Abstract
Patent ductus arteriosus (PDA) constitutes a significant clinical condition, frequently associated with a spectrum of complications that may profoundly compromise the health status of neonates, particularly those born preterm. Multiple predisposing factors—including prematurity, low birth weight, and respiratory insufficiency—have been consistently documented in the scientific literature. In this study, we investigated the influence of genetic polymorphisms in genes associated with the arachidonic acid–prostaglandin metabolic pathway. Specifically, we analyzed polymorphisms in genes encoding phospholipase A2 (rs10798059, rs1549637, rs4375, rs1805017, rs1051931), cyclooxygenase-1 (rs1236913), prostaglandin synthase 2 (rs13283456), and the prostaglandin E2 receptor EP4 (rs4613763). The study cohort comprised 99 preterm neonates born between 24 and 32 weeks of gestation. Genetic analyses were performed to identify polymorphisms in the aforementioned genes. Statistical evaluation demonstrated that selected polymorphic were significantly associated with an increased risk of patent ductus arteriosus development. This study represents a preliminary step toward elucidating the contribution of genetic variability to the pathogenesis of patent ductus arteriosus. Improved understanding of these molecular mechanisms may facilitate the early identification of neonates at increased risk and support the implementation of targeted monitoring and preventive strategies in this high-risk population.
1. Introduction
Prematurity is one of the most well-established risk factors for the development of patent ductus arteriosus (PDA) and its associated hemodynamic complications. Numerous studies have demonstrated an inverse correlation between gestational age and the incidence of PDA. Additional contributing factors include low birth weight, the need for mechanical ventilation, systemic inflammation, low Apgar scores, and perinatal asphyxia. PDA has been implicated in the pathogenesis of several severe neonatal complications, including intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, and retinopathy of prematurity [,]. Furthermore, increased mortality has been reported among neonates diagnosed with PDA.
Identifying individuals at an increased risk of developing patent ductus arteriosus is of particular importance, especially among patients receiving care in neonatal intensive care units. While traditional risk factors remain relevant, contemporary diagnostic approaches should extend beyond the identification of neonates with clinically evident PDA. Efforts should focus on proactively recognizing preterm infants with a heightened predisposition to this condition, including those with underlying genetic susceptibility.
Among neonates, an increased incidence of patent ductus arteriosus has been observed in twins, who have long served as a model for investigating genetic determinants of disease. This association is particularly pronounced in monozygotic twins, suggesting a significant heritable component [,]. Moreover, studies in animal models have identified genetic factors that confer susceptibility to PDA, further supporting the role of inherited predisposition in its pathogenesis [,].
Among the most extensively described mechanisms regulating flow through the patent ductus arteriosus (PDA) is the postnatal increase in oxygen tension, which induces constriction of vascular smooth muscle and, consequently, cessation of flow []. Antagonistic to the vasoconstrictive effect of oxygen is the action of prostaglandins. Prostaglandin E (PGE) plays a central role in maintaining ductal patency in utero. Its biological activity is mediated predominantly through the EP4 receptor, which is primarily expressed in vascular smooth muscle cells. Activation of this receptor stimulates nitric oxide synthase, resulting in smooth muscle relaxation. The postnatal decline in PGE2 concentrations, in conjunction with elevated partial oxygen pressure, contributes to ductal closure [,]. The synthesis of prostaglandins from arachidonic acid is catalyzed principally by cyclooxygenase-1, with the peroxidase site serving as the second catalytic domain [].
The genes analyzed in this study are located on distinct chromosomes and participate in the arachidonic acid–prostaglandin metabolic pathway. The PTGS1 gene, situated on chromosome 9q32–q33.3, encodes cyclooxygenase-1 (COX-1), an enzyme that catalyzes the conversion of arachidonic acid to prostaglandin H2. PTGES2, located on chromosome 9q34.11, encodes prostaglandin E synthase 2, which mediates the subsequent conversion of prostaglandin H2 to prostaglandin E2. The PTGER4 gene, mapped to chromosome 5p13.1, encodes one of the prostaglandin E2 receptors (EP4), which is responsible for transducing its biological effects at the cellular level.
Several genes encoding phospholipase A2 isoforms also contribute to this metabolic pathway. PLA2G4A, located on chromosome 1q31.1, and PLA2G4C, situated on chromosome 19q13.3, encode cytosolic phospholipase A2 enzymes. PLA2G6, mapped to chromosome 22q13.1, and PLA2G7, located on chromosome 6p21.2, represent additional isoforms with distinct regulatory functions. Phospholipase A2 enzymes play a pivotal role in the hydrolysis of ester bonds within membrane phospholipids, facilitating the release of arachidonic acid and other polyunsaturated fatty acids, which serve as key precursors for eicosanoid biosynthesis, including prostaglandins.
The pharmacological management of patent ductus arteriosus includes the use of cyclooxygenase inhibitors, such as ibuprofen and indomethacin, as well as the peroxidase inhibitor paracetamol, alongside the option of surgical ligation []. None of these agents is devoid of systemic effects, particularly in the context of the physiologic immaturity of preterm neonates. Clinical evidence indicates notable interindividual variability in response to pharmacologic treatment. This heterogeneity may be partially explained by genetic polymorphisms within genes encoding enzymatic catalytic domains and prostaglandin receptors.
It is anticipated that elucidating the genetic basis of this variability and its clinical implications will enable more precise selection of pharmacological interventions and optimize therapeutic outcomes.
2. Results
The study group comprised 99 preterm infants born between 27 and 31 weeks of gestation, including 45 females (45.45%) and 54 males (54.55%). Patent ductus arteriosus (PDA) was diagnosed in 36 neonates, of whom 21 met the criteria for hemodynamically significant PDA (HsPDA). Pharmacological treatment was administered in 22 cases (22.22%), using either paracetamol or ibuprofen.
A statistically significant association was observed between the need for mechanical ventilation and the diagnosis of PDA, with affected infants requiring ventilatory support more frequently.
Necrotizing enterocolitis (NEC) was diagnosed in 17 neonates (17.17%), intraventricular hemorrhage (IVH) in 39 neonates (39.39%), bronchopulmonary dysplasia (BPD) in 52 neonates (52.53%), and retinopathy of prematurity (ROP) in 46 neonates (46.46%). In the analyzed cohort, a statistically significant association was found between the occurrence of PDA and the presence of NEC, ROP, and BPD.
The characteristics of the study population are summarized in Table 1. The distribution of individual single nucleotide polymorphisms (SNPs) is presented in Table 2.

Table 1.
The table displays the distribution of clinical variables in relation to the presence of PDA and HsPDA.

Table 2.
Frequency of the studied single nucleotide polymorphisms (SNVs) in the entire cohort (N = 99).
Analysis of the studied polymorphisms in relation to their potential role in promoting delayed closure of the ductus arteriosus (PDA) revealed an increased frequency of PDA among carriers of the rs1051931 polymorphism.
No statistically significant associations were observed for the remaining polymorphisms of the studied genes. Detailed results are presented in Table 3.

Table 3.
The influence of individual SNPs on the occurrence of PDA.
A tendency toward delayed ductal closure was observed in neonates carrying the rs1051931 polymorphism, although the association did not achieve statistical significance (p = 0.099).
For the remaining polymorphisms, no significant effect on the timing of ductus arteriosus closure was identified.
Assessment of the impact of individual polymorphisms on the occurrence of hemodynamically significant PDA (HsPDA) did not reveal any statistically significant associations.
None of the SNPs tested were significant after the Bonferroni correction (p < 0.006) for multiple SNP testing. We did not exclude the rs4375 variant of the PLA2G6 gene from the study despite the absence of HWE, as we considered that preterm infants were not a healthy control group.
Comprehensive results are provided in Table 4.

Table 4.
Association between individual SNPs and HsPDA incidence.
3. Discussion
Ongoing research into genetic determinants may enhance our understanding of the underlying pathophysiological mechanisms and prognostic implications of patent ductus arteriosus, especially in preterm neonates who are inherently more susceptible to hemodynamic instability.
Our study focused on the analysis of polymorphisms in genes encoding enzymes and receptors involved in the prostaglandin metabolism pathway, specifically phospholipase A2, cyclooxygenase-1, prostaglandin synthetase 2, and the EP4 receptor. Genetic material was obtained from neonates born at our institution or in affiliated regional hospitals, resulting in a study cohort characterized by ethnic homogeneity. This methodological feature raises the possibility that the distribution of certain polymorphisms may differ in other European populations or globally, which may, in turn, influence the broader applicability and external validity of our findings.
Among the analyzed genes, the rs1051931 polymorphism was found to be associated with a statistically significant increase in the risk of PDA development; in neonates carrying this variant, the odds ratio (OR) for PDA occurrence was elevated by 2.49-fold. This polymorphism is located within the gene encoding phospholipase A2, an enzyme responsible for catalyzing the conversion of membrane phospholipids into arachidonic acid, a key substrate in the prostaglandin synthesis pathway. The alanine-to-valine substitution affects the gene’s active site, resulting in lower affinity for PAF-AH. Analyzing the available literature, we see that this polymorphism leads to slower degradation of the encoded protein, which translates to longer-lasting activation of proinflammatory signals.
To our knowledge, this is one of the first studies to demonstrate an association between the rs1051931 polymorphism and the incidence of patent ductus arteriosus (PDA) in neonates. The existing literature includes numerous studies investigating the relationship between polymorphisms of this gene and their involvement in the pathogenesis of cardiovascular diseases. These studies primarily focus on the gene’s role in modulating the proinflammatory response and regulating phospholipid metabolism [,,].
Evidence from animal models, particularly in mice, indicates that impaired function of enzymes involved in the prostaglandin metabolism pathway—such as cyclooxygenase-1 (COX-1, Ptgs1) and cyclooxygenase-2 (COX-2)—is associated with a significantly increased mortality rate [,]. Given the high degree of conservation in biochemical pathways across mammalian species, it is plausible that similar mechanisms may be relevant in human neonates, warranting further investigation. A comparable pattern has been observed with dysfunction of the EP4 receptor, which has been linked to an increased incidence of PDA and higher neonatal mortality [,].
The development of hemodynamically significant patent ductus arteriosus (HsPDA) is of critical relevance in clinical practice, as disturbances in systemic perfusion associated with this condition are directly linked to an increased risk of severe complications, including intraventricular hemorrhage, necrotizing enterocolitis, and mortality. Although echocardiographic assessment remains the current gold standard for the evaluation and monitoring of ductal patency, its implementation requires access to advanced imaging equipment and highly trained personnel. Moreover, it often necessitates additional procedures in a patient population composed largely of extremely preterm and clinically fragile neonates.
In the present study, none of the investigated polymorphisms demonstrated a statistically significant association with the development of HsPDA. However, in the case of the rs4375 polymorphism, a trend toward significance was observed under the codominant, dominant, and log-additive genetic models. These preliminary findings highlight the need for further research to elucidate the potential role of this variant in the pathophysiology of HsPDA.
Our study was conducted in a population of preterm infants, including those born at extremely low gestational ages. When evaluating outcomes in this group, it is essential to consider that extremely premature neonates possess a reduced amount of smooth muscle tissue within their vasculature, which may contribute to delayed closure of the ductus arteriosus. The initial phase of ductal closure involves vasoconstriction, which is dependent on the withdrawal of prostaglandin activity.
Although certain genetic predispositions were identified, well-established clinical risk factors—such as prematurity, low birth weight, and the need for mechanical ventilation—remain the most prominent contributors to the development of PDA and HsPDA. At present, the most effective strategy for preventing PDA and its associated complications appears to be the prevention of preterm birth. In cases where premature delivery cannot be avoided, perinatal management in specialized tertiary care centers remains essential to optimize neonatal outcomes.
To our knowledge, this is the first study on the influence of gene polymorphisms on the development of PDA in premature infants. It yielded promising results, shedding new light on the development of this condition in newborns. Given the ethnic homogeneity of the study population—Caucasian—it seems reasonable to expand this study to include a larger number of children from diverse populations in the future.
4. Methods
4.1. Definitions
Patent ductus arteriosus (PDA) is defined in the literature as a persistent vascular connection between the aorta and the pulmonary artery with sustained blood flow beyond the fifth day of postnatal life. Hemodynamic significance was assessed according to the criteria established in current neonatal care guidelines (Table 5). The necessary conditions for the diagnosis of HsDPA is the assessment of the DA width, the flow in the aorta below the DA origin and the end-diastolic flow in the left pulmonary artery.

Table 5.
Outlines the echocardiographic parameters employed in the definition of hemodynamically significant patent ductus arteriosus (HsPDA).
4.2. Diagnosis of PDA
Diagnostic assessment was conducted using echocardiography, which remains the gold standard for the detection and monitoring of the ductus arteriosus. Examinations were performed by appropriately trained medical personnel. The determination of hemodynamic significance was based on the most recent guidelines of the Polish Neonatal Society. Echocardiographic evaluations were carried out using a Samsung V8 ultrasound system equipped with a PA4-12B transducer (Samsung Medison Co., Ltd., Hongcheon, Republic of Korea).
4.3. Study Design and Data Collection
The study included 99 preterm infants born between 27 and 32 weeks of gestation, who were hospitalized in the Neonatal Intensive Care Unit of the Gynecology and Obstetrics Clinical Hospital (GPSK) in Poznań during 2022 and 2023.
Neonates with congenital heart defects requiring surgical intervention, as well as those who died before the fifth day of life, were excluded from the study. Medical data were collected retrospectively from clinical records documented during hospitalization. Blood samples for genetic analysis were obtained during routine blood collection performed for standard diagnostic testing.
4.4. Ethics
Medical data were obtained from available patient records. To minimize the need for additional procedures, blood samples for genetic testing were collected concurrently with routine blood sampling. Efforts were made to reduce the volume of blood required for genotyping to a minimum (0.5 mL). In each case, the parents were informed in advance about the planned testing, its purpose, and provided written informed consent.
To ensure confidentiality, the number of personnel involved in data processing was restricted to the minimum necessary, and all data were encrypted. The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences (Resolution No. 96/22).
4.5. Genetic Testing Methodology
Blood for genetic polymorphism studies was collected in tubes containing EDTA (ethylene diamine tetraacetic acid). The tubes were stored at −20 °C until DNA isolation. DNA isolation from nucleated blood cells was performed using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s recommendations.
The polymorphic presented in the table were marked using polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP). (Table 6) To select restriction enzymes specific for the SNPs we used the NEBcutter (version 3.0—accessed on 8 October 2024, http://nc2.neb.com/NEBcutter2/) (Table 7).

Table 6.
The study included analysis of the following genetic polymorphisms.

Table 7.
The table below contains the forward and reverse primers used for the PCR of each analyzed variant.
The separation of RFLP reaction products was carried out in a 2.5% agarose gel in 1xTBE buffer at 120 V for about 2 h in the presence of a 50 bp standard. Based on the results of electrophoretic separation after visualization in UV light, individual genotypes were determined (Figure 1).
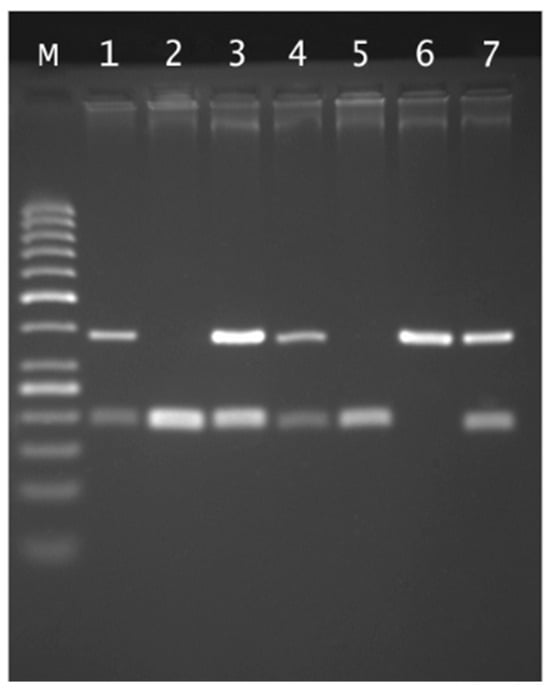
Figure 1.
Agarose gel electrophoresis of rs4375 PLA2G6 genotyping: Lane M, 50 bp marker ladder; lane 6 CC genotype (380 bp); lanes 2 and 5 TT genotypes (199 and 181 bp); lanes 1, 3, 4 and 7 CT genotypes.
4.6. Statistical Analysis
Chi-square tests, with or without Yates’ continuity correction as appropriate, were used to compare dichotomous variables. Odds ratios (ORs) with corresponding 95% confidence intervals (95% CI) were calculated to estimate the strength of associations. A p-value < 0.05 was considered statistically significant.
We calculated the statistical power assuming the incidence of PDA and HsDPA in preterm infants, in our hospital, during the two years of the study, which were 33.6% and 12.1%, respectively. The prevalence in our cohort was 36.4% for PSA and 21.2% for HsPDA. With a sample size of N = 99 and alpha = 0.05, the power of the test was 8.9% for PDA and 74.3% for HsPDA. The significant comparisons were further corrected (Pcorr) using Bonferroni’s correction for multiple testing (0.05/8 = 0.006).
Statistical analyses were performed using GraphPad Software (version 2024) and Statistica (version 10, 2011; StatSoft, Inc., Tulsa, OK, USA).
5. Conclusions
The field of research concerning genetic predispositions to specific disease entities remains in its early stages. Observations of increased disease incidence in twins and in animal models strongly suggest that certain conditions have a substantial genetic component. Our study indicates that specific polymorphisms may contribute to the development of complications such as patent ductus arteriosus, including its hemodynamically significant form. Expanding this line of research through the recruitment of larger patient cohorts—particularly those representing diverse geographic and ethnic backgrounds—may facilitate the development of a robust genomic database. Such a resource could ultimately support the identification of neonates at elevated risk and enable earlier implementation of targeted diagnostic and therapeutic strategies.
Author Contributions
M.M. conceived and designed the study. M.M. and Z.-B.M. collected the data. G.K. performed the statistical analysis. M.M. interpreted the results. M.M. drafted the manuscript. D.S. and A.S.M. critically revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grant numbers 2021/05/x/nz5/01430 from the National Science Center.
Institutional Review Board Statement
The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences (Resolution No. 96/22) and approved on 17 September 2025.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
Due to data privacy regulations, the datasets generated and/or analyzed during the current study are not publicly accessible; however, the data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All authors have no conflicts of interest. There are no financial disclosures to report for any of the authors.
References
- Gournay, V. The ductus arteriosus: Physiology, regulation, and functional and congenital anomalies. Arch. Cardiovasc. Dis. 2011, 104, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; van den Broek, M.; van der Lee, R.; de Boode, W.P. Understanding the pathobiology in patent ductus arteriosus in prematurity—Beyond prostaglandins and oxygen. Pediatr. Res. 2019, 86, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Meraji, S.M.; Houshyar, R.; Radhakrishnan, J.; Mani, A.; Ahangar, M.; Rezaie, T.M.; Taghavinejad, M.-A.; Broumand, B.; Zhao, H.; et al. Finding genetic contributions to sporadic disease: A recessive locus at 12q24 commonly contributes to patent ductus arteriosus. Proc. Natl. Acad. Sci. USA 2002, 99, 15054–15059. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Zhou, G.; Bizzarro, M.J.; Buhimschi, C.; Hussain, N.; Gruen, J.R.; Zhang, H. Genetic contribution to patent ductus arteriosus in the premature newborn. Pediatrics 2009, 123, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Strengers, J.L.; Mentink, M.; Poelmann, R.E.; Patterson, D.F. Histologic studies on normal and persistent ductus arteriosus in the dog. J. Am. Coll. Cardiol. 1985, 6, 394–404. [Google Scholar] [CrossRef]
- Bokenkamp, R.; Gittenberger-De Groot, A.C.; Van Munsteren, C.J.; Grauss, R.W.; Ottenkamp, J.; Deruiter, M.C. Persistent ductus arteriosus in the Brown Norway inbred rat strain. Pediatr. Res. 2006, 60, 407–412. [Google Scholar] [CrossRef][Green Version]
- Heymann, M.A.; Rudolph, A.M. Control of the ductus arteriosus. Physiol. Rev. 1975, 55, 62–78. [Google Scholar] [CrossRef]
- Clyman, R.I. Mechanisms regulating the ductus arteriosus. Biol. Neonate 2006, 89, 330–335. [Google Scholar] [CrossRef]
- Coggins, K.G.; Latour, A.; Ngyuen, M.S.; Audoly, L.; Coffman, T.M.; Koller, B.H. Metabolism of PGE 2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat. Med. 2002, 8, 91–92. [Google Scholar] [CrossRef]
- Rheinlaender, C.; Weber, S.C.; Sarioglu, N.; Strauss, E.; Obladen, M.; Koehne, P. Changing expression of cyclooxygenases and prostaglandin receptor EP4 during development of the human ductus arteriosus. Pediatr. Res. 2006, 60, 270–275. [Google Scholar] [CrossRef]
- Ben-David, Y.; Hallak, M.; Rotschild, A.; Sorokin, Y.; Auslender, R.; Abramovici, H. Indomethacin and fetal ductus arteriosus: Complete closure after cessation of prolonged therapeutic course. Fetal Diagn. Ther. 1996, 11, 341–344. [Google Scholar] [CrossRef]
- Li, L.; Qi, L.; Lv, N.; Gao, Q.; Cheng, Y.; Wei, Y.; Ye, J.; Yan, X.; Dang, A. Association between lipoprotein-associated phospholipase A2 gene polymorphism and coronary artery disease in the Chinese Han population. Ann. Hum. Genet. 2011, 75, 605–611. [Google Scholar] [CrossRef]
- Gu, X.; Lin, W.; Xu, Y.; Che, D.; Tan, Y.; Lu, Z.; Pi, L.; Fu, L.; Zhou, H.; Jiang, Z.; et al. The rs1051931 G>A Polymorphism in the PLA2G7 Gene Confers Resistance to Immunoglobulin Therapy in Kawasaki Disease in a Southern Chinese Population. Front. Pediatr. 2020, 8, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maiolino, G.; Lenzini, L.; Pedon, L.; Cesari, M.; Seccia, T.M.; Frigo, A.C.; Rossitto, G.; Caroccia, B.; Rossi, G.P. Lipoprotein-associated phospholipase A2 single-nucleotide polymorphisms and cardiovascular events in patients with coronary artery disease. J. Cardiovasc. Med. 2015, 16, 29–36, Erratum in J. Cardiovasc. Med. 2015, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Loftin, C.D.; Trivedi, D.B.; Tiano, H.F.; Clark, J.A.; Lee, C.A.; Epstein, J.A.; Morham, S.G.; Breyer, M.D.; Nguyen, M.; Hawkins, B.M.; et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc. Natl. Acad. Sci. USA 2001, 98, 1059–1064. [Google Scholar] [CrossRef]
- Reese, J.; Paria, B.C.; Brown, N.; Zhao, X.; Morrow, J.D.; Dey, S.K. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 9759–9764. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Camenisch, T.; Snouwaert, J.N.; Hicks, E.; Coffman, T.M.; Anderson, P.A.W.; Malouf, N.N.; Koller, B.H. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature 1997, 390, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.Y.; Zhou, Q.; Lin, J.; Chi, L.F.; Chi, W.Z. Interaction between ALOX5AP-SG13S114A/T and COX-2-765G/C increases susceptibility to cerebral infarction in a Chinese population. Genet. Mol. Res. 2013, 12, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, J.P.; Guu, T.W.; Chen, Y.C.; Gałecki, P.; Walczewska, A.; Su, K.P. BanI polymorphism of cytosolic phospholipase A2 gene and somatic symptoms in medication-free acute depressed patients. Prostaglandins Leukot Essent Fat. Acids 2018, 136, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, Q.; Noguti, R.; Bottino, C.M.; Vallada, H. Study of association between genetic polymorphisms of phospholipase A2 enzymes and Alzheimer’s disease. Arq. Neuro-Psiquiatr. 2010, 68, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhuo, X.; Guo, B.; Su, C.; Yan, X.; Yan, J.; Hu, S.; Tie, X.; Chen, Y. Lp-PLA2 variants associated with delayed encephalopathy after acute carbon monoxide poisoning. Int. J. Clin. Exp. Med. 2016, 9, 16393–16398. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).