Bulked Segregant RNA-Seq Reveals Different Gene Expression Patterns and Mutant Genes Associated with the Zigzag Pattern of Tea Plants (Camellia sinensis)
Abstract
:1. Introduction
2. Results
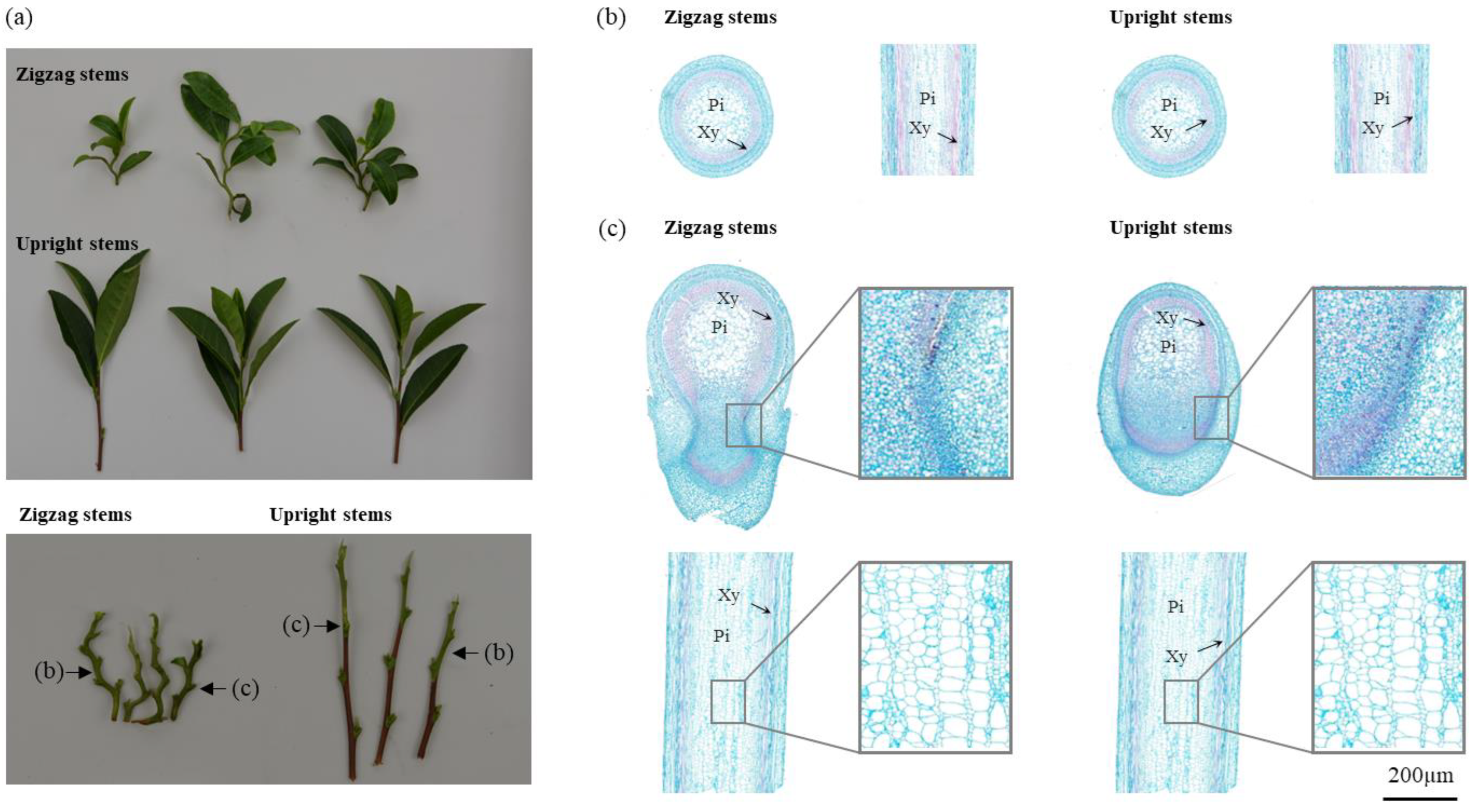
2.1. Phenotypic Characterization and Stem Tissue Sections of Erect and Zigzag-Shaped Shoots in Tea Plants
2.2. RNA Sequencing and Reference Genome Alignment
2.3. DEG Identification and Functional Enrichment Analysis
2.4. SNP Identification and Analysis of the Candidate Gene of Tortuous Stem
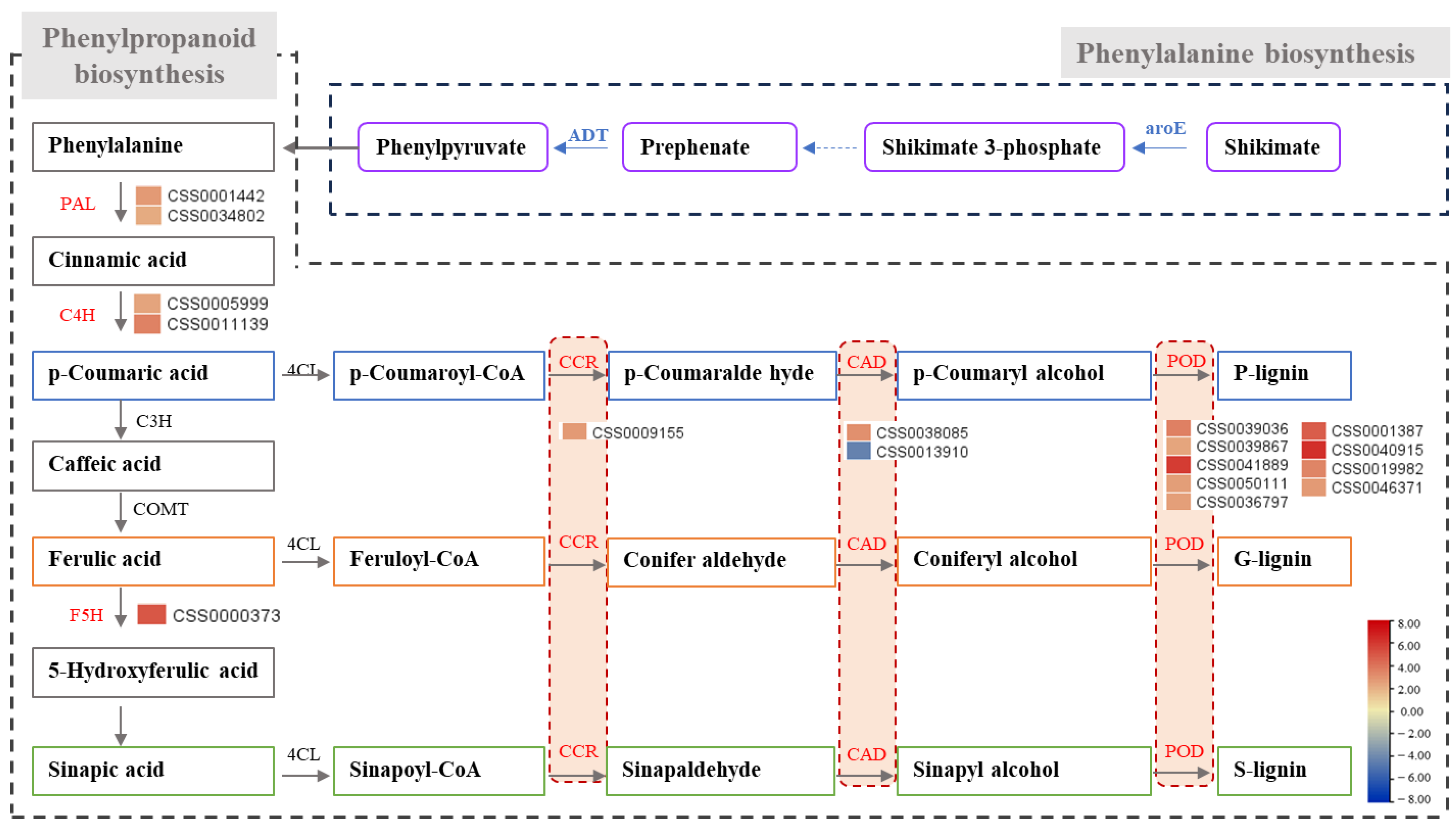
2.5. Analysis of Pathways of and Genes Related to Phenylpropane Biosynthesis
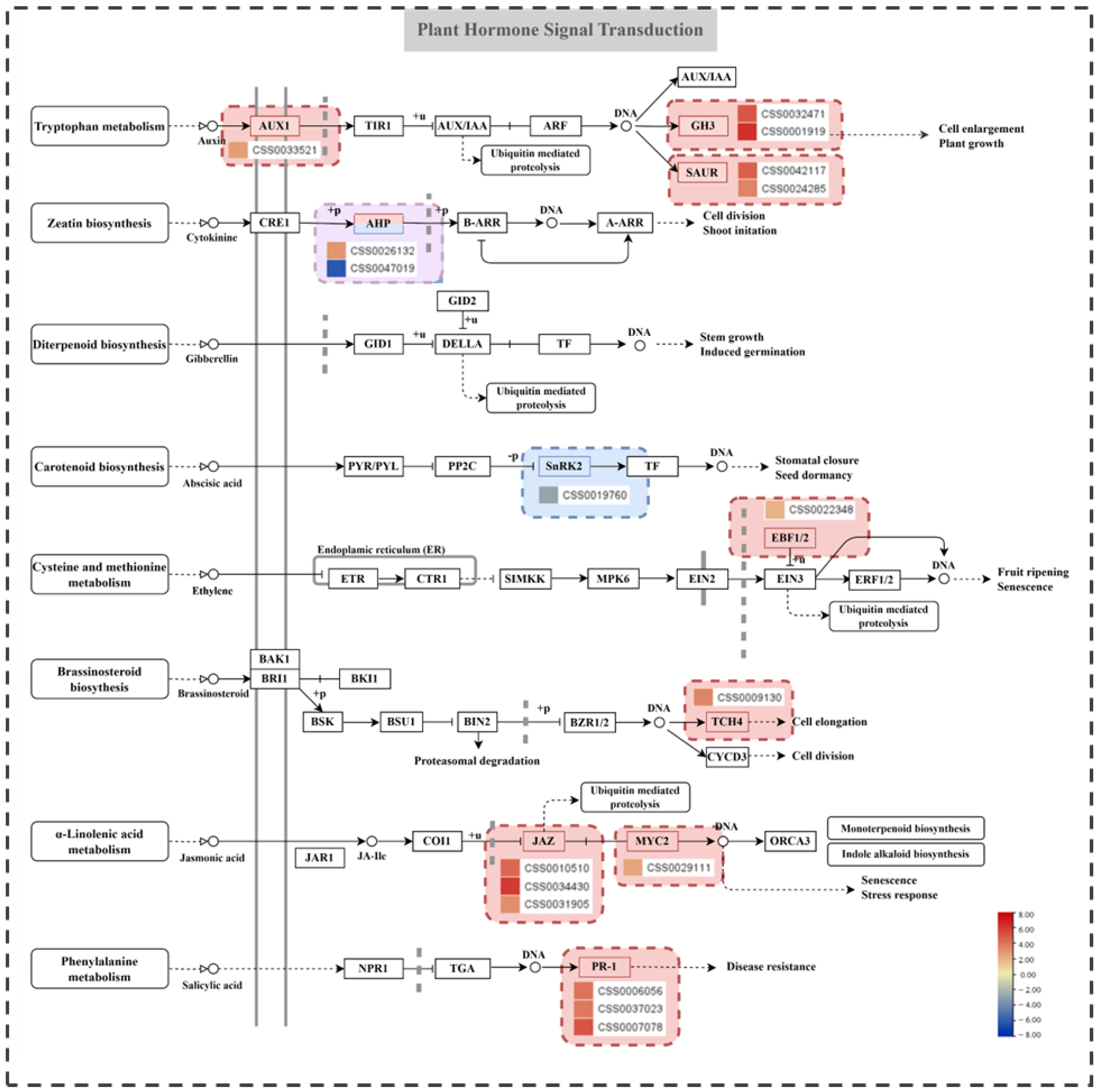
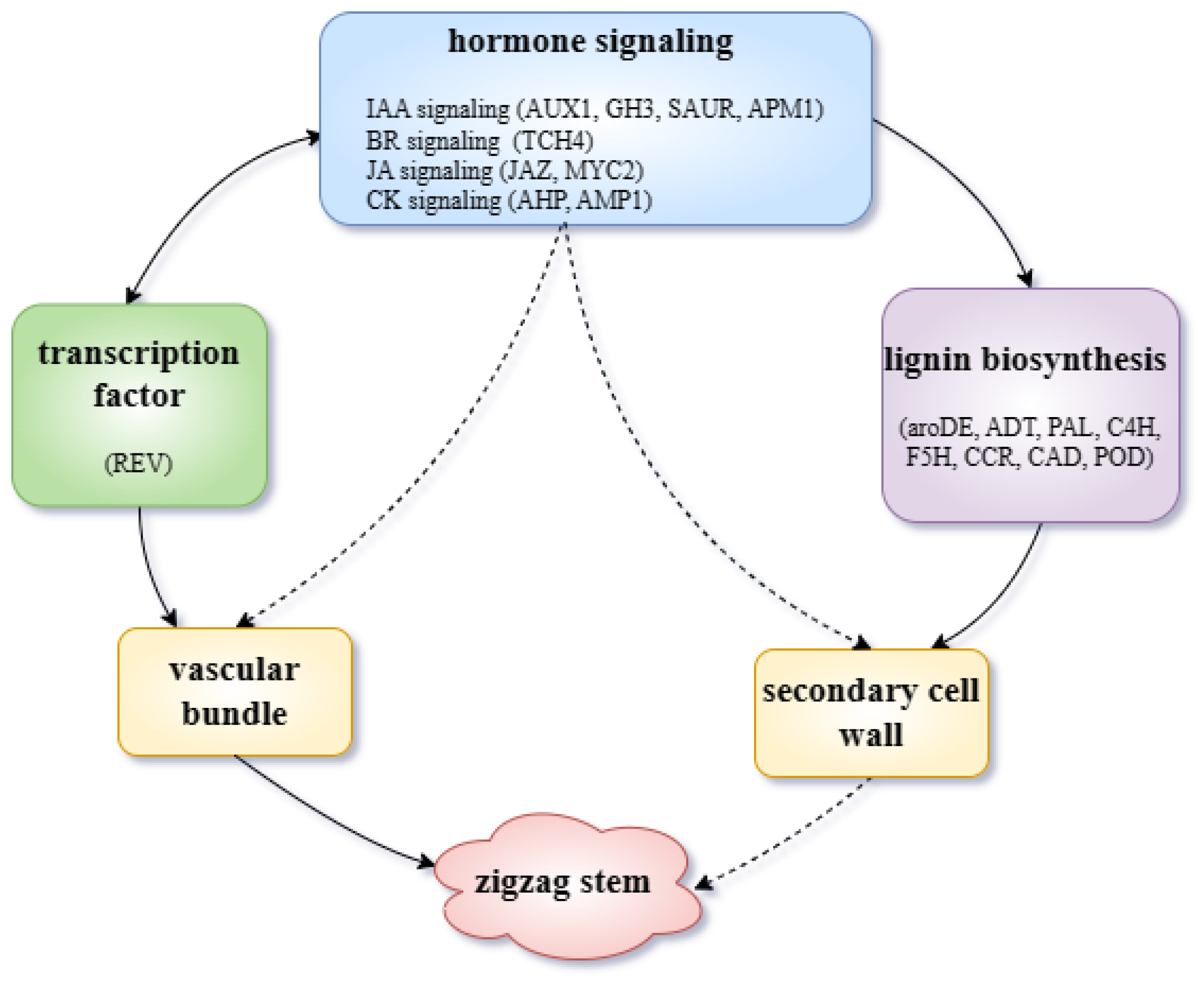
2.6. Analysis of Plant Hormone Signaling Pathways and Related Genes
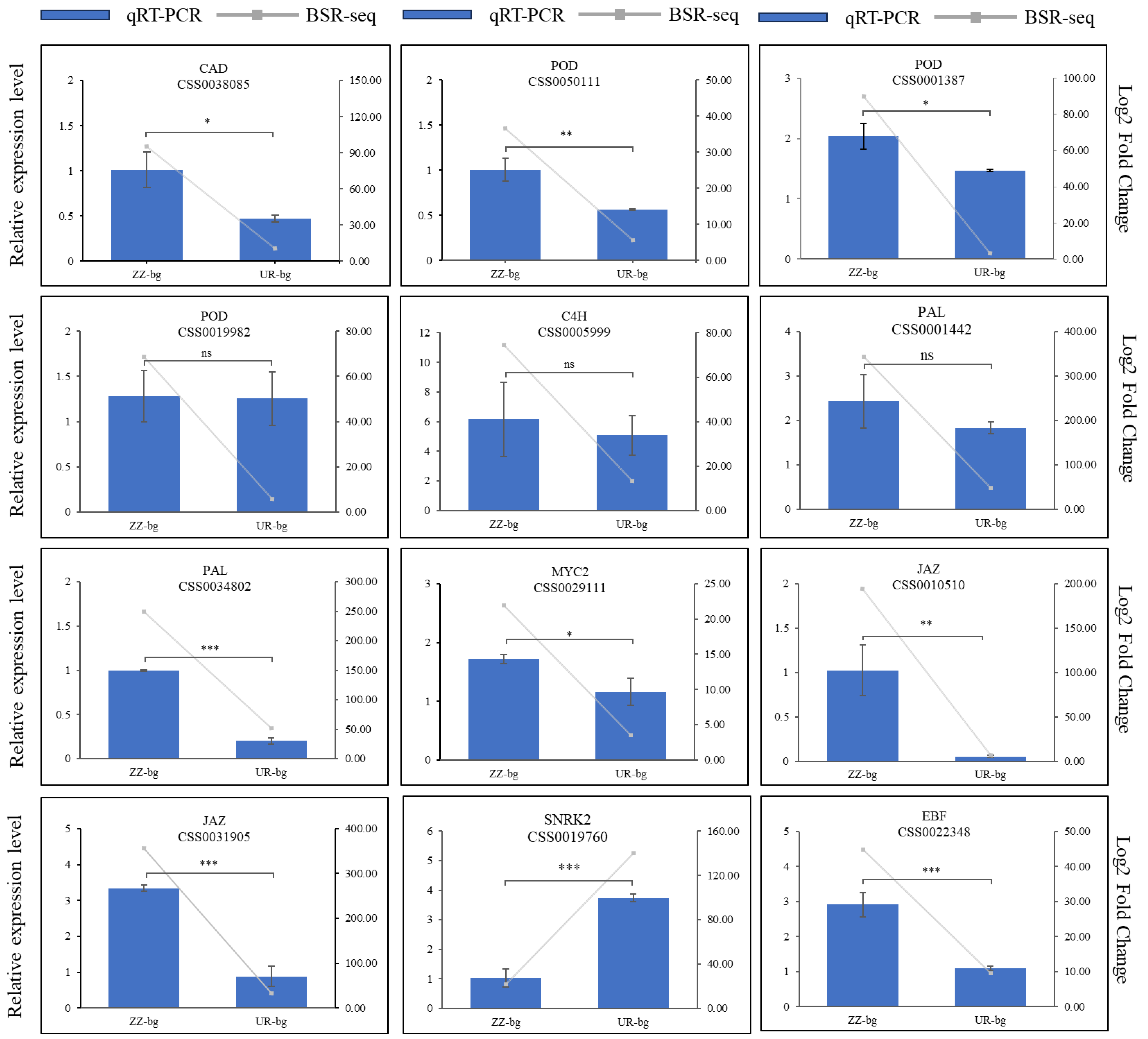
2.7. Gene Expression Validation through Quantitative Real-Time PCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Observation of Tissue Sections
4.3. RNA Extraction, Library Construction, and RNA Sequencing
4.4. SNP Calling, DEG Identification, and Functional Enrichment Analysis
4.5. qRT-PCR Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braybrook, S.A. Plant Development: Lessons from Getting It Twisted. Curr. Biol. 2017, 27, R758–R760. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Mehlenbacher, S.A. Inheritance of Contorted Growth in Hazelnut. Euphytica 1996, 89, 211–213. [Google Scholar] [CrossRef]
- Lin, J.; Gunter, L.E.; Harding, S.A.; Kopp, R.F.; McCord, R.P.; Tsai, C.-J.; Tuskan, G.A.; Smart, L.B. Development of AFLP and RAPD Markers Linked to a Locus Associated with Twisted Growth in Corkscrew Willow (Salix Matsudana ’Tortuosa’). Tree Physiol. 2007, 27, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, P.; Zhuo, X.; Liu, W.; Qiu, L.; Li, L.; Yuan, C.; Sun, L.; Zhang, Z.; Wang, J.; et al. The Chromosome-level Genome Provides Insight into the Molecular Mechanism Underlying the Tortuous-branch Phenotype of Prunus mume. New Phytol. 2022, 235, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, L.; Yan, F.; Liu, Z.; Wang, L.; Zhao, X.; Wang, L.; Zhao, J.; Wang, J.; Liu, M. A Novel Twisted Bud Mutant from Ziziphus jujubaMill. ‘Dongzao’. Sci. Hortic. 2022, 295, 110774. [Google Scholar] [CrossRef]
- Zheng, T.; Li, L.; Zhang, Q. Advances in Research on Tortuous Traits of Plants. Euphytica 2018, 214, 224. [Google Scholar] [CrossRef]
- Kato, T.; Morita, M.T.; Fukaki, H.; Yamauchi, Y.; Uehara, M.; Niihama, M.; Tasaka, M. SGR2, a Phospholipase-like Protein, and ZIG/SGR4, a SNARE, Are Involved in the Shoot Gravitropism of Arabidopsis. Plant Cell 2002, 14, 33–46. [Google Scholar] [CrossRef]
- Klynstra, F.B.; Lycklama, J.C.; Siebers, A.M.; Burggraaf, P.D. On the Anatomy of the Woody Stem of the Twisted Hazel, Corylus Avellana L. ‘Contorta’. Acta Bot. Neerl. 1964, 13, 189–208. [Google Scholar] [CrossRef]
- Lincoln, C.; Britton, J.H.; Estelle, M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 1990, 2, 1071–1080. [Google Scholar] [CrossRef]
- Carland, F.M.; McHale, N.A. LOP1: A Gene Involved in Auxin Transport and Vascular Patterning in Arabidopsis. Development 1996, 122, 1811–1819. [Google Scholar] [CrossRef]
- Boss, P.K.; Thomas, M.R. Association of Dwarfism and Floral Induction with a Grape ‘Green Revolution’ Mutation. Nature 2002, 416, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Malabarba, J.; Reichelt, M.; Pasquali, G.; Mithöfer, A. Tendril Coiling in Grapevine: Jasmonates and a New Role for GABA? J. Plant. Growth Regul. 2019, 38, 39–45. [Google Scholar] [CrossRef]
- Gendron, J.M.; Liu, J.-S.; Fan, M.; Bai, M.-Y.; Wenkel, S.; Springer, P.S.; Barton, M.K.; Wang, Z.-Y. Brassinosteroids Regulate Organ Boundary Formation in the Shoot Apical Meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 21152–21157. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z. Performance, Gene Mapping and Transcriptome Profile of Brachytic Stem in Soybean. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, June 2015. [Google Scholar]
- Guo, W. QTL Mapping and Transcriptome Analysis of Stem Bending Trait in Brassica Napus Stb1 Mutant. Master’s Thesis, Southwest University, Chongqing, China, 2016. [Google Scholar]
- Guan, C.; Xue, Y.; Jiang, P.; He, C.; Zhuge, X.; Lan, T.; Yang, H. Overexpression of PtoCYCD3;3 Promotes Growth and Causes Leaf Wrinkle and Branch Appearance in Populus. IJMS 2021, 22, 1288. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Maruthi, N.M.; Jahn, C.E. CYCD3 D-Type Cyclins Regulate Cambial Cell Proliferation and Secondary Growth in Arabidopsis. EXBOTJ 2015, 66, 4595–4606. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Lowndes, L.; Regan, S.; Beardmore, T. Overexpression of CYCD1;2 in Activation-Tagged Populus Tremula x Populus Alba Results in Decreased Cell Size and Altered Leaf Morphology. Tree Genet. Genomes 2015, 11, 66. [Google Scholar] [CrossRef]
- Emery, J.F.; Floyd, S.K.; Alvarez, J.; Eshed, Y.; Hawker, N.P.; Izhaki, A.; Baum, S.F.; Bowman, J.L. Radial Patterning of Arabidopsis Shoots by Class III HD-ZIP and KANADI Genes. Curr. Biol. 2003, 13, 1768–1774. [Google Scholar] [CrossRef]
- Robischon, M.; Du, J.; Miura, E.; Groover, A. The Populus Class III HD ZIP, popREVOLUTA, Influences Cambium Initiation and Patterning of Woody Stems. Plant Physiol. 2011, 155, 1214–1225. [Google Scholar] [CrossRef]
- Hu, G.; Fan, J.; Xian, Z.; Huang, W.; Lin, D.; Li, Z. Overexpression of SlREV Alters the Development of the Flower Pedicel Abscission Zone and Fruit Formation in Tomato. Plant Sci. 2014, 229, 86–95. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft Genome Sequence of Camellia Sinensis Var. Sinensis Provides Insights into the Evolution of the Tea Genome and Tea Quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Chen, J.-D.; Zheng, C.; Ma, J.-Q.; Jiang, C.-K.; Ercisli, S.; Yao, M.-Z.; Chen, L. The Chromosome-Scale Genome Reveals the Evolution and Diversification after the Recent Tetraploidization Event in Tea Plant. Hortic. Res. 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Yu, F. Tea Germplasm Research in China: Recent Progresses and Prospects. J. Plant Genet. Resour. 2004, 4, 389–392. [Google Scholar] [CrossRef]
- Chen, L.; Apostolides, Z.; Chen, Z. (Eds.) Global Tea Breeding: Achievements, Challenges and Perspectives; Advanced topics in science and technology in China; Zhejiang University Press: Hangzhou, China; Springer: Heidelberg, Germany; New York, NY, USA, 2012; ISBN 978-3-642-31877-1. [Google Scholar]
- Zhang, S.; Jin, J.; Chen, J.; Ercisli, S.; Chen, L. Purine Alkaloids in Tea Plants: Component, Biosynthetic Mechanism and Genetic Variation. Beverage Plant Res 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, R.; Moon, D.-G.; Ercisli, S.; Chen, L. Achievements and Prospects of QTL Mapping and Beneficial Genes and Alleles Mining for Important Quality and Agronomic Traits in Tea Plant (Camellia Sinensis). Beverage Plant Res. 2023, 3, 22. [Google Scholar] [CrossRef]
- Jin, J.-Q.; Qu, F.-R.; Huang, H.; Liu, Q.-S.; Wei, M.-Y.; Zhou, Y.; Huang, K.-L.; Cui, Z.; Chen, J.-D.; Dai, W.-D.; et al. Characterization of Two O-Methyltransferases Involved in the Biosynthesis of O-Methylated Catechins in Tea Plant. Nat. Commun. 2023, 14, 5075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, Y.; Lun, X.; Cao, Y.; Zhang, X.; Jin, M.; Guan, F.; Wang, L.; Zhao, Y.; Zhang, Z. iTRAQ-Based Quantitative Proteomic Analysis of Defense Responses of Two Tea Cultivars to Empoasca Onukii (Matsuda) Feeding. Beverage Plant Res. 2023, 4, e006. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Lu, M.; Wu, Y.; Jing, T.; Zhao, M.; Zhao, Y.; Feng, Y.; Wang, J.; Gao, T.; et al. Salicylic Acid Carboxyl Glucosyltransferase UGT87E7 Regulates Disease Resistance in Camellia sinensis. Plant Physiol. 2022, 188, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yeh, C.-T.; Tang, H.M.; Nettleton, D.; Schnable, P.S. Gene Mapping via Bulked Segregant RNA-Seq (BSR-Seq). PLoS ONE 2012, 7, e36406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wang, Z.; Xu, F.; Lu, G.; Su, Y.; Wu, Q.; Wang, T.; Que, Y.; Xu, L. Screening of Candidate Genes Associated with Brown Stripe Resistance in Sugarcane via BSR-Seq Analysis. IJMS 2022, 23, 15500. [Google Scholar] [CrossRef]
- Hou, X.; Guo, Q.; Wei, W.; Guo, L.; Guo, D.; Zhang, L. Screening of Genes Related to Early and Late Flowering in Tree Peony Based on Bulked Segregant RNA Sequencing and Verification by Quantitative Real-Time PCR. Molecules 2018, 23, 689. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Chen, J.-D.; Wang, S.-L.; Chen, L.; Ma, C.-L.; Yao, M.-Z. Repressed Gene Expression of Photosynthetic Antenna Proteins Associated with Yellow Leaf Variation as Revealed by Bulked Segregant RNA-Seq in Tea Plant Camellia sinensis. J. Agric. Food Chem. 2020, 68, 8068–8079. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, Y.; Qu, F.-R.; Wei, M.-Y.; Zhang, C.-Y.; Liu, H.-R.; Chen, L.; Yao, M.-Z.; Jin, J.-Q. A Novel TcS Allele Conferring the High-Theacrine and Low-Caffeine Traits and Having Potential Use in Tea Plant Breeding. Hortic. Res. 2022, 9, uhac191. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R.; et al. The Reference Genome of Tea Plant and Resequencing of 81 Diverse Accessions Provide Insights into Its Genome Evolution and Adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, D.P.; Ploense, S.; Krogan, N.T.; Berleth, T. AMP1 and MP Antagonistically Regulate Embryo and Meristem Development in Arabidopsis. Development 2007, 134, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.A.; Christendat, D. Structure of Arabidopsis Dehydroquinate Dehydratase-Shikimate Dehydrogenase and Implications for Metabolic Channeling in the Shikimate Pathway. Biochemistry 2006, 45, 7787–7796. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-H.; Corea, O.R.A.; Yang, H.; Bedgar, D.L.; Laskar, D.D.; Anterola, A.M.; Moog-Anterola, F.A.; Hood, R.L.; Kohalmi, S.E.; Bernards, M.A.; et al. Phenylalanine Biosynthesis in Arabidopsis Thaliana. J. Biol. Chem. 2007, 282, 30827–30835. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.R.; Cho, H.-T. Cytoplasm Localization of Aminopeptidase M1 and Its Functional Activity in Root Hair Cells and BY-2 Cells. Mol. Biol. Rep. 2012, 39, 10211–10218. [Google Scholar] [CrossRef] [PubMed]
- Otsuga, D.; DeGuzman, B.; Prigge, M.J.; Drews, G.N.; Clark, S.E. REVOLUTA Regulates Meristem Initiation at Lateral Positions. Plant J. 2001, 25, 223–236. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z.-H. IFL1, a Gene Regulating Interfascicular Fiber Differentiation in Arabidopsis, Encodes a Homeodomain–Leucine Zipper Protein. Plant Cell 1999, 11, 2139–2152. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of Phenylpropanoid Metabolism to Plant Development and Plant–Environment Interactions. JIPB 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.-J. Multifaceted Regulations of Gateway Enzyme Phenylalanine Ammonia-Lyase in the Biosynthesis of Phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Ohta, D.; Sato, R. Lsolation of a cDNA and a Genomic Clone Encoding Cinnamate 4-Hydroxylase from Arabidopsis and Its Expression Manner in Planta. Plant Physiol. 1997, 113, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.Y. Seventy-year major research progress in plant hormones by Chinese scholars. Sci. Sin. Vitae 2019, 49, 1227–1281. (In Chinese) [Google Scholar] [CrossRef]
- Cao, H.; Wang, F.; Lin, H.; Ye, Y.; Zheng, Y.; Li, J.; Hao, Z.; Ye, N.; Yue, C. Transcriptome and Metabolite Analyses Provide Insights into Zigzag-Shaped Stem Formation in Tea Plants (Camellia sinensis). BMC Plant Biol. 2020, 20, 98. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; De Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An Arabidopsis Gene Regulatory Network for Secondary Cell Wall Synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Jarvis, M.C. Comparative Structure and Biomechanics of Plant Primary and Secondary Cell Walls. Front. Plant Sci. 2012, 3, 204. [Google Scholar] [CrossRef]
- Molina, A.; Miedes, E.; Bacete, L.; Rodríguez, T.; Mélida, H.; Denancé, N.; Sánchez-Vallet, A.; Rivière, M.-P.; López, G.; Freydier, A.; et al. Arabidopsis Cell Wall Composition Determines Disease Resistance Specificity and Fitness. Proc. Natl. Acad. Sci. USA 2021, 118, e2010243118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lv, B.; Qiu, J.-L. Being Tough: The Secret Weapon of Plants against Vascular Pathogens. Mol. Plant 2022, 15, 934–936. [Google Scholar] [CrossRef]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax Biosynthesis in Response to Danger: Its Regulation upon Abiotic and Biotic Stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef]
- Kumar, A.P.; Bhasker, K.; Nikhil, B.S.K.; Srinivas, P. Role of Phenylpropanoids and Flavonoids in Plant Defense Mechanism. IJECC 2023, 13, 2951–2960. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R. Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants. Front. Plant Sci. 2019, 10, 476672. [Google Scholar] [CrossRef] [PubMed]
- Jedličková, V.; Ebrahimi Naghani, S.; Robert, H.S. On the Trail of Auxin: Reporters and Sensors. Plant Cell 2022, 34, 3200–3213. [Google Scholar] [CrossRef] [PubMed]
- Traas, J. Molecular Networks Regulating Meristem Homeostasis. Mol. Plant 2018, 11, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Teale, W.; Fleck, C.; Volpers, M.; Ruperti, B.; Palme, K. Modelling Polar Auxin Transport in Developmental Patterning. Plant Biol. 2010, 12, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Habets, M.E.J.; Offringa, R. PIN -driven Polar Auxin Transport in Plant Developmental Plasticity: A Key Target for Environmental and Endogenous Signals. New Phytol. 2014, 203, 362–377. [Google Scholar] [CrossRef]
- Han, H.; Adamowski, M.; Qi, L.; Alotaibi, S.S.; Friml, J. PIN-mediated Polar Auxin Transport Regulations in Plant Tropic Responses. New Phytol. 2021, 232, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Péret, B. AUX/LAX Family of Auxin Influx Carriers—An Overview. Front. Plant Sci. 2012, 3, 225. [Google Scholar] [CrossRef]
- Côté, C.L.; Boileau, F.; Roy, V.; Ouellet, M.; Levasseur, C.; Morency, M.-J.; Cooke, J.E.; Séguin, A.; MacKay, J.J. Gene Family Structure, Expression and Functional Analysis of HD-Zip III Genes in Angiosperm and Gymnosperm Forest Trees. BMC Plant Biol. 2010, 10, 273. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, H.; Cuo, D.; Wu, X.; Duan, R. Genome-Wide Characterization and Expression Profiling of the Relation of the HD-Zip Gene Family to Abiotic Stress in Barley (Hordeum vulgare L.). Plant Physiol. Biochem. 2019, 141, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Adler, H.T.; Parks, D.W.; Comai, L. The REVOLUTA Gene Is Necessary for Apical Meristem Development and for Limiting Cell Divisions in the Leaves and Stems of Arabidopsis thaliana. Development 1995, 121, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, L.; Ren, J.; Zhang, T.; Cui, J.; Bao, Z.; Zhou, W.; Bai, J.; Gong, C. CkREV Regulates Xylem Vessel Development in Caragana Korshinskii in Response to Drought. Front. Plant Sci. 2022, 13, 982853. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol 2010, 11, R106. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]

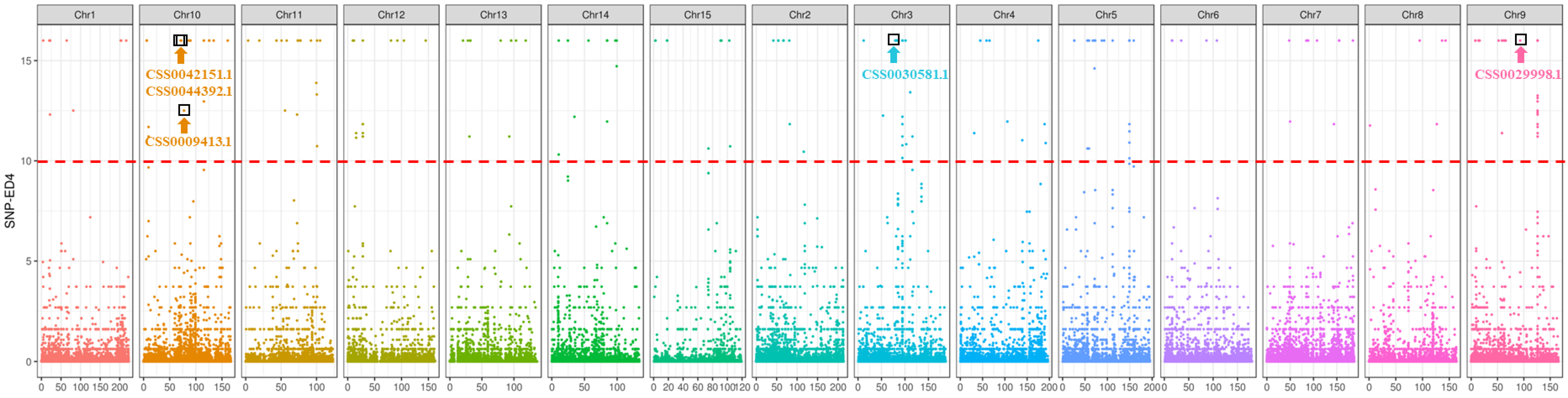
| Gene_id | Function | Mutation Type |
|---|---|---|
| CSS0027659.1 | regulating meristem function; balancing auxin signaling | synonymous |
| CSS0030581.1 | shikimate dehydrogenase | nonsynonymous |
| CSS0009413.1 | arogenate dehydratase/prephenate dehydratase | synonymous |
| CSS0042151.1 | negative regulation of PIN auxin transport proteins | nonsynonymous |
| CSS0044392.1 | regulation of interfascicular fiber and secondary xylem differentiation; determination of vascular patterning and organ polarity | nonsynonymous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.-Y.; Liu, D.-D.; Tang, R.-J.; Gong, Y.; Zhang, C.-Y.; Mei, P.; Ma, C.-L.; Chen, J.-D. Bulked Segregant RNA-Seq Reveals Different Gene Expression Patterns and Mutant Genes Associated with the Zigzag Pattern of Tea Plants (Camellia sinensis). Int. J. Mol. Sci. 2024, 25, 4549. https://doi.org/10.3390/ijms25084549
Ye Y-Y, Liu D-D, Tang R-J, Gong Y, Zhang C-Y, Mei P, Ma C-L, Chen J-D. Bulked Segregant RNA-Seq Reveals Different Gene Expression Patterns and Mutant Genes Associated with the Zigzag Pattern of Tea Plants (Camellia sinensis). International Journal of Molecular Sciences. 2024; 25(8):4549. https://doi.org/10.3390/ijms25084549
Chicago/Turabian StyleYe, Yuan-Yuan, Ding-Ding Liu, Rong-Jin Tang, Yang Gong, Chen-Yu Zhang, Piao Mei, Chun-Lei Ma, and Jie-Dan Chen. 2024. "Bulked Segregant RNA-Seq Reveals Different Gene Expression Patterns and Mutant Genes Associated with the Zigzag Pattern of Tea Plants (Camellia sinensis)" International Journal of Molecular Sciences 25, no. 8: 4549. https://doi.org/10.3390/ijms25084549
APA StyleYe, Y.-Y., Liu, D.-D., Tang, R.-J., Gong, Y., Zhang, C.-Y., Mei, P., Ma, C.-L., & Chen, J.-D. (2024). Bulked Segregant RNA-Seq Reveals Different Gene Expression Patterns and Mutant Genes Associated with the Zigzag Pattern of Tea Plants (Camellia sinensis). International Journal of Molecular Sciences, 25(8), 4549. https://doi.org/10.3390/ijms25084549







