Effects of the Interactions Between Food Additive Titanium Dioxide and Matrices on Genotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Food Additive TiO2 Interacted with Matices
2.2. In Vitro Solubility in Digestive Fluids of Food Additive TiO2 Interacted with Matices
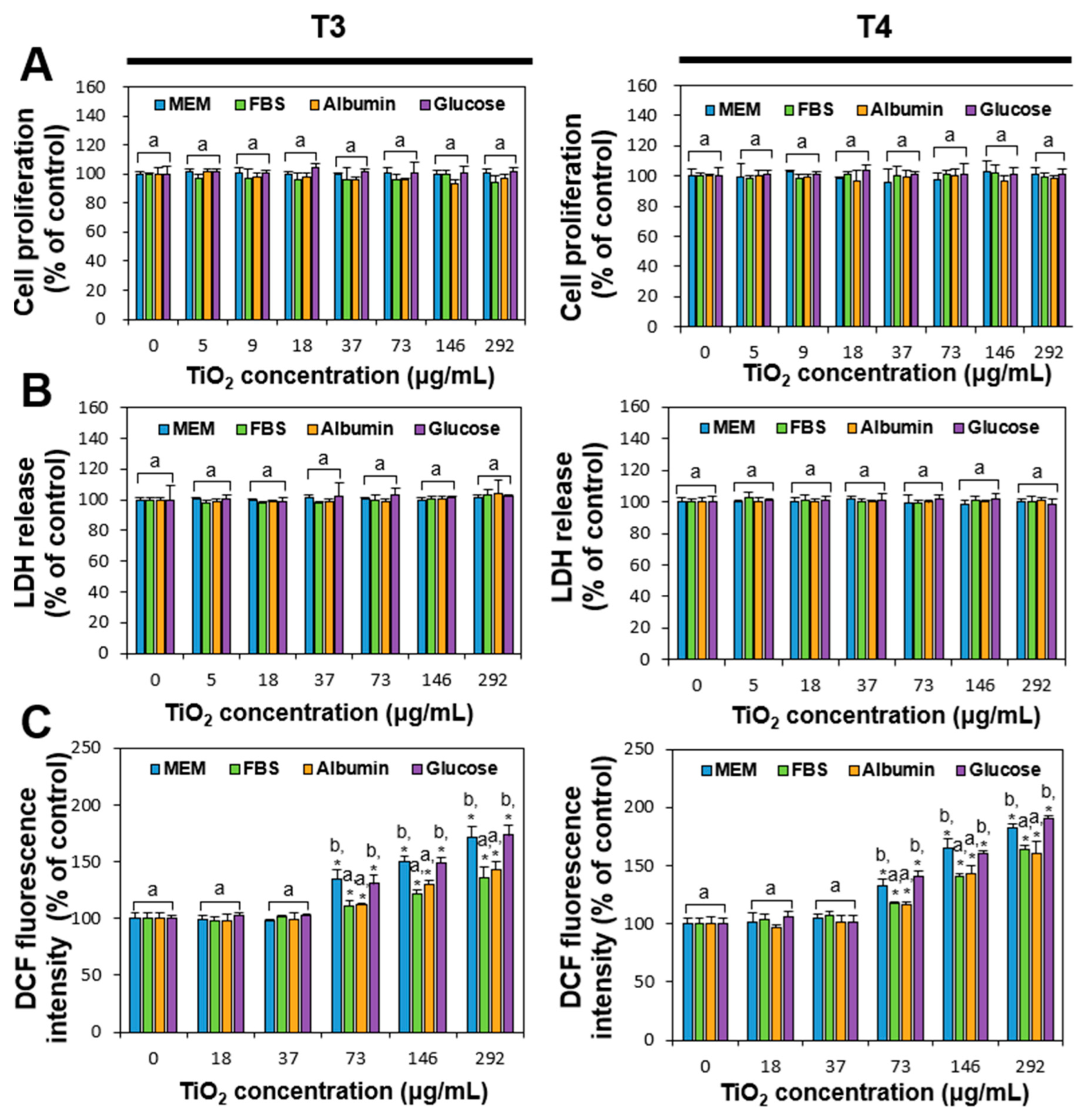
2.3. Cytotoxicity of Food Additive TiO2 Interacted with Matices
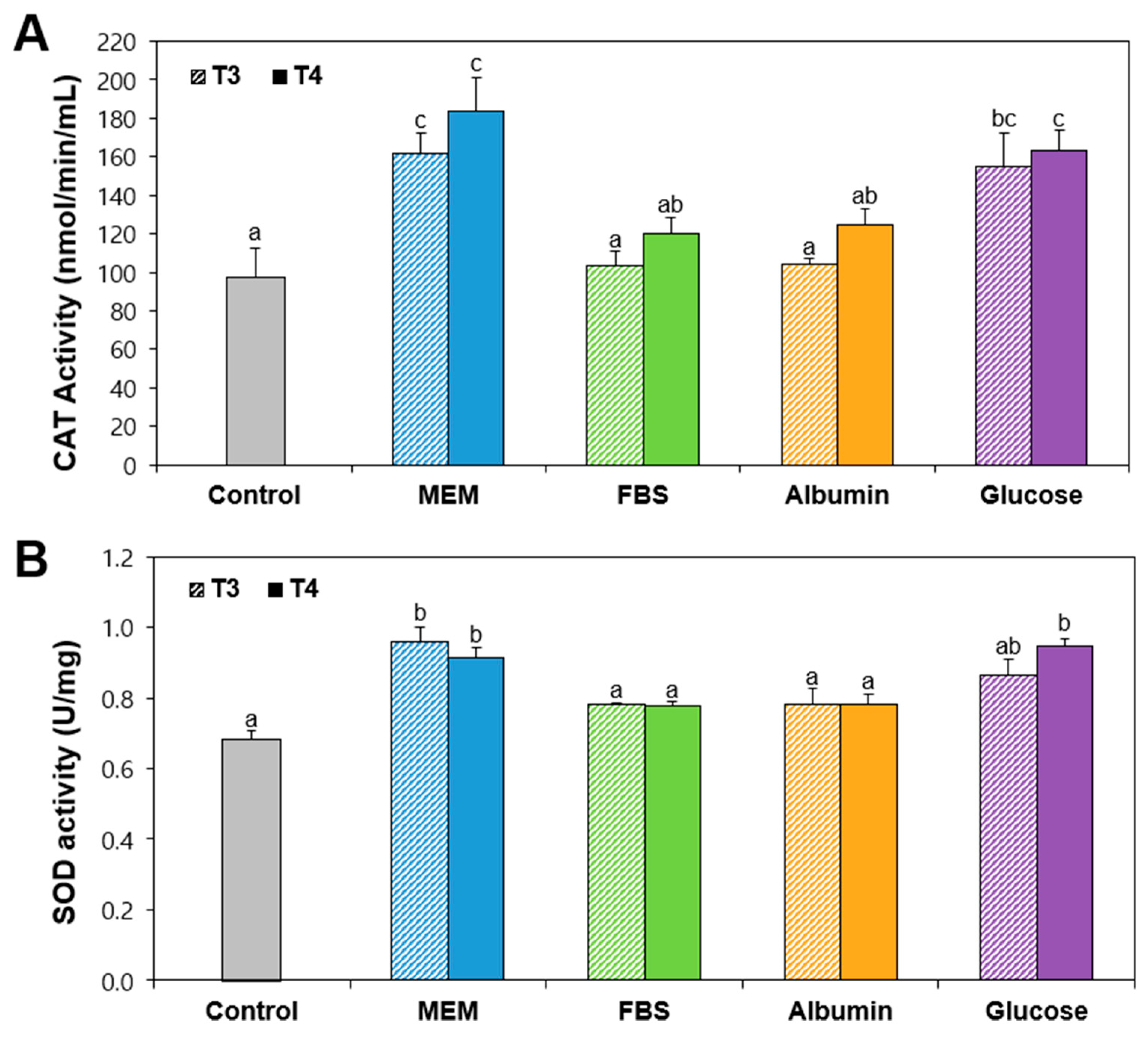
2.4. Antioxidant Enzyme Activity of Food Additive TiO2 Interacted with Matices
2.5. In Vitro Intestinal Transport of Food Additive TiO2 Interacted with Matices
2.6. Ex Vivo Intestinal Absorption of Food Additive TiO2 Interacted with Matices
2.7. DNA Damage Caused by Food Additive TiO2 Interacted with Matices
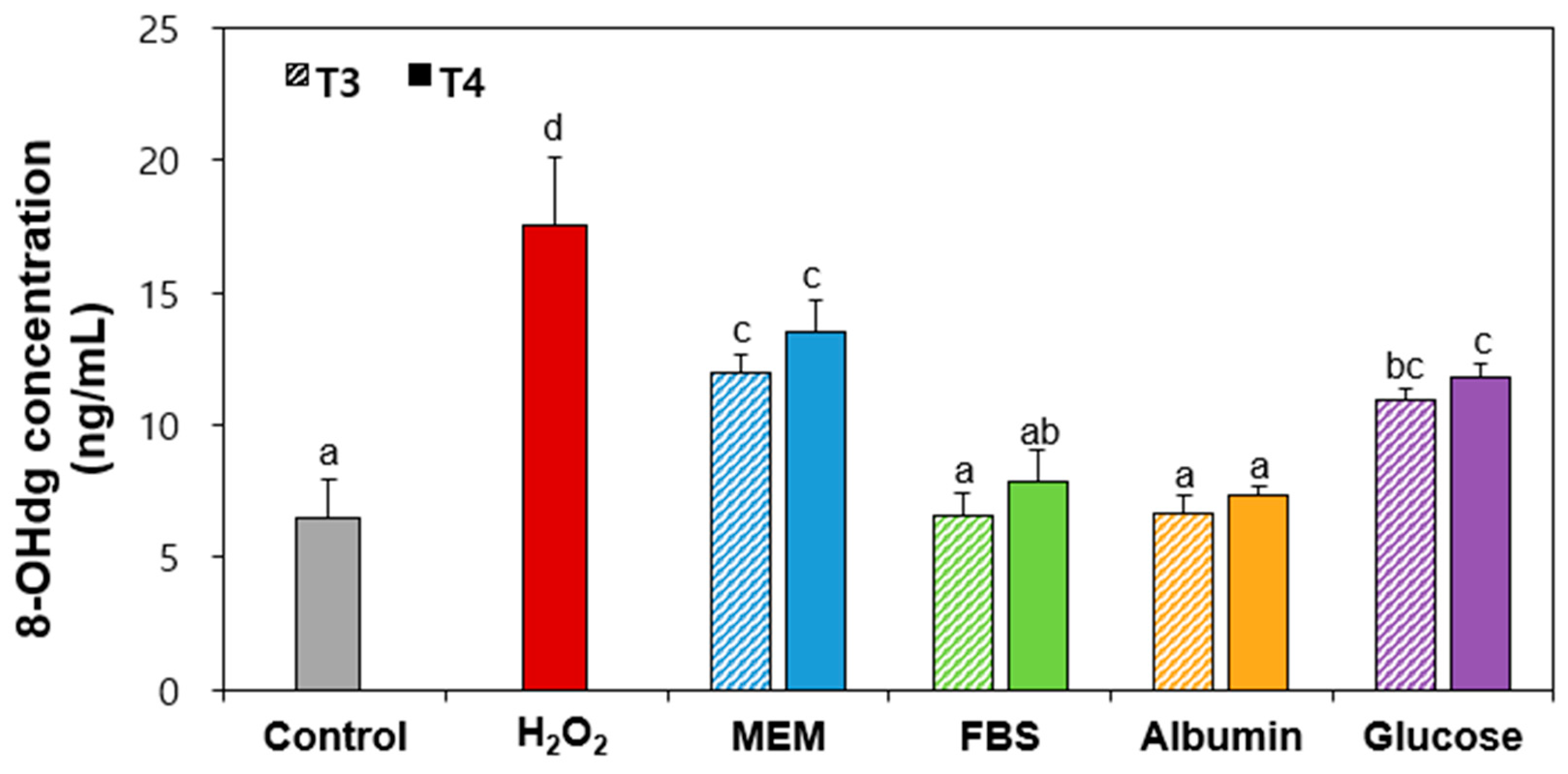
2.8. 8-Hydroxy-2′-Deoxyguanosine (8-OHdg) Production by Food Additive TiO2 Interacted with Matices
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. In Vitro Solubility in Digestive Fluids
3.4. Cell Culture
3.5. Cytotoxicity
3.6. Antioxidatn Enzyme Activity
3.7. In Vitro Intestinal Transport
3.8. Ex Vivo Intestinal Absorption
3.9. ICP-AES Analysis
3.10. Comet Assay
3.11. 8-OHdG Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [PubMed]
- European Commission. European Commission Regulation (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171) (Text with EEA Relevance). 2022. Available online: https://eur-lex.europa.eu/eli/reg/2022/63/oj (accessed on 28 November 2024).
- U.S. Food and Drug Administration (FDA). CFR-Code of Federal Regulations Title 21 CRF 73.575. Available online: https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=17723345ba7c363ef25a68e9776aa2cd&r=PART&n=21y1.0.1.1.27#se21.1.73_1575 (accessed on 28 November 2024).
- National Toxicology Program Official. Bioassay of Titanium Dioxide for Possible Carcinogenicity; National Cancer Institute: Bethesda, MD, USA, 1979.
- Additives, J. Thirteenth Report of the Joint FAO/WHO Expert Committee on Food Additives. Specifications for the Identity and Purity of Food Additives and Their Toxicological Evaluation; World Health Organization: Geneva, Switzerland, 1970. [Google Scholar]
- World Health Organization. Compendium of Food Additive Specifications. In Joint FAO/WHO Expert Committee on Food Additives, Proceedings of the 97th Meeting, Rome, Italy, 31 October–9 November 2023; Food & Agriculture Org.: Rome, Italy, 2024. [Google Scholar]
- Food Standards Australia New Zealand (FSANZ). Review of Titanium Dioxide as a Food Additive. Available online: https://www.foodstandards.gov.au/sites/default/files/consumer/foodtech/Documents/FSANZ_TiO2_Assessment_report.pdf (accessed on 28 November 2024).
- United Kingdom’s Food Standards Authority (FSA). FSA 22-06-19a—Risk Analysis Process Update. 2022. Available online: https://www.food.gov.uk/print/pdf/node/9616 (accessed on 28 November 2024).
- Health Canada. State of the Science of Titanium Dioxide (TiO2) as a Food Additive. 2022. Available online: https://publications.gc.ca/collections/collection_2022/sc-hc/H164-341-2022-eng.pdf (accessed on 28 November 2024).
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front. Nutr. 2019, 6, 446992. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.M.; de Azevedo, W.M.; Dagli, M.L.Z.; Toma, S.H.; de Arruda Leite, A.Z.; Lordello, M.L.; Nishitokukado, I.; Ortiz-Agostinho, C.L.; Duarte, M.I.S.; Ferreira, M.A. Titanium dioxide induced inflammation in the small intestine. World J. Gastroenterol. 2012, 18, 4729. [Google Scholar] [CrossRef]
- Urrutia-Ortega, I.M.; Garduño-Balderas, L.G.; Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; González-Robles, A.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez-Sosa, M.; León-Cabrera, S. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhou, Y.; Zhao, X.; Sheng, L.; Wang, L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomedicine 2017, 12, 6197–6204. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Bian, Q.; Ning, J.; Yong, L.; Ou, T.; Song, Y.; Wei, S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles In Vivo and In Vitro: A Meta-Analysis. Toxics 2023, 11, 882. [Google Scholar] [CrossRef]
- Warheit, D.; Boatman, R.; Brown, S. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Regul. Toxicol. Pharmacol. 2015, 73, 887–896. [Google Scholar]
- Han, H.Y.; Yang, M.J.; Yoon, C.; Lee, G.H.; Kim, D.W.; Kim, T.W.; Kwak, M.; Heo, M.B.; Lee, T.G.; Kim, S. Toxicity of orally administered food-grade titanium dioxide nanoparticles. J. Appl. Toxicol. 2021, 41, 1127–1147. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Fang, J.; Sun, N.; Liu, H.; Feng, Y.; Wang, H.; Yu, Z.; Jia, X. Genotoxicity evaluation of food additive titanium dioxide using a battery of standard in vivo tests. Regul. Toxicol. Pharmacol. 2024, 148, 105586. [Google Scholar] [CrossRef]
- Jo, M.R.; Yu, J.; Kim, H.J.; Song, J.H.; Kim, K.M.; Oh, J.M.; Choi, S.J. Titanium dioxide nanoparticle-biomolecule interactions influence oral absorption. Nanomaterials 2016, 6, 225. [Google Scholar] [CrossRef]
- Mesgari, M.; Aalami, A.H.; Sahebkar, A. Antimicrobial activities of chitosan/titanium dioxide composites as a biological nanolayer for food preservation: A review. Int. J. Biol. Macromol. 2021, 176, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, I.; Greco, G.; Livraghi, S.; Fubini, B. Non-UV-induced radical reactions at the surface of TiO2 nanoparticles that may trigger toxic responses. Chem.-Eur. J. 2009, 15, 4614–4621. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Lee, T.G.; Reipa, V.; Heo, M.B. Titanium dioxide induces apoptosis under UVA irradiation via the generation of lysosomal membrane permeabilization-dependent reactive oxygen species in HaCaT cells. Nanomaterials 2021, 11, 1943. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, M.; Deng, J.; Shi, Y.; Jia, M.; Li, W. Nano-TiO2 regulates the MAPK (ERK, P38) pathway to promote apoptosis and inhibit proliferation of human colon cells. Ecotoxicol. Environ. Saf. 2024, 283, 116973. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar]
- Hwang, J.S.; Yu, J.; Kim, H.M.; Oh, J.M.; Choi, S.J. Food additive titanium dioxide and its fate in commercial foods. Nanomaterials 2019, 9, 1175. [Google Scholar] [CrossRef]
- Duan, S.; Wang, H.; Gao, Y.; Wang, X.; Lyu, L.; Wang, Y. Oral intake of titanium dioxide nanoparticles affect the course and prognosis of ulcerative colitis in mice: Involvement of the ROS-TXNIP-NLRP3 inflammasome pathway. Part. Fibre Toxicol. 2023, 20, 24. [Google Scholar] [CrossRef]
- Bischoff, N.S.; Undas, A.K.; van Bemmel, G.; Briedé, J.J.; van Breda, S.G.; Verhoeven, J.; Verbruggen, S.; Venema, K.; Sijm, D.T.H.M.; de Kok, T.M.; et al. Investigating the ROS Formation and Particle Behavior of Food-Grade Titanium Dioxide (E171) in the TIM-1 Dynamic Gastrointestinal Digestion Model. Nanomaterials 2024, 15, 8. [Google Scholar] [CrossRef]
- Qiaorun, Z.; Honghong, S.; Yao, L.; Bing, J.; Xiao, X.; McClements, D.J.; Chongjiang, C.; Biao, Y. Investigation of the interactions between food plant carbohydrates and titanium dioxide nanoparticles. Food Res. Int. 2022, 159, 111574. [Google Scholar] [CrossRef]
- Gräfe, C.; Weidner, A.; vd Lühe, M.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle–cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Peng, Q.; Wei, X.Q.; Yang, Q.; Zhang, S.; Zhang, T.; Shao, X.R.; Cai, X.X.; Zhang, Z.R.; Lin, Y.F. Enhanced biostability of nanoparticle-based drug delivery systems by albumin corona. Nanomedicine 2015, 10, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, M.K.; Song, J.H.; Jo, M.R.; Yu, J.; Kim, K.M.; Kim, Y.R.; Oh, J.M.; Choi, S.J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B Biointerfaces 2017, 150, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Miao, Y.; Li, W.; Song, G.; Xiao, M.; Liang, X.; Yang, Y. Synthesis of uniform platinum nanoparticles using glucose as dispersant. Nanosci. Nanotechnol. Lett. 2014, 6, 592–595. [Google Scholar] [CrossRef]
- Ferraris, F.; Raggi, A.; Ponti, J.; Mehn, D.; Gilliland, D.; Savini, S.; Iacoponi, F.; Aureli, F.; Calzolai, L.; Cubadda, F. Agglomeration Behavior and Fate of Food-Grade Titanium Dioxide in Human Gastrointestinal Digestion and in the Lysosomal Environment. Nanomaterials 2023, 13, 1908. [Google Scholar] [CrossRef]
- Mbanga, O.; Cukrowska, E.; Gulumian, M. Dissolution of titanium dioxide nanoparticles in synthetic biological and environmental media to predict their biodurability and persistence. In Vitro Toxicol. 2022, 84, 105457. [Google Scholar] [CrossRef]
- Warheit, D.B.; Brown, S.C. What is the impact of surface modifications and particle size on commercial titanium dioxide particle samples?–A review of in vivo pulmonary and oral toxicity studies–Revised 11-6-2018. Toxicol. Lett. 2019, 302, 42–59. [Google Scholar] [CrossRef]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnology 2018, 16, 51. [Google Scholar] [CrossRef]
- Korea Health Industry Development Institute (KHIDI). National Food & Nutrition Statistics. 2017. Available online: https://www.khidi.or.kr/kps/dhraStat/result2?menuId=MENU01653&gubun=&year=2017 (accessed on 29 January 2020).
- Yu, J.; Kim, Y.H.; Choi, S.J. Usage-and daily intake-based cytotoxicity study of frequently used natural food additives in South Korea. Korean J. Food Sci. Technol. 2020, 52, 546–554. [Google Scholar]
- Bhatti, R.; Shakeel, H.; Malik, K.; Qasim, M.; Khan, M.A.; Ahmed, N.; Jabeen, S. Inorganic nanoparticles: Toxic effects, mechanisms of cytotoxicity and phytochemical interactions. Adv. Pharm. Bull. 2021, 12, 757. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Wu, S.; Lv, Z.; Zhang, Q.; Ke, Y. Titanium dioxide nanoparticles induce size-dependent cytotoxicity and genomic DNA hypomethylation in human respiratory cells. RSC Adv. 2017, 7, 23560–23572. [Google Scholar] [CrossRef]
- Gandamalla, D.; Lingabathula, H.; Yellu, N. Nano titanium exposure induces dose-and size-dependent cytotoxicity on human epithelial lung and colon cells. Drug Chem. Toxicol. 2019, 42, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Baek, M.; Chung, H.E.; Choi, S.J. Effects of physicochemical properties of zinc oxide nanoparticles on cellular uptake. J. Phys. Conf. Ser. 2011, 304, 012007. [Google Scholar] [CrossRef]
- Allouni, Z.E.; Høl, P.J.; Cauqui, M.A.; Gjerdet, N.R.; Cimpan, M.R. Role of physicochemical characteristics in the uptake of TiO2 nanoparticles by fibroblasts. Vitr. Toxicol. 2012, 26, 469–479. [Google Scholar] [CrossRef]
- Gu, T.; Yao, C.; Zhang, K.; Li, C.; Ding, L.; Huang, Y.; Wu, M.; Wang, Y. Toxic effects of zinc oxide nanoparticles combined with vitamin C and casein phosphopeptides on gastric epithelium cells and the intestinal absorption of mice. RSC Adv. 2018, 8, 26078–26088. [Google Scholar] [CrossRef]
- Kim, S.B.; Yoo, N.K.; Choi, S.J. Interactions between ZnO nanoparticles and polyphenols affect biological responses. Nanomaterials 2022, 12, 3337. [Google Scholar] [CrossRef]
- Jin, H.R.; Yu, J.; Choi, S.J. Hydrothermal treatment enhances antioxidant activity and intestinal absorption of rutin in tartary buckwheat flour extracts. Foods 2019, 9, 8. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brassinne, F.; Sebaihi, N.; Petry, J.; Cokic, S.M.; Van Landuyt, K.L.; Godderis, L.; Mast, J.; Lison, D.; Hoet, P.H.; et al. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part. Fibre Toxicol. 2020, 17, 10. [Google Scholar] [CrossRef]
- Proquin, H.; Rodriguez-Ibarra, C.; Moonen, C.G.; Urrutia Ortega, I.M.; Briede, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef]
- Vieira, A.; Vital, N.; Rolo, D.; Roque, R.; Goncalves, L.M.; Bettencourt, A.; Silva, M.J.; Louro, H. Investigation of the genotoxicity of digested titanium dioxide nanomaterials in human intestinal cells. Food Chem. Toxicol. 2022, 161, 112841. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Yamada, Y.; Tomonaga, M.; Ichinose, H.; Kamihira, S. Correlation of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a biomarker of oxidative DNA damage, and clinical features of hematological disorders: A pilot study. Leuk. Res. 2000, 24, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.K.; Jeon, Y.R.; Choi, S.J. Determination of Two Differently Manufactured Silicon Dioxide Nanoparticles by Cloud Point Extraction Approach in Intestinal Cells, Intestinal Barriers and Tissues. Int. J. Mol. Sci. 2021, 22, 7035. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Lee, H.I.; Kwon, R.Y.; Choi, S.J. Food Additive Solvents Increase the Dispersion, Solubility, and Cytotoxicity of ZnO Nanoparticles. Nanomaterials 2023, 13, 2573. [Google Scholar] [CrossRef]
- Kwon, R.Y.; Kim, S.B.; Youn, S.M.; Choi, S.-J. Fate Determination and Characterization of Food Additive Silicon Dioxide and Titanium Dioxide in Commercial Foods. Front. Biosci. (Landmark Ed.) 2023, 28, 36. [Google Scholar] [CrossRef]
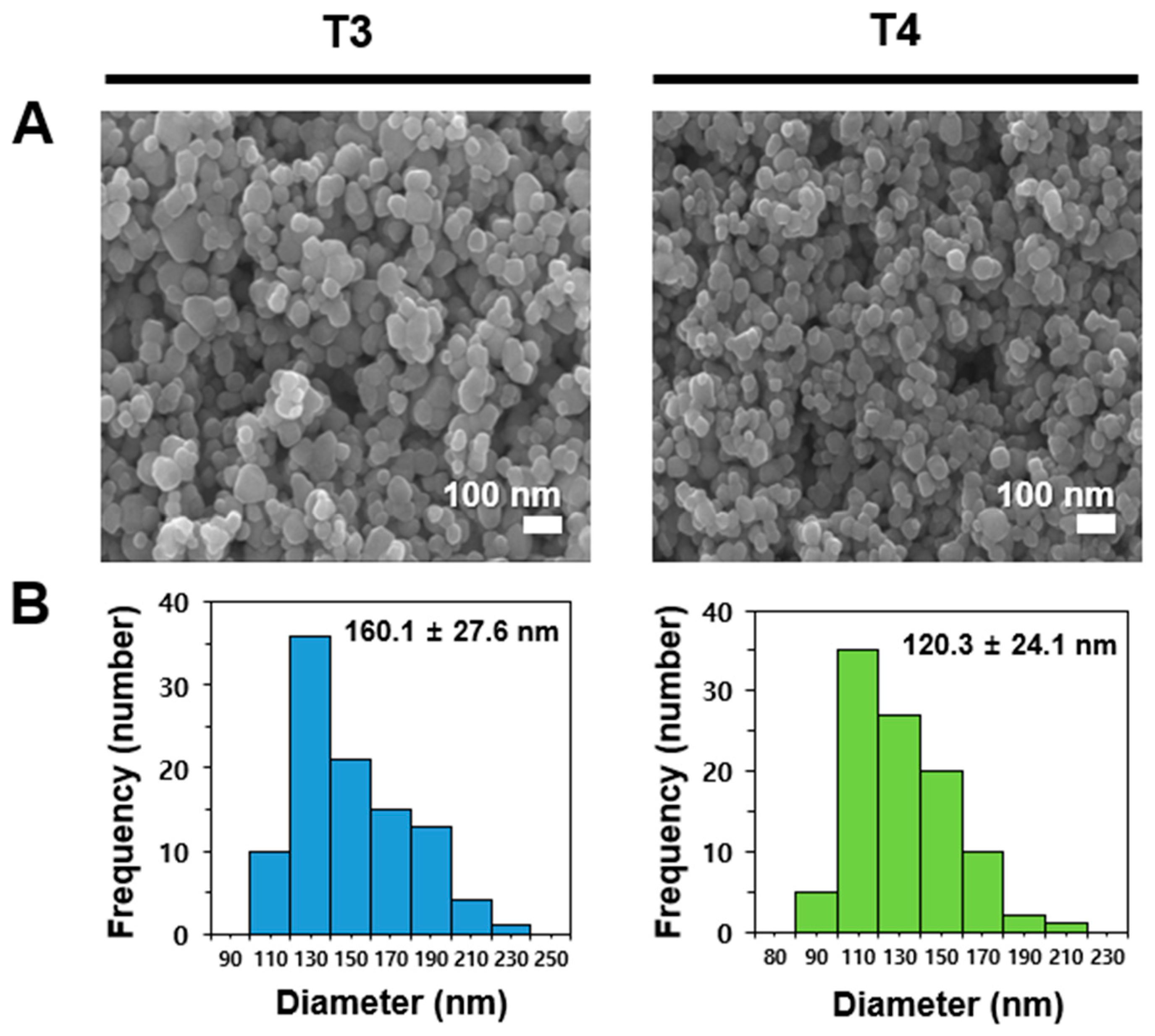



| Samples | Hydrodynamic Diameter (nm) | |||||||
|---|---|---|---|---|---|---|---|---|
| DDW | DDW | MEM | MEM | |||||
| FBS | Albumin | Glucose | FBS | Albumin | Glucose | |||
| T3 | 304.7 ± 0.9 A,a | 404.8 ± 6.6 A,b | 397.5 ± 6.6 A,b | 317.9 ± 6.1 A,a | 440.9 ± 4.3 B,a | 505.6 ± 6.5 B,b | 495.3 ± 6.1 B,b | 456.3 ± 7.6 B,a |
| T4 | 295.7 ± 2.9 A,a | 348.2 ± 1.4 A,b | 342.6 ± 14.8 A,b | 308.1 ± 5.9 A,a | 377.3 ± 2.8 B,a | 437.0 ± 9.2 B,b | 431.9 ± 2.6 B,b | 388.9 ± 1.0 B,a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-M.; Nam, H.-N.; Choi, S.-J. Effects of the Interactions Between Food Additive Titanium Dioxide and Matrices on Genotoxicity. Int. J. Mol. Sci. 2025, 26, 617. https://doi.org/10.3390/ijms26020617
Jeong S-M, Nam H-N, Choi S-J. Effects of the Interactions Between Food Additive Titanium Dioxide and Matrices on Genotoxicity. International Journal of Molecular Sciences. 2025; 26(2):617. https://doi.org/10.3390/ijms26020617
Chicago/Turabian StyleJeong, Su-Min, Han-Na Nam, and Soo-Jin Choi. 2025. "Effects of the Interactions Between Food Additive Titanium Dioxide and Matrices on Genotoxicity" International Journal of Molecular Sciences 26, no. 2: 617. https://doi.org/10.3390/ijms26020617
APA StyleJeong, S.-M., Nam, H.-N., & Choi, S.-J. (2025). Effects of the Interactions Between Food Additive Titanium Dioxide and Matrices on Genotoxicity. International Journal of Molecular Sciences, 26(2), 617. https://doi.org/10.3390/ijms26020617







