Essential Role of COPII Proteins in Maintaining the Contractile Ring Anchoring to the Plasma Membrane during Cytokinesis in Drosophila Male Meiosis
Abstract
:1. Introduction
2. Results
2.1. Multi-Nuclear Cells in the Spermatid Cysts Derived from Spermatocytes That Harbored Silencing of mRNAs Encoding COPII Coatomer Proteins
2.2. Sar1-Dependent COPII Foci Containing Sec13 and Sec23 or Sec31 in Spermatocytes before and during Male Meiosis
2.3. Co-Localization of COPII-Containing Foci with cis-Golgi Marker and Their Dynamic Astral Microtubule-Dependent Distribution in Meiotic Cells
2.4. Sar1 Silencing Did Not Affect Furrowing Initiation
2.5. Sar1 Silencing Led to Abnormal Contractile-Actomyosin and Anilloseptin Ring Localization at the Cell Equator in Telophase I
2.6. Sar1-Dependent Formation of Vesicles Containing DE-Cadherin in Spermatocytes and Their Accumulation in the Cleavage Furrow Sites during Male Meiosis
2.7. COPII Depletion Disrupted Novel Membrane Insertion into CF Sites during Cytokinesis
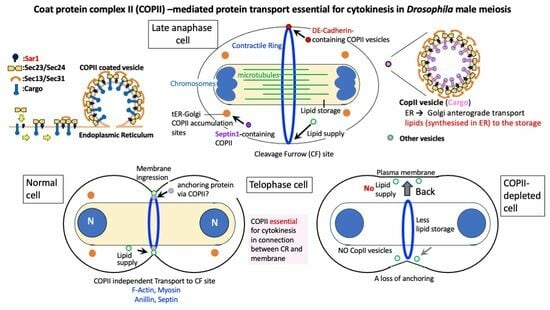
3. Discussion
3.1. COPII-Coated Vesicles Are Indispensable for Cytokinesis in Drosophila Male Meiotic Division
3.2. COPII-Coated Vesicles Are Essential for the Continuous Ingression of the Cleavage Furrow While Coordinating with CR Constriction in Cytokinesis
3.3. Sar1-Dependent DE-Cadherin-Containing Vesicles Accumulate around the CR
3.4. Involvement of COPII Vesicles in the Supply of Membrane Components to Newly Added Plasma Membrane Connected to the Constricting CR
3.5. Comparing the Role of Coatomer-Mediated Transport in Mitosis and Drosophila Male Meiosis and between COPI- and COPII-Mediated Transport in Male Meiosis
4. Materials and Methods
4.1. Drosophila Stocks
4.2. Establishment of the Fly Stock to Express RFP-Tagged Sec13
4.3. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.4. Preparation of Post-Meiotic Spermatid Cysts
4.5. Immunostaining
4.6. In Situ Proximity Ligation Assay (PLA)
4.7. Wheat Germ Agglutinin (WGA) Staining
4.8. Time-Lapse Observation of Male Meiotic Cells
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glotzer, M. Animal cell cytokinesis. Annu. Rev. Cell Dev. Biol. 2001, 17, 351–386. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, M.G.; Bonaccorsi, S.; Williams, B.; Williams, E.V.; Santolamazza, C.; Goldberg, M.L.; Gatti, M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998, 12, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.H.; Matthew, M.S.; Suzuki, T.; Máthé, E.; Yamamoto, M.; Glover, D.M. Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J. Cell Biol. 2004, 166, 49–60. [Google Scholar] [CrossRef] [PubMed]
- D’Avino, P.P.; Takeda, T.; Capalbo, L.; Zhang, W.; Lilley, K.S.; Laue, E.D.; Glover, D.M. Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J. Cell Sci. 2008, 121, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; Sasaki, T.; Kuroda, S.; Itoh, T.; Takai, Y. Regulation of cytoplasmic division of Xenopus embryo by Rho p21 and its inhibitory GDP/GTP exchange protein (Rho GDI). J. Cell Biol. 1993, 1, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Madaule, P.; Reid, T.; Ishizaki, T.; Watanabe, G.; Kakizuka, A.; Saito, S.; Nakao, K.; Jockusch, B.M.; Narumiya, S. p140mDia, a mammalian homolog of Drosophila diaphanous is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997, 16, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Kosako, H.; Yoshida, T.; Matsumura, F.; Ishizaki, T.; Narumiya, S.; Inagaki, M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 2000, 19, 6059–6064. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, M. The Molecular requirements for cytokinesis. Science 2005, 307, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.; Wadsworth, P. Myosin-II-dependent localization and dynamics of F-Actin during cytokinesis. Curr. Biol. 2005, 15, 724–731. [Google Scholar] [CrossRef]
- Carim, S.C.; Hickson, G.R.X. The Rho1 GTPase controls anillo-septin assembly to facilitate contractile ring closure during cytokinesis. iScience 2023, 26, 106903. [Google Scholar] [CrossRef]
- Straight, A.F.; Field, C.M.; Mitchison, T.J. Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol. Biol. Cell 2005, 16, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Aimar, C. Formation of new plasma membrane during the first cleavage cycle in the egg of Xenopus laevis, An immunocytological study. Dev. Growth Differ. 1997, 39, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Shuster, C.B.; Burgess, D.R. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc. Natl. Acad. Sci. USA 2002, 99, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Danilchik, M.V.; Bedrick, S.D.; Brown, E.E.; Ray, K. Furrow microtubules and localized exocytosis in cleaving Xenopus laevis embryos. J. Cell Sci. 2003, 116, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Skop, A.R.; Bergmann, D.; Mohler, W.A.; White, J.G. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr. Biol. 2001, 11, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Prekeris, R. Rabs, Rips, FIPs, and endocytic membrane traffic. Sci. World J. 2003, 3, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Altan-Bonnet, N.; Phair, R.D.; Polishchuk, R.S.; Weigert, R.; Lippincott-Schwartz, J. A role for Arf1 in mitotic Golgi disassembly, chromosome segregation, and cytokinesis. Proc. Natl. Acad. Sci. USA 2003, 100, 13314–13319. [Google Scholar] [CrossRef]
- Albertson, R.; Riggs, B.; Sullivan, W. Membrane traffic: A driving force in cytokinesis. Trends Cell Biol. 2005, 15, 92–101. [Google Scholar] [CrossRef]
- Wilson, G.M.; Fielding, A.B.; Simon, G.C.; Yu, X.; Andrews, P.D.; Hames, R.S.; Frey, A.M.; Peden, A.A.; Gould, G.W.; Prekeris, R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell 2005, 16, 849–860. [Google Scholar] [CrossRef]
- Schweitzer, J.K.; Souza-Schorey, C.D. A requirement for ARF6 during the completion of cytokinesis. Exp. Cell Res. 2005, 311, 74–83. [Google Scholar] [CrossRef]
- Schweitzer, J.K.; Burke, E.E.; Goodson, H.V.; D’Souza-Schorey, C. Endocytosis resumes during late mitosis and is required for cytokinesis. J. Biol. Chem. 2005, 280, 41628–41635. [Google Scholar] [CrossRef] [PubMed]
- McKay, H.F.; Burgess, D.R. ‘Life is a Highway’: Membrane trafficking during cytokinesis. Traffic 2011, 12, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, D.; Yamaguchi, M.; Mori, H.; Inoue, Y.H. COPI-mediated membrane trafficking is required for cytokinesis in Drosophila male meiotic divisions. J. Cell Sci. 2012, 125, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.H.; Miyauchi, C.; Ogata, T.; Kitazawa, D. Dynamic alteration of cellular component of male meiosis in Drosophila. In Meiosis-Molecular Mechanisms and Cytogenetic Diversity; Swan, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 953–979. [Google Scholar]
- Giansanti, M.G.; Fuller, M.T. What Drosophila spermatocytes tell us about the mechanisms underlying cytokinesis. Cytoskeleton 2012, 69, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Dyer, N.; Rebollo, E.; Domínguez, P.; Elkhatib, N.; Chavrier, P.; Daviet, L.; González, C.; González-Gaitán, M. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development 2007, 134, 4437–4447. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, M.G.; Belloni, G.; Gatti, M. Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males. Mol. Biol. Cell 2007, 18, 5034–5047. [Google Scholar] [CrossRef] [PubMed]
- Farkas, R.M.; Giansanti, M.G.; Gatti, M.; Fuller, M.T. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol. Biol. Cell 2003, 14, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Robinett, C.C.; Giansanti, M.G.; Gatti, M.; Fuller, M.T. TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J. Cell Sci. 2009, 122, 4526–4534. [Google Scholar] [CrossRef]
- Vostmeier, C. An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J. 2001, 20, 6742–6750. [Google Scholar]
- Lord, C.; Ferro-Novick, S.; Miller, E.A. The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the Golgi. Cold Spring Harbor Perspect. Biol. 2013, 5, a013367. [Google Scholar] [CrossRef]
- Barlowe, C.; Orci, L.; Yeung, T.; Hosobuchi, M.; Hamamoto, S.; Salama, N.; Rexach, M.F.; Ravazzola, M.; Amherdt, M.; Schekman, R. COPII, a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 1994, 77, 895–907. [Google Scholar] [CrossRef]
- Bi, X.; Corpina, R.A.; Goldberg, J. Structure of the Sec23/24–Sar1 pre-budding complex of the COPII vesicle coat. Nature 2002, 419, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Antonny, B.; Hamamoto, S.; Schekman, R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002, 21, 6105–6113. [Google Scholar] [CrossRef]
- Ivan, V.; Voer, G.D.; Xanthakis, D.; Spoorendonk, K.M.; Kondylis, V.; Rabouille, C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol. Biol. Cell 2008, 19, 4352–4365. [Google Scholar] [CrossRef]
- Gillon, A.D.; Latham, C.F.; Miller, E.A. Vesicle-mediated ER export of proteins and lipids. Mol. Cell Biol. 2012, 1821, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Iolascon, A.; Verissimo, F.; Trede, N.S.; Horsley, W.; Chen, W.; Paw, B.H.; Hopfner, K.P.; Holzmann, K.; Russo, R.; et al. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat. Genet. 2009, 41, 936–940. [Google Scholar] [CrossRef]
- Hayashi, D.; Tanabe, K.; Katsube, H.; Inoue, Y.H. B-type nuclear lamin and the nuclear pore complex Nup107-160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis. Biol. Open 2016, 8, 1011–1021. [Google Scholar] [CrossRef]
- Yang, K.; Liu, M.; Feng, Z.; Rojas, M.; Zhou, L.; Ke, H.; Pastor-Pareja, J.C. ER exit sites in Drosophila display abundant ER-Golgi vesicles and pearled tubes but no megacarriers. Cell Rep. 2021, 36, 109707. [Google Scholar] [CrossRef] [PubMed]
- Kumichel, A.; Kapp, K.; Knust, E.A. Conserved di-basic motif of Drosophila crumbs contributes to efficient ER export. Traffic 2015, 16, 604–616. [Google Scholar] [CrossRef]
- Pagant, S.; Wu, A.; Edwards, S.; Diehl, F.; Miller, E.A. Sec24 is a coincidence detector that simultaneously binds two signals to drive ER export. Curr. Biol. 2015, 25, 403–412. [Google Scholar] [CrossRef]
- Goldbach, P.; Wong, R.; Beise, N.; Sarpal, R.; Trimble, W.S.; Brill, J.A. Stabilization of the actomyosin ring enables spermatocyte cytokinesis in Drosophila. Mol. Biol. Cell 2010, 21, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.C.; Pringle, J.R.; Peifer, M. Evidence for functional differentiation among Drosophila Septins in cytokinesis and cellularization. Mol. Biol. Cell 2000, 11, 3123–3135. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Krauss, M. Septin remodeling during mammalian cytokinesis. Front. Cell Dev. Biol. 2021, 9, 768309. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Robinson, I.M.; Savoian, M.M.; Griffiths, J.R.; Whetton, A.D.; McMahon, H.T.; Glover, D.M. Drosophila F-BAR protein Syndapin contributes to coupling the plasma membrane and contractile ring in cytokinesis. Open Biol. 2013, 3, 130081. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Ohara, N.; Abe, M.; Uchimura, T.; Hosoya, H.; Lee, J.S.; Miki, T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol. Biol. Cell 2006, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yonemura, S. Centralspindlin regulates ECT2 and RhoA accumulation at the Equatorial Cortex during Cytokinesis. J. Cell Sci. 2006, 119, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Großhans, J.P. Cell polarity and E-Cadherin in tissue-scale shape changes in Drosophila embryos. Front. Cell Dev. Biol. 2020, 8, 6199958. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.A.; McKinley, R.F.A.; Harris, T.J.C. Independent Cadherin–Catenin and Bazooka clusters interact to assemble adherens junctions. J. Cell Biol. 2009, 185, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Bulgakova, N.A.; Brown, N.H. Drosophila P120-Catenin is crucial for endocytosis of the dynamic E-Cadherin–Bazooka complex. J. Cell Sci. 2016, 129, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Watanabe, T.; Wang, S.; Kakeno, M.; Matsuzawa, K.; Matsui, T.; Yokoi, K.; Murase, K.; Sugiyama, I.; Ozawa, M.; et al. Numb controls E-Cadherin endocytosis through P120 Catenin with APKC. Mol. Biol. Cell 2011, 22, 3103–3119. [Google Scholar] [CrossRef]
- Kouranti, I.; Sachse, M.; Arouche, N.; Goud, B.; Echard, A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 2006, 16, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, T.; Sekine, K.; Shikanai, M.; Chihama, K.; Tomita, K.; Kubo, K.; Nakajima, K.; Nabeshima, Y.; Hoshino, M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-Cadherin trafficking. Neuron 2010, 67, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Yuan, H.; Salzmann, V.; Fuller, M.T.; Yamashita, Y.M. E-Cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS ONE 2010, 5, e12473. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, R.; Wang, Y.; Zhou, W.; Hu, Y.; Yao, Y.; Li, K.C.X.; Xu, B.; Zhang, J.; Xu, Y.; et al. Manganese regulation of COPII condensation controls circulating lipid homeostasis. Nat. Cell Biol. 2023, 25, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Kunduri, G.; Le, S.; Baena, V.; Vijaykrishna, N.; Harned, A.; Nagashima, K.; Blankenberg, D.; Yoshihiro, I.; Narayan, K.; Bamba, T.; et al. Delivery of ceramide phosphoethanolamine lipids to the cleavage furrow through the endocytic pathway is essential for male meiotic cytokinesis. PLOS Biol. 2022, 20, e3001599. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.T. Spermatogenesis. In The Development of Drosophila; Martinez-Arias, M., Bate, M., Eds.; Cold Spring Harbor Press: New York, NY, USA, 1993; pp. 71–147. [Google Scholar]
- Gorur, A.; Yuan, L.; Kenny, S.J.; Baba, S.; Xu, K.; Schekman, R. COPII-coated membranes function as transport carriers of intracellular procollagen I. J. Cell Biol. 2017, 216, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.T.; Ginsburg, D. Cargo selection in endoplasmic reticulum-to-Golgi transport and relevant diseases. J. Clin. Investig. 2023, 133, e163838. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.J.; Tagiltsev, G.; Briggs, J.A.G. The structure of COPI vesicles and regulation of vesicle turnover. FEBS Lett. 2023, 597, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Stornaiuolo, M.; Lotti, L.V.; Borgese, N.; Torrisi, M.R.; Mottola, G.; Martire, G.; Bonatti, S. KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex. Mol. Biol. Cell 2003, 14, 889–902. [Google Scholar] [CrossRef]
- Kahsai, L.; Millburn, G.H.; Cook, K.R. Phenotypes Associated with Second Chromosome P Element Insertions in Drosophila melanogaster. G3 (Bethesda) 2016, 6, 2665–2670. [Google Scholar] [CrossRef]
- Royou, A.; Field, C.; John, C.S.; Sullivan, W.; Karess, R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol. Biol. Cell 2004, 2, 1059–1524. [Google Scholar]
- O’Neill, R.; Clark, D.V. Partial functional diversification of Drosophila melanogaster Septin genes Sep2 and Sep5. G3 Genes Genomes Genet. 2016, 6, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lakonishok, M.; Gelfand, V.I. Gatekeeper function for Short stop at the ring canals of the Drosophila ovary. Curr. Biol. 2021, 31, 3207–3220.e4. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, D.; Matsuo, T.; Kaizuka, K.; Miyauchi, C.; Hayashi, D.; Inoue, Y.H. Orbit/CLASP is required for myosin accumulation at the cleavage furrow in Drosophila male meiosis. PLoS ONE 2014, 5, e93669. [Google Scholar] [CrossRef]
- Oka, S.; Hira, J.; Yasukawa, T.; Nakahara, Y.; Inoue, Y.H. A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 2015, 16, 485–501. [Google Scholar] [CrossRef]
- Tanabe, K.; Awane, R.; Shoda, T.; Yamazoe, K.; Inoue, Y.H. Mutations in mxc tumor-suppressor gene induce chromosome instability in Drosophila male meiosis. Cell Struct. Funct. 2019, 44, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, K.; Inoue, Y.H. Cyclin B export to the cytoplasm via the Nup62 subcomplex and subsequent rapid nuclear import are required for the initiation of Drosophila male meiosis. Cells 2023, 12, 2611. [Google Scholar] [CrossRef] [PubMed]
- Flesch, F.M.; Voorhout, W.F.; Colenbrander, B.; van Golde, L.M.; Gadella, B.M. Use of lectins to characterize plasma membrane preparations from boar spermatozoa: A novel technique for monitoring membrane purity and quantity. Biol. Reprod. 1998, 59, 1530–1539. [Google Scholar] [CrossRef]
- Kinoshita, S.; Takarada, K.; Kinoshita, Y.; Inoue, Y.H. Drosophila hemocytes recognize lymph gland tumors of mxc mutants and activate the innate immune pathway in a reactive oxygen species-dependent manner. Biol. Open 2022, 11, bio059523. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, Y.; Kaizuka, K.; Inoue, Y.H. Essential Role of COPII Proteins in Maintaining the Contractile Ring Anchoring to the Plasma Membrane during Cytokinesis in Drosophila Male Meiosis. Int. J. Mol. Sci. 2024, 25, 4526. https://doi.org/10.3390/ijms25084526
Matsuura Y, Kaizuka K, Inoue YH. Essential Role of COPII Proteins in Maintaining the Contractile Ring Anchoring to the Plasma Membrane during Cytokinesis in Drosophila Male Meiosis. International Journal of Molecular Sciences. 2024; 25(8):4526. https://doi.org/10.3390/ijms25084526
Chicago/Turabian StyleMatsuura, Yoshiki, Kana Kaizuka, and Yoshihiro H. Inoue. 2024. "Essential Role of COPII Proteins in Maintaining the Contractile Ring Anchoring to the Plasma Membrane during Cytokinesis in Drosophila Male Meiosis" International Journal of Molecular Sciences 25, no. 8: 4526. https://doi.org/10.3390/ijms25084526
APA StyleMatsuura, Y., Kaizuka, K., & Inoue, Y. H. (2024). Essential Role of COPII Proteins in Maintaining the Contractile Ring Anchoring to the Plasma Membrane during Cytokinesis in Drosophila Male Meiosis. International Journal of Molecular Sciences, 25(8), 4526. https://doi.org/10.3390/ijms25084526