Abstract
COVID-19 severity is frequently linked to exacerbated inflammation, with the inflammasome pathway playing a key role in activating inflammatory interleukins. This observational post-mortem study evaluated the expression of inflammasome-associated molecules in patients who died from COVID-19 during the second wave. Minimally invasive autopsies were performed on patients from the first (n = 24) and second (n = 18) waves. Lung tissue samples underwent immunohistochemical staining for ACE-2, TLR-4, NF-κB, TNF-α, NOX4, NLRP3, ASC, CASPASE-1, IL-1β, IL-18, GSDMD, and CASPASE-9. Additionally, genetic polymorphisms within inflammasome-related genes were assessed via real-time polymerase chain reaction. Lung tissue expressions of TLR-4, NLRP3, and IL-18 were significantly higher in patients from the second wave compared to those from the first, with expression levels of 26.3 versus 12.1, 13.9 versus 6.4, and 25.6 versus 3.8, respectively. The A allele at rs4648090 of NFKB1 and the T allele at rs317155 of NOX4 were associated with increased corresponding protein expression by factors of 5.1 and 8.9, respectively. Notably, IL-18 demonstrated substantial immunological relevance, correlating strongly with elevated expression linked to these genetic variants in second wave cases. These findings suggest that the inflammasome pathway harbors biologically meaningful molecules implicated in severe COVID-19, meriting further investigation for their potential as diagnostic or therapeutic targets.
1. Introduction
Several host-related risk factors have been identified as contributors to severe disease and increased mortality in coronavirus infections [,]. Current research indicates that the most severe COVID-19 cases typically involve clinical features such as lymphopenia, hypoalbuminemia, elevated lactate dehydrogenase, increased C-reactive protein, ferritin, and D-dimer levels. These alterations often accompany an exaggerated inflammatory response termed the “cytokine storm” [,,]. Elevated cytokine concentrations contribute to the pathogenesis of severe acute respiratory syndrome (SARS) caused by SARS-CoV-2, where the virus triggers intense inflammation that can exacerbate clinical decline and increase the risk of fatality.
Central to inflammation is the activation of the inflammasome, a multi-protein intracellular complex that orchestrates inflammatory responses and induces a form of programmed cell death known as pyroptosis [,]. The inflammasome responds to diverse stimuli, including pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), nucleic acids, and pore-forming toxins. Key molecules such as NLRP3, ASC, pro-caspase-1, pro-IL-1β, and IL-18 have established roles in inflammasome activation [,,]. Extensive research has implicated the inflammasome pathway in numerous diseases, and during the COVID-19 pandemic, new and repurposed drugs targeting this pathway, including disulfiram and biologics like Anakinra® and Canakinumab®, were evaluated for modulating the inflammatory cascade related to pyroptosis and endothelial dysfunction [,,]. These molecular components represent promising candidates for further study.
Genetic variation across the human genome, particularly single-nucleotide polymorphisms (SNPs), can significantly influence gene expression and function. While many SNPs exert minimal effects, select polymorphisms may affect disease susceptibility, clinical outcomes, and response to treatment [,,]. Consequently, genetic variants may serve as important components in future biomarker discovery and understanding individual risk profiles.
Biomarkers—both genetic and protein—offer measurable insights into biological states, facilitating diagnosis, prognosis, and prediction of treatment responses. The expression of inflammasome-related proteins may be modulated by specific polymorphisms, underscoring the potential of these molecules as indicators of severity and therapeutic targets in COVID-19. Given that the second wave of COVID-19 in Brazil yielded higher mortality, this study hypothesizes that differential expression and polymorphisms in inflammasome pathway genes among deceased patients could highlight molecules of biological relevance for future pandemics. Accordingly, this study aims to investigate the relationship between SNPs in inflammasome-associated genes and protein expression levels in post-mortem lung tissues from COVID-19 patients, using the second wave as a reference for outcome analysis.
2. Results
2.1. Sociodemographic and Clinical Information
Table 1 compares demographic and clinical variables between the first and second COVID-19 waves. Patients in the first wave were significantly older, with a median age of 72.5 years versus 55.5 years in the second wave (p < 0.0001). Mechanical ventilation duration was longer in the second wave group (median 14.5 days) compared to the first wave (9.5 days, p = 0.040). No significant difference was observed in hospital stay length until death (p = 0.430).

Table 1.
Comparison between COVID-19 (1st WAVE) and COVID-19 (2nd WAVE) according to demographic and clinical findings.
2.2. Immunohistochemistry Findings
Immunohistochemistry revealed significantly elevated expressions of TLR-4, NLRP3, and IL-18 in lung tissue from second wave patients compared to the first (median percentages: 26.3 vs. 12.1, 13.9 vs. 6.4, and 25.6 vs. 3.8, respectively). After Bonferroni correction for multiple comparisons, only IL-18 maintained statistical significance. Other markers such as ACE-2 and GSDMD showed higher expression in the second wave but without statistical significance, while NF-κB and NOX4 expression were lower in the second wave (Table 2, Figure 1).

Table 2.
Comparison between COVID-19 (1st WAVE) and COVID-19 (2nd WAVE) groups according to immunohistochemical findings.
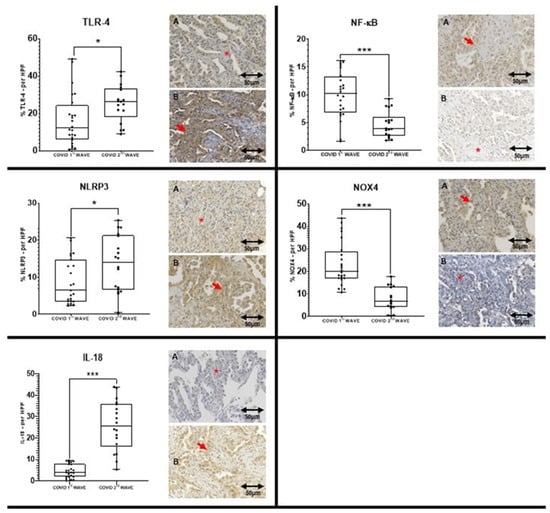
Figure 1.
Comparison between the COVID 1st WAVE (A) and COVID 2nd WAVE (B) regarding TLR-4, NLRP3, IL-18, NF-kB and NOX4 expressions. The left Panel shows the expression of three markers that are significantly higher in the COVID 2nd WAVE group compared to the COVID 1st WAVE group. The right Panel shows the expression of two markers that are significantly higher in the COVID 1st WAVE group compared to the COVID 2nd WAVE group. The symbol “***” in the graph means p ≤ 0.001, while “*” in the graph means p ≤ 0.05 and in the image appoint to an area without immunoexpression. The red arrows are appointed to an area with immunoexpression. The slide images were obtained at 40× magnification.
2.3. Genotyping Findings
Table 3 summarizes genotype frequencies across COVID-19 waves. Significant differences were noted with elevated homozygosity for ACE2 rs4646188 AA (p = 0.000), rs4646156 AA (p = 0.028), and rs2048683 GG (p = 0.028) polymorphisms in the second wave. For TLR4 rs10759932 CT genotype, a higher frequency was observed in the second wave (p = 0.000). After Bonferroni correction (p < 0.001), significance persisted for ACE2 rs4646188 and TLR4 rs10759932. No significant results were found in the first and second wave of patients in grouping (dominant and recessive analysis (see Supplementary Tables S1 and S2).

Table 3.
Distribution of genotype frequencies in COVID-19 groups (1st and 2nd waves).
Further genotype clustering analyses linked the NFKB1 rs4648090 AA genotype and NOX4 rs317155 TT + TC cluster with higher protein expression in second wave lung tissues (p = 0.022 and p = 0.008, respectively), although these associations lost significance after strict correction. IL-18-related polymorphisms (rs1946518, rs187238) showed expression trends with biological plausibility despite lacking statistical significance (Table 4).

Table 4.
Immunohistochemical expression in genotyping groups (dominant model) in COVID 2nd wave.
The main analysis of this study was conducted to verify a possible association between genotypes and high tissue expression using genotype clustering models (dominant and recessive) in the first and second waves. The AA genotype for rs4648090 [G/A] of NFKB1 (p = 0.022) and the TT + TC cluster for rs317155 [T/C] of the NOX4 gene (p = 0.008) were significantly associated with the highest expression of the proteins encoded by these genes in the cohort of patients affected by the second wave of COVID-19 (Table 4); however, before the Bonferroni correction (p < 0.001), this association was lost. Considering that in the first round of analysis only IL-18 remained significant after statistical correction, the SNPs in the IL18 gene, despite not presenting statistical significance, could acquire some biological plausibility. The highest expressions of IL-18 can be associated with the GG + GT (rs1946518 G/T), and CC + CG (rs187238 C/G) grouping genotypes, respectively. No significant results were found in the first wave of patients in this analysis (see Supplementary Tables S3 and S4).
Multivariate analyses were performed, but no significance was found for any of the variables.
3. Discussion
This study aimed to further elucidate marked differences in the expression of inflammasome-related proteins and associated genetic polymorphisms between COVID-19 fatal cases from the first and second pandemic waves in Brazil. Our findings contribute to understanding the molecular underpinnings that may explain the increased severity and mortality characterizing the second wave.
The COVID 1st WAVE group exhibited a higher mean age with mostly older patients. In contrast, the COVID 2nd WAVE group predominantly comprised younger patients, which aligns with the findings reported in the extant literature []. The age is a major risk factor for severe COVID-19, since older patients are more vulnerable due to immune senescence and chronic low-grade inflammation [], every ten years the risk of death rises by 1.5 times [,]. Despite the higher risk, the second wave of COVID-19 in Brazil was three times deadlier than the first, affecting the entire population []. This demonstrates that other major aspects were involved in the severity of the disease. The clinical variables not addressed in relation to the 1st and 2nd waves in this sample were not part of the analyses of this article as they have already been the subject of discussion in other articles of the group [,,,,,,,,,].
Immunohistochemical analysis (Table 2) revealed significantly elevated lung tissue expression of TLR-4, NLRP3, and IL-18 during the second wave, with IL-18 expression showing robust statistical significance even after stringent Bonferroni correction. This pronounced IL-18 elevation aligns with the literature recognizing IL-18 as a critical pro-inflammatory cytokine driving pulmonary inflammation and systemic cytokine storm in severe COVID-19 [,]. The enhanced TLR-4 expression mirrors its role as a pattern recognition receptor that can be activated by SARS-CoV-2 components and oxidized phospholipids, stimulating innate immune responses and perpetuating inflammation [,,,]. Similarly, increased NLRP3 levels reflect inflammasome assembly and activation, consistent with its established contribution to IL-1β and IL-18 maturation and subsequent pyroptotic cell death in infected tissues [,,,,].
ACE-2 and GSDMD also demonstrated higher expression in the second wave, although without statistical significance. ACE-2 plays a dual role as the viral entry receptor and regulator of inflammatory pathways [,,,,,,,]. Elevated ACE-2 expression in post-mortem tissues may indicate enhanced viral tropism or compensatory mechanisms during later disease stages. GSDMD mediates pyroptotic membrane pore formation, further amplifying inflammasome downstream effects [,]. Conversely, NF-κB and NOX4 levels were paradoxically reduced in the second wave. Given NF-κB’s role in transcriptional regulation of inflammatory genes and NOX4’s involvement in reactive oxygen species generation, their reduced expression may represent negative feedback or cell exhaustion phenomena arising during sustained inflammation [,,,,,,,].
Genetic analysis (Table 3) uncovered higher frequencies of homozygous genotypes for ACE2 rs4646188 AA, rs4646156 AA, and rs2048683 GG in second wave patients, polymorphisms previously implicated in susceptibility to severe pulmonary and cardiovascular pathologies [,,,,]. The enrichment of TLR4 rs10759932 CT genotype aligns with reports linking this variant to altered immune responses in chronic lung diseases [,]. Clustering analyses (Table 4) further demonstrated that NFKB1 rs4648090 AA and NOX4 rs317155 TT + TC genotypes associate with increased protein expression, underscoring the genetic influence on inflammasome pathway activity. Though statistical significance was lost after correction, these results present biologically plausible mechanisms by which host genetics exacerbate inflammasome-mediated injury.
The observed shift toward younger patients with prolonged ventilation during the second wave points to changes in clinical phenotype potentially driven by viral evolution and immune landscape remodeling [,]. Enhanced inflammasome activation likely exacerbates lung tissue damage through sustained cytokine release and pyroptosis, contributing to increased mortality despite demographic differences [,].
Our data help support a model in which inflammasome activation may contribute as a central factor in the severe pathology of COVID-19, intensified in the second wave by both increased cytokine expression and host genetic predisposition. This reinforces the promise of inflammasome components, particularly IL-18, as future biomarkers and potential therapeutic targets [,,,]. Future interventions aimed at modulating inflammasome activation have the potential to improve outcomes, especially for genetically susceptible individuals.
This study’s limitations include sample size constraints with associated power limitations, especially for genetic analyses, and the inability to temporally track longitudinal changes due to the post-mortem study design. Nonetheless, the integrated immunohistochemical and genetic approach offers valuable insights into host–pathogen interactions driving COVID-19 mortality across epidemic waves.
4. Materials and Methods
4.1. Study Population
This study was conducted following the Declaration of Helsinki and approved by the National Research Ethics Committee (CONEP; Project ID: 30188020.7.1001.0020). Families of deceased COVID-19 patients provided informed consent for minimally invasive post-mortem lung biopsies. The cohort consisted of 42 patients admitted to the intensive care unit at Hospital Marcelino Champagnat, Curitiba, Brazil. Samples included 24 patients from the first COVID-19 wave (June–August 2020) and 18 from the second wave (April–May 2021). All patients had SARS-CoV-2 confirmed by RT-PCR performed on nasopharyngeal swabs during hospitalization. Patients with negative SARS-CoV-2 tests or without consent were excluded.
4.2. Tissue Processing and Immunohistochemistry
Post-mortem lung biopsy fragments were formalin-fixed, paraffin-embedded, and assembled into tissue microarray (TMA) blocks. Sections (4 μm) were mounted on positively charged slides. Immunohistochemistry was performed using primary monoclonal antibodies against ACE-2, TLR-4, NF-κB, TNF-α, NOX4, NLRP3, ASC, CASPASE-1, IL-1β, IL-18, GSDMD, and CASPASE-9. Primary antibody incubation occurred overnight at 2–8 °C. Visualization employed horseradish peroxidase conjugated polymer detection with diaminobenzidine substrate, followed by Harris hematoxylin counterstain. Positive control reactivity confirmed assay specificity. The authors will indicate only those markers that show significantly higher tissue expression in the second wave group, as the first wave was recently reported for these samples [].
4.3. Morphometric Analysis
Slides were digitally scanned (Axio Scan Z1, Carl Zeiss—ZEISS, Jena, Germany) producing approximately 500 high-power field images per sample. Non-representative images (e.g., bronchioles, artifacts) were excluded. This process was performed by a single researcher, ensuring the homogeneity of the selection. Thirty representative images per case were randomly selected for quantification. Image ProPlus® software version 4 (Media Cybernetics, Rockville, MD, USA) semi-automatically measured immunostained area, expressed as a percentage of total tissue area, averaged per patient.
4.4. DNA Extraction and Genotyping
Genomic DNA was extracted from paraffin-embedded tissue using phenol-chloroform deparaffinization. DNA purity was assessed spectrophotometrically (OD 260/280). SNP selection targeted (Available at: https://snpinfo.niehs.nih.gov accessed on 30 July 2024) twelve inflammasome pathway genes (ACE2, TLR4, NFKB1, TNFA, NOX4, NLRP3, ASC, CASP1, IL1B, IL18, GSDMD, CASP9) based on SNPinfo database (parameters tuned for a CEU population proxy for the southern Brazilian population. The Brazilian population is heterogeneous and has overlapping genotypes due to miscegenation, but the authors used the CEU population due to its greater proximity to the population of southern Brazil where the study was carried out. Tag SNPs with functional relevance from literature were included. Genotyping was performed by real-time PCR using TaqMan® probes on an Applied Biosystems 7500 system (Foster City, CA, USA). Genotypes were called automatically based on fluorescent signal ratios; homozygous genotypes produce single fluorophore signals, and heterozygotes display dual signals. Ten percent of samples were re-run to assess reproducibility.
4.5. Statistical Analysis
Categorical variables were represented as counts and percentages, continuous variables as medians with ranges. Differences between waves were assessed by Pearson’s chi-square, Fisher’s exact, or Mann–Whitney tests as appropriate. Logistic regression examined genotype-phenotype associations. Bonferroni adjustment corrected for multiple comparisons. Statistical significance was set at p < 0.05 before correction. For the multivariate analysis, the binary logistic regression model was used and included clinical variables, IHQ and SNP for each gene. A simplified Bonferroni correction was applied, based on dividing the significant p-value by the number of variables analyzed in the expression and genotypes. Analyses leveraged SPSS v20.0 (IBM, Chicago, IL, USA) and GraphPad Prism 8.0 (La Jolla, CA, USA).
5. Conclusions
With the COVID-19 pandemic now concluded, our study offers valuable insights into the molecular mechanisms underlying fatal outcomes during its course, particularly in the second epidemic wave. We demonstrated that lung tissues from patients who died in this wave exhibit increased expression of inflammasome-associated proteins, especially IL-18, TLR-4, and NLRP3, and that specific genetic polymorphisms in NFKB1 and NOX4 correlate with enhanced inflammasome activation. These results emphasize the role of host genetics and immune dysregulation in determining COVID-19 severity and mortality.
Our findings provide a theoretical foundation for developing targeted biomarkers and therapeutic strategies aimed at inflammasome components, which may have broader applications in managing hyperinflammation in future infectious or inflammatory diseases. The molecular distinctions identified between epidemic waves underline the dynamic interplay between viral evolution and host response, underscoring the importance of ongoing monitoring and personalized interventions beyond the pandemic context.
In summary, enhanced inflammasome activation driven by molecular and genetic factors highlights important aspects of fatal COVID-19 in the second wave, highlighting IL-18 and related genetic markers (rs4648090 NFKB1 and rs317155 NOX4) as molecules of key interest for prognostic and therapeutic consideration in future severe respiratory diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26209993/s1.
Author Contributions
Conceptualization, L.d.N. and C.M.-S.; methodology, L.d.N. and C.M.-S.; software, S.N.; validation, L.d.N. and C.M.-S.; formal analysis, C.B.V.d.P., M.L.V.A., A.C.d.A. and S.N.; investigation, T.R.d.S., L.B.C., L.V.B., M.C. and N.d.A.; data curation, T.R.d.S., L.B.C., L.V.B., M.C., N.d.A., C.B.V.d.P., M.L.V.A., A.C.d.A., S.N., L.d.N. and C.M.-S.; writing—review and editing, T.R.d.S., L.B.C., C.B.V.d.P., M.L.V.A., A.C.d.A., S.N., L.d.N. and C.M.-S.; visualization, T.R.d.S. and L.B.C.; supervision, L.d.N. and C.M.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was indirectly funded through scholarships by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brasil—Finance Code 001; and Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná (FA)—Brasil—CP 23/2023.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Research Ethics Committee (Conselho Nacional de Ética em Pesquisa—CONEP), under protocol numbers 3.944.734—CAAE: 30188020.7.1001.0020/2020, approval date: 13 August 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We are grateful to Pequeno Principe Complex, CAPES and FA for their support. During the preparation of this manuscript, the authors used DeepL (https://www.deepl.com/pt-BR/write accessed on 8 October 2025) for the purposes of improve grammar and clarity. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE2 | Angiotensin-converting enzyme 2 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| CARD | Caspase Recruitment Domain |
| CONEP | National Research Ethics Committee |
| DAB | 3,3-diaminobenzidine |
| DAD | Diffuse alveolar damage |
| DNA | Deoxyribonucleic acid |
| GSDMD | Gasdermin D |
| HRP | Horseradish peroxidase |
| ICU | Intensive Care Unit |
| IHQ | Immunohistochemistry |
| IL | Interleucin |
| NETs | Neutrophil extracellular traps |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer |
| NLRP3 | Nod (Nucleotide-binding oligomerization domain)-, LRR (Leucine-rich repeat)- and pyrin domain-containing protein 3 |
| PAMP | Pathogen-Associated Molecular Patterns |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SNP | Single-Nucleotide Polymorphism |
| TLR | Toll-Like receptor |
| TMA | Tissue Microarray |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Khreefa, Z.; Barbier, M.T.; Koksal, A.R.; Love, G.; Del Valle, L. Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas. Cells 2023, 12, 262. [Google Scholar] [CrossRef]
- Kartsonaki, C.; Baillie, J.K.; Barrio, N.G.; Baruch, J.; Beane, A.; Blumberg, L.; Bozza, F.; Broadley, T.; Burrell, A.; Carson, G.; et al. Characteristics and outcomes of an international cohort of 600000 hospitalized patients with COVID-19. Int. J. Epidemiol. 2023, 52, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Baas, T.; Taubenberger, J.K.; Chong, P.Y.; Chui, P.; Katze, M.G. SARS-CoV virus-host interactions and comparative etiologies of acute respiratory distress syndrome as determined by transcriptional and cytokine profiling of formalin-fixed paraffin-embedded tissues. J. Interf. Cytokine Res. 2006, 26, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; De Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Favaloro, E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb. Haemost. 2020, 120, 876–877. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, W.; Meng, G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 2011, 11, 549–554. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology, 9th ed.; Garland Science/Taylor & Francis Group: New York, NY, USA, 2017. [Google Scholar]
- Sagoo, P.; Garcia, Z.; Breart, B.; Lemaître, F.; Michonneau, D.; Albert, M.L.; Levy, Y.; Bousso, P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med. 2016, 22, 64–71. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Fillmore, N.; Bell, S.; Shen, C.; Nguyen, V.; La, J.; Dubreuil, M.; Strymish, J.; Brophy, M.; Mehta, G.; Wu, H.; et al. Disulfiram use is associated with lower risk of COVID-19: A retrospective cohort study. PLoS ONE 2021, 16, e0259061. [Google Scholar] [CrossRef]
- Potere, N.; Del Buono, M.G.; Caricchio, R.; Cremer, P.C.; Vecchié, A.; Porreca, E.; Dalla Gasperina, D.; Dentali, F.; Abbate, A.; Bonaventura, A. Interleukin-1 and the NLRP3 inflammasome in COVID-19: Pathogenetic and therapeutic implications: IL-1 and NLRP3 inflammasome in COVID-19. EBioMedicine 2022, 85, 104299. [Google Scholar] [CrossRef]
- Chanock, S. Candidate genes and single nucleotide polymorphisms (SNPs) in the study of human disease. Dis. Markers 2001, 17, 89–98. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Bernig, T.; Hanisch, M.; Koehl, U.; Behl, M.; Reinhardt, D.; Creutzig, U.; Klingebiel, T.; Chanock, S.J.; Schwabe, D. Common genetic variants in the interleukin-6 and chitotriosidase genes are associated with the risk for serious infection in children undergoing therapy for acute myeloid leukemia. Leukemia 2005, 19, 1745–1750. [Google Scholar] [CrossRef]
- Pertovaara, M.; Antonen, J.; Hurme, M. Th2 cytokine genotypes are associated with a milder form of primary Sjögren’s syndrome. Ann. Rheum. Dis. 2006, 65, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Iftimie, S.; Lopez-Azcona, A.F.; Vallverdu, I.; Hernandez-Flix, S.; De Febrer, G.; Parra, S.; Hernandez-Aguilera, A.; Riu, F.; Joven, J.; Andreychuk, N.; et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS ONE 2021, 16, e0248029. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Minervini, G.; Giordano, M. COVID-19 and Frailty. Vaccines 2023, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Veiga, V.C.; Cavalcanti, A.B. Age, host response, and mortality in COVID-19. Eur. Respir. J. 2023, 62, 2300796. [Google Scholar] [CrossRef]
- Moura, E.C.; Cortez-Escalante, J.; Cavalcante, F.V.; Barreto, I.C.H.C.; Sanchez, M.N.; Santos, L.M.P. Covid-19: Evolução temporal e imunização nas três ondas epidemiológicas, Brasil, 2020–2022. Rev. Saude Publica 2022, 56, 105. [Google Scholar] [CrossRef]
- Miggiolaro, A.F.R.S.; Motta, J.S., Jr.; de Paula, C.B.V.; Nagashima, S.; Malaquias, M.A.S.; Carstens, L.B.; Moreno-Amaral, A.N.; Baena, C.P.; de Noronha, L. Covid-19 cytokine storm in pulmonary tissue: Anatomopathological and immunohistochemical findings. Respir. Med. Case Reports 2020, 31, 101292. [Google Scholar] [CrossRef]
- Nagashima, S.; Mendes, M.C.; Martins, A.P.C.; Borges, N.H.; Godoy, T.M.; Dos Santos Miggiolaro, A.F.R.S.; Dos Santos Dezidério, F.; Machado-Souza, C.; De Noronha, L. Endothelial Dysfunction and Thrombosis in Patients With COVID-19—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2404–2407. [Google Scholar] [CrossRef]
- Paula, C.B.V.; Azevedo, M.L.V.; Nagashima, S.; Martins, A.P.C.; Malaquias, M.A.S.; Miggiolaro, A.F.R.S.; Motta, J.S., Jr.; Avelino, G.; Carmo, L.A.P.; Carstens, L.B.; et al. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci. Rep. 2020, 10, 18689. [Google Scholar] [CrossRef]
- Nagashima, S.; Dutra, A.A.; Arantes, M.P.; Zeni, R.C.; Klein, C.K.; de Oliveira, F.C.; Piper, G.W.; Brenny, I.D.; Pereira, M.R.C.; Stocco, R.B.; et al. COVID-19 and Lung Mast Cells: The Kallikrein–Kinin Activation Pathway. Int. J. Mol. Sci. 2022, 23, 1714. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, M.A.S.; Gadotti, A.C.; Motta, J.S., Jr.; Martins, A.P.C.; Azevedo, M.L.V.; Benevides, A.P.K.; Cézar-Neto, P.; Carmo, L.A.P.; Zeni, R.C.; Raboni, S.M.; et al. The role of the lectin pathway of the complement system in SARS-CoV-2 lung injury. Transl. Res. 2021, 231, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Carstens, L.B.; Campos D’amico, R.; Fernandes de Moura, K.; Morais de Castro, E.; Centenaro, F.; Silva Barbosa, G.; Silva, G.V.C.; Brenny, I.; D’Agostini, J.C.H.; Hlatchuk, E.C.; et al. Lung Inflammasome Activation in SARS-CoV-2 Post-Mortem Biopsies. Int. J. Mol. Sci. 2022, 23, 13033. [Google Scholar] [CrossRef]
- Barbosa, L.V.; Prá, D.M.M.; Nagashima, S.; Pereira, M.R.C.; Stocco, R.B.; Silva, F.L.F.; Cruz, M.R.; Dallagassa, D.; Stupak, T.J.; Götz, G.W.X.R.; et al. Immune Response Gaps Linked to SARS-CoV-2 Infection: Cellular Exhaustion, Senescence, or Both? Int. J. Mol. Sci. 2022, 23, 13734. [Google Scholar] [CrossRef]
- Collete, M.; dos Santos, T.R.; de Araújo, N.; Martins, A.P.C.; Nagashima, S.; de Paula, C.B.V.; Machado-Souza, C.; de Noronha, L. Neutrophil Extracellular Trap Markers in Post Mortem Lung Biopsies from COVID-19 Patients. Int. J. Mol. Sci. 2025, 26, 8059. [Google Scholar] [CrossRef]
- Hartmann, C.; Miggiolaro, A.F.R.S.; Motta, J.S., Jr.; Carstens, L.B.; Paula, C.B.V.; Grobe, S.F.; Nunes, L.H.S.; Marques, G.L.; Libby, P.; Moura, L.Z.; et al. The Pathogenesis of COVID-19 Myocardial Injury: An Immunohistochemical Study of Postmortem Biopsies. Front. Immunol. 2021, 12, 748417. [Google Scholar] [CrossRef]
- Miggiolaro, A.F.R.S.; da Silva, F.P.G.; Wiedmer, D.B.; Godoy, T.M.; Borges, N.H.; Piper, G.W.; Oricil, A.G.G.; Klein, C.K.; Hlatchuk, E.C.; Dagostini, J.C.H.; et al. COVID-19 and Pulmonary Angiogenesis: The Possible Role of Hypoxia and Hyperinflammation in the Overexpression of Proteins Involved in Alveolar Vascular Dysfunction. Viruses 2023, 15, 706. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyazaki, N.; Oashi, K.; Teramoto, S.; Shiratori, M.; Hashimoto, M.; Ohmichi, M.; Abe, S. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J. Allergy Clin. Immunol. 2001, 107, 331–336. [Google Scholar] [CrossRef]
- Hoshino, T.; Kato, S.; Oka, N.; Imaoka, H.; Kinoshita, T.; Takei, S.; Kitasato, Y.; Kawayama, T.; Imaizumi, T.; Yamada, K.; et al. Pulmonary inflammation and emphysema: Role of the cytokines IL-18 and IL-13. Am. J. Respir. Crit. Care Med. 2007, 176, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Birra, D.; Benucci, M.; Landolfi, L.; Merchionda, A.; Loi, G.; Amato, P.; Licata, G.; Quartuccio, L.; Triggiani, M.; Moscato, P. COVID 19: A clue from innate immunity. Immunol. Res. 2020, 68, 161. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Banerjee, M.; Berk, M. Genetic gateways to COVID-19 infection: Implications for risk, severity, and outcomes. FASEB J. 2020, 34, 8787–8795. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, M.; Netea, M.G.; Rezaei, N. Targeting of Toll-like receptors: A decade of progress in combating infectious diseases. Lancet Infect. Dis. 2011, 11, 702–712. [Google Scholar] [CrossRef]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-cov-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2020, 218, e20201707. [Google Scholar] [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef]
- Marshall, R. The Pulmonary Renin-Angiotensin System. Curr. Pharm. Des. 2003, 9, 715–722. [Google Scholar] [CrossRef]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Mei, D.; Wong, W.S.F. Targeting the renin–angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Oakes, J.M.; Fuchs, R.M.; Gardner, J.D.; Lazartigues, E.; Yue, X. Nicotine and the renin-angiotensin system. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2018, 315, R895–R906. [Google Scholar] [CrossRef]
- Singh, H.O.; Choudhari, R.; Nema, V.; Khan, A.A. ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb. Pathog. 2021, 150, 104621. [Google Scholar] [CrossRef] [PubMed]
- Li YDer Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Gintoni, I.; Adamopoulou, M.; Yapijakis, C. The Impact of ACE and ACE2 Gene Polymorphisms in Pulmonary Diseases including COVID-19. In Vivo 2022, 36, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pei, J.; Lai, Y.; Guan, T.; Zeyaweiding, A.; Maimaiti, T.; Zhao, H.; Shen, Y. Association of ACE2 variant rs4646188 with the risks of atrial fibrillation and cardioembolic stroke in Uygur patients with type 2 diabetes. BMC Cardiovasc. Disord. 2021, 21, 103. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, C.; Guan, T.; Li, Y.; Lai, Y.; Li, F.; Zhao, H.; Maimaiti, T.; Zeyaweiding, A. Association of ACE2 genetic polymorphisms with hypertension-related target organ damages in south Xinjiang. Hypertens. Res. 2019, 42, 681–689. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Santoro, M.G.; Rossi, A.; Amici, C. NF-κB and virus infection: Who controls whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Ribeiro, L.R.; Gouveia, M.I.M.; Marcelino, B.R.; Santos, C.S.; Lima, K.V.B.; Lima, L.N.G.C. Hyperinflammatory Response in COVID-19: A Systematic Review. Viruses 2023, 15, 553. [Google Scholar] [CrossRef]
- Koh, H.M.; Jang, B.G.; Hyun, C.L.; Kim, D.C. Prognostic value of Musashi 2 (MSI2) in cancer patients: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 969632. [Google Scholar] [CrossRef]
- Chen, F.; Haigh, S.; Barman, S.; Fulton, D.J.R. From form to function: The role of Nox4 in the cardiovascular system. Front. Physiol. 2012, 3, 412. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; Meir, E.G.V.; Lambeth, J.D. Homologs of gp91 phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, X.; Liu, Q.; Song, H.; He, B.; Shi, P.; Zhang, Q.; Li, X.; Wang, J. Functional variations of the TLR4 gene in association with chronic obstructive pulmonary disease and pulmonary tuberculosis. BMC Pulm. Med. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.H.M.; Costa, E.L.V.; Zimmermann, N.A.; Gois, L.S.O.; Anjos, M.V.A.; Lima, F.G.; Andrade, P.S.; Joelsons, D.; Ho, Y.L.; Sales, F.C.S.; et al. Temporal trends of severity and outcomes of critically ill patients with COVID-19 after the emergence of variants of concern: A comparison of two waves. PLoS ONE 2024, 19, e0299607. [Google Scholar] [CrossRef]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Corrêa, T.D.; Midega, T.D.; Cordioli, R.L.; Barbas, C.S.V.; Rabello Filho, R.; da Silva, B.C.; Silva Júnior, M.; Nawa, R.K.; de Carvalho, F.R.T.; de Matos, G.F.J.; et al. Clinical characteristics and outcomes of patients with COVID-19 admitted to the intensive care unit during the first and second waves of the pandemic in Brazil: A single-center retrospective cohort study. Einstein 2023, 21, eAO0233. [Google Scholar] [CrossRef]
- Wlodek, E.; Kirkpatrick, R.B.; Andrews, S.; Noble, R.; Schroyer, R.; Scott, J.; Watson, C.J.E.; Clatworthy, M.; Harrison, E.M.; Wigmore, S.J.; et al. A pilot study evaluating GSK1070806 inhibition of interleukin-18 in renal transplant delayed graft function. PLoS ONE 2021, 16, e0247972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).