H-VISTA Immunohistochemistry Score Is Associated with Advanced Stages in Cutaneous and Ocular Melanoma
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, P.-W.; Chang, J.W.-C. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed. J. 2019, 42, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, N.; Derakhshani, A.; Shadbad, M.A.; Argentiero, A.; Racanelli, V.; Kazemi, T.; Mokhtarzadeh, A.; Brunetti, O.; Silvestris, N.; Baradaran, B. The Role of V-Domain Ig Suppressor of T Cell Activation (VISTA) in Cancer Therapy: Lessons Learned and the Road Ahead. Front. Immunol. 2021, 12, 676181. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Lai, W.V.; Adusumilli, P.S.; Desmeules, P.; Frosina, D.; Jungbluth, A.; Ni, A.; Eguchi, T.; Travis, W.D.; Ladanyi, M.; et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod. Pathol. 2020, 33, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tagliamento, M.; Agostinetto, E.; Borea, R.; Brandão, M.; Poggio, F.; Addeo, A.; Lambertini, M. VISTA: A Promising Target for Cancer Immunotherapy? ImmunoTargets Ther. 2021, 10, 185–200. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Croteau, W.; Noelle, R.J.; Lines, J.L. VISTA: A novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 2019, 42, 101308. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Sempere, L.F.; Broughton, T.; Wang, L.; Noelle, R. VISTA Is a Novel Broad-Spectrum Negative Checkpoint Regulator for Cancer Immunotherapy. Cancer Immunol. Res. 2014, 2, 510–517. [Google Scholar] [CrossRef]
- Yum, J.-E.I.; Hong, Y.-K. Terminating Cancer by Blocking VISTA as a Novel Immunotherapy: Hasta la vista, baby. Front. Oncol. 2021, 11, 658488. [Google Scholar] [CrossRef] [PubMed]
- Broughton, T.W.K.; ElTanbouly, M.A.; Schaafsma, E.; Deng, J.; Sarde, A.; Croteau, W.; Li, J.; Nowak, E.C.; Mabaera, R.; Smits, N.C.; et al. Defining the Signature of VISTA on Myeloid Cell Chemokine Responsiveness. Front. Immunol. 2019, 10, 2641. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Knecht, M.; Mollaee, M.; Zhong, Z.; Erkes, D.A.; McCue, P.A.; Chervoneva, I.; Berger, A.C.; Lo, J.A.; Fisher, D.E.; et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020, 30, 510–524.e6. [Google Scholar] [CrossRef]
- Blando, J.; Sharma, A.; Higa, M.G.; Zhao, H.; Vence, L.; Yadav, S.S.; Kim, J.; Sepulveda, A.M.; Sharp, M.; Maitra, A.; et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumours WHO Classification of Tumours, 4th ed.; IARC: Lyon, France, 2018; Volume 11.
- Hussain, M.R.M.; Baig, M.; Mohamoud, H.S.A.; Ulhaq, Z.; Hoessli, D.C.; Khogeer, G.S.; Al-Sayed, R.R.; Al-Aama, J.Y. BRAF gene: From human cancers to developmental syndromes. Saudi J. Biol. Sci. 2014, 22, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, M.; Giunta, E.F.; Tortora, M.; Curvietto, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Rosanova, M.; De Placido, P.; Pietroluongo, E.; et al. BRAF Gene and Melanoma: Back to the Future. Int. J. Mol. Sci. 2021, 22, 3474. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.-J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Eberhart, C.G.; Kivelä, T.T. WHO Classification of Tumours of the Eye: WHO Classification of Tumours; IARC: Lyon, France, 2018; Volume 12.
- Singh, M.; Durairaj, P.; Yeung, J. Uveal Melanoma: A Review of the Literature. Oncol. Ther. 2018, 6, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, L.F.; Yan, S.; Li, Z.; Fisher, J.L.; Cheng, C.; Noelle, R.J.; Angeles, C.V.; Turk, M.J.; Ernstoff, M.S. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol. Immunother. 2018, 67, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Pilotto, S.; Teixidó, C.; Viteri, S.; González-Cao, M.; Riso, A.; Morales-Espinosa, D.; Molina, M.A.; Chaib, I.; Santarpia, M.; et al. Melanoma: Oncogenic drivers and the immune system. Ann. Transl. Med. 2015, 3, 265. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, Y.J.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E.; Lee, W.J. The prognostic significance of VISTA and CD33-positive myeloid cells in cutaneous melanoma and their relationship with PD-1 expression. Sci. Rep. 2020, 10, 14372. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Lancellotta, V.; Fionda, B.; Mangoni, M.; Casà, C.; Di Stefani, A.; Pagliara, M.M.; D’aviero, A.; Schinzari, G.; Chiesa, S.; et al. Immunotherapy and radiotherapy in melanoma: A multidisciplinary comprehensive review. Hum. Vaccines Immunother. 2022, 18, 1903827. [Google Scholar] [CrossRef]
- Theurich, S.; Rothschild, S.I.; Hoffmann, M.; Fabri, M.; Sommer, A.; Garcia-Marquez, M.; Thelen, M.; Schill, C.; Merki, R.; Schmid, T.; et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol. Res. 2016, 4, 744–754. [Google Scholar] [CrossRef]
- Fionda, B.; Pagliara, M.M.; Lancellotta, V.; Caputo, C.G.; Casà, C.; Sammarco, M.G.; Placidi, E.; Cornacchione, P.; Boselli, F.; Iezzi, R.; et al. Radiological and clinical findings in uveal melanoma treated by plaque interventional radiotherapy (brachytherapy): Visual atlas and literature review on response assessment. J. Contemp. Brachytherapy 2022, 14, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, M.M.; Tagliaferri, L.; Savino, G.; Fionda, B.; D’aviero, A.; Lanza, A.; Lancellotta, V.; Midena, G.; Gambacorta, M.A.; Blasi, M.A. High-Dose-Rate Interstitial Brachytherapy (Interventional Radiotherapy) for Conjunctival Melanoma with Orbital Extension. Ocul. Oncol. Pathol. 2021, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, H.; Jackett, L.A.; Menzies, A.M.; Gide, T.N.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Negative immune checkpoint regulation by VISTA: A mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017, 30, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska, A.; Sobocki, B.K.; Sakowicz-Burkiewicz, M.; Jereczek-Fossa, B.A.; Alterio, D.; Szot, O.; Korwat, A.; Pęksa, R. VISTA H-Score Is Significantly Associated with a 5-Year DFS Rate in Oral Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 1619. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, S.; Ren, Y.; Tai, R.; Liu, H.; Wang, Y.; Li, J.; Wang, F.; Xing, J.; Zhang, Y.; et al. Chemotherapy induces immune checkpoint VISTA expression in tumor cells via HIF-2alpha. Biochem. Pharmacol. 2023, 210, 115492. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, P.; Mihajlovic, M.; Djordjevic-Jocic, J.; Vlajkovic, S.; Cekic, S.; Stefanovic, V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013, 6, 1230–1244. [Google Scholar] [PubMed]
- Salah, N.e.I.I.; Marnissi, F.; Lakhdar, A.; Karkouri, M.; ElBelhadji, M.; Badou, A. The immune checkpoint VISTA is associated with prognosis in patients with malignant uveal melanoma. Front. Immunol. 2023, 14, 1225140. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, X.; Zhou, Y.; Mao, F.; Lin, Y.; Wu, H.; Sun, Q. VISTA expression on immune cells correlates with favorable prognosis in patients with triple-negative breast cancer. Front. Oncol. 2021, 10, 583966. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef]
- Ghouzlani, A.; Lakhdar, A.; Rafii, S.; Karkouri, M.; Badou, A. The immune checkpoint VISTA exhibits high expression levels in human gliomas and associates with a poor prognosis. Sci. Rep. 2021, 11, 21504. [Google Scholar] [CrossRef]
- Zong, L.; Mo, S.; Sun, Z.; Lu, Z.; Yu, S.; Chen, J.; Xiang, Y. Analysis of the immune checkpoint V-domain Ig-containing suppressor of T-cell activation (VISTA) in endometrial cancer. Mod. Pathol. 2022, 35, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Xiong, Y.-L.; Shi, X.-G.; Zhao, Y.-B.; Shi, A.-P.; Zheng, K.-F.; Liu, Y.-J.; Jiang, T.; Ma, N.; Zhao, J.-B. IGSF11 and VISTA: A pair of promising immune checkpoints in tumor immunotherapy. Biomark. Res. 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.M.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J. Clin. Oncol. 2021, 39, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Scolyer, R.A.; Rumcheva, P.; Moncrieff, M.; Murali, R.; McCarthy, S.W.; Saw, R.P.; Thompson, J.F. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 2012, 30, 2678–2683. [Google Scholar] [CrossRef]
- Tinca, A.C.; Moraru, R.; Cocuz, I.G.; Șincu, M.C.; Niculescu, R.; Sabău, A.H.; Chiorean, D.M.; Szoke, A.R.; Morariu, S.-H.; Cotoi, O.S. Actualities in the Morphology and Immunohistochemistry of Cutaneous and Ocular Melanoma: What Lies Ahead? A Single-Centre Study. Biomedicines 2022, 10, 2500. [Google Scholar] [CrossRef]
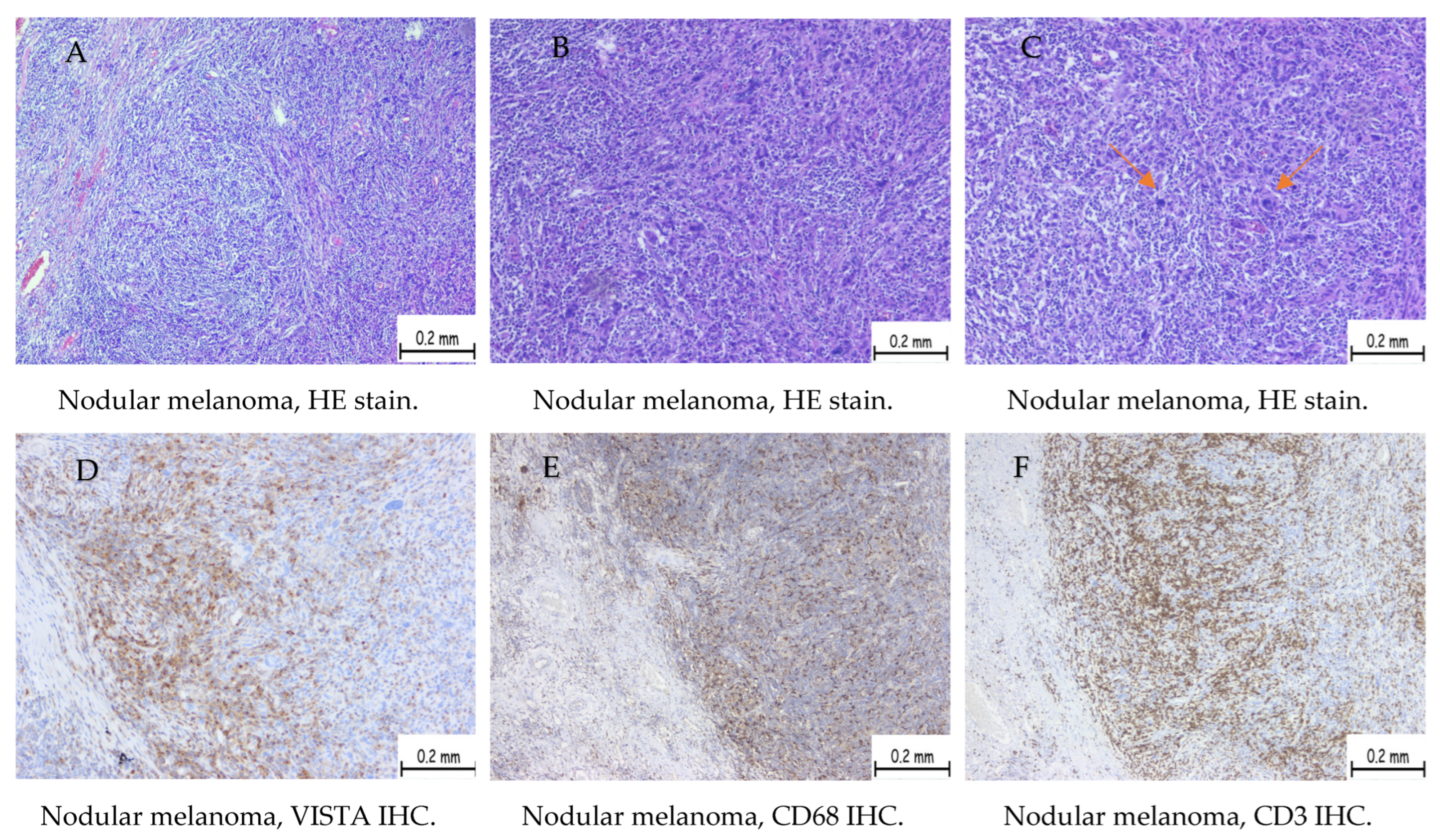
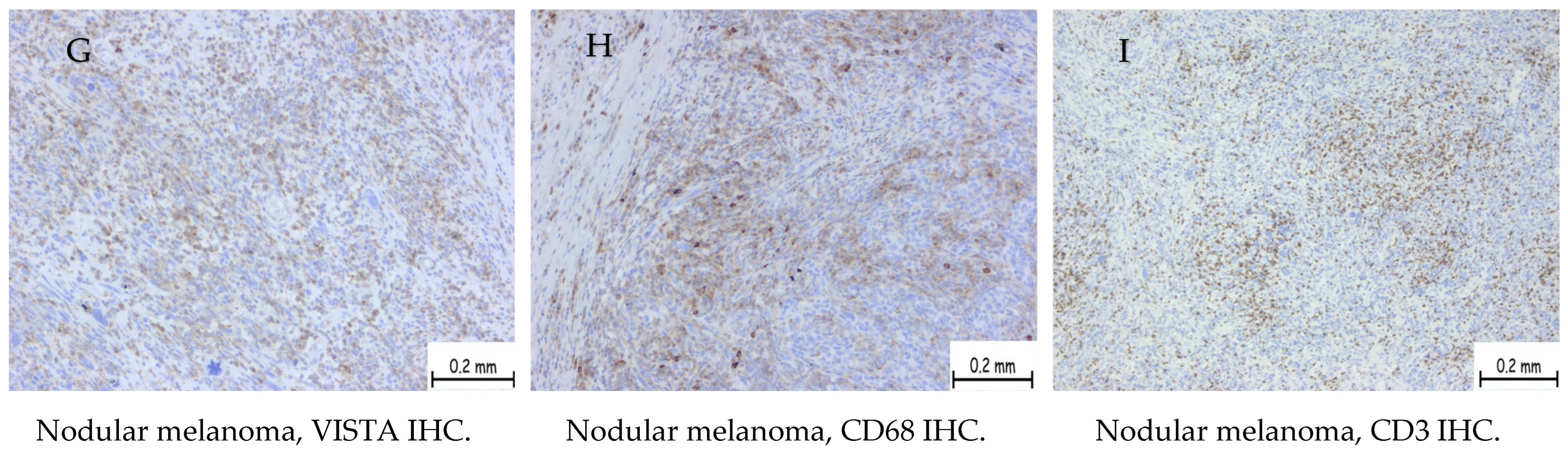
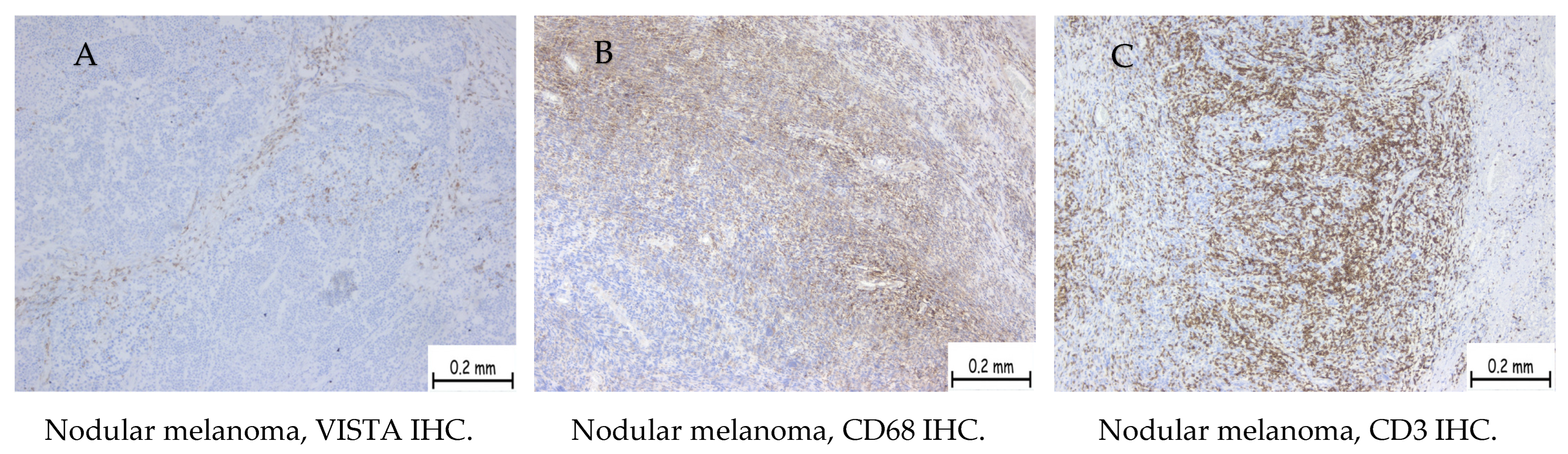

| Positive Cases | Nodular Melanoma | Superficial Spreading Melanoma | Uveal Melanoma |
|---|---|---|---|
| Total | 56 | 9 | 8 |
| High score | 41 | 0 | 3 |
| Low score | 15 | 9 | 5 |
| No. Cases | Tumor Stage | Positive Cases | Vista High H-Score | Vista Low H-Score |
|---|---|---|---|---|
| 3 | pT1a | 0 | 0 | 0 |
| 2 | pT2a | 1 | 1 | 0 |
| 7 | pT3a | 7 | 2 | 5 |
| VISTA+ | CD4+ | CD8+ | |
|---|---|---|---|
| High score | 41 | 10 | 31 |
| Low score | 15 | 5 | 10 |
| Low score | 9 | 9 | 0 |
| Total | 65 | 24 | 41 |
| Parameter | Positive VISTA Expression Patients n = 65 | Negative VISTA Expression Patients n = 27 | * p Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Age (years) | 64 (53–76) | 60 (49–68) | 0.11 | - | - | ||
| Advanced stage | 52 | 80 | 4 | 14.8 | <0.001 | 23.0 | 6.6–66.9 |
| Ki-67 proliferation index ≥ 25 | 25 | 38.4 | 1 | 3.7 | 0.001 | 16.2 | 2.5–173.6 |
| Pagetoid spread | 21 | 32.3 | 6 | 22.2 | 0.4 | 1.6 | 0.6–4.8 |
| Clark levels 3 to 5 | 58 | 89.2 | 20 | 74.0 | 0.1 | 2.9 | 0.9–8.8 |
| Inflammatory cells (moderate/high) | 59 | 90.7 | 1 | 3.7 | <0.001 | 255.7 | 34.2–2647 |
| Breslow thickness > 2 mm | 52 | 80 | 4 | 14.8 | <0.001 | 23.0 | 6.6–66.9 |
| Tumor ulceration | 36 | 55.3 | 7 | 25.9 | 0.01 | 3.5 | 1.2–8.8 |
| In situ component | 16 | 24.6 | 4 | 14.8 | 0.4 | 1.8 | 0.6–5.6 |
| Mitotic count (No) | 12 (5–20) | 6 (3–8) | <0.001 | - | - | ||
| Positive surgical margins | 7 | 10.7 | 1 | 3.7 | 0.4 | 3.1 | 0.5–36.6 |
| Perineural invasion | 2 | 3.0 | 2 | 7.4 | 0.7 | 0.3 | 0.06–2.6 |
| Lymphovascular invasion | 8 | 12.3 | 1 | 3.7 | 0.3 | 3.6 | 0.4–41.9 |
| Microsatelitosis | 3 | 4.6 | 1 | 3.7 | 0.7 | 1.2 | 0.1–16.9 |
| Metastasis | 10 | 15.3 | 1 | 3.7 | 0.2 | 4.7 | 0.7–53.1 |
| Parameter | High Levels VISTA Expression n = 42 | Low Levels VISTA Expression n = 23 | * p Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Age (years) | 72 (60–79) | 57 (47–66) | <0.001 | - | - | ||
| Advanced stage | 41 | 97.6 | 11 | 47.8 | <0.001 | 44.7 | 6.0–487.3 |
| Ki-67 proliferation index ≥ 25 | 19 | 45.2 | 6 | 26.0 | 0.18 | 2.3 | 0.7–7.4 |
| Pagetoid spread | 8 | 19.0 | 13 | 56.5 | 0.004 | 0.1 | 0.06–0.5 |
| Clark levels 3 to 5 | 40 | 95.2 | 18 | 76.2 | 0.09 | 5.5 | 0.9–29.1 |
| Inflammatory cells (moderate/high) | 41 | 97.6 | 18 | 76.2 | 0.03 | 11.3 | 1.3–136.7 |
| Breslow thickness > 2 mm | 39 | 92.8 | 13 | 56.5 | 0.001 | 10.0 | 2.5–36.3 |
| Tumor ulceration | 30 | 71.4 | 6 | 26.0 | <0.001 | 7.0 | 2.0–21.8 |
| In situ component | 7 | 16.6 | 9 | 39.1 | 0.06 | 0.3 | 0.09–1.0 |
| Mitotic count (No) | 14 (5–70) | 9 (4–16) | 0.05 | - | - | ||
| Positive surgical margins | 5 | 11.9 | 2 | 8.6 | 0.9 | 1.4 | 0.2–7.5 |
| Perineural invasion | 1 | 2.3 | 1 | 4.3 | 0.7 | 0.5 | 0.02–10.6 |
| Lymphovascular invasion | 6 | 14.2 | 2 | 8.6 | 0.7 | 1.7 | 0.3–9.0 |
| Microsatelitosis | 2 | 4.7 | 1 | 4.3 | 0.5 | 1.1 | 0.1–16.6 |
| Metastasis | 8 | 19.0 | 2 | 8.6 | 0.4 | 2.4 | 0.4–12.3 |
| Parameter | High VISTA Expression Levels n = 42 | Low VISTA Expression Levels n = 23 | Negative Expression of VISTA n = 27 | * p Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age | 72 (60–79) | 57 (47–66) | 60 (49–68) | 0.001 | |||
| Advanced stage | 41 | 97.6 | 11 | 47.8 | 4 | 14.8 | <0.001 |
| Ki-67 proliferation index ≥ 25 | 19 | 45.2 | 6 | 26.0 | 1 | 3.7 | <0.001 |
| Pagetoid spread | 8 | 19.0 | 13 | 56.5 | 6 | 22.2 | 0.004 |
| Clark levels 3 to 5 | 40 | 95.2 | 18 | 76.2 | 20 | 74.0 | 0.03 |
| Inflammatory cells (moderate/high) | 41 | 97.6 | 18 | 76.2 | 1 | 3.7 | <0.001 |
| Breslow thickness > 2 mm | 39 | 92.8 | 13 | 56.5 | 4 | 14.8 | <0.001 |
| Tumor ulceration | 30 | 71.4 | 6 | 26.0 | 7 | 25.9 | <0.001 |
| In situ component | 7 | 16.6 | 9 | 39.1 | 4 | 14.8 | 0.05 |
| Mitotic count (No) | 14 (5–70) | 9 (4–16) | 6 (3–8) | <0.001 | |||
| Positive surgical margins | 5 | 11.9 | 2 | 8.6 | 1 | 0.4 | 0.4 |
| Perineural invasion | 1 | 2.3 | 1 | 4.3 | 2 | 0.7 | 0.6 |
| Lymphovascular invasion | 6 | 14.2 | 2 | 8.6 | 1 | 0.3 | 0.3 |
| Microsatelitosis | 2 | 4.7 | 1 | 4.3 | 1 | 0.7 | 0.9 |
| Metastasis | 8 | 19.0 | 2 | 8.6 | 1 | 0.2 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinca, A.C.; Szoke, A.R.; Lazar, B.A.; Szász, E.A.; Tomuț, A.N.; Sabău, A.H.; Cocuz, I.-G.; Cotoi, T.-C.; Niculescu, R.; Chiorean, D.M.; et al. H-VISTA Immunohistochemistry Score Is Associated with Advanced Stages in Cutaneous and Ocular Melanoma. Int. J. Mol. Sci. 2024, 25, 4335. https://doi.org/10.3390/ijms25084335
Tinca AC, Szoke AR, Lazar BA, Szász EA, Tomuț AN, Sabău AH, Cocuz I-G, Cotoi T-C, Niculescu R, Chiorean DM, et al. H-VISTA Immunohistochemistry Score Is Associated with Advanced Stages in Cutaneous and Ocular Melanoma. International Journal of Molecular Sciences. 2024; 25(8):4335. https://doi.org/10.3390/ijms25084335
Chicago/Turabian StyleTinca, Andreea Cătălina, Andreea Raluca Szoke, Bianca Andreea Lazar, Emőke Andrea Szász, Alexandru Nicușor Tomuț, Adrian Horațiu Sabău, Iuliu-Gabriel Cocuz, Titiana-Cornelia Cotoi, Raluca Niculescu, Diana Maria Chiorean, and et al. 2024. "H-VISTA Immunohistochemistry Score Is Associated with Advanced Stages in Cutaneous and Ocular Melanoma" International Journal of Molecular Sciences 25, no. 8: 4335. https://doi.org/10.3390/ijms25084335
APA StyleTinca, A. C., Szoke, A. R., Lazar, B. A., Szász, E. A., Tomuț, A. N., Sabău, A. H., Cocuz, I.-G., Cotoi, T.-C., Niculescu, R., Chiorean, D. M., Ungureanu, I. A., Turdean, S. G., & Cotoi, O. S. (2024). H-VISTA Immunohistochemistry Score Is Associated with Advanced Stages in Cutaneous and Ocular Melanoma. International Journal of Molecular Sciences, 25(8), 4335. https://doi.org/10.3390/ijms25084335








