Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes
Abstract
:1. Innovation
2. Introduction
3. Results
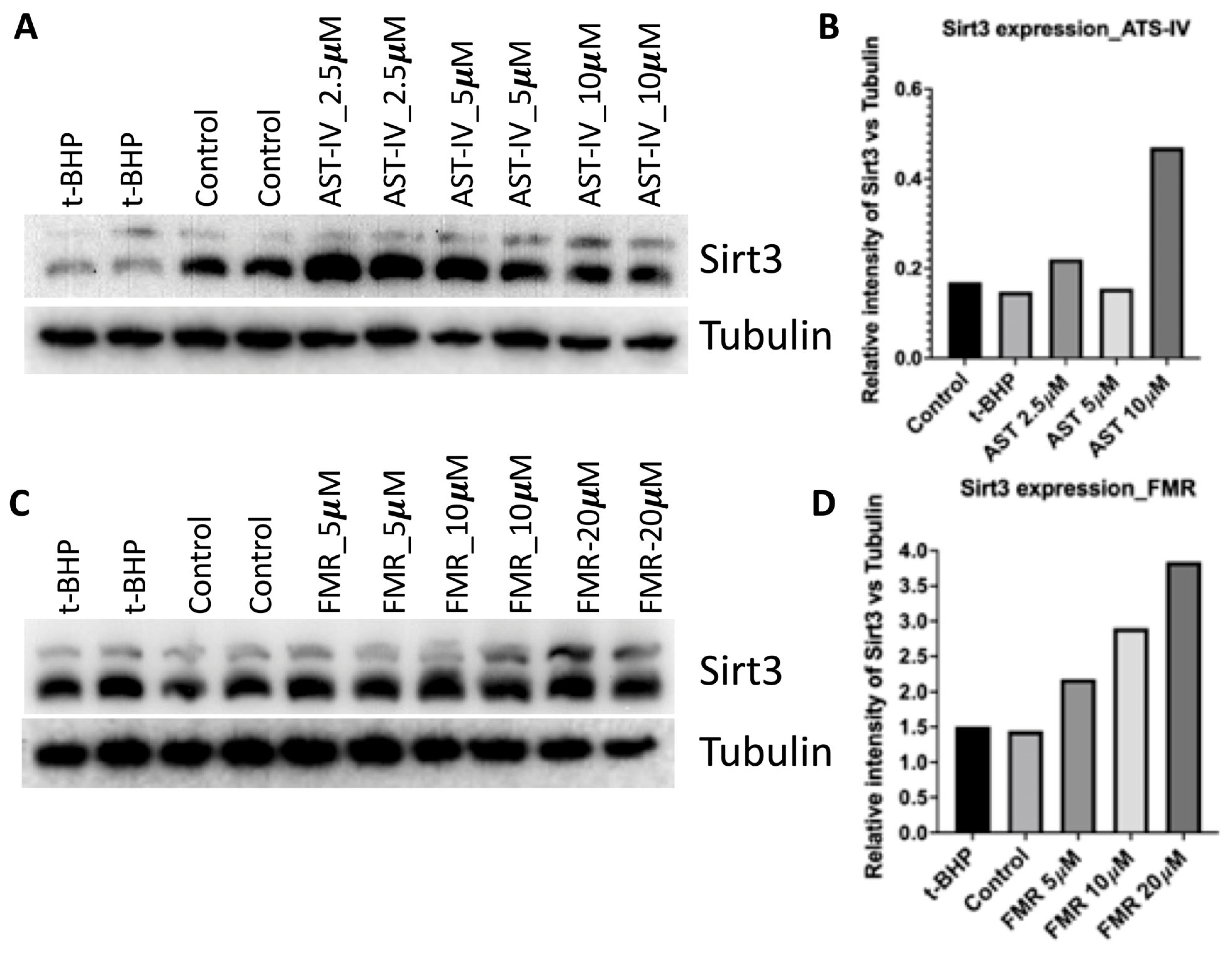
3.1. AST-IV and FMR Induced SIRT3 Activity and Expression in AML12 Cells
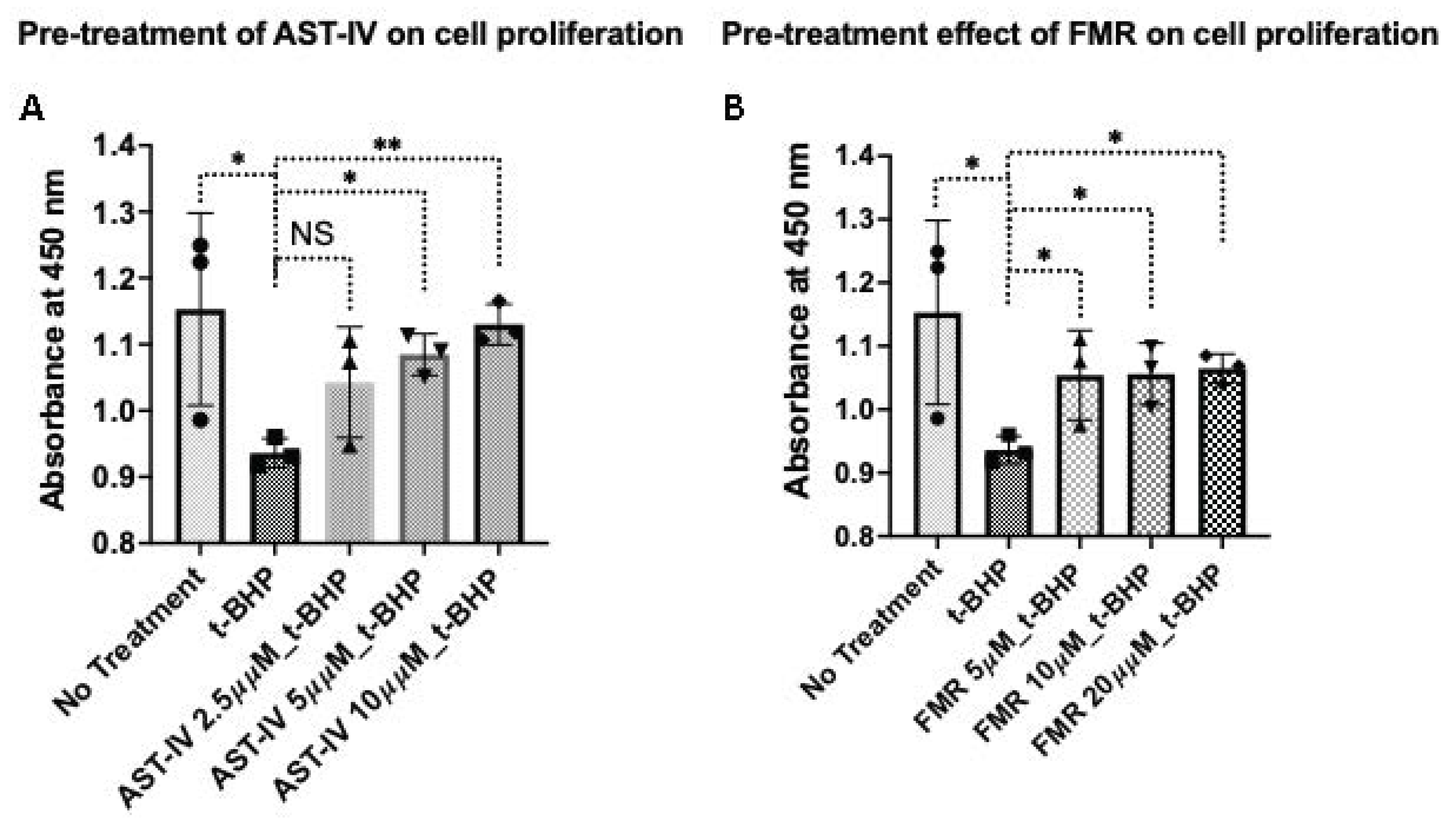
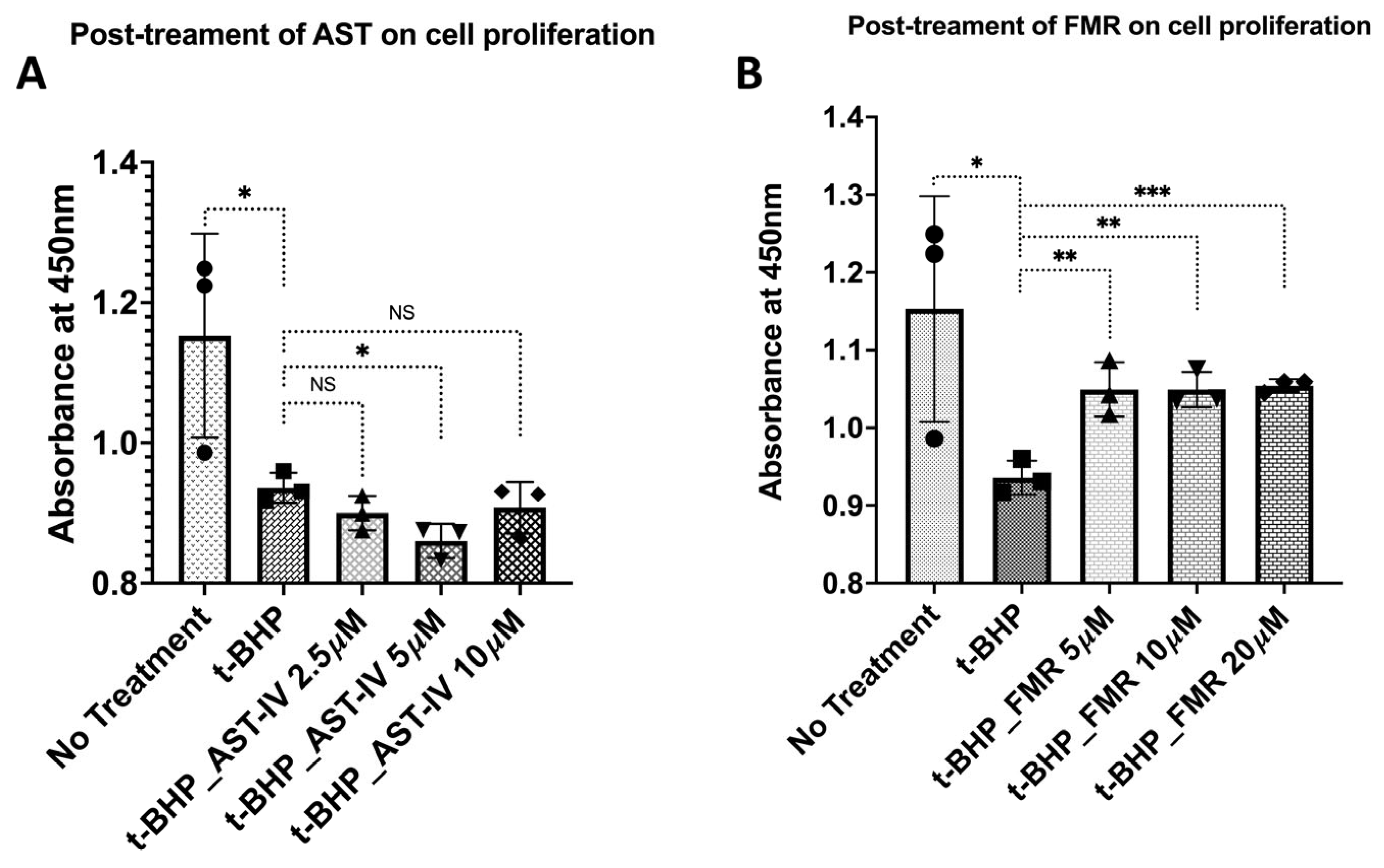
3.2. Rescue Effect of AST-IV and FMR on t-BHP-Induced Oxidative Injury in AML12 Hepatocytes Assessed by Proliferation and Viability
3.2.1. Rescue Effects of AST-IV and FMR on Cell Proliferation
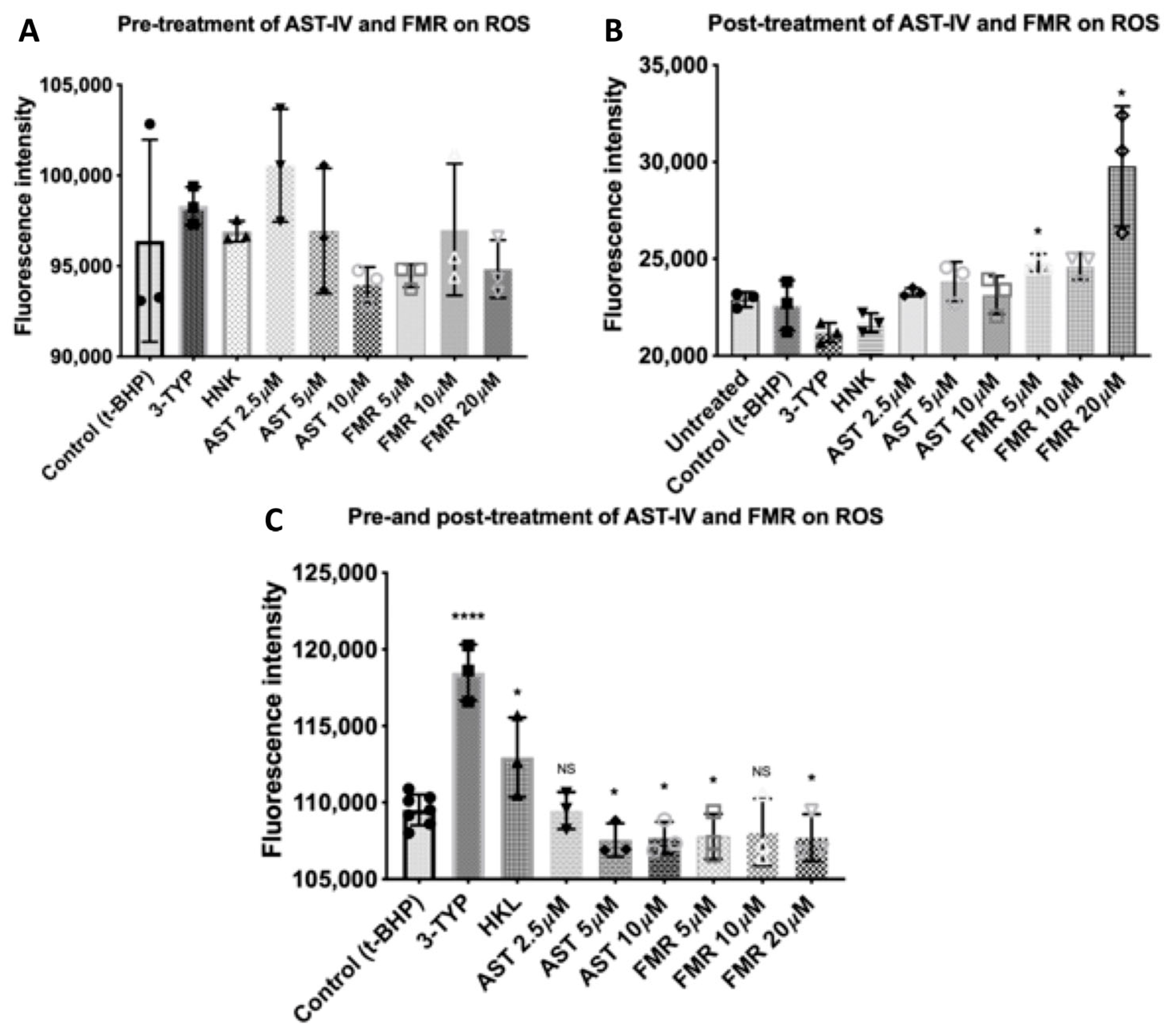
3.2.2. Rescue Effects of AST-IV and FMR on ROS Levels
3.3. Cellular Antioxidant Defense Capacity of AST-IV and Formononetin
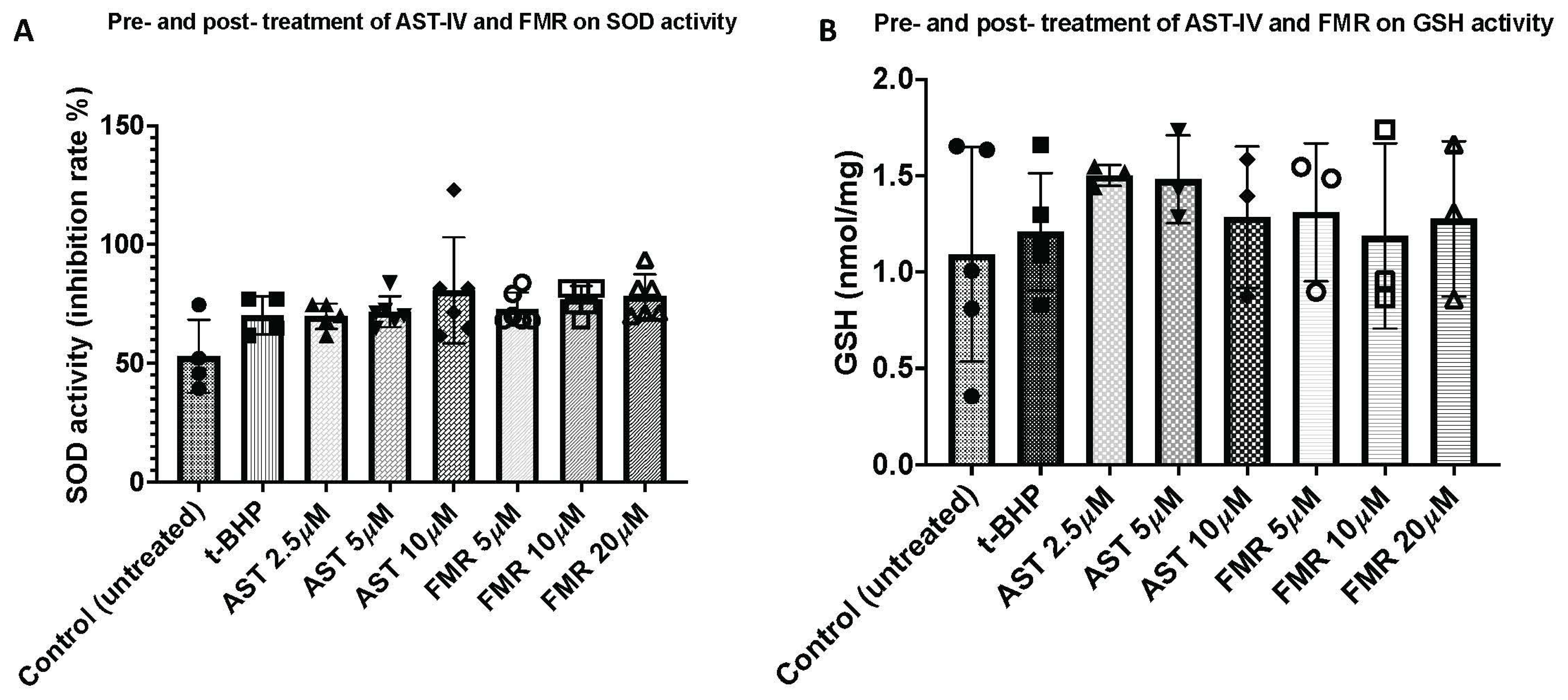
3.3.1. SOD Antioxidant Activity
3.3.2. Glutathione (GSH) Antioxidant Activity
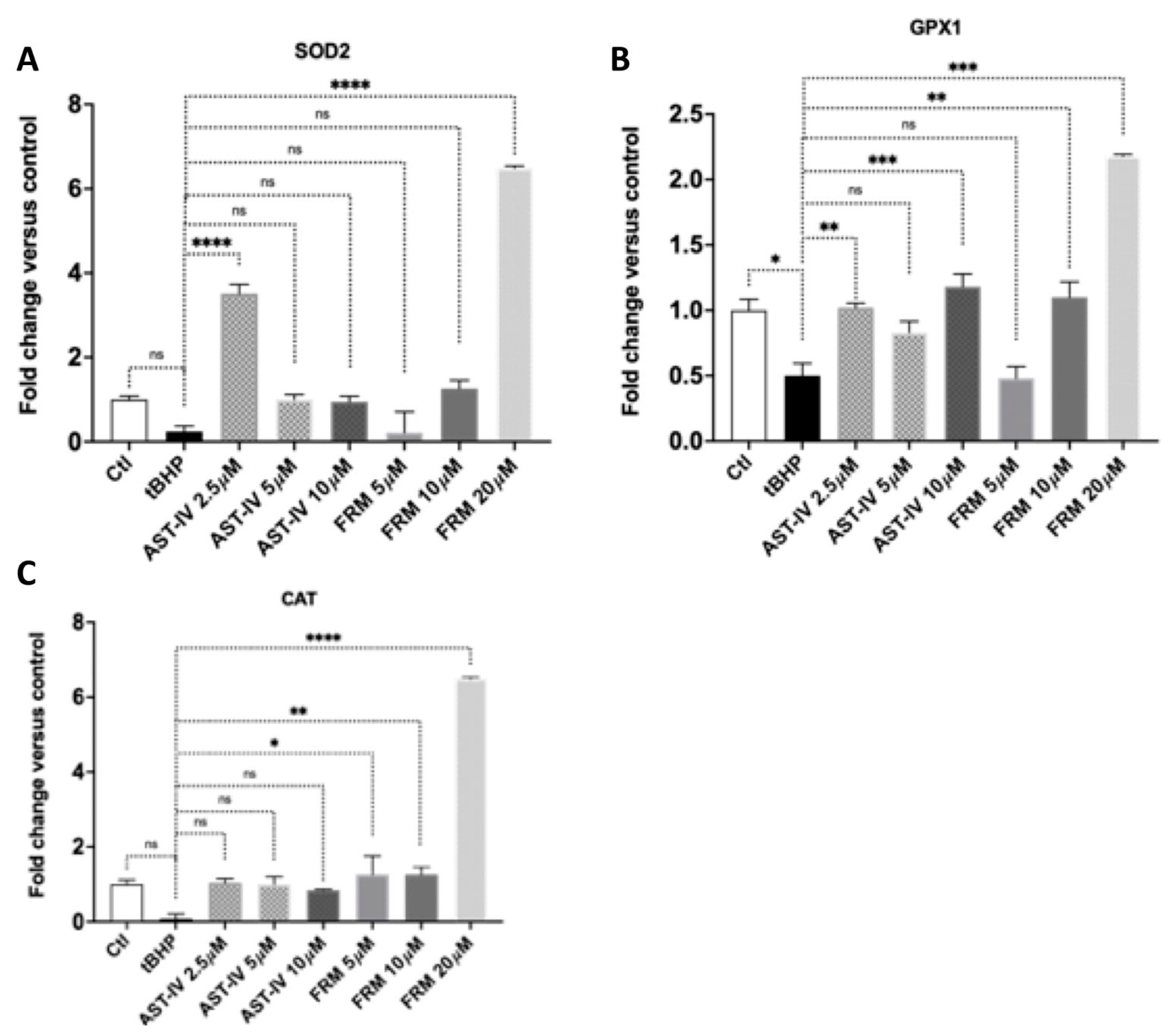
3.3.3. AST-IV and FMR Promote Antioxidant Gene Expression
3.4. AST-IV and FMR Prevent Mitochondrial Damage in Oxidation-Injured Hepatocytes via Enhancing Mitochondrial Mass, Membrane Potential, and Biogenesis
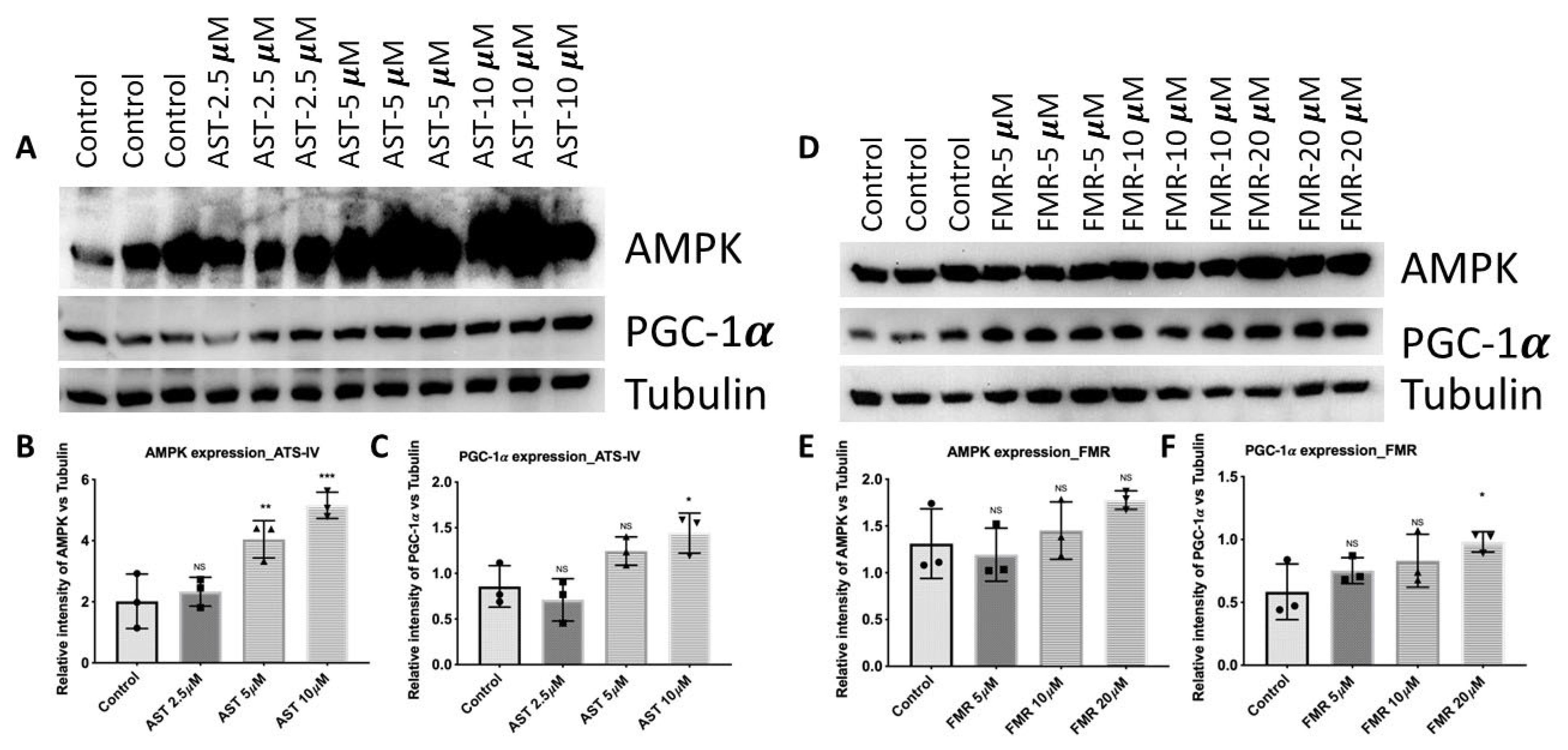
3.4.1. AST-IV and FMR Enhance Mitochondrial Biogenesis via Activating AMPK and PGC-1α
3.4.2. AST-IV and FMR Enhance Mitochondrial Membrane Potential and Mitochondrial Content
4. Discussion
5. Conclusions
6. Materials and Methods
6.1. Electronic Laboratory Notebook Was Not Used
Testing Agents
6.2. Cell Culture and Treatment Conditions
6.3. Cell Proliferation and Viability Assay
6.4. ROS Measurement
6.5. SOD Activity Assay
6.6. Glutathione GSH Measurement
6.7. Immunoblotting
6.8. RNA Extraction and Real-Time PCR
6.9. Mitochondrial Membrane Potential
6.10. Statistics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Nonstandard Abbreviations
| AML12 | Alpha mouse liver 12 |
| AMPK | AMP-activated protein kinase |
| AST-IV | Astragaloside |
| Cat | Catalase |
| FMR | Formononetin |
| GDH | Glutamate dehydrogenase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione (glutathione disulfide) |
| GPX1 | Glutathione peroxidase 1 |
| IDH2 | Isocitrate dehydrogenase 2 |
| IL-13 | Interleukin 13 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MnSOD | Manganese superoxide dismutase |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PGC-1α | Peroxisome proliferator-activated receptor γ coactivator 1α |
| ROS | Reactive oxygen species |
| SIRT3 | Sirtuin-3 |
| Sirt1 | Sirtuin-1 |
| SirT | Sirtuins |
| SOD | Superoxide dismutase |
| Sod2 | Superoxide dismutase 2 |
| t-BHP | tert-Butyl hydroperoxide |
References
- Zhang, X.; Wu, X.; Hu, Q.; Wu, J.; Wang, G.; Hong, Z.; Ren, J.; Lab for, T.; Surgical, I. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019, 236, 116464. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Hu, R.; Koyama, Y.; Liang, S.; Rosenthal, S.B.; Yamamoto, G.; Karin, D.; Baglieri, J.; Ma, H.Y.; Xu, J.; et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J. Hepatol. 2019, 71, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free. Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- van de Ven, R.A.H.; Santos, D.; Haigis, M.C. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.; Kim, H.S.; Flynn, C.R.; Hill, S.; Hayes McDonald, W.; et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 2010, 40, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Yu, W.; Dittenhafer-Reed, K.E.; Denu, J.M. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012, 287, 14078–14086. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, C.; Gertz, M.; Papatheodorou, P.; Kachholz, B.; Becker, C.F.; Steegborn, C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J. Mol. Biol. 2008, 382, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.; Zhang, T.; Tong, Q.; Ye, R.D.; Lin, L. SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity. Cell Death Dis. 2017, 8, e3158. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, Z.; Linghu, K.G.; Liu, J.; Gan, L.; Lin, L. Small molecule-driven SIRT3-autophagy-mediated NLRP3 inflammasome inhibition ameliorates inflammatory crosstalk between macrophages and adipocytes. Br. J. Pharmacol. 2020, 177, 4645–4665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, A.; Li, D.; Wang, Y.; Lin, L. Pinocembrin from Penthorum chinense Pursh suppresses hepatic stellate cells activation through a unified SIRT3-TGF-beta-Smad signaling pathway. Toxicol. Appl. Pharmacol. 2018, 341, 38–50. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wu, X.; Zhao, Z.; Jian, C.; Li, M.; Qin, X. Astragaloside IV Alleviates Depression in Rats by Modulating Intestinal Microbiota, T-Immune Balance, and Metabolome. J. Agric. Food Chem. 2024, 72, 259–273. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-kappaB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- Yimam, M.; Jiao, P.; Hong, M.; Jia, Q. A Standardized Composition from Extracts of Myristica Fragrans, Astragalus Membranaceus, and Poria Cocos Protects Liver from Acute Ethanol Insult. J. Med. Food 2016, 19, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of Astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Hu, R.; Zhao, J.; Li, Y.; Li, Q.; Zhang, X. Metabolomics combined with network pharmacology reveals the protective effect of astragaloside IV on alcoholic liver disease. Phytomedicine 2024, 135, 156032. [Google Scholar] [CrossRef]
- Shen, Q.; Fang, J.; Guo, H.; Su, X.; Zhu, B.; Yao, X.; Wang, Y.; Cao, A.; Wang, H.; Wang, L. Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic. Biol. Med. 2023, 203, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.B.; Gao, J.; Chai, Y.H.; Li, W.; Luo, Y.F.; Chen, Y.Z. Astragaloside alleviates alcoholic fatty liver disease by suppressing oxidative stress. Kaohsiung J. Med. Sci. 2021, 37, 718–729. [Google Scholar] [CrossRef]
- Wu, S.; Wen, F.; Zhong, X.; Du, W.; Chen, M.; Wang, J. Astragaloside IV ameliorate acute alcohol-induced liver injury in mice via modulating gut microbiota and regulating NLRP3/caspase-1 signaling pathway. Ann. Med. 2023, 55, 2216942. [Google Scholar] [CrossRef]
- Ding, M.; Bao, Y.; Liang, H.; Zhang, X.; Li, B.; Yang, R.; Zeng, N. Potential mechanisms of formononetin against inflammation and oxidative stress: A review. Front. Pharmacol. 2024, 15, 1368765. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, D.; Sun, J.; Zhang, Q.; Qiao, Y.; Zhu, Y.; Niu, J.; Ren, Q.; Zhou, L.; Wen, A.; et al. Formononetin Inhibits Hepatic I/R-Induced Injury through Regulating PHB2/PINK1/Parkin Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 6481192. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Ma, Y.F.; Huang, Y.F. Inhibiting effects of three components of Astragalus membranaceus on oxidative stress in Chang Liver cells. Zhongguo Zhong Yao Za Zhi 2015, 40, 318–323. [Google Scholar] [PubMed]
- Alauddin; Chaturvedi, S.; Malik, M.Y.; Azmi, L.; Shukla, I.; Naseem, Z.; Rao, C.; Agarwal, N.K. Formononetin and biochanin A protects against ritonavir induced hepatotoxicity via modulation of NfkappaB/pAkt signaling molecules. Life Sci. 2018, 213, 174–182. [Google Scholar] [CrossRef]
- Yi, L.; Cui, J.; Wang, W.; Tang, W.; Teng, F.; Zhu, X.; Qin, J.; Wuniqiemu, T.; Sun, J.; Wei, Y.; et al. Formononetin Attenuates Airway In fl ammation and Oxidative Stress in Murine Allergic Asthma. Front. Pharmacol. 2020, 11, 533841. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Tian, N.; Wang, T.; Shi, Y.; Xu, J.; Wu, B. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-kappaB p65 axis. Sci. Rep. 2019, 9, 323. [Google Scholar]
- Wang, X.; Gao, Y.; Tian, N.; Zhu, Z.; Wang, T.; Xu, J.; Wu, B.; Zhang, N. Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-kappaB p65 subunit. Drug Des. Dev. Ther. 2018, 12, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Ameliorates Diabetic Neuropathy by Increasing Expression of SIRT1 and NGF. Chem. Biodivers. 2020, 17, e2000162. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, X.; Xie, A. Formononetin ameliorates IL-13-induced inflammation and mucus formation in human nasal epithelial cells by activating the SIRT1/Nrf2 signaling pathway. Mol. Med. Rep. 2021, 24, 832. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, C.; Sang, X.; Peng, M.; Yang, Q.; Ning, Y.; Lv, Q.; Shan, Q.; Hao, M.; Wang, K.; et al. Targeting Sirtuin1 to treat aging-related tissue fibrosis: From prevention to therapy. Pharmacol. Ther. 2022, 229, 107983. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Jiang, H.C.; Wang, A.; Bu, F.T.; Jia, P.C.; Zhu, S.; Zhu, L.; Huang, C.; Li, J. Hesperetin derivative-16 attenuates CCl(4)-induced inflammation and liver fibrosis by activating AMPK/SIRT3 pathway. Eur. J. Pharmacol. 2022, 915, 174530. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.A.; Kim, M.; Lee, S.; Oh, M.; Moon, J.; Nam, J.W.; Choi, H.; Mun, Y.J.; Roh, S.S. Gardeniae Fructus Attenuates Thioacetamide-Induced Liver Fibrosis in Mice via Both AMPK/SIRT1/NF-kappaB Pathway and Nrf2 Signaling. Antioxidants 2021, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, E.; Colell, A.; Morales, A.; Mari, M. Relevance of SIRT1-NF-kappaB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int. J. Mol. Sci. 2020, 21, 3858. [Google Scholar] [CrossRef]
- Wang, J.; Nisar, M.; Huang, C.; Pan, X.; Lin, D.; Zheng, G.; Jin, H.; Chen, D.; Tian, N.; Huang, Q.; et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab. J. Gastroenterol. 2018, 19, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.M.; Corum, D.; Beeson, C.C.; Schnellmann, R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Zamzami; Marchetti, P.; Castedo, M.; Decaudin, D.; Macho, A.; Hirsch, T.; Susin, S.A.; Petit, P.X.; Mignotte, B.; Kroemer, G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995, 182, 367–377. [Google Scholar] [CrossRef]
- Preiser, J.C. Oxidative stress. JPEN J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Weydert, C.J. and J.J. Cullen, Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef] [PubMed]


| Gene | NCBI Ref. Seq. (NM) | Primers | Primer Sequences (5′ -> 3′) |
|---|---|---|---|
| SOD2 | NM_013671 | F | TGG ACA AAC CTG AGC CCT AAG |
| R | CCC AAA GTC ACG CTT GAT AGC | ||
| CAT | NM_009804 | F | GGA GGC GGG AAC CCA ATA G |
| R | GTG TGC CAT CTC GTC AGT GAA | ||
| GPX1 | NM_008160 | F | CCA CCG TGT ATG CCT TCT CC |
| R | AGA GAG ACG CGA CAT TCT CAA T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, Q.-A.; Tran, G.V.; Velic, S.; Xiong, H.-M.; Kaur, J.; Moosavi, Z.; Nguyen, P.; Duong, N.; Luu, V.T.; Singh, G.; et al. Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes. Int. J. Mol. Sci. 2025, 26, 774. https://doi.org/10.3390/ijms26020774
Tran Q-A, Tran GV, Velic S, Xiong H-M, Kaur J, Moosavi Z, Nguyen P, Duong N, Luu VT, Singh G, et al. Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes. International Journal of Molecular Sciences. 2025; 26(2):774. https://doi.org/10.3390/ijms26020774
Chicago/Turabian StyleTran, Quoc-Anh, Grant Van Tran, Sanel Velic, Hou-Mai Xiong, Jaspreet Kaur, Zuhurr Moosavi, Phuong Nguyen, Nhi Duong, Vy Tran Luu, Gurjot Singh, and et al. 2025. "Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes" International Journal of Molecular Sciences 26, no. 2: 774. https://doi.org/10.3390/ijms26020774
APA StyleTran, Q.-A., Tran, G. V., Velic, S., Xiong, H.-M., Kaur, J., Moosavi, Z., Nguyen, P., Duong, N., Luu, V. T., Singh, G., Bui, T., Rose, M., & Ho, L. (2025). Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes. International Journal of Molecular Sciences, 26(2), 774. https://doi.org/10.3390/ijms26020774






