Molecular Mechanism of Vine Tea Dihydromyricetin Extract on Alleviating Glucolipid Metabolism Disorder in db/db Mice: Based on Liver RNA-Seq and TLR4/MyD88/NF-κB Pathway
Abstract
1. Introduction
2. Results
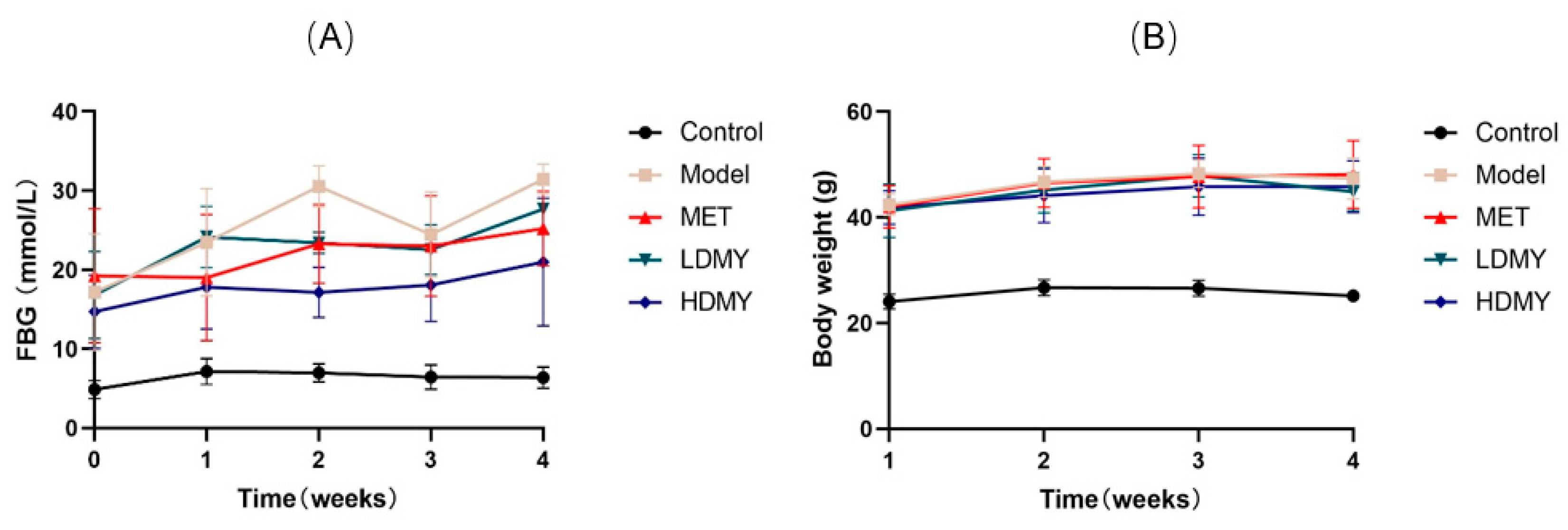
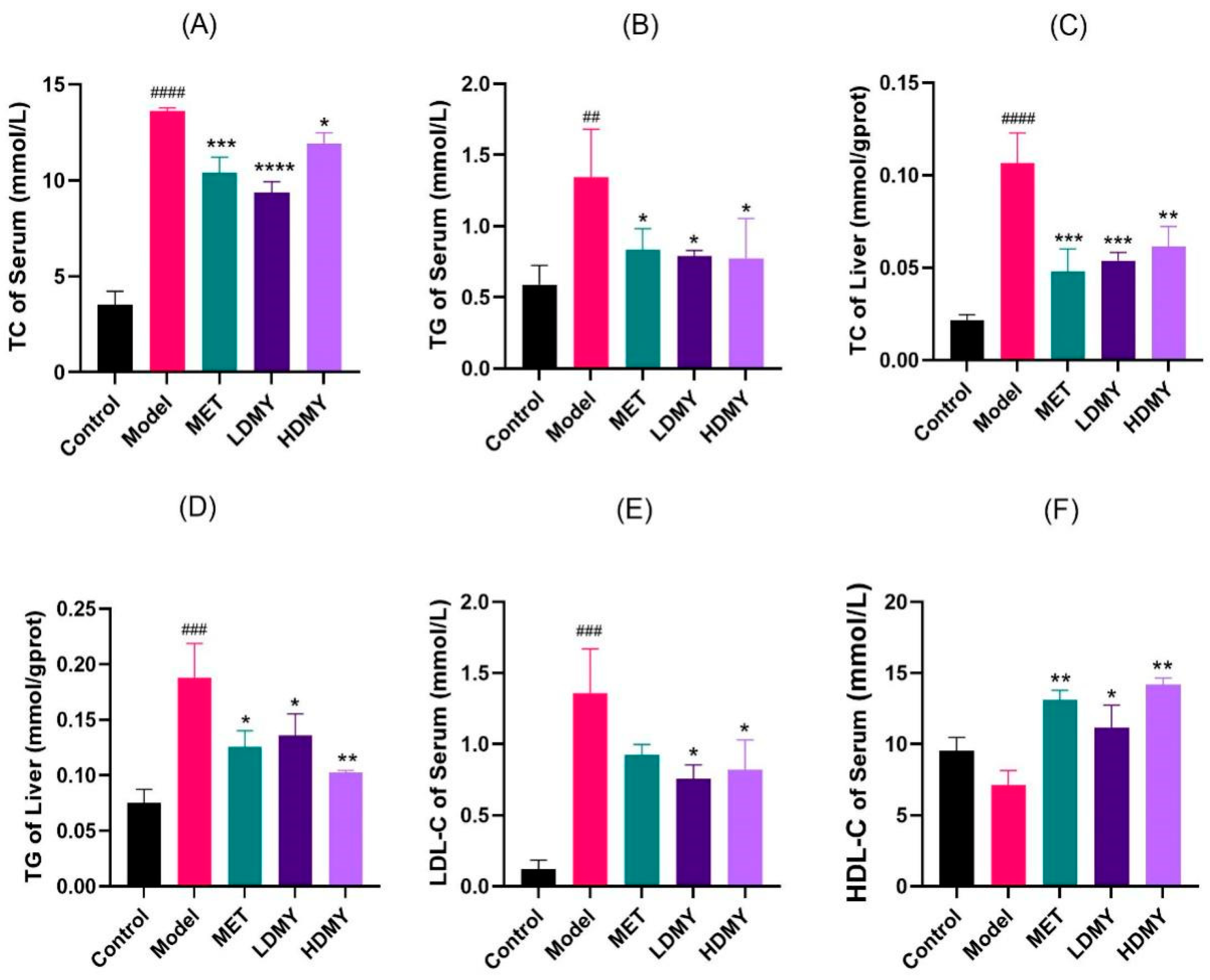
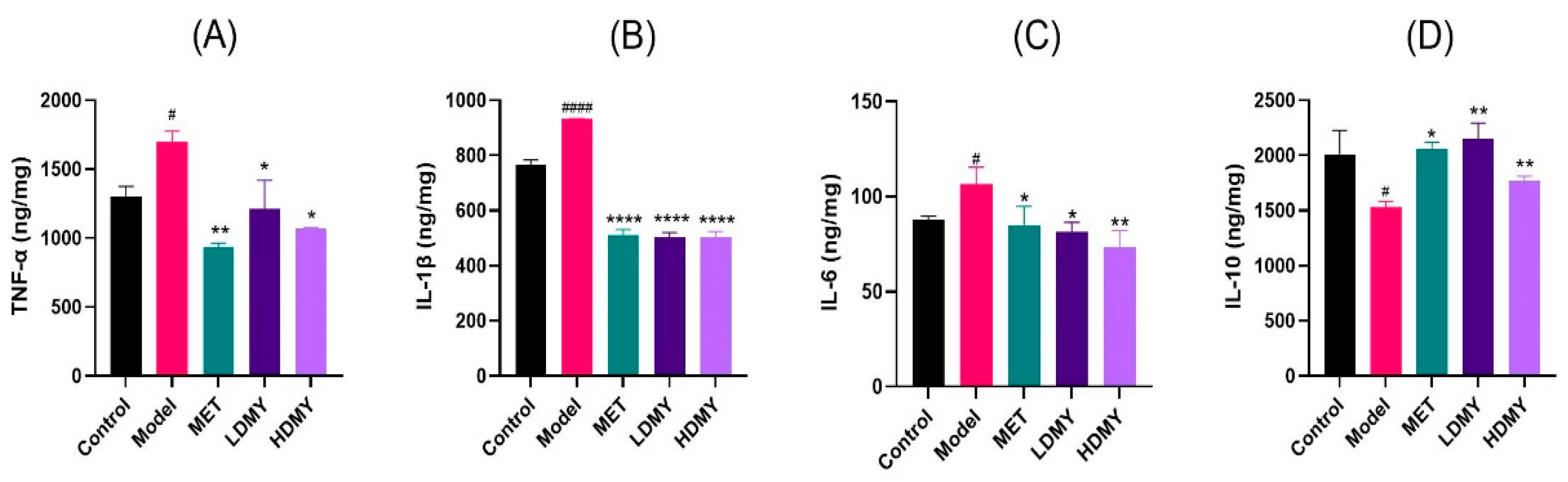
2.1. Effects of VDMY on Blood Biochemical Indicators in db/db Mice
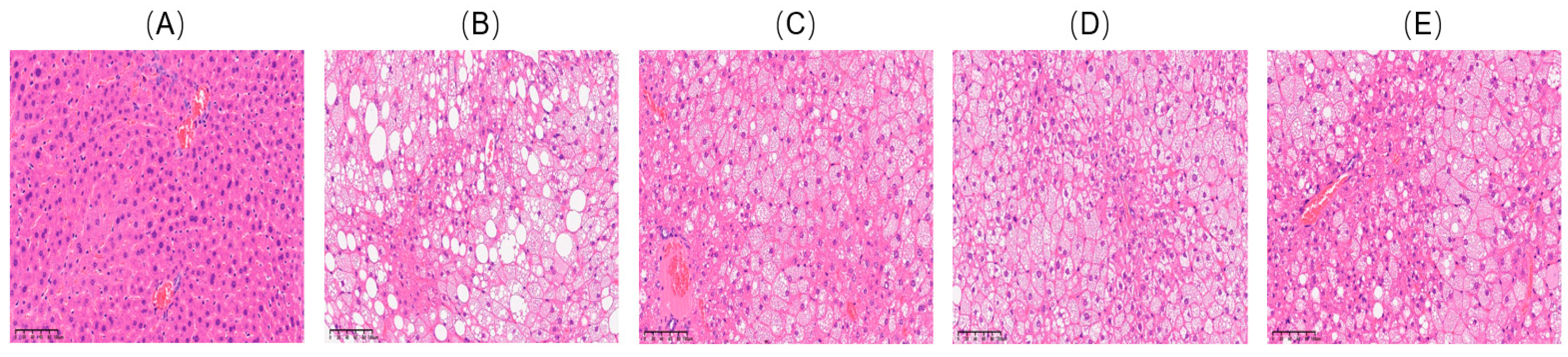
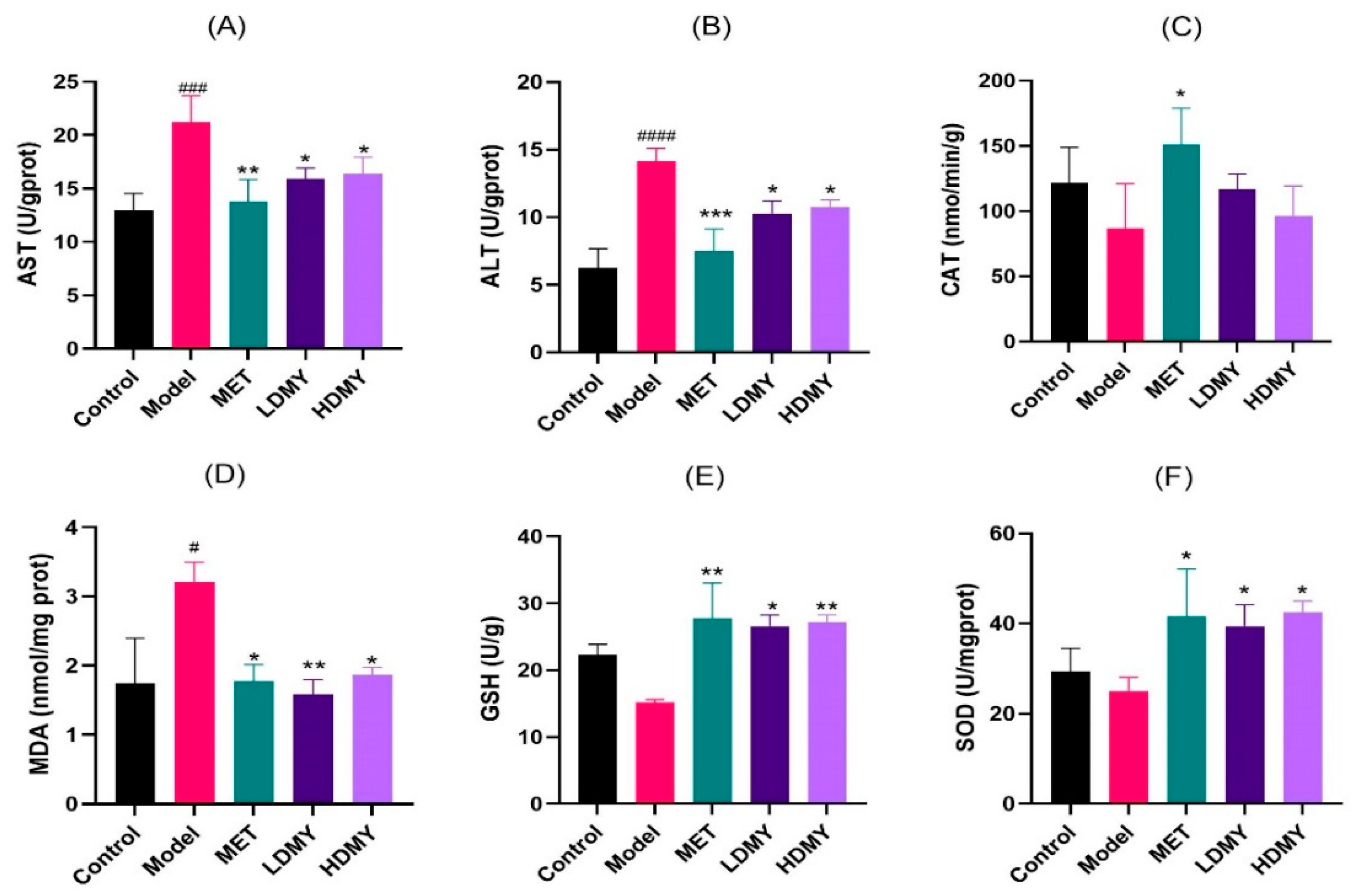
2.2. Effects of VDMY on Liver Injury and Oxidative Stress in db/db Mice
2.3. Effects of VDMY on the Liver Transcriptome of db/db Mice
2.3.1. Differential Gene Analysis
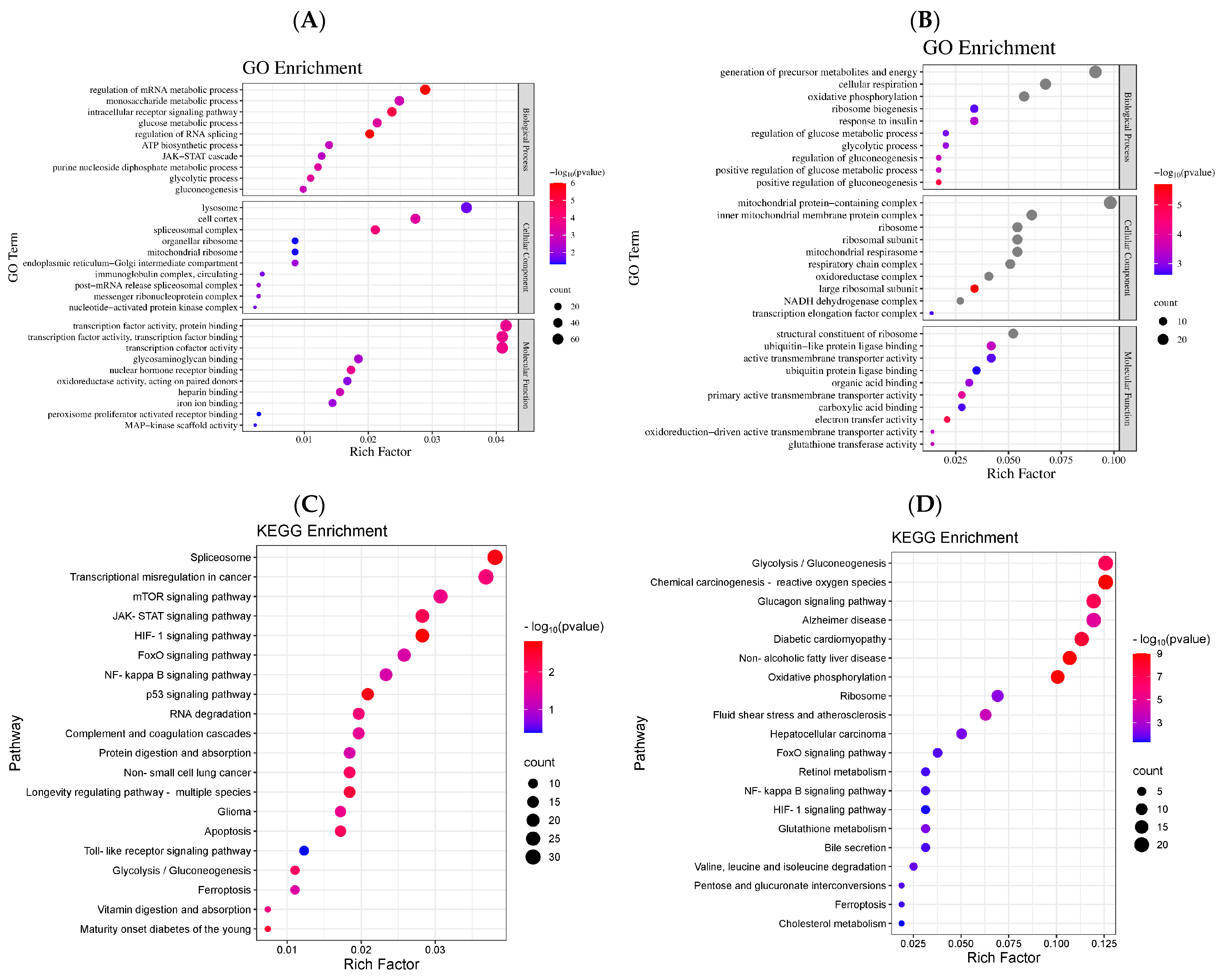
2.3.2. Differential Gene GO Enrichment Analysis
2.3.3. Differential Gene KEGG Pathway Enrichment Analysis
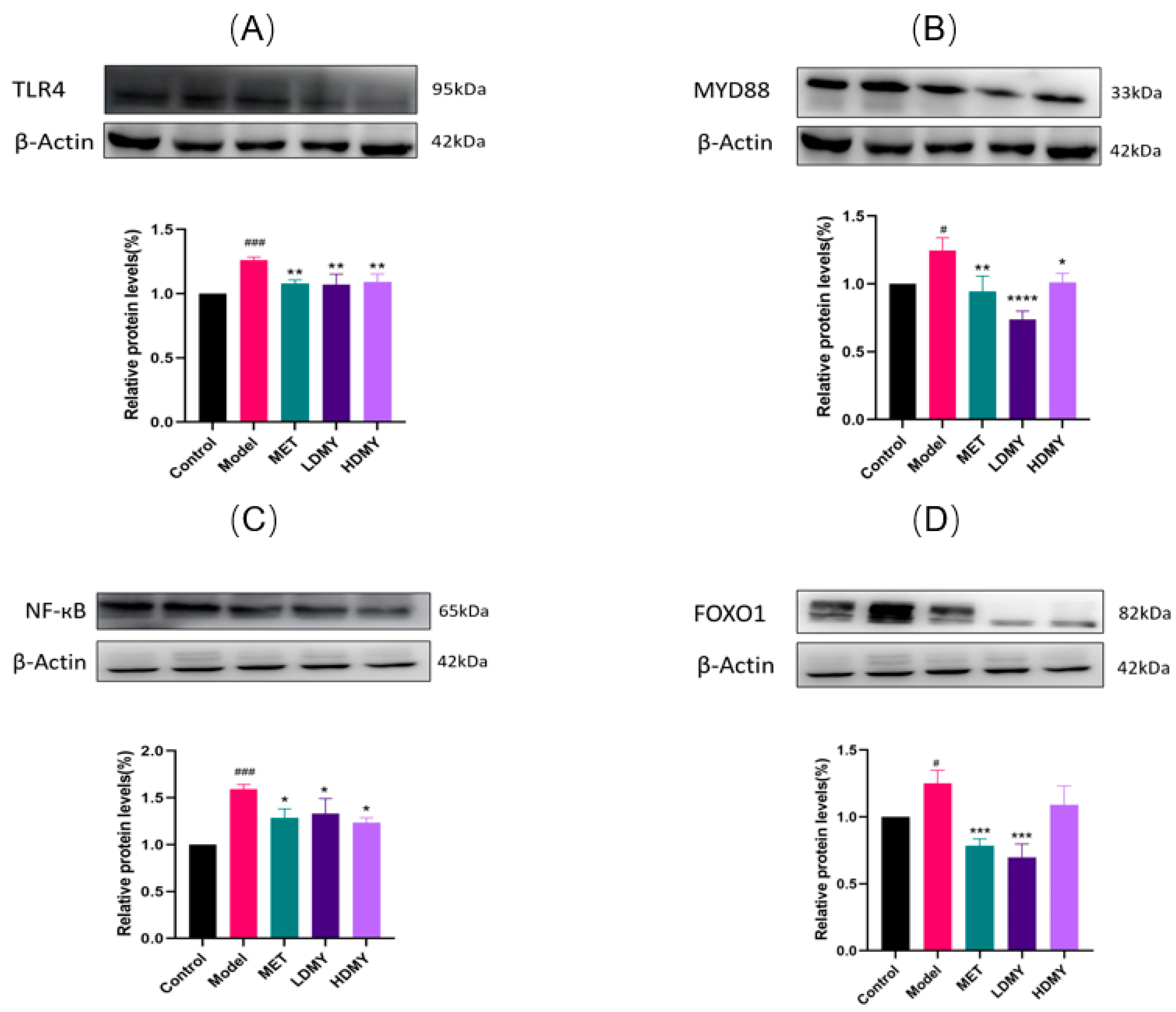
2.4. Effects of VDMY on the TLR4/MyD88/NF-κB and FOXO1 Signaling Pathways in db/db Mice Liver
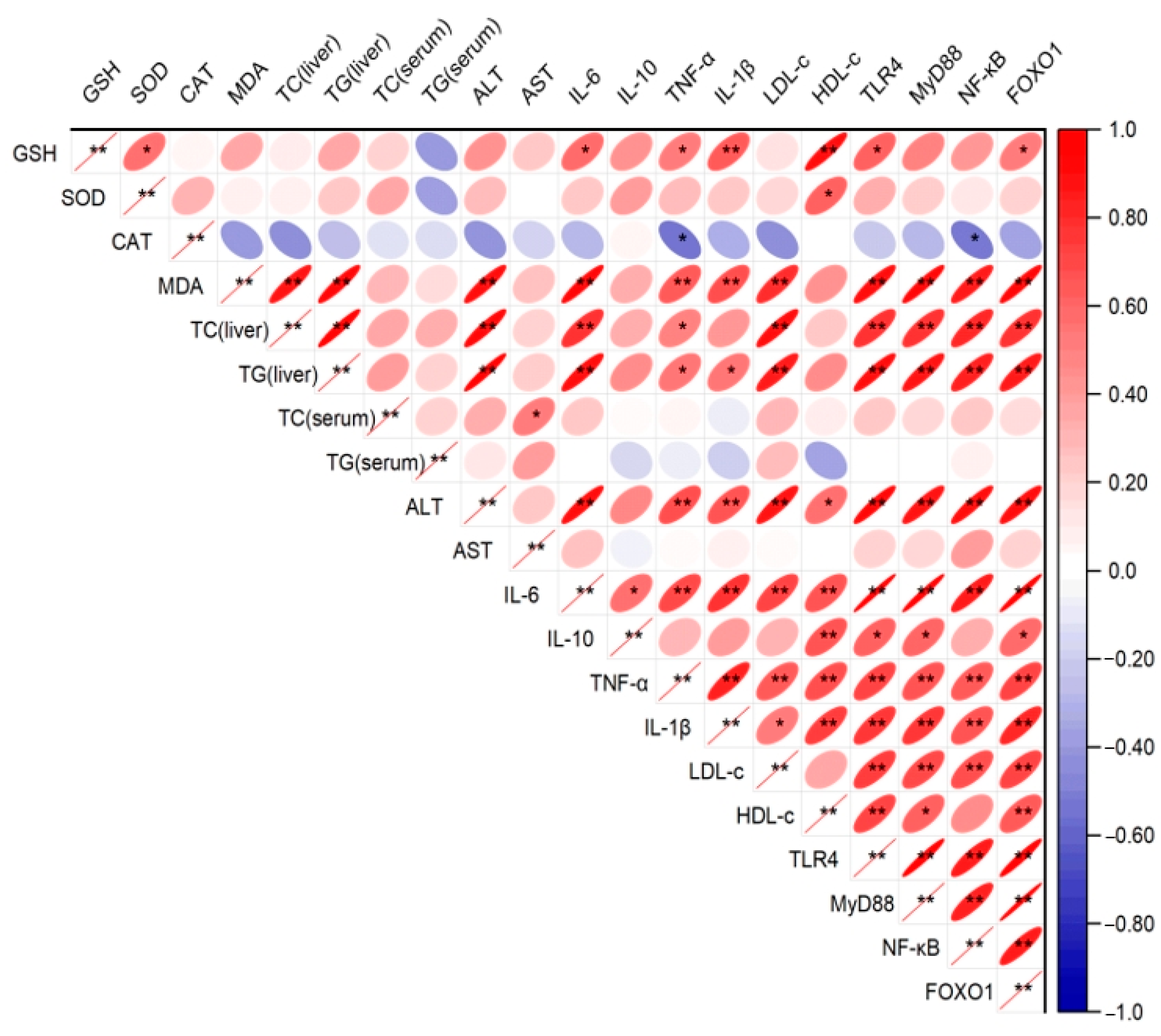
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animal Models
4.3. Serum and Liver Biochemical Indicators
4.4. Organizational Pathology Analysis
4.5. Liver RNA Sequencing
4.6. GO Enrichment and KEGG Pathway Analysis
4.7. Western Blotting
4.8. Correlation Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Zhang, Z.; Wang, S.; Cai, J.; Guo, J. Hypothalamus-pituitary-adrenal Axis in Glucolipid metabolic disorders. Rev. Endocr. Metab. Disord. 2020, 21, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Feng, Y.; Peng, M.; Cai, J. A narrative review on the mechanism of natural flavonoids in improving glucolipid metabolism disorders. Phytother. Res. 2024, 38, 4202–4229. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, S.; Shi, C.; Wang, S.; Wang, X.; Lü, X. A review on the potential use of natural products in overweight and obesity. Phytother. Res. 2022, 36, 1990–2015. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Liu, X.; Chen, S.; Yi, Y.; Wen, X.; Li, T.; Qin, S. Extract of Gardenia jasminoides Ellis Attenuates High-Fat Diet-Induced Glycolipid Metabolism Disorder in Rats by Targeting Gut Microbiota and TLR4/Myd88/NF-κB Pathway. Antioxidants 2024, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Zhang, F.; Liu, F.; Zhao, J.; Huang, X.; Luo, D.; Guo, J. Metaflammation in glucolipid metabolic disorders: Pathogenesis and treatment. Biomed. Pharmacother. 2023, 161, 114545. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Spelleken, M.; Röhrig, K.; Hauner, H.; Eckel, J. Tumor necrosis factor-alpha acutely inhibits insulin signaling in human adipocytes: Implication of the p80 tumor necrosis factor receptor. Diabetes 1998, 47, 515–522. [Google Scholar] [CrossRef]
- Sun, S.Y.; Yang, W.Y.; Tan, Z.; Zhang, X.Y.; Shen, Y.L.; Guo, Q.W.; Su, G.M.; Chen, X.; Lin, J.; Fang, D.Z. Serum Levels of Free Fatty Acids in Obese Mice and Their Associations with Routine Lipid Profiles. Diabetes, Metab. Syndr. Obes. Targets Ther. 2022, 15, 331–343. [Google Scholar] [CrossRef]
- Zielinska-Blizniewska, H.; Sitarek, P.; Merecz-Sadowska, A.; Malinowska, K.; Zajdel, K.; Jablonska, M.; Sliwinski, T.; Zajdel, R. Plant Extracts and Reactive Oxygen Species as Two Counteracting Agents with Anti- and Pro-Obesity Properties. Int. J. Mol. Sci. 2019, 20, 4556. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-kappaκB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation[J/OL]. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D.-X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis grossedentata. Antioxidants 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Song, Y.; Zeng, C.; Zhang, H.; Lv, C.; Shi, M.; Qin, S. Molecular Mechanism Underlying the Regulatory Effect of Vine Tea on Metabolic Syndrome by Targeting Redox Balance and Gut Microbiota. Front. Nutr. 2022, 9, 802015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, G.; Tian, M.; Pu, Q.; Qin, M. Optimization of the Ultrasonic-Assisted Extraction of Bioactive Flavonoids from Ampelopsis grossedentata and Subsequent Separation and Purification of Two Flavonoid Aglycones by High-Speed Counter-Current Chromatography. Molecules 2016, 21, 1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, L.; Sun, D.; Zhang, S.; Zhu, Y.; Xu, P. Supercritical carbon dioxide extraction of bioactive compounds from Ampelopsis grossedentata stems: Process optimization and antioxidant activity. Int. J. Mol. Sci. 2011, 12, 6856–6870. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; He, K.; Li, T.; Cui, S.; Tang, M.; Kang, S.; Ma, W.; Song, L. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci. Rep. 2020, 10, 21416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, S.; Xu, Y.; Yao, A.; Liao, Q.; Han, L.; Zou, Z.; Zhang, X. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017, 38, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, Y.; Yin, Z.; Zhang, Q. Physicochemical properties of dihydromyricetin and the effects of ascorbic acid on its stability and bioavailability. J. Sci. Food Agric. 2020, 101, 3862–3869. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Zeng, Y.; Tang, K.; Chen, X.; Zhang, W.; Le Xu, X. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis 2017, 262, 39–50. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, J.; Dong, L.; Dang, X.; Wang, L.; Cheng, L.; Huang, Y. Dihydromyricetin Attenuates Metabolic Syndrome And Improves Insulin Sensitivity By Upregulating Insulin Receptor Substrate-1 (Y612) Tyrosine Phosphorylation In db/db Mice. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, P.; Liu, S.; Sun, Y.; Chen, C.; Long, J.; Lin, Y.; Liang, J.; Wang, H.; Zhang, L.; et al. Dihydromyricetin treats pulmonary hypertension by modulating CKLF1/CCR5 axis-induced pulmonary vascular cell pyroptosis. Biomed. Pharmacother. 2024, 180, 117614. [Google Scholar] [CrossRef]
- Ling, H.; Zhu, Z.; Yang, J.; He, J.; Yang, S.; Wu, D.; Feng, S.; Liao, D. Dihydromyricetin improves type 2 diabetes-induced cognitive impairment via suppressing oxidative stress and enhancing brain-derived neurotrophic factor-mediated neuroprotection in mice. Acta Biochim. Biophys. Sin. 2018, 50, 298–306. [Google Scholar] [CrossRef]
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808. [Google Scholar] [CrossRef]
- Silva, J.; Yu, X.; Moradian, R.; Folk, C.; Spatz, M.H.; Kim, P.; Bhatti, A.A.; Davies, D.L.; Liang, J. Dihydromyricetin Protects the Liver via Changes in Lipid Metabolism and Enhanced Ethanol Metabolism. Alcohol. Clin. Exp. Res. 2020, 44, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Spatz, M.H.; Folk, C.; Chang, A.; Cadenas, E.; Liang, J.; Davies, D.L. Dihydromyricetin improves mitochondrial outcomes in the liver of alcohol-fed mice via the AMPK/Sirt-1/PGC-1α signaling axis. Alcohol 2020, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Zhang, H.; Zeng, T. Dihydromyricetin inhibits oxidative stress and apoptosis in oxygen and glucose deprivation/reoxygenation-induced HT22 cells by activating the Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 23, 397. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid. Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; He, X.; Chen, K.; Sakao, K.; Hou, D.-X. Ameliorative effects and molecular mechanisms of vine tea on western diet-induced NAFLD. Food Funct. 2020, 11, 5976–5991. [Google Scholar] [CrossRef] [PubMed]
- Hinder, L.M.; Vivekanandan-Giri, A.; McLean, L.L.; Pennathur, S.; Feldman, E.L. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J. Endocrinol. 2012, 216, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lv, Q.; Yi, F.; Song, Y.; Le, L.; Jiang, B.; Xu, L.; Xiao, P. Dietary Supplementation of Vine Tea Ameliorates Glucose and Lipid Metabolic Disorder via Akt Signaling Pathway in Diabetic Rats. Molecules 2019, 24, 1866. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, M.; Lang, H.; Zhou, Y.; Mi, M. Dihydromyricetin enhances glucose uptake by inhibition of MEK/ERK pathway and consequent down-regulation of phosphorylation of PPARγ in 3T3-L1 cells. J. Cell. Mol. Med. 2018, 22, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, T.; Liang, X.; Hu, Q.; Huang, J.; Zhou, Y.; Chen, M.; Zhang, Q.; Zhu, J.; Mi, M. Dihydromyricetin improves skeletal muscle insulin resistance by inducing autophagy via the AMPK signaling pathway. Mol. Cell. Endocrinol. 2015, 409, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.M.; Ahmed, A.-S.F.; El-Daly, M.; Amin, A.H.; El-Tahawy, N.F.; Wagdy, A.; Hollenberg, M.D.; Taye, A. Dihydromyricetin protects against high glucose-induced endothelial dysfunction: Role of HIF-1α/ROR2/NF-κB. Biomed. Pharmacother. 2022, 153, 113308. [Google Scholar] [CrossRef] [PubMed]
- Sunil, B.; Ashraf, A.P. Dyslipidemia in Pediatric Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2020, 20, 53. [Google Scholar] [CrossRef]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free. Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Dimova, M.; Tugai, A.; Tugai, T.; Iutynska, G.; Dordevic, D.; Kushkevych, I. Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact. Int. J. Mol. Sci. 2022, 23, 11415. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Gaddam, N.; Chandler, V.; Chakraborty, S. Oxidative Stress–Induced Liver Damage and Remodeling of the Liver Vasculature. Am. J. Pathol. 2023, 193, 1400–1414. [Google Scholar] [CrossRef]
- Wu, B.; Lin, J.; Luo, J.; Han, D.; Fan, M.; Guo, T.; Tao, L.; Yuan, M.; Yi, F. Dihydromyricetin Protects against Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Mice. BioMed Res. Int. 2017, 2017, 3764370. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, F.; Huang, B.; Zheng, W.; Jiang, Y.; Cai, G.; Liu, D.; Hu, C.Y.; Wang, C. Dihydromyricetin Ameliorates Inflammation-Induced Insulin Resistance via Phospholipase C-CaMKK-AMPK Signal Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 8542809. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Shen, Y.; Ge, H.; Xiong, W.; He, L.; Liu, L.; Yin, C.; Wei, X.; Gao, Y. Dihydromyricetin Alleviates Diabetic Neuropathic Pain and Depression Comorbidity Symptoms by Inhibiting P2X7 Receptor. Front. Psychiatry 2019, 10, 770. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, Y.; Lang, H.; Wang, X.; Chen, K.; Gong, X.; Zhou, M.; Ran, L.; Zhu, J.; Mi, M. Dihydromyricetin prevents obesity-induced slow-twitch-fiber reduction partially via FLCN/FNIP1/AMPK pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wan, J.; Lang, H.; Si, M.; Zhu, J.; Zhou, Y.; Mi, M. Dihydromyricetin delays the onset of hyperglycemia and ameliorates insulin resistance without excessive weight gain in Zucker diabetic fatty rats. Mol. Cell. Endocrinol. 2017, 439, 105–115. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.L.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 2016, 5, 1438. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2014, 72, 557–581. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Liu, X.; Yi, Y.; Chen, S.; Zhang, Y.; Fan, W.; Lv, C.; Qin, S. Molecular Mechanism of Vine Tea Dihydromyricetin Extract on Alleviating Glucolipid Metabolism Disorder in db/db Mice: Based on Liver RNA-Seq and TLR4/MyD88/NF-κB Pathway. Int. J. Mol. Sci. 2025, 26, 2169. https://doi.org/10.3390/ijms26052169
Zhou X, Liu X, Yi Y, Chen S, Zhang Y, Fan W, Lv C, Qin S. Molecular Mechanism of Vine Tea Dihydromyricetin Extract on Alleviating Glucolipid Metabolism Disorder in db/db Mice: Based on Liver RNA-Seq and TLR4/MyD88/NF-κB Pathway. International Journal of Molecular Sciences. 2025; 26(5):2169. https://doi.org/10.3390/ijms26052169
Chicago/Turabian StyleZhou, Xixin, Xin Liu, Yuhang Yi, Shiyun Chen, Yi Zhang, Wei Fan, Chenghao Lv, and Si Qin. 2025. "Molecular Mechanism of Vine Tea Dihydromyricetin Extract on Alleviating Glucolipid Metabolism Disorder in db/db Mice: Based on Liver RNA-Seq and TLR4/MyD88/NF-κB Pathway" International Journal of Molecular Sciences 26, no. 5: 2169. https://doi.org/10.3390/ijms26052169
APA StyleZhou, X., Liu, X., Yi, Y., Chen, S., Zhang, Y., Fan, W., Lv, C., & Qin, S. (2025). Molecular Mechanism of Vine Tea Dihydromyricetin Extract on Alleviating Glucolipid Metabolism Disorder in db/db Mice: Based on Liver RNA-Seq and TLR4/MyD88/NF-κB Pathway. International Journal of Molecular Sciences, 26(5), 2169. https://doi.org/10.3390/ijms26052169









