Water-Insoluble, Thermostable, Crosslinked Gelatin Matrix for Soft Tissue Implant Development
Abstract
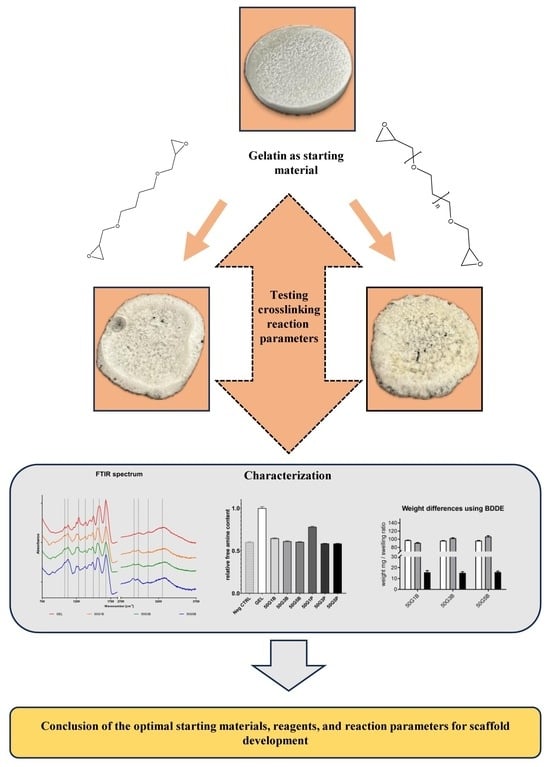
:1. Introduction
2. Results
2.1. Degradation of Native Gelatin in Solutions with Different pH Values
2.2. Weight Differences and Swelling Ratio
2.3. Enzymatic Degradation Differences
2.4. FTIR Spectroscopy
2.5. Free Primary Amine Content
2.6. Tensile Strength
2.7. Compression Test
3. Discussion
4. Materials and Methods
4.1. Hydrogel Preparation
4.2. Degradation of Native Gelatin in Different pH Values
4.3. Weight Differences and Swelling Ratio Measurements
4.4. Enzymatic Degradation Measurement
4.5. Structural Analysis Using FTIR Spectroscopy
4.6. Free Primary Amine Content
4.7. Compression Test
4.8. Tensile Strength
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.S.; Smoak, M.M.; Melchiorri, A.J.; Mikos, A.G. An Overview of the Tissue Engineering Market in the United States from 2011 to 2018. Tissue Eng. Part A 2019, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Kállay-Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, A.; Benyo, Z.; Hornyak, I. Overview of Tissue Engineering Patent Strategies and Patents from 2010 to 2020, Including Outcomes. Tissue Eng. Part B Rev. 2022, 28, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, A.; Kardos, D.; Lacza, Z.; Hornyák, I. A Practical Guide to Class IIa Medical Device Development. Appl. Sci. 2020, 10, 3638. [Google Scholar] [CrossRef]
- Hinsenkamp, A.; Fulop, A.; Hricisak, L.; Pal, E.; Kun, K.; Majer, A.; Varga, V.; Lacza, Z.; Hornyak, I. Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling. J. Funct. Biomater. 2022, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. From Collagen to Gelatine. In Gelatine Handbook; Wiley-VCH: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar]
- Shih, F.-Y.; Su, I.-J.; Chu, L.-L.; Lin, X.; Kuo, S.-C.; Hou, Y.-C.; Chiang, Y.-T. Development of Pectin-Type B Gelatin Polyelectrolyte Complex for Curcumin Delivery in Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 3625. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Luo, L.-J.; Ma, D.H.-K. Effect of Cross-Linking Density on the Structures and Properties of Carbodiimide-Treated Gelatin Matrices as Limbal Stem Cell Niches. Int. J. Mol. Sci. 2018, 19, 3294. [Google Scholar] [CrossRef] [PubMed]
- Gilsenan, P.M.; Ross-Murphy, S.B. Viscoelasticity of thermoreversible gelatin gels from mammalian and piscine collagens. J. Rheol. 2000, 44, 871–883. [Google Scholar] [CrossRef]
- Bikuna-Izagirre, M.; Aldazabal, J.; Paredes, J. Gelatin Blends Enhance Performance of Electrospun Polymeric Scaffolds in Comparison to Coating Protocols. Polymers 2022, 14, 1311. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.J.; Tsou, T.L.; Wang, H.J.; Hsu, S.H. Characterization of chitosan-gelatin scaffolds for dermal tissue engineering. J. Tissue Eng. Regen. Med. 2013, 7, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Masutani, E.M.; Kinoshita, C.K.; Tanaka, T.T.; Ellison, A.K.; Yoza, B.A. Increasing Thermal Stability of Gelatin by UV-Induced Cross-Linking with Glucose. Int. J. Biomater. 2014, 2014, 979636. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, B.; Parvin, T.N.; Alam, M.M.; Chanda, K.; Mm, B. Insights on Chemical Crosslinking Strategies for Proteins. Molecules 2022, 27, 8124. [Google Scholar] [CrossRef]
- Hinsenkamp, A.; Ezsias, B.; Pal, E.; Hricisak, L.; Fulop, A.; Besztercei, B.; Somkuti, J.; Smeller, L.; Pinke, B.; Kardos, D.; et al. Crosslinked Hyaluronic Acid Gels with Blood-Derived Protein Components for Soft Tissue Regeneration. Tissue Eng. Part. A 2021, 27, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Czerner, M.; Prudente, M.; Martucci, J.F.; Rueda, F.; Fasce, L.A. Mechanical behavior of cold-water fish gelatin gels crosslinked with 1,4-butanediol diglycidyl ether. J. Appl. Polym. Sci. 2020, 137, 48985. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Crosslinking and modification of dermal sheep collagen using 1, 4-butanediol diglycidyl ether. J. Biomed. Mater. Res. 1999, 46, 424–433. [Google Scholar] [CrossRef]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells. Int. J. Mol. Sci. 2018, 19, 3341. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Ahmad, T.; Zain, N.M.; Awang, S.R. Identification of bovine, porcine and fish gelatin signatures using chemometrics fuzzy graph method. Sci. Rep. 2021, 11, 9793. [Google Scholar] [CrossRef] [PubMed]
- Rusch, K.C. Load-compression behavior of brittle foams. J. Appl. Polym. Sci. 1970, 14, 1263–1276. [Google Scholar] [CrossRef]
- La Gatta, A.; Papa, A.; Schiraldi, C.; De Rosa, M. Hyaluronan dermal fillers via crosslinking with 1,4-butandiol diglycidyl ether: Exploitation of heterogeneous reaction conditions. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.; Hendriks, M.; Cahalan, P.T.; Feijen, J. The kinetics of 1,4-butanediol diglycidyl ether crosslinking of dermal sheep collagen. J. Biomed. Mater. Res. 2000, 51, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, J.W.; Braden, R.L.; Osborn, K.G.; Christman, K.L. Modulating In Vivo Degradation Rate of Injectable Extracellular Matrix Hydrogels. J. Mater. Chem. B 2016, 4, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J. Perspective article: The fate of collagen implants in tissue defects. Wound Repair. Regen. 2000, 8, 5–12. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Gu, Z.; Ma, Q.; Hu, X. Exploring the Structural Transformation Mechanism of Chinese and Thailand Silk Fibroin Fibers and Formic-Acid Fabricated Silk Films. Int. J. Mol. Sci. 2018, 19, 3309. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589–600. [Google Scholar] [CrossRef]
- Kircher, R.; Mross, S.; Hasse, H.; Münnemann, K. Functionalized Controlled Porous Glasses for Producing Radical-Free Hyperpolarized Liquids by Overhauser DNP. Molecules 2022, 27, 6402. [Google Scholar] [CrossRef] [PubMed]
- Deflores, L.P.; Ganim, Z.; Nicodemus, R.A.; Tokmakoff, A. Amide I’-II’ 2D IR spectroscopy provides enhanced protein secondary structural sensitivity. J. Am. Chem. Soc. 2009, 131, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Parker, F.S. Amides and Amines. In Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine; Parker, F.S., Ed.; Springer: Boston, MA, USA, 1971; pp. 165–172. [Google Scholar]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef] [PubMed]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, V.; Smeller, L.; Várdai, R.; Kocsis, B.; Zsoldos, I.; Cruciani, S.; Pala, R.; Hornyák, I. Water-Insoluble, Thermostable, Crosslinked Gelatin Matrix for Soft Tissue Implant Development. Int. J. Mol. Sci. 2024, 25, 4336. https://doi.org/10.3390/ijms25084336
Varga V, Smeller L, Várdai R, Kocsis B, Zsoldos I, Cruciani S, Pala R, Hornyák I. Water-Insoluble, Thermostable, Crosslinked Gelatin Matrix for Soft Tissue Implant Development. International Journal of Molecular Sciences. 2024; 25(8):4336. https://doi.org/10.3390/ijms25084336
Chicago/Turabian StyleVarga, Viktória, László Smeller, Róbert Várdai, Bence Kocsis, Ibolya Zsoldos, Sara Cruciani, Renzo Pala, and István Hornyák. 2024. "Water-Insoluble, Thermostable, Crosslinked Gelatin Matrix for Soft Tissue Implant Development" International Journal of Molecular Sciences 25, no. 8: 4336. https://doi.org/10.3390/ijms25084336