Glycoscience in Advancing PD-1/PD-L1-Axis-Targeted Tumor Immunotherapy
Abstract
:1. Introduction
2. PD-1/PD-L1 Axis
3. Glycosylation
3.1. N-glycosylation and O-glycosylation
3.1.1. N-glycosylation
3.1.2. O-glycosylation
3.2. Modifications That Can Extend the Structure of Glycoconjugates
3.2.1. Fucosylation
3.2.2. Sialylation
4. Glycoscience in Advancing PD-1/PD-L1 Blockade Therapies
4.1. Targeting Glycan
4.2. Targeting Glycosylation
4.3. Targeting Glycoside Hydrolases and Glycosyltransferases (GTs)
4.4. Optimizing PD-1/PD-L1-Blocking Therapeutic Antibodies
4.4.1. Monoclonal Antibodies Targeting Glycosylated PD-L1 (gPD-L1) and PD-1 (gPD-1)
4.4.2. Antibody–Drug Conjugates (ADCs)
4.4.3. Glycoengineered Therapeutic Antibodies
4.4.4. Lysosome-Targeting Chimeras (LYTACs)
4.5. As Predictive Biomarkers
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, C.-W.; Chung, E.M.; Yang, R.; Kim, Y.-S.; Park, A.H.; Lai, Y.-J.; Yang, Y.; Wang, Y.-H.; Liu, J.; et al. Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity. Cancer Res. 2020, 80, 2298–2310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Franc, V.; Heck, A.J.R. Glycoproteomics: A Balance between High-Throughput and In-Depth Analysis. Trends Biotechnol. 2017, 35, 598–609. [Google Scholar] [CrossRef]
- RodrÍguez, E.; Schetters, S.T.T.; van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, H.; Chai, S.; Xu, L.; Lin, B.; Yi, W.; Wu, L. O-GlcNAcylation promotes tumor immune evasion by inhibiting PD-L1 lysosomal degradation. Proc. Natl. Acad. Sci. USA 2023, 120, e2216796120. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.W.T.; Baghdassarian, H.M.; Kellman, B.P.; Bao, B.; Sorrentino, J.T.; Liang, C.; Kuo, C.-C.; Masson, H.O.; Lewis, N.E. Systems glycobiology for discovering drug targets, biomarkers, and rational designs for glyco-immunotherapy. J. Biomed. Sci. 2021, 28, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, C.; Han, J.; Zhu, J.; Liu, S.; Jin, T. PD-1/PD-L1 Axis as a Potential Therapeutic Target for Multiple Sclerosis: A T Cell Perspective. Front. Cell. Neurosci. 2021, 15, 716747. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006, 27, 195–201. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004, 574, 37–41. [Google Scholar] [CrossRef]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. 2001, 98, 13866–13871. [Google Scholar] [CrossRef]
- Gao, M.; Shi, J.; Xiao, X.; Yao, Y.; Chen, X.; Wang, B.; Zhang, J. PD-1 regulation in immune homeostasis and immunotherapy. Cancer Lett. 2024, 588, 216726. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Schütz, F.; Stefanovic, S.; Mayer, L.; von Au, A.; Domschke, C.; Sohn, C. PD-1/PD-L1 Pathway in Breast Cancer. Oncol. Res. Treat. 2017, 40, 294–297. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hirano, F.; Kaneko, K.; Tamura, H.; Dong, H.; Wang, S.; Ichikawa, M.; Rietz, C.; Flies, D.B.; Lau, J.S.; Zhu, G.; et al. Blockade of B7-H1 and PD-1 by Monoclonal Antibodies Potentiates Cancer Therapeutic Immunity. Cancer Res. 2005, 65, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Raglow, Z.; McKenna, M.K.; Bonifant, C.L.; Wang, W.; Pasca di Magliano, M.; Stadlmann, J.; Penninger, J.M.; Cummings, R.D.; Brenner, M.K.; Markovitz, D.M. Targeting glycans for CAR therapy: The advent of sweet CARs. Mol. Ther. 2022, 30, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Wiederschain, G.Y. Essentials of glycobiology. Biochemistry 2009, 74, 1056. [Google Scholar] [CrossRef]
- Saraswathy, N.; Ramalingam, P. 16-Glycoproteomics. In Concepts and Techniques in Genomics and Proteomics; Saraswathy, N., Ramalingam, P., Eds.; Woodhead Publishing: Sebastopol, CA, USA, 2011; pp. 213–218. [Google Scholar] [CrossRef]
- Čaval, T.; Alisson-Silva, F.; Schwarz, F. Roles of glycosylation at the cancer cell surface: Opportunities for large scale glycoproteomics. Theranostics 2023, 13, 2605–2615. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Z.; Liu, W.; Liu, Z. Current knowledge and potential intervention of hexosamine biosynthesis pathway in lung cancer. World J. Surg. Oncol. 2023, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Vasconcelos-dos-Santos, A.; Oliveira, I.A.; Lucena, M.C.; Mantuano, N.R.; Whelan, S.A.; Dias, W.B.; Todeschini, A.R. Biosynthetic Machinery Involved in Aberrant Glycosylation: Promising Targets for Developing of Drugs Against Cancer. Front. Oncol. 2015, 5, 138. [Google Scholar] [CrossRef]
- Cha, J.-H.; Chan, L.-C.; Li, C.-W.; Hsu, J.L.; Hung, M.-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Li, C.-W.; Lim, S.-O.; Xia, W.; Lee, H.-H.; Chan, L.-C.; Kuo, C.-W.; Khoo, K.-H.; Chang, S.-S.; Cha, J.-H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef]
- Li, C.-W.; Lim, S.-O.; Chung, E.M.; Kim, Y.-S.; Park, A.H.; Yao, J.; Cha, J.-H.; Xia, W.; Chan, L.-C.; Kim, T.; et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell 2018, 33, 187–201.e110. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Chen, B.; Liu, W.; Li, Y.; Ma, B.; Shang, S.; Tan, Z. Impact of N-Linked Glycosylation on Therapeutic Proteins. Molecules 2022, 27, 8859. [Google Scholar] [CrossRef]
- Breitling, J.; Aebi, M. N-Linked Protein Glycosylation in the Endoplasmic Reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013359. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N. A sugar-coated switch for cellular growth and arrest. Nat. Chem. Biol. 2007, 3, 307–309. [Google Scholar] [CrossRef]
- Ren, X.; Lin, S.; Guan, F.; Kang, H. Glycosylation Targeting: A Paradigm Shift in Cancer Immunotherapy. Int. J. Biol. Sci. 2024, 20, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhang, H.; Chai, Y.; Song, H.; Tong, Z.; Wang, Q.; Qi, J.; Wong, G.; Zhu, X.; Liu, W.J.; et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat. Commun. 2017, 8, 14369. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tan, S.; Jin, W.; Guan, J.; Wang, Q.; Sun, H.; Qi, J.; Yan, J.; Chai, Y.; Wang, Z.; et al. N-glycosylation of PD-1 promotes binding of camrelizumab. EMBO Rep. 2020, 21, e51444. [Google Scholar] [CrossRef]
- Duan, Z.; Shi, R.; Gao, B.; Cai, J. N-linked glycosylation of PD-L1/PD-1: An emerging target for cancer diagnosis and treatment. J. Transl. Med. 2024, 22, 705. [Google Scholar] [CrossRef] [PubMed]
- Haas, Q.; Boligan, K.F.; Jandus, C.; Schneider, C.; Simillion, C.; Stanczak, M.A.; Haubitz, M.; Seyed Jafari, S.M.; Zippelius, A.; Baerlocher, G.M.; et al. Siglec-9 Regulates an Effector Memory CD8+ T-cell Subset That Congregates in the Melanoma Tumor Microenvironment. Cancer Immunol. Res. 2019, 7, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, L.; Ge, D.; Tan, L.; Cao, B.; Fan, H.; Xue, L. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8+ T cells. Cancer Lett. 2021, 500, 98–106. [Google Scholar] [CrossRef]
- Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press Copyright 2015–2017 by The Consortium of Glycobiology Editors, La Jolla, California; All Rights Reserved; Cold Spring Harbor: Long Island, NY, USA, 2015. [Google Scholar]
- Okada, M.; Chikuma, S.; Kondo, T.; Hibino, S.; Machiyama, H.; Yokosuka, T.; Nakano, M.; Yoshimura, A. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 2017, 20, 1017–1028. [Google Scholar] [CrossRef]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Pearce, O.M.T.; Läubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2015, 26, 111–128. [Google Scholar] [CrossRef]
- Manni, M.; Läubli, H. Targeting glyco-immune checkpoints for cancer therapy. Expert Opin. Biol. Ther. 2021, 21, 1063–1071. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; van der Vlag, J.; Heise, T.; et al. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell–Mediated Tumor Immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Rodrigues Mantuano, N.; Kirchhammer, N.; Sanin, D.E.; Jacob, F.; Coelho, R.; Everest-Dass, A.V.; Wang, J.; Trefny, M.P.; Monaco, G.; et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci. Transl. Med. 2022, 14, eabj1270. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, L.A.M.; Santegoets, K.C.M.; Kers-Rebel, E.D.; Bossmann, S.A.J.F.H.; Ter Laan, M.; Granado, D.; Adema, G.J. Glioma-Associated Sialoglycans Drive the Immune Suppressive Phenotype and Function of Myeloid Cells. Pharmaceutics 2024, 16, 953. [Google Scholar] [CrossRef]
- Lubbers, J.; Rodriguez, E.; van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.-D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, M.; Xu, X.; Zhang, Y.; Liu, Y.; Zhao, M.; Li, P.; Chen, J.; Fukuda, T.; Gu, J.; et al. Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. Eur. J. Immunol. 2020, 50, 1820–1833. [Google Scholar] [CrossRef]
- Liao, C.; An, J.; Yi, S.; Tan, Z.; Wang, H.; Li, H.; Guan, X.; Liu, J.; Wang, Q. FUT8 and Protein Core Fucosylation in Tumours: From Diagnosis to Treatment. J. Cancer 2021, 12, 4109–4120. [Google Scholar] [CrossRef]
- Ibarlucea-Benitez, I.; Weitzenfeld, P.; Smith, P.; Ravetch, J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2107424118. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, N.; Li, J.; He, L.; Yang, Z.; Lu, J.; Xiong, G.; Yu, C.; Wang, S. High affinity monoclonal antibody targeting Siglec-15 for cancer immunotherapy. J. Clin. Transl. Res. 2021, 7, 739–749. [Google Scholar] [PubMed]
- Choi, H.; Ho, M.; Adeniji, O.S.; Giron, L.; Bordoloi, D.; Kulkarni, A.J.; Puchalt, A.P.; Abdel-Mohsen, M.; Muthumani, K. Development of Siglec-9 Blocking Antibody to Enhance Anti-Tumor Immunity. Front. Oncol. 2021, 11, 778989. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological function of sialic acid and sialylation in human health and disease. Cell Death Discov. 2024, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.; Kouverianou, E.; Rooney, C.M.; McHugh, B.J.; Howie, S.E.M.; Gregory, C.D.; Forbes, S.J.; Henderson, N.C.; Zetterberg, F.R.; Nilsson, U.J.; et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 2019, 79, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Compagno, D.; Tiraboschi, C.; Garcia, J.D.; Rondón, Y.; Corapi, E.; Velazquez, C.; Laderach, D.J. Galectins as Checkpoints of the Immune System in Cancers, Their Clinical Relevance, and Implication in Clinical Trials. Biomolecules 2020, 10, 750. [Google Scholar] [CrossRef]
- Martinez-Perez, A.; Granda-Diaz, R.; Aguilar-Garcia, C.; Sordo-Bahamonde, C.; Gonzalez, S. Deciphering LAG-3: Unveiling molecular mechanisms and clinical advancements. Biomark. Res. 2024, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Hegewisch-Becker, S.; Mendez, G.; Chao, J.; Nemecek, R.; Feeney, K.; Cutsem, E.V.; Al-Batran, S.-E.; Mansoor, W.; Maisey, N.; Cid, R.P.; et al. First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study. J. Clin. Oncol. 2024, 42, 2080–2093. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Dong, W.; Lin, M.; He, J.; Zhang, X.; Tian, T.; Yang, Y.; Chen, K.; Lei, Q.-Y.; et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc. Natl. Acad. Sci. USA 2022, 119, e2114851119. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Yang, W.-H.; Xia, W.; Wei, Y.; Chan, L.-C.; Lim, S.-O.; Li, C.-W.; Kim, T.; Chang, S.-S.; Lee, H.-H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e607. [Google Scholar] [CrossRef] [PubMed]
- Verdura, S.; Cuyas, E.; Cortada, E.; Brunet, J.; Lopez-Bonet, E.; Martin-Castillo, B.; Bosch-Barrera, J.; Encinar, J.A.; Menendez, J.A. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging 2020, 12, 8–34. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, Z.; Hao, X.; Dandreo, L.J.; He, L.; Zhang, Y.; Wang, F.; Wu, X.; Xu, L. Niclosamide improves cancer immunotherapy by modulating RNA-binding protein HuR-mediated PD-L1 signaling. Cell Biosci. 2023, 13, 192. [Google Scholar] [CrossRef]
- Lin, M.C.; Chuang, Y.T.; Wu, H.Y.; Hsu, C.L.; Lin, N.Y.; Huang, M.C.; Lou, P.J. Targeting tumor O-glycosylation modulates cancer–immune-cell crosstalk and enhances anti-PD-1 immunotherapy in head and neck cancer. Mol. Oncol. 2023, 18, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Ju, H.; Fan, J.; Shi, X.; Cheng, Y.; Cang, X.; Zheng, Z.; Duan, X.; Yi, W. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat. Commun. 2020, 11, 36. [Google Scholar] [CrossRef]
- Xu, T.; Liu, J.; Xia, Y.; Wang, Z.; Li, X.; Gao, Q. Integrated analysis reveals the participation of IL4I1, ITGB7, and FUT7 in reshaping the TNBC immune microenvironment by targeting glycolysis. Ann. Med. 2021, 53, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, H.; Zhang, K.; Zhu, J.; Wang, Z.; Long, Y.; He, Y.; Feng, F.; Liu, W.; Ye, F.; et al. OGT as potential novel target: Structure, function and inhibitors. Chem. Biol. Interact. 2022, 357, 109886. [Google Scholar] [CrossRef] [PubMed]
- Trapannone, R.; Rafie, K.; van Aalten, D.M. O-GlcNAc transferase inhibitors: Current tools and future challenges. Biochem. Soc. Trans. 2016, 44, 88–93. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Wang, R.; Jiao, S.; Wang, S.; Zhang, J.; Zhang, M. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Commun. Biol. 2019, 2, 392. [Google Scholar] [CrossRef]
- Liu, J.; Wang, G.; Liu, L.; Wu, R.; Wu, Y.; Fang, C.; Zhou, X.; Jiao, J.; Gu, Y.; Zhou, H.; et al. Study of the interactions of a novel monoclonal antibody, mAb059c, with the hPD-1 receptor. Sci. Rep. 2019, 9, 17830. [Google Scholar] [CrossRef] [PubMed]
- Shivatare, S.S.; Shivatare, V.S.; Wong, C.-H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next generation of antibody–drug conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef]
- Yamazoe, S.; Kotapati, S.; Hogan, J.M.; West, S.M.; Deng, X.A.; Diong, S.J.; Arbanas, J.; Nguyen, T.A.; Jashnani, A.; Gupta, D.; et al. Impact of Drug Conjugation on Thermal and Metabolic Stabilities of Aglycosylated and N-Glycosylated Antibodies. Bioconjug. Chem. 2022, 33, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-R.; Wang, X.-Y.; Jiang, L.; Li, D.-W.; Qian, R.-C. Sialidase-Conjugated “NanoNiche” for Efficient Immune Checkpoint Blockade Therapy. ACS Appl. Bio Mater. 2021, 4, 5735–5741. [Google Scholar] [CrossRef]
- Wu, Y.T.; Fang, Y.; Wei, Q.; Shi, H.; Tan, H.; Deng, Y.; Zeng, Z.; Qiu, J.; Chen, C.; Sun, L.; et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2214278119. [Google Scholar] [CrossRef]
- Muller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Ha, S.J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6, 36956. [Google Scholar] [CrossRef] [PubMed]
- Goletz, C.; Lischke, T.; Harnack, U.; Schiele, P.; Danielczyk, A.; Rühmann, J.; Goletz, S. Glyco-Engineered Anti-Human Programmed Death-Ligand 1 Antibody Mediates Stronger CD8 T Cell Activation Than Its Normal Glycosylated and Non-Glycosylated Counterparts. Front. Immunol. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Scallon, B.J.; Tam, S.H.; McCarthy, S.G.; Cai, A.N.; Raju, T.S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 2007, 44, 1524–1534. [Google Scholar] [CrossRef]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef]
- Ahn, G.; Banik, S.M.; Miller, C.L.; Riley, N.M.; Cochran, J.R.; Bertozzi, C.R. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 2021, 17, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-y.; Yang, Y.; Zhang, R.-s.; Ge, R.-x.; Xie, S.-b. Targeted degradation of membrane and extracellular proteins with LYTACs. Acta Pharmacol. Sin. 2024, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Yu, L.; Huang, X.; Wang, X.; Han, D.; Yang, Y.; Liu, Z. Covalent LYTAC Enabled by DNA Aptamers for Immune Checkpoint Degradation Therapy. J. Am. Chem. Soc. 2023, 145, 24506–24521. [Google Scholar] [CrossRef]
- Su, L.-Y.; Tian, Y.; Zheng, Q.; Cao, Y.; Yao, M.; Wang, S.; Xu, W.; Xi, C.; Clocchiatti, A.; Nie, G.; et al. Anti-tumor immunotherapy using engineered bacterial outer membrane vesicles fused to lysosome-targeting chimeras mediated by transferrin receptor. Cell Chem. Biol. 2024, 31, 1219–1230.e1215. [Google Scholar] [CrossRef]
- Pance, K.; Gramespacher, J.A.; Byrnes, J.R.; Salangsang, F.; Serrano, J.-A.C.; Cotton, A.D.; Steri, V.; Wells, J.A. Modular cytokine receptor-targeting chimeras for targeted degradation of cell surface and extracellular proteins. Nat. Biotechnol. 2023, 41, 273–281. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Yuan, R.; Shen, A.; Wang, P.; Li, H.; Zhang, J.; Tian, C.; Jiang, Z.; Li, W.; et al. A co-assembly platform engaging macrophage scavenger receptor A for lysosome-targeting protein degradation. Nat. Commun. 2024, 15, 1663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Brahma, R.K.; Zhu, L.; Feng, J.; Hu, S.; Qian, L.; Du, S.; Yao, S.Q.; Ge, J. Insulin-like Growth Factor 2 (IGF2)-Fused Lysosomal Targeting Chimeras for Degradation of Extracellular and Membrane Proteins. J. Am. Chem. Soc. 2023, 145, 24272–24283. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Zheng, X.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Qian, Y.; Cui, P.; et al. Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 562315. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hsu, J.-M.; Sun, L.; Wang, S.-C.; Hung, M.-C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Hu, Z.; Sheng, X.; Sun, Z.; Yang, L.; Shu, H.; Liu, X.; Yan, G.; Zhang, L.; Liu, C.; et al. Glyco-signatures in patients with advanced lung cancer during anti-PD-1/PD-L1 immunotherapy. Acta Biochim. Biophys. Sin. 2024, 56, 1099–1107. [Google Scholar] [CrossRef]
- Sato, Y.; Nakata, K.; Kato, Y.; Shima, M.; Ishii, N.; Koji, T.; Taketa, K.; Endo, Y.; Nagataki, S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N. Engl. J. Med. 1993, 328, 1802–1806. [Google Scholar] [CrossRef]
- Yi, X.; Yu, S.; Bao, Y. Alpha-fetoprotein-L3 in hepatocellular carcinoma: A meta-analysis. Clin. Chim. Acta 2013, 425, 212–220. [Google Scholar] [CrossRef]
- Lee, H.-H.; Wang, Y.-N.; Xia, W.; Chen, C.-H.; Rau, K.-M.; Ye, L.; Wei, Y.; Chou, C.-K.; Wang, S.-C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178.e164. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, J.; Lubman, D.M.; Gao, C. Aberrant glycosylation and cancer biomarker discovery: A promising and thorny journey. Clin. Chem. Lab. Med. 2019, 57, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Yu, H.; Han, M.; Tan, Y.; Wu, M.; Zhang, J.; Wu, Y.; Zhang, Q. Analysis of Tumor Glycosylation Characteristics and Implications for Immune Checkpoint Inhibitor’s Efficacy for Breast Cancer. Front. Immunol. 2022, 13, 830158. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.-H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy—current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
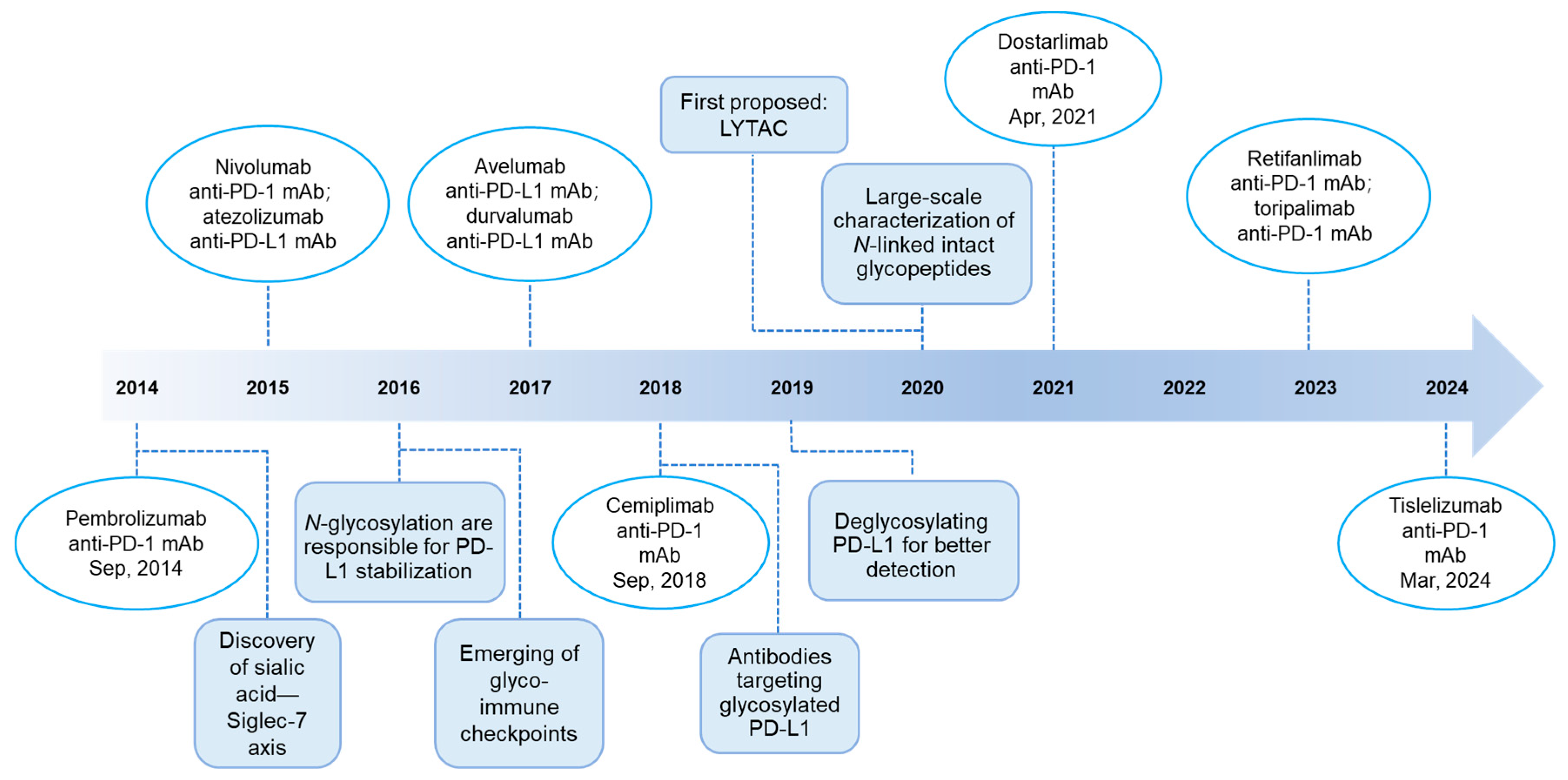

| Target | Generic Name | Trade Name | Company | Approved |
|---|---|---|---|---|
| PD-1 | Nivolumab | Opdivo, BMS-936558, MDX1106 | Bristol-Meyers Squibb | Melanoma; NSCLC |
| Pembrolizumab | Keytruda, MK-3475, Lambrolizumab | Merck | Melanoma; NSCLC; MPM; HNSCC; cHL; PMBCL; Urothelial Cancer; dMMR CRC; Gastric Cancer; Esophageal Cancer; Cervical Cancer; HCC; BTC; MCC; RCC; EC; TMB-H Cancer; CSCC; TNBC | |
| Cemiplimab | Libtayo, REGN2810 | Sanofi | CSCC; BCC; NSCLC | |
| Dostarlimab | Jemperili, TSR-042 | GlaxoSmithKline | EC; dMMR recurrent or advanced Solid Tumors | |
| Retifanlimab | Zynyz, INCMGA00012 | Incyte | MCC | |
| Toripalimab | Loqtorzi, sintilimab | Junshi Biosciences | NPC | |
| Tislelizumab | Tevimbra, BGB-A317 | BeiGene | ESCC | |
| PD-L1 | Atezolizumab | Tecentriq, MPDL3280A | Roche | NSCLC; SCLC; HCC; Melanoma; ASPS |
| Avelumab | Bavencio, MSB0010718C | Merck, Pfizer | MCC; UC; RCC | |
| Durvalumab | Imfinzi, MEDI4736 | AstraZeneca | NSCLC; ES-SCLC; BTC; uHCC; dMMR EC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Hong, S. Glycoscience in Advancing PD-1/PD-L1-Axis-Targeted Tumor Immunotherapy. Int. J. Mol. Sci. 2025, 26, 1238. https://doi.org/10.3390/ijms26031238
Sun Q, Hong S. Glycoscience in Advancing PD-1/PD-L1-Axis-Targeted Tumor Immunotherapy. International Journal of Molecular Sciences. 2025; 26(3):1238. https://doi.org/10.3390/ijms26031238
Chicago/Turabian StyleSun, Qiyue, and Senlian Hong. 2025. "Glycoscience in Advancing PD-1/PD-L1-Axis-Targeted Tumor Immunotherapy" International Journal of Molecular Sciences 26, no. 3: 1238. https://doi.org/10.3390/ijms26031238
APA StyleSun, Q., & Hong, S. (2025). Glycoscience in Advancing PD-1/PD-L1-Axis-Targeted Tumor Immunotherapy. International Journal of Molecular Sciences, 26(3), 1238. https://doi.org/10.3390/ijms26031238





