Nutritional Support: The Use of Antioxidants in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Antioxidant Effect of Minerals
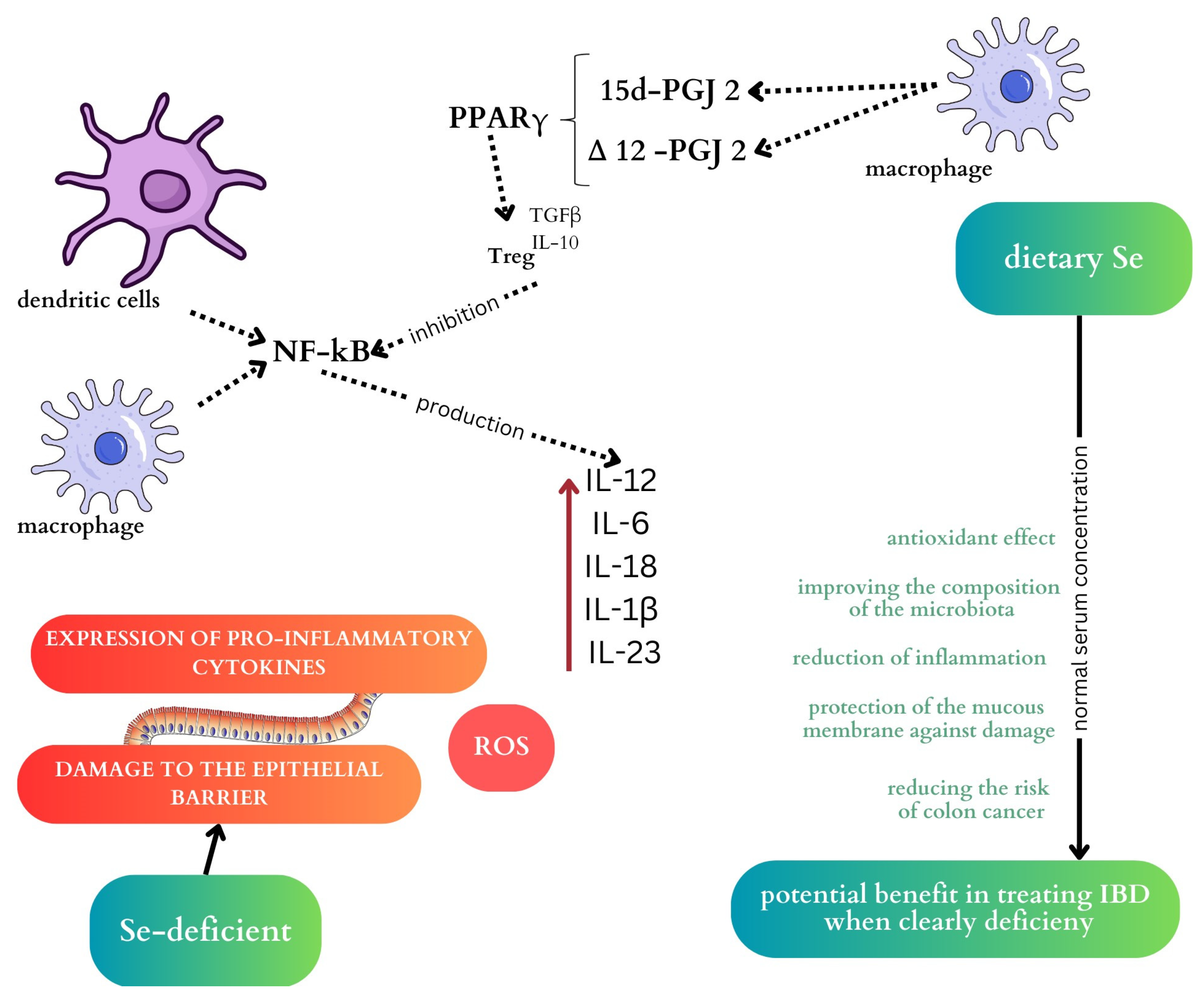
2.1. Selenium
2.1.1. The Effect of Selenium on the Microbiome
2.1.2. Selenium and Inflammatory Bowel Disease
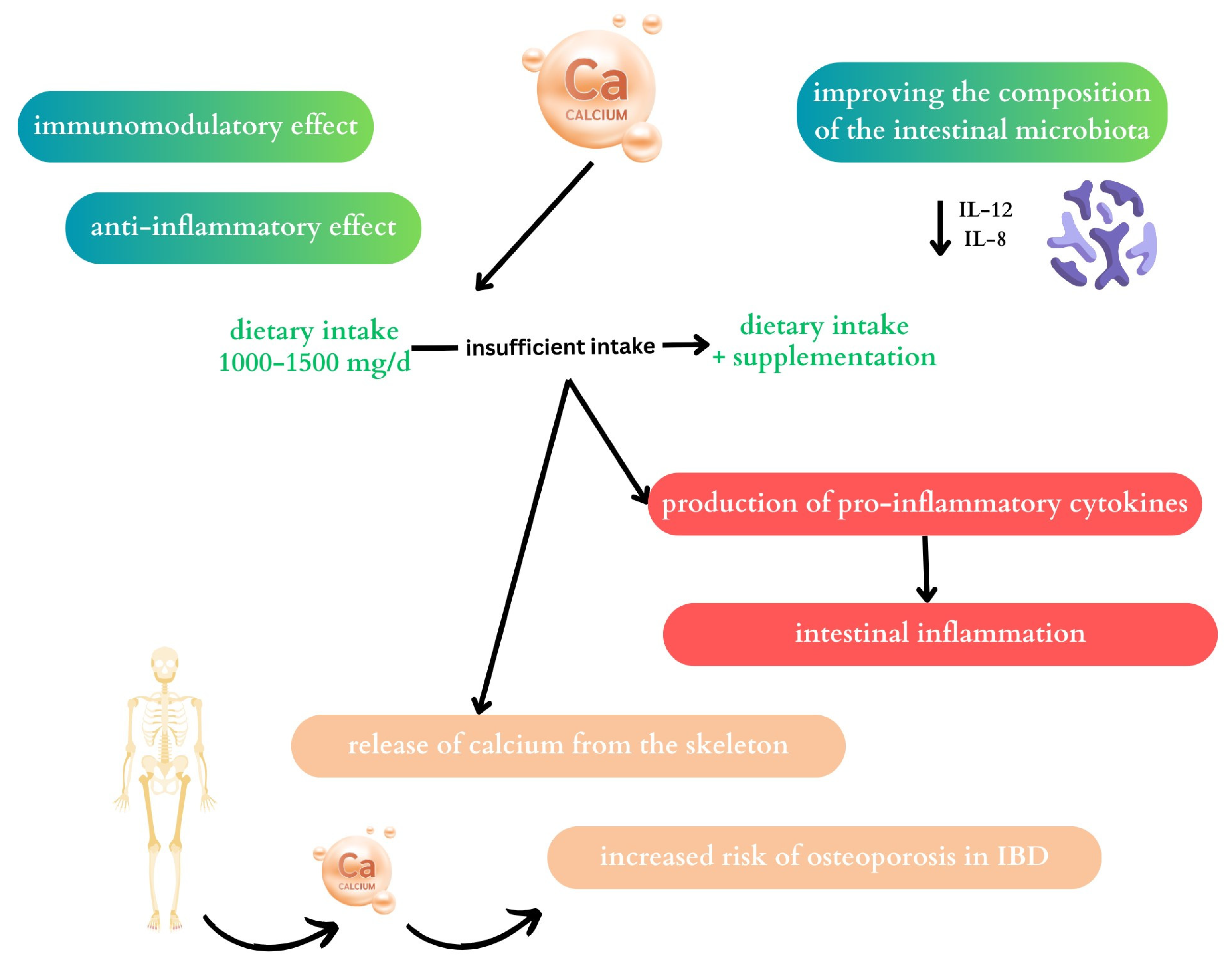
2.2. Calcium
2.2.1. The Effect of Calcium on the Microbiome
2.2.2. Calcium and Inflammatory Bowel Disease
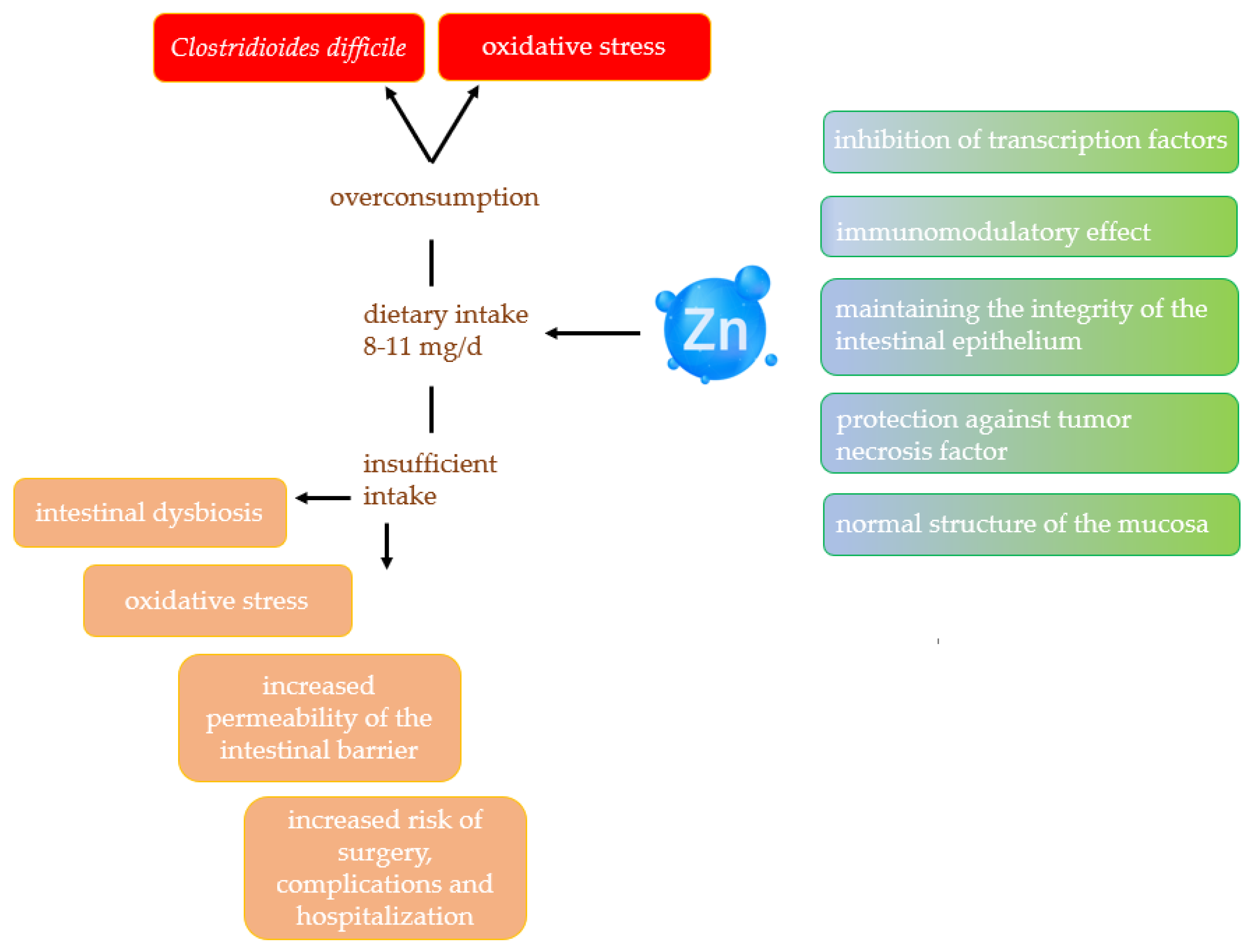
2.3. Zinc
2.3.1. Effect of Zinc on Gut Microbiota
2.3.2. Zinc and Inflammatory Bowel Disease
2.4. Magnesium
2.4.1. Effect of Magnesium on Gut Microbiota
2.4.2. Magnesium and Inflammatory Bowel Disease
2.5. Iron
2.5.1. Effect of Iron on Gut Microbiota
2.5.2. Iron and Inflammatory Bowel Disease
3. Limitations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 1, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Buie, M.J.; Quan, J.; Windsor, J.W.; Coward, S.; Hansen, T.M.; King, J.A.; Kotze, P.G.; Gearry, R.B.; Ng, S.C.; Mak, J.W.Y.; et al. Global Hospitalization Trends for Crohn’s Disease and Ulcerative Colitis in the 21st Century: A Systematic Review with Temporal Analyses. Clin. Gastroenterol. Hepatol. 2023, 21, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Allin, K.H.; Petralia, F.; Colombel, J.F.; Jess, T. Multiomics to elucidate inflammatory bowel disease risk factors and pathways. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Wang, R.; Ren, F.; Wang, X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines 2022, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Kikut, J.; Konecka, N.; Ziętek, M.; Kulpa, D.; Szczuko, M. Diet supporting therapy for inflammatory bowel diseases. Eur. J. Nutr. 2021, 60, 2275–2291. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Malard, E.; Valable, S.; Bernaudin, M.; Pérès, E.; Chatre, L. The Reactive Species Interactome in the Brain. Antioxid. Redox Signal. 2021, 35, 1176–1206. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites 2023, 13, 573. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 4, 685317. [Google Scholar] [CrossRef] [PubMed]
- Selenium. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ (accessed on 15 December 2023).
- Silva Junior, E.C.; Wadt, L.H.O.; Silva, K.E.; Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Carvalho, G.S.; Carvalho, T.S.; Reis, A.R.; Lopes, G.; et al. Natural variation of selenium in Brazil nuts and soils from the Amazon region. Chemosphere 2017, 188, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Yang, S.; Li, T.; Huang, X.; Dong, Y.; Wang, P.; Huang, J. Advances in the Study of the Mechanism by Which Selenium and Selenoproteins Boost Immunity to Prevent Food Allergies. Nutrients 2022, 14, 3133. [Google Scholar] [CrossRef]
- Swart, R.; Schutte, A.E.; van Rooyen, J.M.; Mels, C.M.C. Serum selenium levels, the selenoprotein glutathione peroxidase and vascular protection: The SABPA study. Food Res. Int. 2018, 104, 69–76. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 1, 113–119. [Google Scholar] [CrossRef]
- Ma, C.; Hoffmann, P.R. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin. Cell Dev. Biol. 2021, 115, 54–61. [Google Scholar] [CrossRef]
- Lee, S.; Lee, E.K.; Kang, D.H.; Lee, J.; Hong, S.H.; Jeong, W.; Kang, S.W. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp. Mol. Med. 2021, 53, 1080–1091. [Google Scholar] [CrossRef]
- Fredericks, G.J.; Hoffmann, P.R. Selenoprotein K and protein palmitoylation. Antioxid. Redox Signal. 2015, 23, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.Z.; Kerkadi, A.; Agouni, A. Selenium and Health: An Update on the Situation in the Middle East and North Africa. Nutrients 2019, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.H.; Wang, T.; Regmi, N.; Chen, X.; Huang, K.; Liao, S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.X.; Cen, S.; Li, P.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Chen, W. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ. Sci. Technol. 2018, 5, 724–730. [Google Scholar] [CrossRef]
- Souza Silveira, B.K.; Rocha, D.M.U.P.; Stampini Duarte Martino, H.; Grancieri, M.; Gomes, M.J.C.; Cuquetto Mantovani, H.; Bressan, J.; Hermsdorff, H.H.M. Daily Cashew and Brazil Nut Consumption Modifies Intestinal Health in Overweight Women on Energy-Restricted Intervention: A Randomized Controlled Trial (Brazilian Nuts Study). J. Nutr. 2024, 154, 962–977. [Google Scholar] [CrossRef]
- Cai, J.; Su, W.; Chen, X.; Zheng, H. Advances in the study of selenium and human intestinal bacteria. Front. Nutr. 2022, 14, 1059358. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Effect of gut microflora on nutritional availability of selenium. Food Chem. 2020, 30, 126537. [Google Scholar] [CrossRef]
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G71–G77. [Google Scholar] [CrossRef]
- Barra, N.G.; Anhê, F.F.; Cavallari, J.F.; Singh, A.M.; Chan, D.Y.; Schertzer, J.D. Micronutrients impact the gut microbiota and blood glucose. J. Endocrinol. 2021, 28, 1–21. [Google Scholar] [CrossRef]
- Andoh, A.; Hirashima, M.; Maeda, H.; Hata, K.; Inatomi, O.; Tsujikawa, T.; Sasaki, M.; Takahashi, K.; Fujiyama, Y. Serum selenoprotein-P levels in patients with inflammatory bowel disease. Nutrition 2005, 21, 574–579. [Google Scholar] [CrossRef]
- Barrett, C.W.; Singh, K.; Motley, A.K.; Lintel, M.K.; Matafonova, E.; Bradley, A.M.; Ning, W.; Poindexter, S.V.; Parang, B.; Reddy, V.K.; et al. Dietary selenium deficiency exacerbates DSS-induced epithelial injury and AOM/DSS-induced tumorigenesis. PLoS ONE 2013, 8, e67845. [Google Scholar] [CrossRef] [PubMed]
- Hiller, F.; Oldorff, L.; Besselt, K.; Kipp, A.P. Differential Acute Effects of Selenomethionine and Sodium Selenite on the Severity of Colitis. Nutrients 2015, 7, 2687–2706. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Kheyri, Z. The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view. Nutrition 2021, 85, 111153. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, J.; Godefroy, E.; Papargyris, L.; Sarrabayrouse, G.; Tabiasco, J.; Bridonneau, C.; Yazdanbakhsh, K.; Sokol, H.; Altare, F.; Jotereau, F. Faecalibacterium prausnitzii Skews Human DC to Prime IL10-Producing T Cells Through TLR2/6/JNK Signaling and IL-10, IL-27, CD39, and IDO-1 Induction. Front. Immunol. 2019, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Yoon, H.; Lim, S.; Sung, M.K.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. Risk Factors for Vitamin D, Zinc, and Selenium Deficiencies in Korean Patients with Inflammatory Bowel Disease. Gut Liver 2017, 11, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Stochel-Gaudyn, A.; Fyderek, K.; Kościelniak, P. Serum trace elements profile in the pediatric inflammatory bowel disease progress evaluation. J. Trace Elem. Med. Biol. 2019, 55, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J. Dig. Dis. 2019, 20, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Nettleford, S.K.; Prabhu, K.S. Selenium and Selenoproteins in Gut Inflammation—A Review. Antioxidants 2018, 7, 36. [Google Scholar] [CrossRef]
- Scientific Opinion on Dietary Reference Values for Calcium. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4101 (accessed on 15 December 2023).
- Calcium. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional (accessed on 15 December 2023).
- Wongdee, K.; Rodrat, M.; Teerapornpuntakit, J. Factors inhibiting intestinal calcium absorption: Hormones and luminal factors that prevent excessive calcium uptake. J. Physiol. Sci. 2019, 69, 683–696. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.; Sommerfeld, V.; Huber, K.; Rodehutscord, M.; Stefanski, V. Immunomodulatory Effects of Dietary Phosphorus and Calcium in Two Strains of Laying Hens. Animals 2021, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, H.; Han, J. Anti-inflammatory effects of calcium citrate in RAW 264.7cells via suppression of NF-κB activation. Environ. Toxicol. Pharmacol. 2015, 39, 27–34. [Google Scholar] [CrossRef]
- Wang, K.; Yang, A.; Peng, X.; Lv, F.; Wang, Y.; Cui, Y.; Wang, Y.; Zhou, J.; Si, H. Linkages of Various Calcium Sources on Immune Performance, Diarrhea Rate, Intestinal Barrier, and Post-gut Microbial Structure and Function in Piglets. Front. Nutr. 2022, 17, 921773. [Google Scholar] [CrossRef]
- Rosa, M.J.; Lee, L.G.; Wright, R.J. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Yang, J.S.; Kim, K.A.; Yoon, K.Y.; Song, D.G.; Erdene-Ochir, E.; Kang, K.; Pan, C.H.; Ko, G. Improvement in host metabolic homeostasis and alteration in gut microbiota in mice on the high-fat diet: A comparison of calcium supplements. Food Res. Int. 2020, 136, 109495. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Fuhren, J.; Schwalbe, M.; Boekhorst, J.; Rösch, C.; Schols, H.A.; Kleerebezem, M. Dietary calcium phosphate strongly impacts gut microbiome changes elicited by inulin and galacto-oligosaccharides consumption. Microbiome 2021, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2016, 60, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Guo, Z.; Kwok, L.Y.; Zhao, Z.; Wang, K.; Li, Y.; Sun, Z.; Zhao, J.; Zhang, H. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur. J. Nutr. 2023, 62, 965–976. [Google Scholar] [CrossRef]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Malik, T.F.; Aurelio, D.M. Extraintestinal Manifestations of Inflammatory Bowel Disease. Updated 6 March 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568797/ (accessed on 15 December 2023).
- Vernia, P.; Loizos, P.; Di Giuseppantonio, I.; Amore, B.; Chiappini, A.; Cannizzaro, S. Dietary calcium intake in patients with inflammatory bowel disease. J. Crohns Colitis 2014, 8, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liao, Y.; Ouyang, Y.; Wu, Z.; Li, Z.; Lin, J.; Zhang, K.; Wang, X.; Cen, Z.; Ma, W.; et al. The effects and predictive value of calcium and magnesium concentrations on nutritional improvement, inflammatory response and diagnosis in patients with Crohn’s disease. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2023, 36, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Algieri, F.; Rodriguez-Nogales, A.; Garrido-Mesa, J.; Camuesco, D.; Vezza, T.; Garrido-Mesa, N.; Utrilla, P.; Rodriguez-Cabezas, M.E.; Pischel, I.; Galvez, J. Intestinal anti-inflammatory activity of calcium pyruvate in the TNBS model of rat colitis: Comparison with ethyl pyruvate. Biochem. Pharmacol. 2016, 103, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 1, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Włodarek, D.; Lange, E. Współczesna Dietoterapia; PZWL: Warszawa, Polska, 2023; p. 165, EAN 9788301227548. [Google Scholar]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Vegetarian diets across the lifecycle: Impact on zinc intake and status. Adv. Food Nutr. Res. 2015, 74, 93–131. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Zinc. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Zinc-Consumer (accessed on 1 April 2024).
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.S.; Campa, A.; Li, Y.; Fleetwood, C.; Stewart, T.; Ramamoorthy, V.; Baum, M.K. Low Plasma Zinc Is Associated with Higher Mitochondrial Oxidative Stress and Faster Liver Fibrosis Development in the Miami Adult Studies in HIV Cohort. J. Nutr. 2017, 147, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc Homeostasis in Platelet-Related Diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef]
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Bakiu, R.; Pucciarelli, S.; Miceli, C.; Vallesi, A.; Irato, P.; Santovito, G. Molecular Characterization, Protein–Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena thermophila. Antioxidants 2020, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, G.; Ferruzza, S.; Canali, R.; Leoni, G.; Zalewski, P.D.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J. Nutr. Biochem. 2013, 24, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Milutinovic, A.S.; Milovanovic, T.; Stojkovic Lalosevic, M.; Stojanovic, M.; Pavlovic, S.; Popovic, D. Genetic Aspects of Micronutrients Important for Inflammatory Bowel Disease. Life 2022, 12, 1623. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- Islam, T.; Albracht-Schulte, K.; Ramalingam, L.; Schlabritz-Lutsevich, N.; Hee Park, O.; Zabet-Moghaddam, M.; Kalupahana, N.S.; Moustaid-Moussa, N. Anti-inflammatory mechanisms of polyphenols in adipose tissue: Role of gut microbiota, intestinal barrier integrity and zinc homeostasis. J. Nutr. Biochem. 2023, 115, 109242. [Google Scholar] [CrossRef]
- Zackular, J.P.; Moore, J.L.; Jordan, A.T.; Juttukonda, L.J.; Noto, M.J.; Nicholson, M.R.; Crews, J.D.; Semler, M.W.; Zhang, Y.; Ware, L.B.; et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016, 22, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Chovolou, Y.; Watjen, W.; Kampkotter, A.; Kahl, R. Resistance to tumor necrosis factor-alpha (TNF-alpha)-induced apoptosis in rat hepatoma cells expressing TNF-alpha is linked to low antioxidant enzyme expression. J. Biol. Chem. 2003, 278, 29626–29632. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Di Leo, V.; Ferronato, A.; D’Odorico, A.; D’Incà, R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, V.; D’Incà, R.; Barollo, M.; Tropea, A.; Fries, W.; Mazzon, E.; Irato, P.; Cecchetto, A.; Sturniolo, G.C. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig. Liver Dis. 2001, 33, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Balsiger, L.M.; Maurizi, V.; Rinninella, E.; Gasbarrini, A.; Giostra, N.; Santori, P.; Abenavoli, L.; Rasetti, C. Zinc and gut microbiota in health and gastrointestinal disease under the COVID-19 suggestion. Biofactors 2022, 48, 294–306. [Google Scholar] [CrossRef]
- Ishihara, J.; Arai, K.; Kudo, T.; Nambu, R.; Tajiri, H.; Aomatsu, T.; Abe, N.; Kakiuchi, T.; Hashimoto, K.; Sogo, T.; et al. Serum Zinc and Selenium in Children with Inflammatory Bowel Disease: A Multicenter Study in Japan. Dig. Dis. Sci. 2022, 67, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Moon, N.; Figgins, B.; Altshuler, E.; Pham, A.; Kamel, A.Y. Concurrent zinc and vitamin B6 deficiencies in acutely exacerbated inflammatory bowel disease: Case reports. Nutr. Clin. Pract. 2022, 37, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium—An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Magnesium. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 2 April 2024).
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U. Magnesium and Drugs. Int. J. Mol. Sci. 2019, 20, 2094. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Ederveen, T.H.A.; van der Wijst, J.; Overmars-Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Agus, Z.S. Mechanisms and causes of hypomagnesemia. Curr. Opin. Nephrol. Hypertens. 2016, 25, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Petito, V.; Di Agostini, A.; Arduini, D.; Hamersma, W.; Pietropaolo, G.; Luongo, F.; Arena, V.; Stigliano, E.; Lopetuso, L.R.; et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Upregulation of the Transient Receptor Potential Melastatin 6 Channel. Inflamm. Bowel Dis. 2018, 24, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Liu, M.; Jeong, E.M.; Liu, H.; Xie, A.; So, E.Y.; Shi, G.; Jeong, G.E.; Zhou, A.; Dudley, S.C., Jr. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 2019, 4, 123182. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef]
- García-Legorreta, A.; Soriano-Pérez, L.A.; Flores-Buendía, A.M.; Medina-Campos, O.N.; Noriega, L.G.; Granados-Portillo, O.; Nambo-Venegas, R.; Tovar, A.R.; Mendoza-Vargas, A.; Barrera-Oviedo, D.; et al. Effect of Dietary Magnesium Content on Intestinal Microbiota of Rats. Nutrients 2020, 12, 2889. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shi, H.; Qian, C.; Han, H.; Lu, K.; Tao, R.; Gu, R.; Zhao, Y.; Wei, Z.; Lu, Y. Modulation of Gut Microbiota by Magnesium Isoglycyrrhizinate Mediates Enhancement of Intestinal Barrier Function and Amelioration of Methotrexate-Induced Liver Injury. Front. Immunol. 2022, 13, 874878. [Google Scholar] [CrossRef]
- Cao, S.; Huang, K.; Wen, X.; Gao, J.; Cui, B.; Yao, K.; Zhan, X.; Hu, S.; Wu, Q.; Xiao, H.; et al. Dietary supplementation with potassium-magnesium sulfate modulates the antioxidant capacity, immunity, and gut microbiota in weaned piglets. Front. Microbiol. 2022, 13, 961989. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Trapani, V.; Petito, V.; Reddel, S.; Pietropaolo, G.; Graziani, C.; Masi, L.; Gasbarrini, A.; Putignani, L.; Scaldaferri, F.; et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota. Nutrients 2021, 13, 4188. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Ichikawa, N.; Sasaki, H.; Shibata, S. The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients 2021, 13, 152. [Google Scholar] [CrossRef]
- Aljewicz, M.; Siemianowska, E.; Cichosz, G.; Tońska, E. The effect of probiotics (Lactobacillus rhamnosus HN001, Lactobacillus paracasei LPC-37, and Lactobacillus acidophilus NCFM) on the availability of minerals from Dutch-type cheese. J. Dairy Sci. 2014, 97, 4824–4831. [Google Scholar] [CrossRef]
- Bergillos-Meca, T.; Cabrera-Vique, C.; Artacho, R.; Moreno-Montoro, M.; Navarro-Alarcón, M.; Olalla, M.; Giménez, R.; Seiquer, I.; Ruiz-López, M.D. Does Lactobacillus plantarum or ultrafiltration process improve Ca, Mg, Zn and P bioavailability from fermented goats’ milk? Food Chem. 2015, 187, 314–321. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef]
- Geerling, B.J.; Badart-Smook, A.; Stockbrügger, R.W.; Brummer, R.J. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur. J. Clin. Nutr. 2000, 54, 514–521. [Google Scholar] [CrossRef]
- Gilca-Blanariu, G.E.; Trifan, A.; Ciocoiu, M.; Popa, I.V.; Burlacu, A.; Balan, G.G.; Olteanu, A.V.; Stefanescu, G. Magnesium—A Potential Key Player in Inflammatory Bowel Diseases? Nutrients 2022, 14, 1914. [Google Scholar] [CrossRef] [PubMed]
- Soare, I.; Sirbu, A.; Diculescu, M.M.; Mateescu, B.R.; Tieranu, C.; Martin, S.; Barbu, C.G.; Ionescu, M.; Fica, S. Lean mass, magnesium, faecal calprotectin and glucocorticoid exposure as risk factors for low bone mineral density in inflammatory bowel disease patients. Endocr. Connect. 2021, 10, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmiercza, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Blood and iron. J. Mol. Med. 2015, 93, 469–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samek, G.; Gogga, P. Prevention of iron deficiency in persons on vegan diet. Med. Og. Nauk. Zdr. 2022, 28, 33–39. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- National Institutes of Health (NIH) Office of Dietary Supplements (ODS). Iron. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/?fbclid=IwAR1jOEVSHAaaaciW5F0_2xPs7vXt92CnL-hjghXXwdhZhlj3kPQhdZ4nrfE (accessed on 15 December 2023).
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- Husmann, F.M.D.; Zimmermann, M.B.; Herter-Aeberli, I. The Effect of Prebiotics on Human Iron Absorption: A Review. Adv. Nutr. 2022, 13, 2296–2304. [Google Scholar] [CrossRef]
- Chifman, J.; Laubenbacher, R.; Torti, S.V. A systems biology approach to iron metabolism. Adv. Exp. Med. Biol. 2014, 844, 201–225. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tang, R.; Wang, J.; Wan, D.; Yin, Y.; Xie, L. Gut microbiota bridges the iron homeostasis and host health. Sci. China Life Sci. 2023, 66, 1952–1975. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef]
- Puga, A.M.; Samaniego-Vaesken, M.L.; Montero-Bravo, A.; Ruperto, M.; Partearroyo, T.; Varela-Moreiras, G. Iron Supplementation at the Crossroads of Nutrition and Gut Microbiota: The State of the Art. Nutrients 2022, 14, 1926. [Google Scholar] [CrossRef]
- Malesza, I.J.; Bartkowiak-Wieczorek, J.; Winkler-Galicki, J.; Nowicka, A.; Dzięciołowska, D.; Błaszczyk, M.; Gajniak, P.; Słowińska, K.; Niepolski, L.; Walkowiak, J.; et al. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review. Nutrients 2022, 14, 3478. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, J.; Zhang, Q.; Chen, G.; Yin, S.; Luo, Z.; Zeng, W.; Yu, J.; Lan, P.; He, Z. Dietary iron modulates gut microbiota and induces SLPI secretion to promote colorectal tumorigenesis. Gut Microbes 2023, 15, 2221978. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Wu, A.; Yu, B.; Zhang, T.; Lai, X.; Chen, J.; Yan, H.; Zheng, P.; Luo, Y.; Luo, J.; et al. Iron overload induces colitis by modulating ferroptosis and interfering gut microbiota in mice. Sci. Total Environ. 2023, 905, 167043. [Google Scholar] [CrossRef]
- Mahadea, D.; Adamczewska, E.; Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Iron Deficiency Anemia in Inflammatory Bowel Diseases—A Narrative Review. Nutrients 2021, 13, 4008. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Patel, D.; Khan, N. Iron deficiency anemia in IBD: An overlooked comorbidity. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv. Exp. Med. Biol. 2021, 1301, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Yuan, J.; Wei, Z.; Mao, F.; Zhang, Z. Role of ferroptosis in the pathogenesis and as a therapeutic target of inflammatory bowel disease (Review). Int. J. Mol. Med. 2023, 51, 53. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; He, Y.; Lin, L.; Chen, P.; Chen, M.; Zhang, S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Ma, J.; Ma, J.; Liu, J.; Wang, F.; Tang, X. Inhibiting Ferroptosis: A Novel Approach for Ulcerative Colitis Therapeutics. Oxidative Med. Cell. Longev. 2022, 2022, 9678625. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Yilmaz, B.; Li, H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, N.; Cui, M.; Zhang, M. Role of epigenetic modifications mediated by vitamins and trace elements in inflammatory bowel disease. Epigenomics 2023, 15, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wu, X.; Lu, S.; Du, J.; Long, Y.; Zhu, Y.; Qin, H. Engineered procyanidin-Fe nanoparticle alleviates intestinal inflammation through scavenging ROS and altering gut microbiome in colitis mice. Front. Chem. 2023, 11, 1089775. [Google Scholar] [CrossRef] [PubMed]
| Selenoptrotein | Functions |
|---|---|
| GPXs | Promote hydroperoxide metabolism and reduce damage to the body [17]. |
| GPX1 | Key regulatory role in the cell apoptosis signalling pathway (mainly, in which damaged mitochondria produce ROS above a physiological level; involved in the activation and differentiation of T cells [17,21]. |
| TXNRD | Acts as a regulatory factor and regulated target in macrophage gene expression [17]. |
| DIO | Deficiency interferes with the conversion of T4 to T3 and may affect systemic selenium levels [17]. |
| MSRB1 | Responsible for DC-mediated T cell activation and Th1 differentiation; acts as an oxidoreductase [17]. |
| SPS2 | A catalyst for selenophosphate production, which is used to synthesise selenoproteins [20] |
| SELENOK | Promotes Ca2+ flow in immune cell activation; interacts with DHHC6; the SELENOK promotes palmioylation of proteins catalysed by DHHC6 [17,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarmakiewicz-Czaja, S.; Ferenc, K.; Sokal-Dembowska, A.; Filip, R. Nutritional Support: The Use of Antioxidants in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 4390. https://doi.org/10.3390/ijms25084390
Jarmakiewicz-Czaja S, Ferenc K, Sokal-Dembowska A, Filip R. Nutritional Support: The Use of Antioxidants in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2024; 25(8):4390. https://doi.org/10.3390/ijms25084390
Chicago/Turabian StyleJarmakiewicz-Czaja, Sara, Katarzyna Ferenc, Aneta Sokal-Dembowska, and Rafał Filip. 2024. "Nutritional Support: The Use of Antioxidants in Inflammatory Bowel Disease" International Journal of Molecular Sciences 25, no. 8: 4390. https://doi.org/10.3390/ijms25084390
APA StyleJarmakiewicz-Czaja, S., Ferenc, K., Sokal-Dembowska, A., & Filip, R. (2024). Nutritional Support: The Use of Antioxidants in Inflammatory Bowel Disease. International Journal of Molecular Sciences, 25(8), 4390. https://doi.org/10.3390/ijms25084390







