA StyleWu, X., Cunningham, K. P., Bruening-Wright, A., Pandey, S., & Larsson, H. P. (2024). Loose Coupling between the Voltage Sensor and the Activation Gate in Mammalian HCN Channels Suggests a Gating Mechanism. International Journal of Molecular Sciences, 25(8), 4309. https://doi.org/10.3390/ijms25084309




