Gene Therapy: An Historical Overview for Familial Hearing Loss
Abstract
1. Introduction
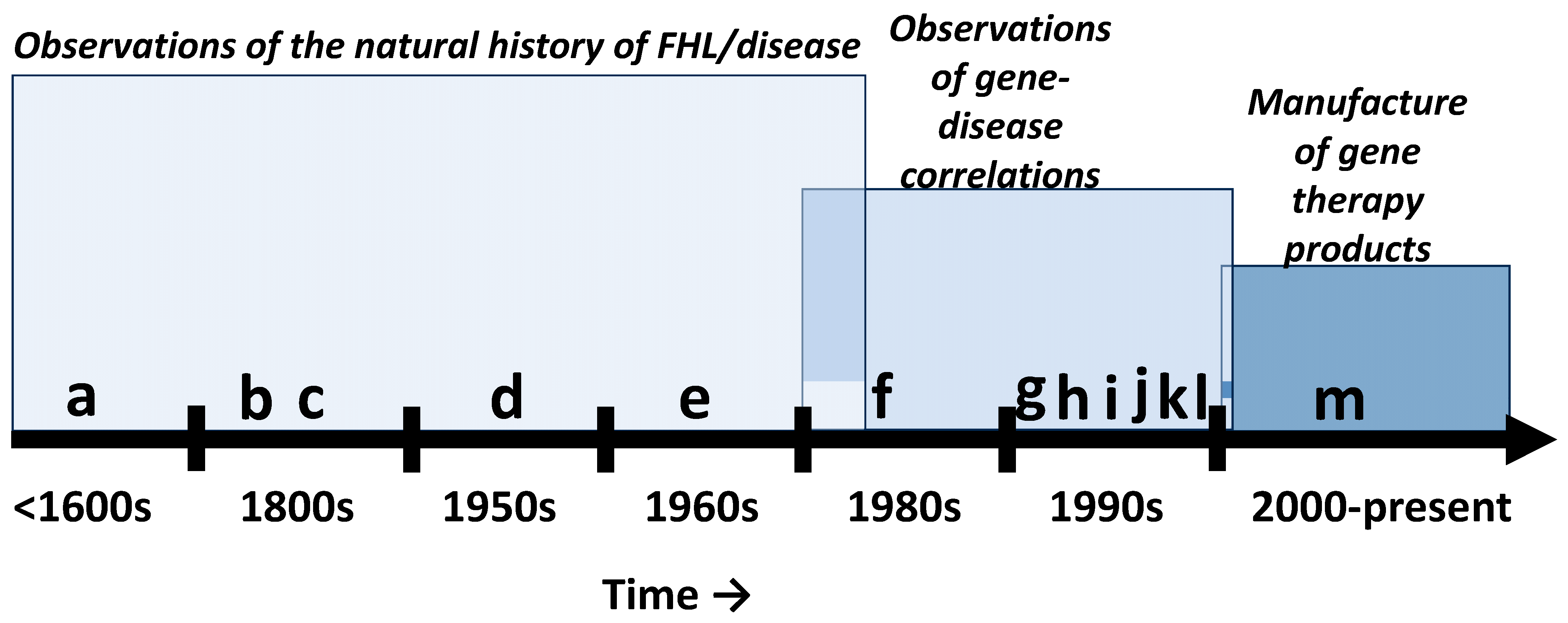
2. Historical Context
2.1. Basic Science Phase
2.2. Pre-Commercialization Phase
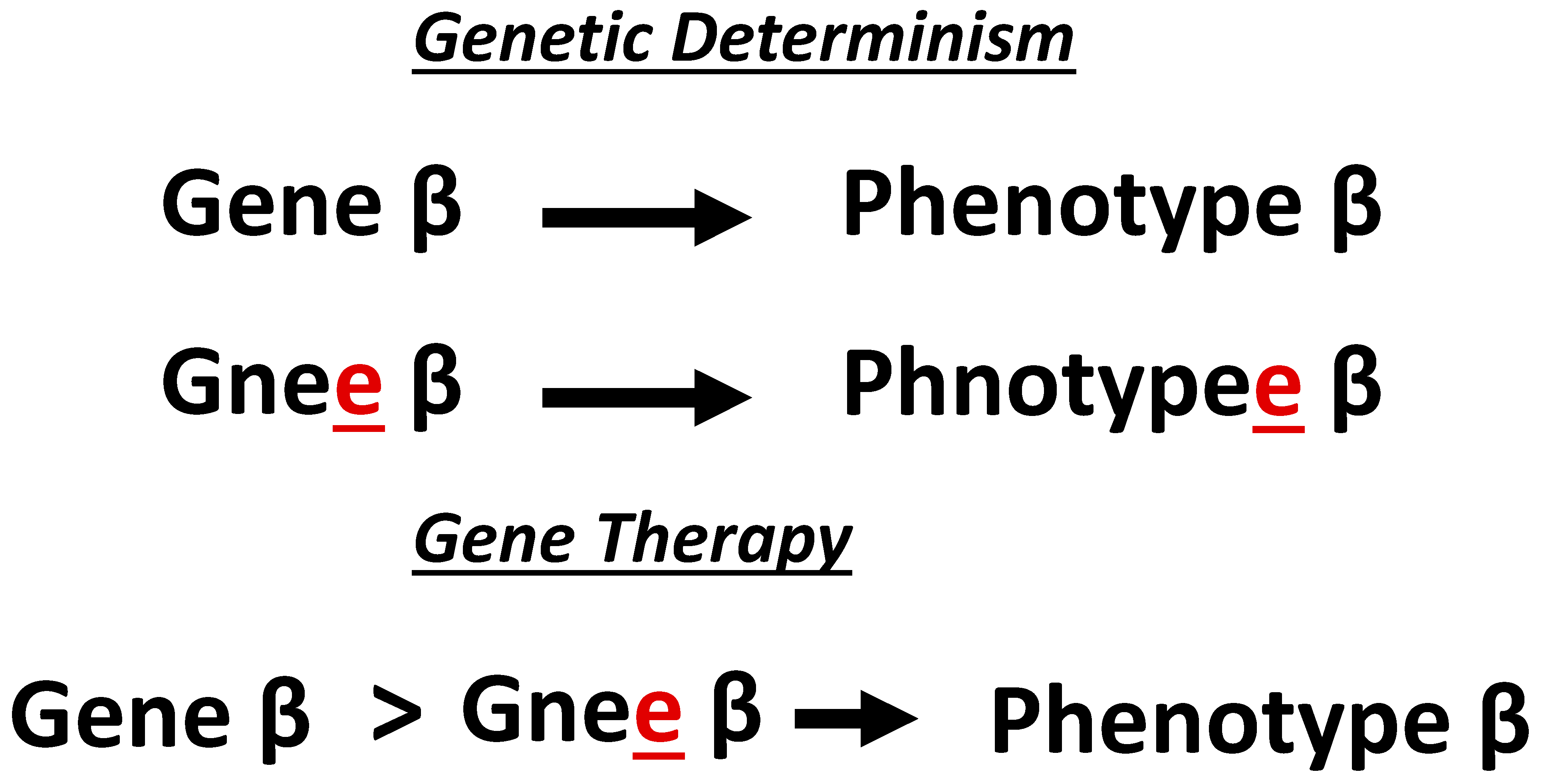
2.3. Commercialization Phase
- (1)
- Affordability encompasses the excessively high price for individual gene therapies and the limits of insurance coverage. Additionally, long-term medical costs of the morbidities that develop over years after gene therapy is known to be a highly significant contributor to affordability.
- (2)
- Assessment-of-value encompasses the perceived value for individual patients and their families, as well as perceived value within individual countries, cultures, and healthcare systems/agencies.
- (3)
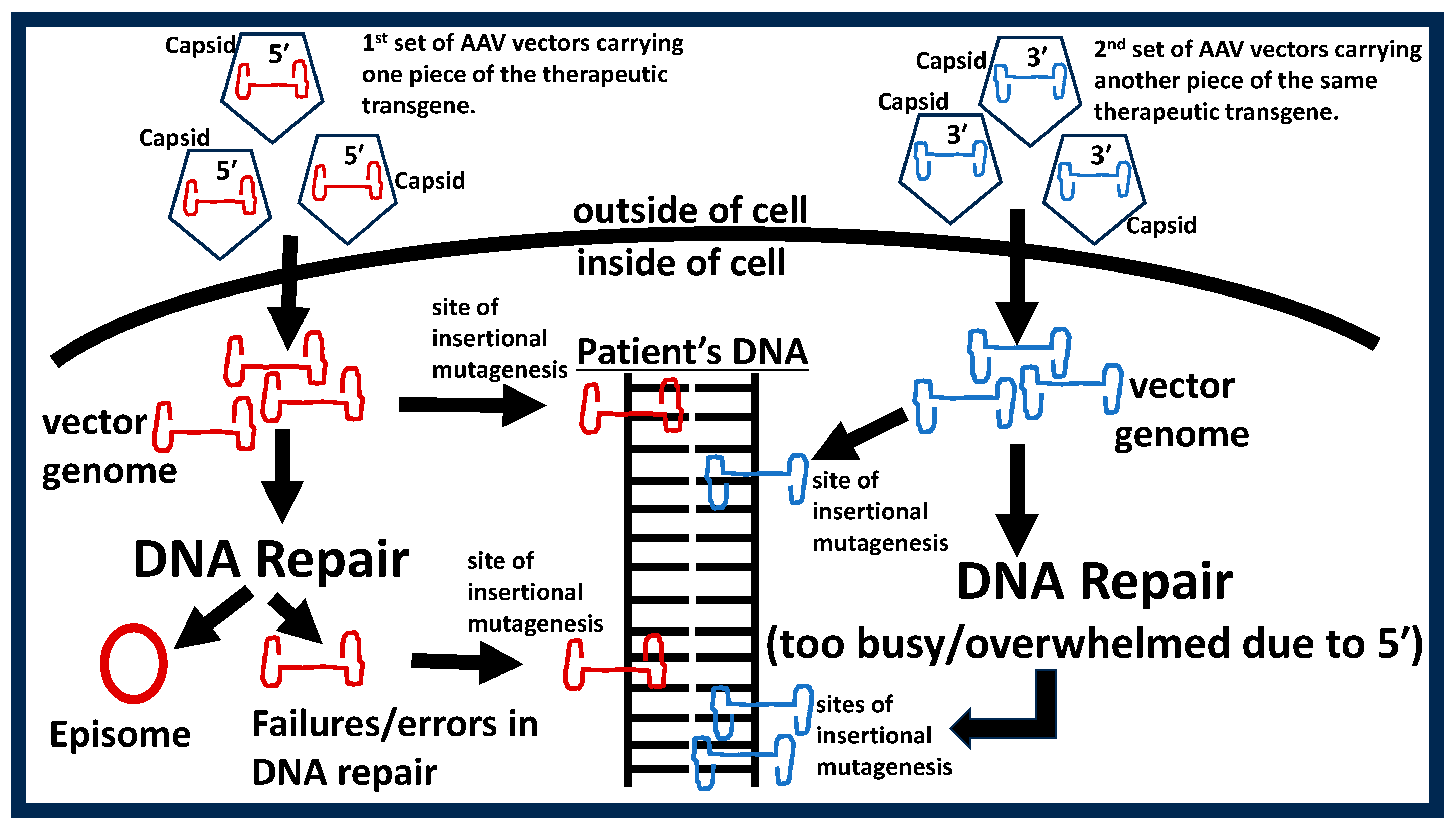
- Development-of-therapy relates to the fact that all manufactured gene therapy products yield some degree of uncertainty in manufacturing precision/chemistry. This includes patients treated with vectors that mistakenly omit the therapeutic transgene and vectors with incorrect, misaligned, or mutated transgenes and/or vector DNA sequences. The penultimate consequence of these unintended manufacturing uncertainties is a high-level failure rate (in terms of efficacy and safety) in clinical trials which again limits patient accessibility.
- (4)
- Ethical/social factors encompass negative religious, cultural, political, and socioeconomic beliefs and misinformation against gene therapy. Furthermore, disparities in costs and standards-of-care within and between countries creates heterogenous barriers for different populations and perpetuate notions of “treatment tourists”.
- (5)
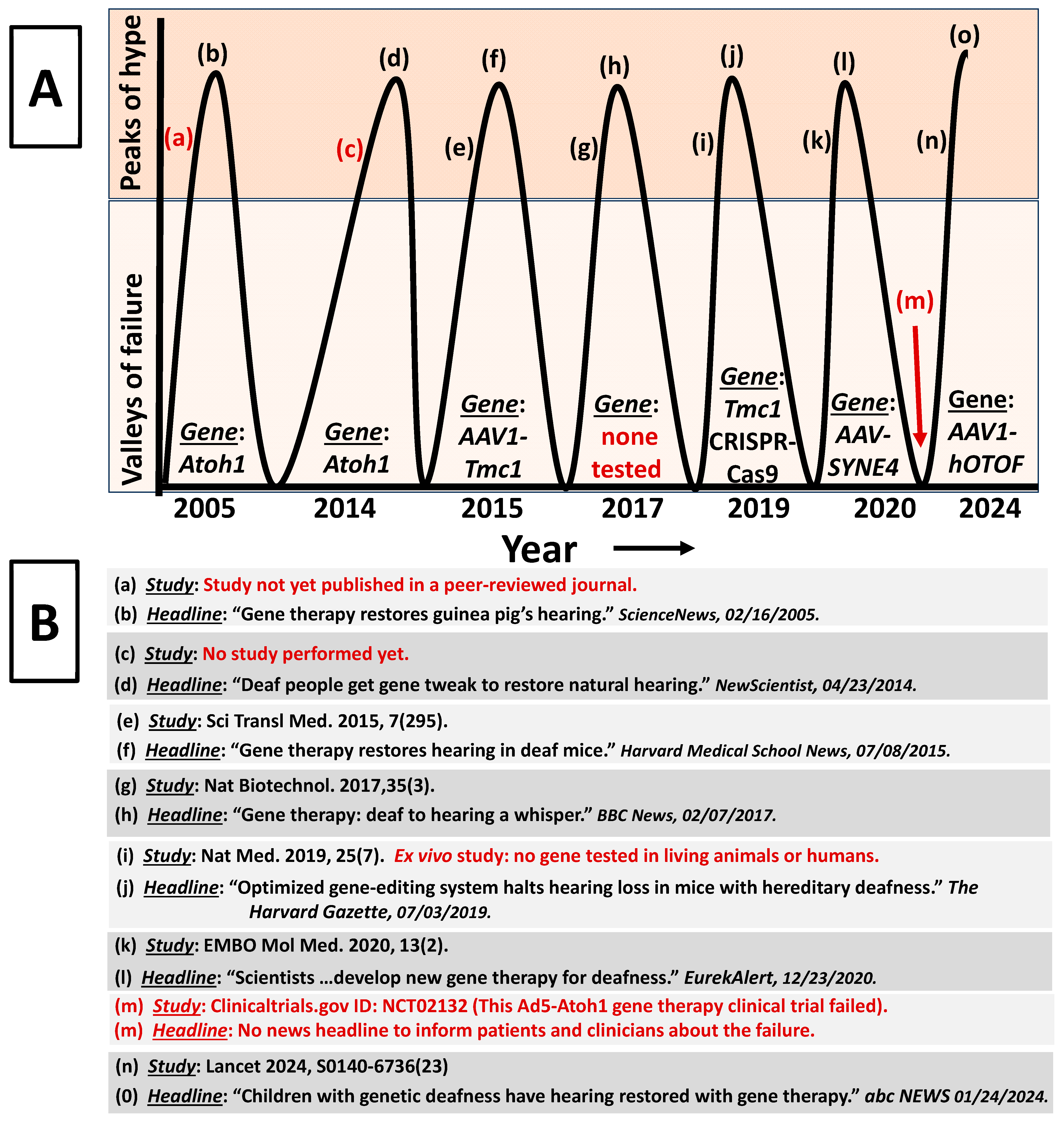
- Evidence generation relates to the fact that most gene therapy clinical trials do not meet established scientific standards for credible evidence which then limits patients’ access to therapies. For instance, clinical trials and the data produced from the trials need to exhibit specific characteristics to be considered credible, such as (but not limited to) randomization of patients/independent sampling; inclusion of a control arm/group; double/triple-blinding; placebo; statistical power; constrained multiplicity and parametric data homoscedasticity and Gaussian distribution. Interestingly, gene therapy researchers and clinicians typically justify the absence of the above standards with two colloquial excuses. One is that “the field is too new”; however, human gene therapy can be traced back to the Shope papilloma virus in 1973 (>5 decades old). The second is “gene therapy research is just too difficult”. Yet across the biomedical sciences, difficulty has never been an appropriate excuse for sloppy research. The consequences of lowering scientific standards for gene therapy research is exemplified by many perverse and dire examples in the literature. In the X-linked myotubular myopathy field, lowered standards resulted in a death rate that increased to 30% with the more recent death of another patient (NCT03199469). In the Duchenne muscular dystrophy (DMD) field, cardiovascular field, and several other fields, years of studies with lowered scientific standards perpetuated the notion that gene therapy can cure DMD, cardiovascular diseases and other genetic diseases [39,40]. However, when consortiums of institutions and researchers finally conducted scientifically rigorous studies, it was revealed that the gene therapies were never cures. This ultimately led to decades of wasted resources, time, unnecessary toxic exposures, and untimely deaths for study subjects.
- (6)
- Operational implementation encompasses barriers endemic to both patients and healthcare providers. For instance, patients need to have access to a hospital equipped with specialized pre- and post-therapy administration protocols, methods, facilities, and personnel. This limits access for many patients and burdens others who would have to travel great distances. Healthcare providers need to be formally trained to provide the specialized patientcare needed before and after therapy administration. Such formal training includes specialized safety and rescue procedures that are bespoke to the acute and chronic toxic outcomes for each gene therapy product as well as training on managing unintended deaths and life-threatening adverse events from the therapy. Furthermore, the high administrative burdens (added medical record keeping, proper patient consent, health insurance forms, etc.) further compound the situation, which all serve to limit the number of providers willing to serve patients.
- (7)
- Regulatory hurdles encompass the philosophies, practices, and procedures of regulatory bodies such as the US Food and Drug Administration (FDA), European Medicine Agency (EMA), Health Canada, Russian Ministry of Health, and the State Food and Drug Administration of China (SFDA). Here, a major barrier to patient access is the disparities between regulatory bodies. For instance, gene therapies approved by the EMA may not receive approval from the FDA and others. Additionally, approval of a given therapy does not mean widespread use or acceptance by patients and healthcare providers. Furthermore, the level of safety and quality control is different among the regulatory bodies, and some allow for parallel (less regulated) pathways to achieve therapy approval. Even more frustrating is the fact that disparities exist within regulatory bodies, for example, member states within the European Union. Interestingly, regulatory bodies within individual countries can be segregated by whether they prioritize clinical benefit from gene therapy, costs associated with gene therapy, or both.
- Are the risks of morbidity and mortality associated with gene therapy reasonable in the context of deafness/hearing loss?
- Can clinicians really claim that the therapy is safe and effective, given the current publication bias and poor-quality clinical trials (see discussion below)?
- Given current hyperbolized claims regarding gene therapy (see discussion below), are clinicians adequately consenting their patients before gene therapy trials?
- Given the well-known efficacy of hearing aids and cochlear implants, is it reasonable for clinicians to bias counseling towards gene therapy or position gene therapy as having similar levels of efficacy as hearing aids and cochlear implants?
- Should clinicians support parents who would rather wait for gene therapy to become available rather than manage the hearing loss with cochlear implants?
3. Scientist, Clinician and Patient Appraisal of Gene Therapy Claims
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- World Report on Hearing. Available online: https://www.who.int/publications-detail-redirect/world-report-on-hearing (accessed on 18 July 2022).
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; ElHafeez, S.A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global Incidence, Prevalence, Years Lived with Disability (YLDs), Disability-Adjusted Life-Years (DALYs), and Healthy Life Expectancy (HALE) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Myers, C.A.; Kolberg, E.; Deal, J.A.; Lin, F.R.; Powell, D.S. Mental Health, Cognition, and Hearing Loss in Older Adults. In Health and Hearing; World Scientific: London, UK, 2022; pp. 393–443. ISBN 9789811264993. [Google Scholar]
- Sloan-Heggen, C.M.; Bierer, A.O.; Shearer, A.E.; Kolbe, D.L.; Nishimura, C.J.; Frees, K.L.; Ephraim, S.S.; Shibata, S.B.; Booth, K.T.; Campbell, C.A.; et al. Comprehensive Genetic Testing in the Clinical Evaluation of 1119 Patients with Hearing Loss. Hum. Genet. 2016, 135, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, H.; Booth, K.T.; Ephraim, S.S.; Crone, B.; Black-Ziegelbein, E.A.; Marini, R.J.; Shearer, A.E.; Sloan-Heggen, C.M.; Kolbe, D.; Casavant, T.; et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am. J. Hum. Genet. 2018, 103, 484–497. [Google Scholar] [CrossRef]
- Lv, J.; Wang, H.; Cheng, X.; Chen, Y.; Wang, D.; Zhang, L.; Cao, Q.; Tang, H.; Hu, S.; Gao, K.; et al. AAV1-hOTOF Gene Therapy for Autosomal Recessive Deafness 9: A Single-Arm Trial. Lancet 2024, 403, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tan, F.; Zhang, L.; Lu, L.; Zhang, S.; Zhai, Y.; Lu, Y.; Qian, X.; Dong, W.; Zhou, Y.; et al. AAV-Mediated Gene Therapy Restores Hearing in Patients with DFNB9 Deafness. Adv. Sci. 2024, 11, 2306788. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Animal Models for Understanding Hearing Loss. In Health and Hearing; World Scientific: London, UK, 2023; pp. 44–78. ISBN 9789811264993. [Google Scholar]
- Noble, D. Claude Bernard, the First Systems Biologist, and the Future of Physiology. Exp. Physiol. 2008, 93, 16–26. [Google Scholar] [CrossRef]
- Backwell, L.; Marsh, J.A. Diverse Molecular Mechanisms Underlying Pathogenic Protein Mutations: Beyond the Loss-of-Function Paradigm. Annu. Rev. Genom. Hum. Genet. 2022, 23, 475–498. [Google Scholar] [CrossRef]
- Griffith, F. The Significance of Pneumococcal Types. Epidemiol. Infect. 1928, 27, 113–159. [Google Scholar] [CrossRef] [PubMed]
- Alloway, J.L. The Transformation In Vitro of R Pneumococci into S Forms of Different Specific Types by the Use of Filtered Pneumococcus Extracts. J. Exp. Med. 1932, 55, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; MacLeod, C.M.; McCarty, M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types. Inductions of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III. J. Exp. Med. 1979, 149, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Tatum, E.L.; Lederberg, J. Gene Recombination in the Bacterium Escherichia Coli. J. Bacteriol. 1947, 53, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Pfuderer, P. Use of Viruses as Carriers of Added Genetic Information. Nature 1968, 219, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Temin, H.M. Mixed Infection with Two Types of Rous Sarcoma Virus. Virology 1961, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D.; Crick, F.H.C. The Structure of Dna. Cold Spring Harb. Symp. Quant. Biol. 1953, 18, 123–131. [Google Scholar] [CrossRef]
- Matthaei, J.H.; Jones, O.W.; Martin, R.G.; Nirenberg, M.W. Characteristics and Composition of Rna Coding Units. Proc. Natl. Acad. Sci. USA 1962, 48, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Beadle, G.W.; Tatum, E.L. Genetic Control of Biochemical Reactions in Neurospora. Proc. Natl. Acad. Sci. USA 1941, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Arber, W.; Linn, S. DNA Modification and Restriction. Annu. Rev. Biochem. 1969, 38, 467–500. [Google Scholar] [CrossRef] [PubMed]
- Lehman, I.R. DNA Ligase: Structure, Mechanism, and Function. Science 1974, 186, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Szybalska, E.H.; Szybalski, W. Genetics of Human Cell Line. IV. DNA-Mediated Heritable Transformation of a Biochemical Trait. Proc. Natl. Acad. Sci. USA 1962, 48, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Westphal, H.; Srinivasan, P.R.; Dulbecco, R. The Integrated State of Viral DNA in SV40-Transformed Cells. Proc. Natl. Acad. Sci. USA 1968, 60, 1288–1295. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. A Preliminary Report. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Merrouche, Y.; Negrier, S.; Bain, C.; Combaret, V.; Mercatello, A.; Coronel, B.; Moskovtchenko, J.F.; Tolstoshev, P.; Moen, R.; Philip, T. Clinical Application of Retroviral Gene Transfer in Oncology: Results of a French Study with Tumor-Infiltrating Lymphocytes Transduced with the Gene of Resistance to Neomycin. J. Clin. Oncol. 1995, 13, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Lowenthal, A.; Terheggen, H.G.; Columbo, J.P. Induction of Arginase Activity with the Shope Papilloma Virus in Tissue Culture Cells from an Argininemic Patient. J. Exp. Med. 1973, 137, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D. NIH Censure for Dr Martin Cline. Nature 1981, 291, 369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Howe, S.J.; Mansour, M.R.; Schwarzwaelder, K.; Bartholomae, C.; Hubank, M.; Kempski, H.; Brugman, M.H.; Pike-Overzet, K.; Chatters, S.J.; de Ridder, D.; et al. Insertional Mutagenesis Combined with Acquired Somatic Mutations Causes Leukemogenesis Following Gene Therapy of SCID-X1 Patients. J. Clin. Investig. 2008, 118, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional Oncogenesis in 4 Patients after Retrovirus-Mediated Gene Therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Hacein-Bey, S.; Basile, G.d.S.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.-L.; et al. Gene Therapy of Human Severe Combined Immunodeficiency (SCID)-X1 Disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Fehse, B.; Roeder, I. Insertional Mutagenesis and Clonal Dominance: Biological and Statistical Considerations. Gene Ther. 2008, 15, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Gross, D.-A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Sung, C.Y.W.; Grati, M.; Chien, W. Immune Responses in the Mammalian Inner Ear and Their Implications for AAV-Mediated Inner Ear Gene Therapy. Hear. Res. 2023, 432, 108735. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Foster, G.R.; Coppens, M.; Thomsen, H.; Dolmetsch, R.; Heijink, L.; Monahan, P.E.; Pipe, S.W. Molecular Evaluation and Vector Integration Analysis of HCC Complicating AAV Gene Therapy for Hemophilia B. Blood Adv. 2023, 7, 4966–4969. [Google Scholar] [CrossRef]
- Blaese, R.M.; Culver, K.W.; Miller, A.D.; Carter, C.S.; Fleisher, T.; Clerici, M.; Shearer, G.; Chang, L.; Chiang, Y.; Tolstoshev, P.; et al. T Lymphocyte-Directed Gene Therapy for ADA− SCID: Initial Trial Results After 4 Years. Science 1995, 270, 475–480. [Google Scholar] [CrossRef]
- Wirth, T.; Parker, N.; Ylä-Herttuala, S. History of Gene Therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Sepodes, B.; Martins, A.P. Patient Access to Gene Therapy Medicinal Products: A Comprehensive Review. BMJ Innov. 2021, 7, 123–134. [Google Scholar] [CrossRef]
- van Overbeeke, E.; Michelsen, S.; Toumi, M.; Stevens, H.; Trusheim, M.; Huys, I.; Simoens, S. Market Access of Gene Therapies across Europe, USA, and Canada: Challenges, Trends, and Solutions. Drug Discov. Today 2021, 26, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Muntoni, F. AAV Gene Therapy for Duchenne Muscular Dystrophy: Lessons Learned from a Phase 3 Trial. Gene Ther. 2024, 31, 541–543. [Google Scholar] [CrossRef]
- Hedman, M.; Hartikainen, J.; Ylä-Herttuala, S. Progress and Prospects: Hurdles to Cardiovascular Gene Therapy Clinical Trials. Gene Ther. 2011, 18, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Arabi, F.; Mansouri, V.; Ahmadbeigi, N. Gene Therapy Clinical Trials, Where Do We Go? An Overview. Biomed. Pharmacother. 2022, 153, 113324. [Google Scholar] [CrossRef]
- Cheng, Y.-F. Atoh1 Regulation in the Cochlea: More than Just Transcription. J. Zhejiang Univ. Sci. B 2019, 20, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.G.M.; Wolpert, S.; Saeed, S.; Middelink, L.M.; Edge, A.S.B.; Blackshaw, H.; Pastiadis, K.; Bibas, A.G. A Phase I/IIa Safety and Efficacy Trial of Intratympanic Gamma-Secretase Inhibitor as a Regenerative Drug Treatment for Sensorineural Hearing Loss. Nat. Commun. 2024, 15, 1896. [Google Scholar] [CrossRef]
- Landegger, L.D.; Pan, B.; Askew, C.; Wassmer, S.J.; Gluck, S.D.; Galvin, A.; Taylor, R.; Forge, A.; Stankovic, K.M.; Holt, J.R.; et al. A Synthetic AAV Vector Enables Safe and Efficient Gene Transfer to the Mammalian Inner Ear. Nat. Biotechnol. 2017, 35, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tan, F.; Zhang, L.; Lu, L.; Wang, H.; Li, W.; Liu, W.; Fu, X.; He, Z.; Ding, X.; et al. Clinical Practice Guidelines for Gene Therapy to Treat Hereditary Hearing Loss. Interdiscip. Med. 2024, 2, e20240008. [Google Scholar] [CrossRef]
- Guthrie, O.W. DNA Repair Proteins and Telomerase Reverse Transcriptase in the Cochlear Lateral Wall of Cisplatin-Treated Rats. J. Chemother. 2009, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Localization and Distribution of Neurons That Co-Express Xeroderma Pigmentosum-A and Epidermal Growth Factor Receptor within Rosenthal’s Canal. Acta Histochem. 2015, 117, 688–695. [Google Scholar] [CrossRef]
- Guthrie, O.W. Noise Induced DNA Damage Within the Auditory Nerve. Anat. Rec. 2017, 300, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W.; Carrero-Martínez, F.A. Real-Time Quantification of Xeroderma Pigmentosum mRNA from the Mammalian Cochlea. Ear Hear. 2010, 31, 714–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, L.; Guthrie, O.W. Effects of Acute Noise Exposure on DNA Damage Response Genes in the Cochlea, Cortex, Heart and Liver. Exp. Mol. Pathol. 2020, 114, 104401. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Preincision Complex-I from the Excision Nuclease Reaction among Cochlear Spiral Limbus and Outer Hair Cells. J. Mol. Histol. 2008, 39, 617–625. [Google Scholar] [CrossRef]
- Guthrie, O.W. Noise Stress Induces an Epidermal Growth Factor Receptor/Xeroderma Pigmentosum-A Response in the Auditory Nerve. J. Histochem. Cytochem. 2017, 65, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W.; Li-Korotky, H.-S.; Durrant, J.D.; Balaban, C. Cisplatin Induces Cytoplasmic to Nuclear Translocation of Nucleotide Excision Repair Factors among Spiral Ganglion Neurons. Hear. Res. 2008, 239, 79–91. [Google Scholar] [CrossRef]
- Rosas, L.E.; Grieves, J.L.; Zaraspe, K.; Perle, K.M.L.; Fu, H.; McCarty, D.M. Patterns of scAAV Vector Insertion Associated with Oncogenic Events in a Mouse Model for Genotoxicity. Mol. Ther. 2012, 20, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Dynamic Compartmentalization of DNA Repair Proteins within Spiral Ganglion Neurons in Response to Noise Stress. Int. J. Neurosci. 2012, 122, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Preservation of Neural Sensitivity after Noise-Induced Suppression of Sensory Function. J. Am. Acad. Audiol. 2016, 27, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W. Functional Consequences of Inducible Genetic Elements from the P53 SOS Response in a Mammalian Organ System. Exp. Cell Res. 2017, 359, 50–61. [Google Scholar] [CrossRef]
- Guthrie, O.W. Noise Stress Abrogates Structure-Specific Endonucleases within the Mammalian Inner Ear. Int. J. Mol. Sci. 2024, 25, 1749. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, O.W.; Gearhart, C.A.; Fulton, S.; Fechter, L.D. Carboxy Alkyl Esters of Uncaria Tomentosa Augment Recovery of Sensorineural Functions Following Noise Injury. Brain Res. 2011, 1407, 97–106. [Google Scholar] [CrossRef]
- Guthrie, O.W.; Xu, H. Reduced Phosphorylation of Histone Variant H2Ax in the Organ of Corti Is Associated with Otoprotection from Noise Injury. Otolaryngol. Open Access 2013, 3, 131. [Google Scholar] [CrossRef]
- Rose, M.L. Deaf and Dumb in Ancient Greece. Disabil. Stud. Read. 2006, 17–31. [Google Scholar]
- Stephens, D. Deafness and Its Treatment in Ancient Civilisations. Audiol. Med. 2006, 4, 85–93. [Google Scholar] [CrossRef]
- Mahase, E. Alzheimer’s Disease: Lecanemab Gets Full FDA Approval and Black Box Safety Warning. BMJ 2023, 382, p1580. [Google Scholar] [CrossRef] [PubMed]
- Maulden, A. Ignoring the Experts: Implications of the FDA’s Aduhelm Approval. Am. J. Law. Med. 2022, 48, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Askew, C.; Rochat, C.; Pan, B.; Asai, Y.; Ahmed, H.; Child, E.; Schneider, B.L.; Aebischer, P.; Holt, J.R. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 2015, 7, 295. [Google Scholar] [CrossRef]
- Pan, B.; Askew, C.; Galvin, A.; Heman-Ackah, S.; Asai, Y.; Indzhykulian, A.A.; Jodelka, F.M.; Hastings, M.L.; Lentz, J.J.; Vandenberghe, L.H.; et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat. Biotechnol. 2017, 35, 264–272. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Nist-Lund, C.; Pan, B.; Asai, Y.; Karavitaki, K.D.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Solanes, P.; Spataro, S.; et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 2019, 25, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Taiber, S.; Cohen, R.; Yizhar-Barnea, O.; Sprinzak, D.; Holt, J.R.; Avraham, K.B. Neonatal AAV gene therapy rescues hearing in a mouse model of SYNE4 deafness. EMBO Mol. Med. 2020, 13, e13259. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, C. Hearing Repaired: Gene Therapy Restores Guinea Pigs’ Hearing. Available online: https://www.sciencenews.org/article/hearing-repaired-gene-therapy-restores-guinea-pigs-hearing (accessed on 22 March 2024).
- Deaf People Get Gene Tweak to Restore Natural Hearing. Available online: https://www.newscientist.com/article/mg22229662-400-deaf-people-get-gene-tweak-to-restore-natural-hearing/ (accessed on 22 March 2024).
- Scientists at Tel Aviv University Develop New Gene Therapy for Deafness. Available online: https://www.eurekalert.org/news-releases/517082 (accessed on 22 March 2024).
- This Ad5-Atoh1 Gene Therapy Clinical Trial Failed. Available online: https://clinicaltrials.gov/study/NCT02132130 (accessed on 22 March 2024).
- Schünemann, H.J.; Reinap, M. GRADE: A Transparent Approach for Evidence-Based Recommendations and Decisions in Health. In Global Health Essentials; Raviglione, M.C.B., Tediosi, F., Villa, S., Casamitjana, N., Plasència, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 541–548. ISBN 978-3-031-33851-9. [Google Scholar]
- Barnett, M.K.; Macnamara, B.N. Individual Responses versus Aggregate Group-Level Results: Examining the Strength of Evidence for Growth Mindset Interventions on Academic Performance. J. Intell. 2023, 11, 104. [Google Scholar] [CrossRef]
- Buckinx, F.; Aubertin-Leheudre, M. Nutrition to Prevent or Treat Cognitive Impairment in Older Adults: A GRADE Recommendation. J. Prev. Alzheimer’s Dis. 2021, 8, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gough, D. Appraising Evidence Claims. Rev. Res. Educ. 2021, 45, 1–26. [Google Scholar] [CrossRef]
- Adachi, N.; Ito, K.; Sakata, H.; Yamasoba, T. Etiology and One-Year Follow-up Results of Hearing Loss Identified by Screening of Newborn Hearing in Japan. Otolaryngol. Head. Neck Surg. 2010, 143, 97–100. [Google Scholar] [CrossRef]
- Talero-Gutiérrez, C.; Carvajalino-Monje, I.; de Samper, B.S.; Ibáñez-Pinilla, M. Delayed Auditory Pathway Maturation in the Differential Diagnosis of Hypoacusis in Young Children. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Bauer, D.E.; Chiarle, R. Assessing and Advancing the Safety of CRISPR-Cas Tools: From DNA to RNA Editing. Nat. Commun. 2023, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Gao, X. Multiplex Base- and Prime-Editing with Drive-and-Process CRISPR Arrays. Nat. Commun. 2022, 13, 2771. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. Shoot the Messenger: RNA Editing Is Here. Nat. Biotechnol. 2023, 41, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. A Personal View of the Project. The Code of Codes: Scientific and Social Issues in the Human Genome Project; Harvard University Press: Cambridge, MA, USA, 1992; pp. 164–173. [Google Scholar]
- Collins, F. The Language of Life: DNA and the Revolution in Personalised Medicine; Profile Books: London, UK, 2010; ISBN 978-1-84765-209-6. [Google Scholar]
- Roche, J.P.; Huang, B.Y.; Castillo, M.; Bassim, M.K.; Adunka, O.F.; Buchman, C.A. Imaging Characteristics of Children With Auditory Neuropathy Spectrum Disorder. Otol. Neurotol. 2010, 31, 780. [Google Scholar] [CrossRef]
- Westerhof, J.P.; Rademaker, J.; Weber, B.P.; Becker, H. Congenital Malformations of the Inner Ear and the Vestibulocochlear Nerve in Children with Sensorineural Hearing Loss: Evaluation with CT and MRI. J. Comput. Assist. Tomogr. 2001, 25, 719. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Lv, J.; Cheng, X.; Cao, Q.; Wang, D.; Zhang, L.; Zhu, B.; Shen, M.; Xu, C.; et al. Bilateral Gene Therapy in Children with Autosomal Recessive Deafness 9: Single-Arm Trial Results. Nat. Med. 2024, 30, 1898–1904. [Google Scholar] [CrossRef]

| General Variant Descriptions (applies to g, c, n, and r; only g used in the examples below): | |
| g.23A>C | A to C change (substitution) at nucleotide 23 in genomic DNA sequence. |
| g.23_33del | The 23rd to 33rd nucleotide sequences have been deleted. |
| g.23_33inv | The 23rd to 33rd nucleotide sequences have been replaced with nucleotides (2 minimum) that are the reverse-complement of the normal sequence (e.g., normal = CTCGA and reverse-complement = TCGAG). |
| g.23_33dup | The 23rd to 33rd nucleotide sequences are duplicates of previous nucleotides (3′ of original sequence). |
| g.23_33insGCAT | The nucleotides GCAT have been abnormally inserted between the 23rd and 33rd nucleotide sequences immediately before the normal sequence (5′ of the original sequence). |
| g.23_33con53_63 | The nucleotides between the 23rd and 33rd nucleotide sequences have been deleted and replaced (converted) with a copy of nucleotide sequences between the 53rd and 63rd nucleotide sequences (deletion–insertion). |
| g.23_33delinsGC | Two (must be more than one) normal nucleotides have been deleted between the 23rd and 33rd nucleotides and replaced with GC (insertion). |
| DNA Sequence Variations (applies to g and c; also see general variant description above): | |
| g.23A[12] | A repetitive stretch of DNA starting at the 23rd nucleotide with the number of repeats in brackets. |
| c.[23C>T; 63G>C] | Two variants on one molecule (cis). |
| c.[23C>T]; [63G>C] | Two variants on two different molecules (trans). |
| g.[23C>T(;) 63G>C] | Two variants with unknown phase. |
| g.23A= | Variant screen performed but no change detected. |
| RNA Sequence Variations (applies to r; also see general variant description above): | |
| r.23a>u | The 23rd nucleotide sequence within RNA is normally an “a” but it has been changed to a “u”. |
| r.[23a>u, 63_73del] | Two RNA variants (substitution and deletion) resulted from a single DNA change. |
| Protein Sequence Variations: | |
| p.T23* | A termination codon (Ter or *) exist at the 23rd amino acid (also known as. nonsense change). |
| p.A23S | The 23rd amino acid (A) is substituted with S (also known as missense change). |
| p.(A23S) | The protein change is predicted (no empirical proof). |
| p.A23_S33del | Deletion of amino acids 23 to 33. |
| p.A23_S33dup | Duplication of amino acids 23 to 33. |
| p.A23_S24insWT | Insertion of the W and T amino acids at the 23 and 24 place. |
| p.A23_S24delinsWT | Deletion-insertion (indel). |
| p.(A23fs) | Frame shift (fs). |
| p.A23SextW-12 | Extension. |
| p.A23[22] | Repetitive amino acid stretch, 22 repeats starting with the 23 amino acids. |
| p.[A23*;S33W] | Two variants on one molecule (cis). |
| p.[A23*];[S33W] | Two variants on two different molecules (trans). |
| p.[A23*(;)S33W] | Two variants with unknown phase. |
| p.(T23=) | No predicted consequence at protein level. |
| ASHA Continuing Education Course: “Gene Therapy: Current Promises and Future Challenges”. https://apps.asha.org/eweb/OLSDynamicPage.aspx?Webcode=olsdetails&title=Gene+Therapy%3A+Current+Promises+and+Future+Challenges (accessed on 20 March 2024) Note: audiovisual course that provides a modern and balanced perspective on gene therapy for students, clinicians, and educators. |
| ASHA Voices: “The Limits of Our Genes”. https://leader.pubs.asha.org/do/10.1044/2022-0324-podcast-gene-therapy-three/full/ (accessed on 20 March 2024). Note: Podcast that presents how genes work and what can be realistically expected from gene therapy. Appropriate for clinicians, their patients, students, and the public. |
| “Animal Models for Understanding Hearing Loss”. In Health and Hearing, pp. 44–78. 2024. https://doi.org/10.1142/9789811265006_0002 [8] Note: a modern treatise on the systems biology of human hearing loss, models of hearing loss (including genetic hearing loss), therapies for hearing loss and how to appraise hearing loss research. Appropriate for students, clinicians, researchers, and educators. |
| Hereditary Hearing Loss Homepage. https://hereditaryhearingloss.org/ (accessed on 20 March 2024) Note: website that provides an up-to-date overview of the genetics of hereditary hearing impairment for researchers and clinicians working in the field. |
| Online Mendelian Inheritance in Man® https://omim.org/ (accessed on 20 March 2024) Note: a comprehensive database of all known genetic disorders (including hearing loss). Both genotype and clinical phenotype is provided for each disorder. Appropriate for students, patients, concerned parents, clinicians, scientists, and educators. |
| HGVS Nomenclature. https://hgvs-nomenclature.org/stable/ (accessed on 20 March 2024) Note: the HGVS Nomenclature curates an Internationally Recognized standard for the description of DNA, RNA, and protein sequence variants that is used to convey variants in clinical reports and to share variants in publications and databases. Appropriate for clinicians, researchers, educators, authors, and students. |
| Deafness Variation Database. https://deafnessvariationdatabase.org/ (accessed on 20 March 2024) Note: a comprehensive guide to genetic variation in genes known to be associated with deafness. Appropriate for genetic researchers, students, and clinicians. |
| RefSeq: NCBI Reference Sequence Database. https://www.ncbi.nlm.nih.gov/refseq/ (accessed on 20 March 2024) Note: a comprehensive, integrated, non-redundant, well-annotated set of reference sequences including genomic, transcript, and protein. It is appropriate for students, researchers, authors, and educators. |
| The Human Protein Atlas. https://www.proteinatlas.org/ (accessed on 20 March 2024) Note: a comprehensive map of all human proteins in cells, tissues, and organs. It is appropriate for students, researchers, authors, and educators. |
| Study or News Report: | ||
|---|---|---|
| Claim: | ||
| Quality | Description | Quality Score (1 or 0) |
| Human Participants | Humans were treated with the therapy. | 1 |
| Randomization | The required number of human participants were selected randomly and then randomly assigned to groups, such as control groups (groups of participants who do not receive the therapy) and treated groups (groups of participants who receive the therapy). Therefore, each participant had an equal chance of being selected for the study and assigned to any group. | 1 |
| Double-blind | Neither the researchers nor the participants were aware of the type of treatment administered to the participants. Therefore, the researchers are not able to bias the results by selecting only patients who have certain attributes (such as conductive hearing loss, fluctuating hearing, etc.) that would interfere with the true effect of the therapy. | 1 |
| Control | A group or condition that served as a natural standard by not being exposed to the therapy, surgery, injections, drugs, and/or any experimental procedures. In general, a control group does not receive the therapy. | 1 |
| Placebo | A group or condition that served as an artificial standard by not being exposed to the real therapy but exposed to a fake therapy that is almost indistinguishable from the real therapy. Also, the placebo group or condition must be exposed to the same procedures (surgery, injections, drugs, etc.) as that of the real treatment group/condition. Therefore, the placebo is a condition that has no therapeutic value. | 1 |
| Dose–response | The therapeutic effect (e.g., restoration of hearing) systematically increases as the therapy increases. This means that as the dose or frequency of the treatment increases then the hearing loss should simultaneously decrease. | 1 |
| Meaningful Effect | The therapeutic effect (e.g., restoration of hearing) is large enough to be significant to the communication abilities of the participants in real-world social situations and to mandate changes in clinical management. This is important because changes that are statistically significant can be clinically useless. Therefore, patient outcomes from a given therapy must be meaningful to the patients and directly impact their day-to-day activities. | 1 |
| Safety | The therapy was safe for 100% of the participants, monitored over years. No gene therapy can be considered safe without several years of monitoring for cancer development, immunotoxicity and many more side-effects. | 1 |
| Confounds | The absence of other variables (drugs, surgery, etc.) that could drive a meaningful effect, instead of (or in addition to) the gene therapy. This means that the researchers were not able to bias the results of the clinical trial by including, drugs, procedures, or other therapies that would impact the hearing loss and lead to the false assumption that the therapy restored hearing. | 1 |
| Blinded Analyses | The analysis of the data was conducted by research personnel who were unaware of which data belonged to which group, condition, and placebo. This means that the researchers could not bias the data analysis to find positive effects of the therapy when there is no positive effect. | 1 |
| Strength-of-the-claim score (underline one) → Fair Mediocre Poor | ||
| To calculate strength-of-the-claim: (1st) Count the number of ones, then divide by 10, then multiply 100 = percent (%). (2nd) A strength-of-the-claim score of 100% means fair; 99–71% means mediocre; and ≤70 means poor. | ||
| Study or News Report: Lancet 2024, S0140-6736(23)02874-X. https://doi.org/10.1016/S0140-6736(23)02874-X [6]. | ||
| Claim: AAV1-hOTOF gene therapy recovered hearing in deaf children. | ||
| Quality | Description | Quality Score (1 or 0) |
| Human Participants | YES | 1 |
| Randomization | NO | 0 |
| Double-blind | NO | 0 |
| Control | NO | 0 |
| Placebo | NO | 0 |
| Dose–response | NO | 0 |
| Meaningful Effect | NO | 0 |
| Safety | NO | 0 |
| Confounds | NO | 0 |
| Blinded Analyses | NO | 0 |
| Strength-of-the-claim score (underline one) → Fair Mediocre Poor | ||
| To calculate strength-of-the-claim: (1st) Count the number of ones, then divide by 10, then multiply 100 = percent (%). (2nd) A strength-of-the-claim score of 100% means fair; 99–71% means mediocre; and ≤70 means poor. | ||
| Gene Therapies | Advantages | Disadvantages | Remarks |
|---|---|---|---|
| Routine gene therapy | Seeks to match pathogenic effects (amorphic, hypomorphic, hypermorphic, neomorphic, antimorphic, etc.) from the gene variant to gene therapy strategies (replacement, addition, suppression, editing, etc.) to achieve precision/personalized medicine. | Pathogenic effects that result from pathogenic variants are context dependent, such that the same variant is associated with different pathogenic effects (even normal effects) in different patients/organs/tissues/cells. Therefore, the pathogenic effect from each pathogenic variant must be verified within the target cells/tissues for each patient before selecting a gene therapy strategy, yet this is rarely if ever done and might be impossible in some situations. | All gene therapy technologies (whether gene or RNA based) will never consistently achieve their desired therapeutic outcomes because genes and RNA cannot faithfully control the functions of proteins (malfunctioning proteins are believed to cause genetic diseases so gene therapies seek to correct the proteins by correcting the genes or RNA). This is because a protein’s function is dependent on the environmental context in which the protein is staged. For example, a given protein (p53, ATM, XPC, RPA, XPA, XPF, XPG, etc.) can contribute to both cell death and cell survival depending on how it is post-translationally modified, whether it is localized to the nucleus vs. cytoplasm, the spatio-temporal specifics of its pulsing dynamics, what other proteins it interacts with, etc. Given the unique nature of gene therapies and the circumstances around patient treatment with such therapies, it is well-known that the Placebo Effect (improvement in a patient’s condition from a fake treatment) is particularly prominent in all successful gene therapy clinical trials yet gene-centric scientists, clinicians, and the media choose to overlook or not quantify the magnitude of the placebo effect. |
| CRISPR-Cas9 | Deliberately creates DNA damage within a patient’s genome to make permanent corrections to the patient’s genes. | Falsely assume that the patient’s DNA repair pathways will: (1) correctly repair the CRISPR-Cas9 induced DNA damage, (2) all target cells have the same DNA repair capacity/efficiency, (3) the consequences of failed DNA repair will not result in cancers, toxic immune responses and increased susceptibilities to future/other types of diseases. Yet, adjuvant DNA repair therapies are never used. | |
| RNA-, Base- and prime-editing | Uses engineered or natural enzymes to facilitate the editing of DNA or RNA. | All engineered and natural enzymes are vulnerable to Star-Activity (the enzyme doing things that we cannot predict) which can occur due to a slight change in temperature, pH, salt/metal concentration, the unit number of enzymes, and over 1000 additional variables. This means that any given enzymes will cut or make changes in areas where it is deleterious to do so and will perform differently in different cells/tissues/organs/patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guthrie, O.W. Gene Therapy: An Historical Overview for Familial Hearing Loss. Int. J. Mol. Sci. 2025, 26, 1469. https://doi.org/10.3390/ijms26041469
Guthrie OW. Gene Therapy: An Historical Overview for Familial Hearing Loss. International Journal of Molecular Sciences. 2025; 26(4):1469. https://doi.org/10.3390/ijms26041469
Chicago/Turabian StyleGuthrie, O’neil W. 2025. "Gene Therapy: An Historical Overview for Familial Hearing Loss" International Journal of Molecular Sciences 26, no. 4: 1469. https://doi.org/10.3390/ijms26041469
APA StyleGuthrie, O. W. (2025). Gene Therapy: An Historical Overview for Familial Hearing Loss. International Journal of Molecular Sciences, 26(4), 1469. https://doi.org/10.3390/ijms26041469





