Effect of Fenugreek Extract on Testosterone Propionate-Induced Benign Prostatic Hyperplasia
Abstract
1. Introduction
2. Results
2.1. Identification of Phenolic Compound in FCT
2.2. FCT Decreased Prostate Weight, DHT Levels, and 5a-Reductase 2 Expression in Rats Induced with BPH
2.3. FCT Regulated Apoptosis Signaling via Androgen Receptor in BPH-Induced Rats
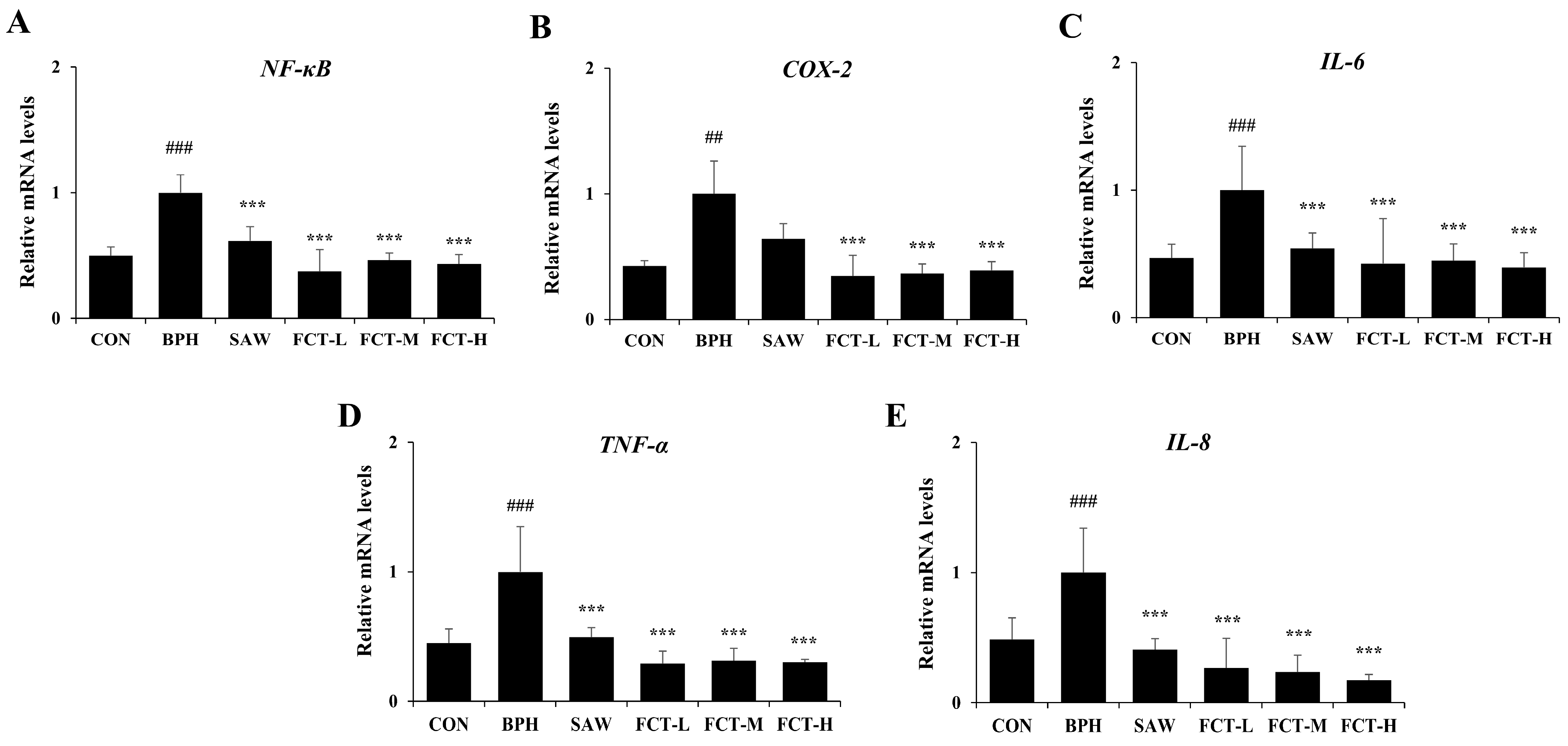
2.4. FCT Reduced the mRNA Expression of NF-κB and Pro-Inflammatory Cytokines in BPH-Induced Rats
2.5. FCT Inhibited Cell Proliferation and Decreased the mRNA Expression Levels of AR, Steroid 5 Alpha-Reductase 2 (SRD5A2), and Bcl-2 in BPH-1 Cells
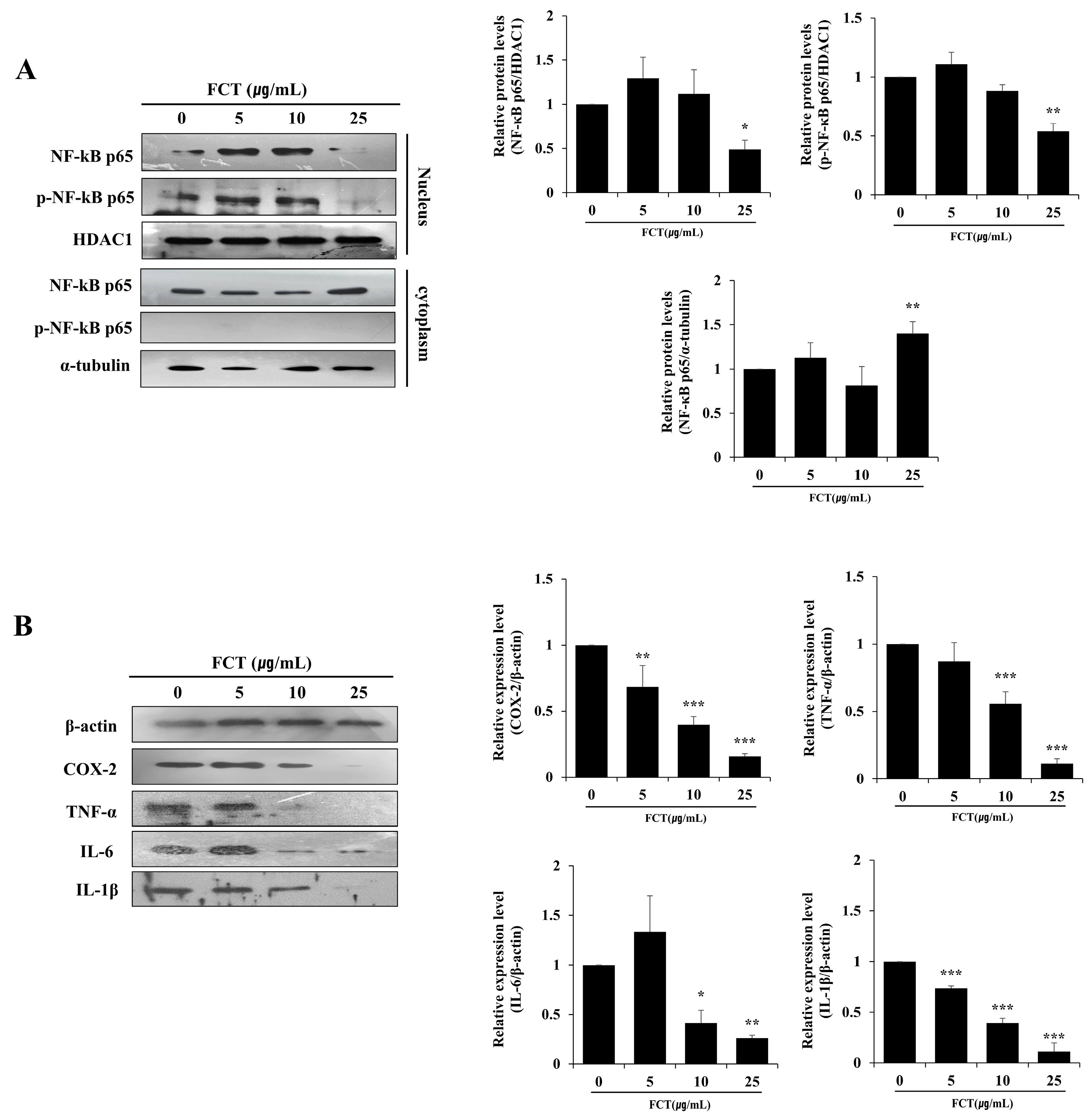
2.6. FCT Suppressed the Protein Expression of NF-κB p65, COX-2, TNF-A, IL-6, and IL-1β in Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Fenugreek Seed Extracts
4.2. Liquid Chromatography
4.3. Cell Proliferation
4.4. Separation of the Nuclear and the Cytoplasmic Fractions in BPH-1 Cells
4.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.6. Animals
4.7. Castration
4.8. H&E Staining
4.9. Immunohistochemistry (IHC)
4.10. Western Blot Analysis
4.11. Chemical Analysis of Serum
4.12. ELISA in Serum and Prostate
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | Benign Prostatic Hyperplasia |
| DHT | Dihydrotestosterone |
| T | Testosterone |
| TP | Testosterone Propionate |
| SD | Sprague–Dawley |
| FCT | Fenugreek seed extract (Forceterone®) |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor Necrosis Factor-α |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | Bcl-2 associated protein X |
| NF-κB | Nuclear factor κB |
| pNF-κB | Phosphorylated NF-κB |
| PCNA | Proliferating Cell Nuclear Antigen |
| AR | Androgen Receptor |
References
- Passos, G.R.; Ghezzi, A.C.; Antunes, E.; de Oliveira, M.G.; Mónica, F.Z. The Role of Periprostatic Adipose Tissue on Prostate Function in Vascular-Related Disorders. Front. Pharmacol. 2021, 12, 626155. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, X.; Hu, Z.; Dong, C. Longitudinal Association between the Dynamic Nature of Depression with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia (LUTS/BPH). BMC Public Health 2024, 24, 1121. [Google Scholar] [CrossRef]
- Chess-Williams, R.; McDermott, C.; Sellers, D.J.; West, E.G.; Mills, K.A. Chronic Psychological Stress and Lower Urinary Tract Symptoms. Low. Urin. Tract Symptoms 2021, 13, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Glavač, N.K.; Nagy, M.; Protti, M.; et al. Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef] [PubMed]
- Miernik, A.; Gratzke, C. Current Treatment for Benign Prostatic Hyperplasia. Dtsch. Arztebl. Int. 2020, 117, 843–854. [Google Scholar] [CrossRef]
- Shin, Y.S.; Karna, K.K.; Choi, B.R.; Park, J.K. Finasteride and Erectile Dysfunction in Patients with Benign Prostatic Hyperplasia or Male Androgenetic Alopecia. World J. Men’s Health 2019, 37, 157–165. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Qin, X.H.; Hu, C.Y. The Efficacy and Safety of Dutasteride and Finasteride in Patients with Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis. Transl. Androl. Urol. 2022, 11, 313–324. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Barbagallo, F.; La Vignera, S.; Calogero, A.E. Endocrinology of the Aging Prostate: Current Concepts. Front. Endocrinol. 2021, 12, 554078. [Google Scholar] [CrossRef]
- Deslypere, J.P.; Young, M.; Wilson, J.D.; McPhaul, M.J. Testosterone and 5 Alpha-Dihydrotestosterone Interact Differently with the Androgen Receptor to Enhance Transcription of the MMTV-CAT Reporter Gene. Mol. Cell. Endocrinol. 1992, 88, 15–22. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, R.Y. Review of the Roles and Interaction of Androgen and Inflammation in Benign Prostatic Hyperplasia. Mediat. Inflamm. 2020, 2020, 7958316. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. Effects of Inflammatory Responses, Apoptosis, and STAT3/NF-ΚB- and Nrf2-Mediated Oxidative Stress on Benign Prostatic Hyperplasia Induced by a High-Fat Diet. Aging 2019, 11, 5570–5578. [Google Scholar] [CrossRef]
- Quiles, M.T.; Arbós, M.A.; Fraga, A.; De Torres, I.M.; Reventós, J.; Morote, J. Antiproliferative and Apoptotic Effects of the Herbal Agent Pygeum Africanum on Cultured Prostate Stromal Cells from Patients with Benign Prostatic Hyperplasia (BPH). Prostate 2010, 70, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Visuvanathan, T.; Than, L.T.L.; Stanslas, J.; Chew, S.Y.; Vellasamy, S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants 2022, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Zhang, B.; Zhu, J.; Zhang, Q.; Hu, Y.; Wang, S.; Wang, Y.; Cao, H.; Xiao, J. Advances on Application of Fenugreek Seeds as Functional Foods: Pharmacology, Clinical Application, Products, Patents and Market. Crit. Rev. Food Sci. Nutr. 2020, 60, 2342–2352. [Google Scholar] [CrossRef]
- Sevrin, T.; Boquien, C.Y.; Gandon, A.; Grit, I.; de Coppet, P.; Darmaun, D.; Alexandre-Gouabau, M.C. Fenugreek Stimulates the Expression of Genes Involved in Milk Synthesis and Milk Flow through Modulation of Insulin/GH/IGF-1 Axis and Oxytocin Secretion. Genes 2020, 11, 1208. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek Cultivation with Emphasis on Historical Aspects and Its Uses in Traditional Medicine and Modern Pharmaceutical Science. Mini-Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef]
- Paneru, D.; Tellez-Isaias, G.; Bottje, W.G.; Asiamah, E.; Abdel-Wareth, A.A.; Salahuddin, M.; Lohakare, J. Modulation of Immune Response and Cecal Microbiota by Dietary Fenugreek Seeds in Broilers. Vet. Sci. 2024, 11, 57. [Google Scholar] [CrossRef]
- Al-Timimi, L.A.N. Antibacterial and Anticancer Activities of Fenugreek Seed Extract. Asian Pac. J. Cancer Prev. 2019, 20, 3771–3776. [Google Scholar] [CrossRef]
- Rao, A.; Grant, R. The Effect of Trigonella foenum-graecum Extract on Prostate-Specific Antigen and Prostate Function in Otherwise Healthy Men with Benign Prostate Hyperplasia. Phytother. Res. 2020, 34, 634–639. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, J.; Xiao, Y.; Luo, J.; Xu, L.; Chen, Z. Global Burden of Benign Prostatic Hyperplasia in Males Aged 60-90 Years from 1990 to 2019: Results from the Global Burden of Disease Study 2019. BMC Urol. 2024, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of Inflammation in Benign Prostatic Hyperplasia. Rev. Urol. 2011, 13, 147. [Google Scholar] [PubMed]
- Sudeep, H.V.; Venkatakrishna, K.; Amrutharaj, B.; Anitha Shyamprasad, K. A Phytosterol-Enriched Saw Palmetto Supercritical CO2 Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating the Inflammatory and Apoptotic Proteins in a Rat Model. BMC Complement. Altern. Med. 2019, 19, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, T.; Li, M.; Song, Y.; Li, J.; Xue, Z.; Li, J.; Bu, D.; Liu, W.; Zeng, Q.; et al. Pao Pereira Extract Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Inhibiting 5α-Reductase. Sci. Rep. 2019, 9, 19703. [Google Scholar] [CrossRef]
- Mostafa, F.; Mantawy, E.M.; Azab, S.S.; El-Demerdash, E. The Angiotensin Converting Enzyme Inhibitor Captopril Attenuates Testosterone-Induced Benign Prostatic Hyperplasia in Rats; a Mechanistic Approach. Eur. J. Pharmacol. 2019, 865, 172729. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Li, W.; Zhang, K.; Yuan, Z.; Cai, Y.; Xu, K.; Zhou, J.; Du, Z. Curcuma Oil Ameliorates Benign Prostatic Hyperplasia through Suppression of the Nuclear Factor-Kappa B Signaling Pathway in Rats. J. Ethnopharmacol. 2021, 279, 113703. [Google Scholar] [CrossRef]
- Bhatt, D.; Ghosh, S. Regulation of the NF-ΚB-Mediated Transcription of Inflammatory Genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-KappaB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Sethi, G.; Sung, B.; Aggarwal, B.B. Nuclear Factor-KappaB Activation: From Bench to Bedside. Exp. Biol. Med. 2008, 233, 21–31. [Google Scholar] [CrossRef]
- Wang, W.; Bergh, A.; Damber, J.E. Chronic Inflammation in Benign Prostate Hyperplasia Is Associated with Focal Upregulation of Cyclooxygenase-2, Bcl-2, and Cell Proliferation in the Glandular Epithelium. Prostate 2004, 61, 60–72. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Cui, J.; Xie, S.; Wan, H.; Luo, Y.; Guo, G. Vitexin, a Fenugreek Glycoside, Ameliorated Obesity-Induced Diabetic Nephropathy via Modulation of NF-ΚB/IkBα and AMPK/ACC Pathways in Mice. Biosci. Biotechnol. Biochem. 2021, 85, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Y.; Li, S.; Zhen, Y.L.; Wang, Y.N.; Shao, X.; Luo, Z.G. Cardioprotection of Vitexin on Myocardial Ische-mia/Reperfusion Injury in Rat via Regulating Inflammatory Cytokines and MAPK Pathway. Am. J. Chin. Med. 2013, 41, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Lodra, E.H.; Effendi, M.C.; Pematasari, N.; Dradjat, R.S. Fenugreek Seed Ethanolic Extract Improves Alveolar Bone Parameters by Attenuating Inflammation in Ovariectomized Rats. J. Inflamm. Res. 2023, 16, 4933–4940. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Effect of Glycosides Based Standardized Fenugreek Seed Extract in Bleomycin-Induced Pulmonary Fibrosis in Rats: Decisive Role of Bax, Nrf2, NF-ΚB, Muc5ac, TNF-α and IL-1β. Chem. Biol. Interact. 2015, 237, 151–165. [Google Scholar] [CrossRef]
- Xu, J.; Qian, D.; Jiang, S.; Zhang, X.; Shang, E.; Guo, J.; Duan, J. Application of Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry to Determine the Metabolites of Orientin Produced by Human Intestinal Bacteria. J. Chromatogr. B 2014, 944, 123–127. [Google Scholar] [CrossRef]
- Dong, P.; Shi, L.; Wang, S.; Jiang, S.; Li, H.; Dong, F.; Xu, J.; Dai, L.; Zhang, J. Rapid Profiling and Identification of Vitexin Metabolites in Rat Urine, Plasma, and Faeces after Oral Administration Using a UHPLC-Q-Exactive Orbitrap Mass Spectrometer Coupled with Multiple Data-Mining Methods. Curr. Drug Metab. 2021, 22, 185–197. [Google Scholar]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Zheng, S.; Geng, D.; Liu, S.; Wang, Q.; Liu, S.; Wang, R. A Newly Isolated Human Intestinal Bacterium Strain Capable of Deglycosylating Flavone C-Glycosides and Its Functional Properties. Microb. Cell Fact. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.; Kim, S.; Kim, J.H.; Bae, B.S.; Koo, G.B.; So, S.H.; Lee, J.; Lee, Y.H. Effects of Red Ginseng Oil (KGC11o) onTestosterone-Propionate-Induced Benign Prostatic Hyperplasia. J. Ginseng Res. 2022, 46, 473–480. [Google Scholar] [CrossRef]
- OECD. Test No. 441: Hershberger Bioassay in Rats: A Short-Term Screening Assay for (Anti)Androgenic Properties; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2009. [Google Scholar]
- Lee, J.Y.; Kim, S.; Kim, H.; Yeon, S.H.; Kim, S.Y.; Son, R.H.; Park, C.L.; Lee, Y.H. Improvement in Testosterone Production by Acorus gramineus for the Alleviation of Andropause Symptoms. J. Med. Food 2024, 27, 740–748. [Google Scholar] [CrossRef]
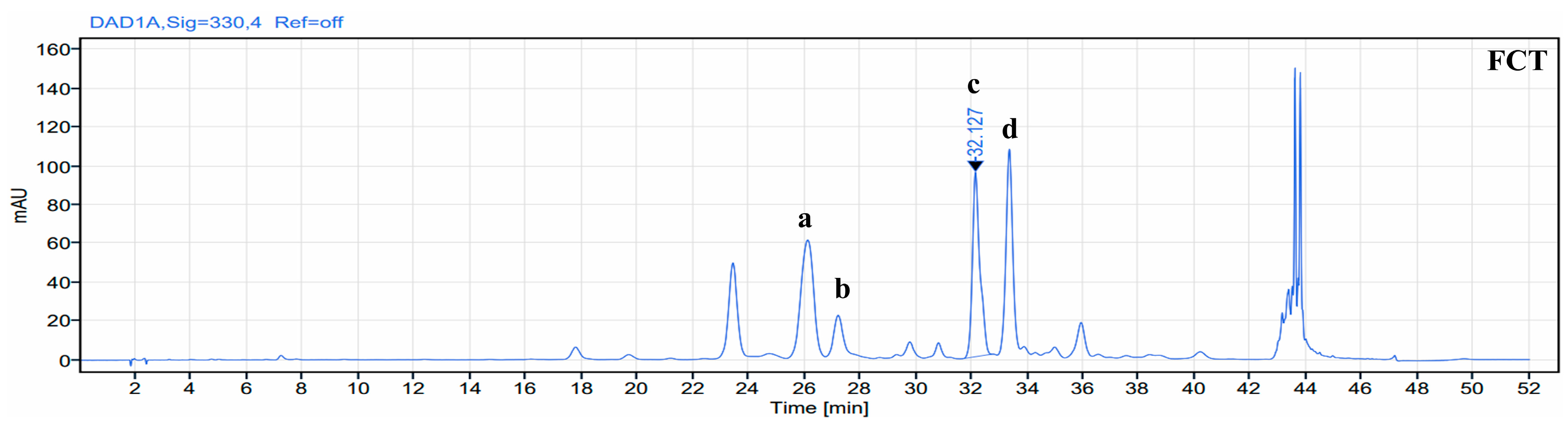
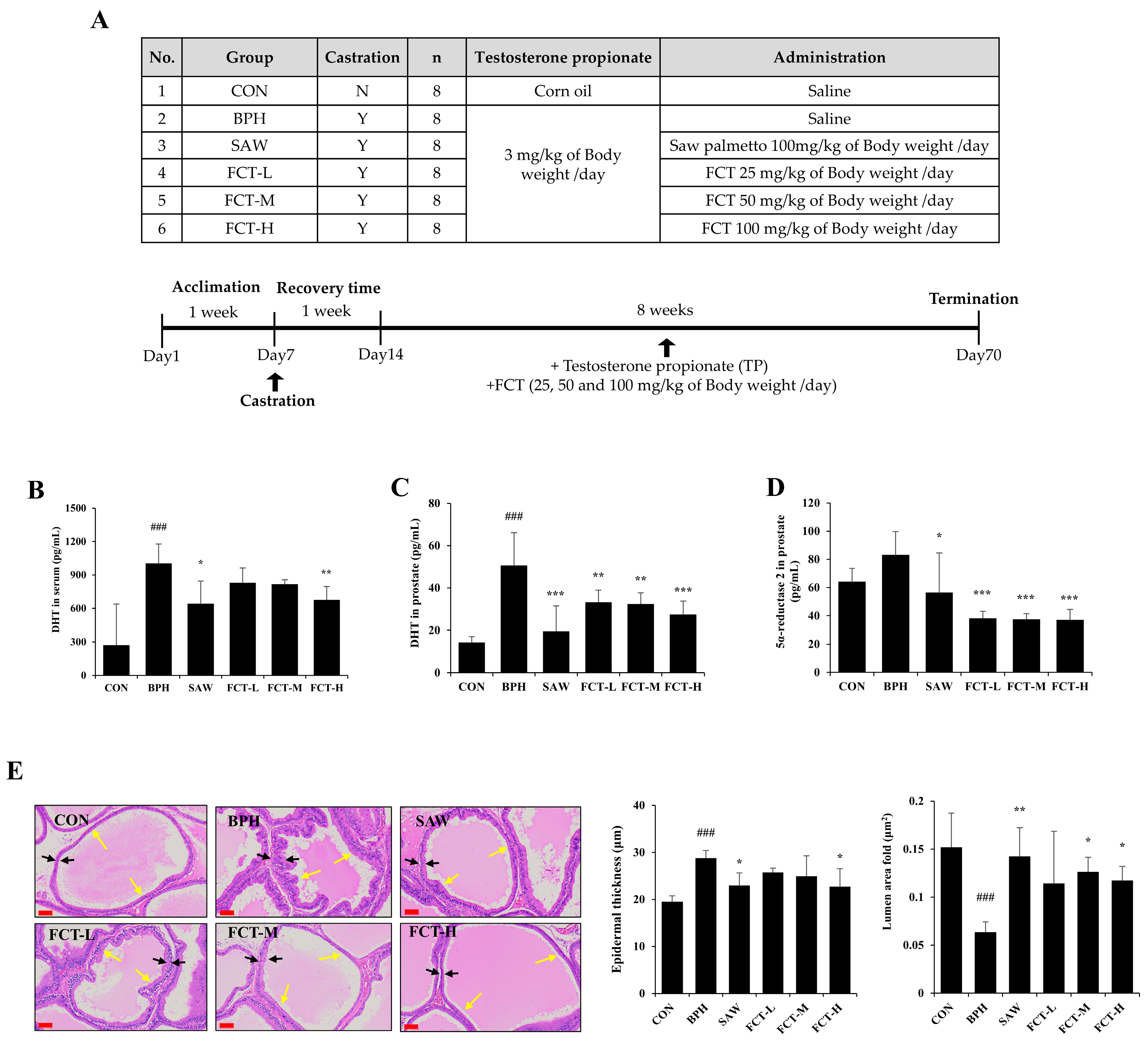


| Group | Prostate Weight (g) | Prostate Ratio 1 |
|---|---|---|
| CON | 0.66 ± 0.15 | 0.16 ± 0.03 |
| BPH | 1.23 ± 0.18 ### | 0.33 ± 0.05 ### |
| SAW | 1.00 ± 0.15 * | 0.24 ± 0.05 ** |
| FCT-L | 1.05 ± 0.13 | 0.28 ± 0.05 |
| FCT-M | 0.96 ± 0.18 * | 0.26 ± 0.06 * |
| FCT-H | 0.91 ± 0.22 ** | 0.24 ± 0.06 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Bang, J.; Kim, J.; Baek, K.-S.; Oh, D.; Lee, Y.-H. Effect of Fenugreek Extract on Testosterone Propionate-Induced Benign Prostatic Hyperplasia. Int. J. Mol. Sci. 2025, 26, 1261. https://doi.org/10.3390/ijms26031261
Lee JY, Bang J, Kim J, Baek K-S, Oh D, Lee Y-H. Effect of Fenugreek Extract on Testosterone Propionate-Induced Benign Prostatic Hyperplasia. International Journal of Molecular Sciences. 2025; 26(3):1261. https://doi.org/10.3390/ijms26031261
Chicago/Turabian StyleLee, Jeong Yoon, Jiyoung Bang, Jinhak Kim, Kwang-Soo Baek, Dongchan Oh, and Yoo-Hyun Lee. 2025. "Effect of Fenugreek Extract on Testosterone Propionate-Induced Benign Prostatic Hyperplasia" International Journal of Molecular Sciences 26, no. 3: 1261. https://doi.org/10.3390/ijms26031261
APA StyleLee, J. Y., Bang, J., Kim, J., Baek, K.-S., Oh, D., & Lee, Y.-H. (2025). Effect of Fenugreek Extract on Testosterone Propionate-Induced Benign Prostatic Hyperplasia. International Journal of Molecular Sciences, 26(3), 1261. https://doi.org/10.3390/ijms26031261







