Combined Therapy Targeting MET and Pro-HGF Activation Shows Significant Therapeutic Effect Against Liver Metastasis of CRPC
Abstract
1. Introduction
2. Results
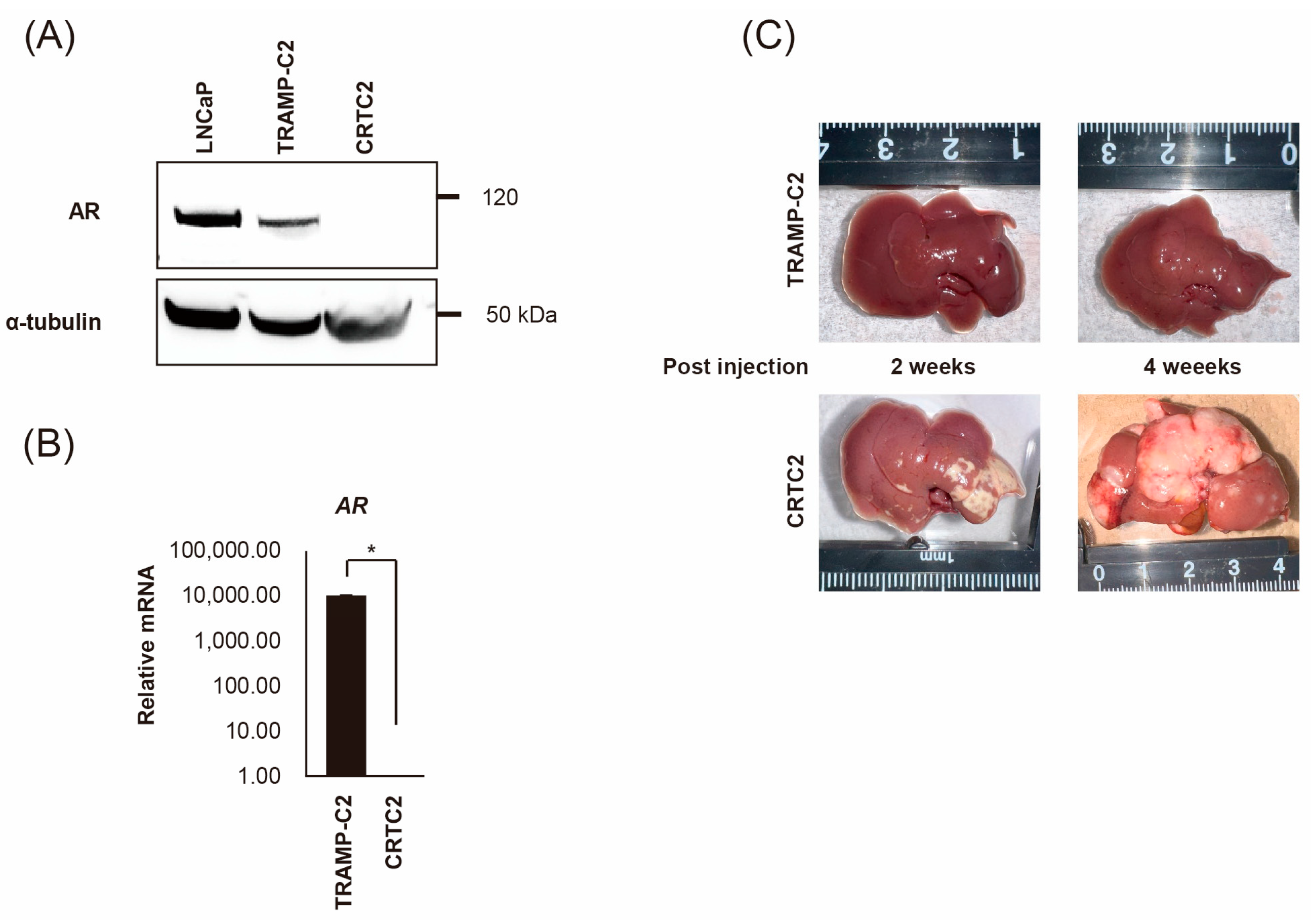
2.1. Establishment of Mouse CRPC Cell Line (CRTC2) and Mouse Liver Metastasis Model
2.2. Comparison of mRNA Expression Between Non-Metastatic and Metastatic CRTC2
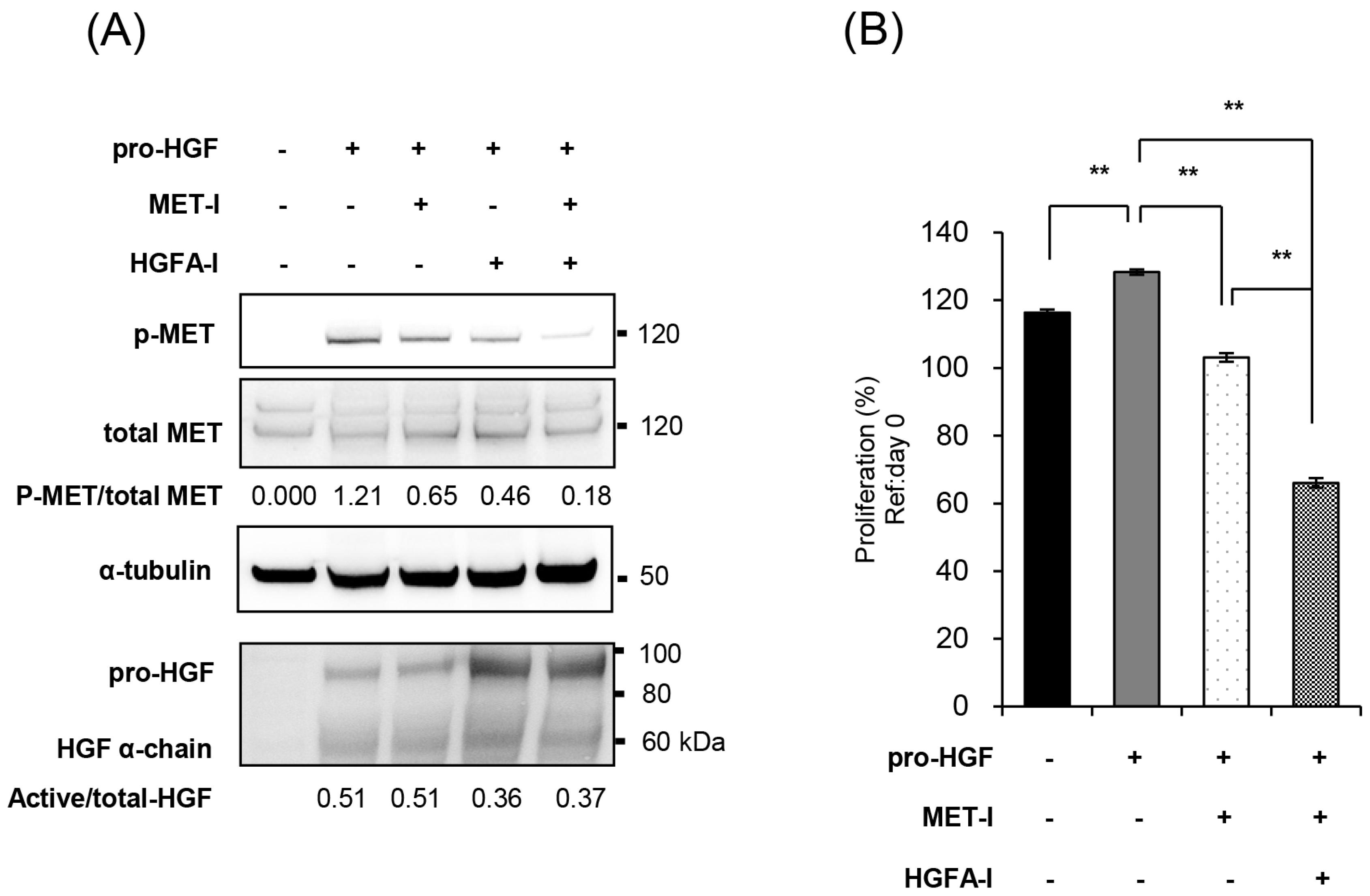
2.3. Inhibition of MET and HGF-Activation in CRTC2 Cells, In Vitro Analysis
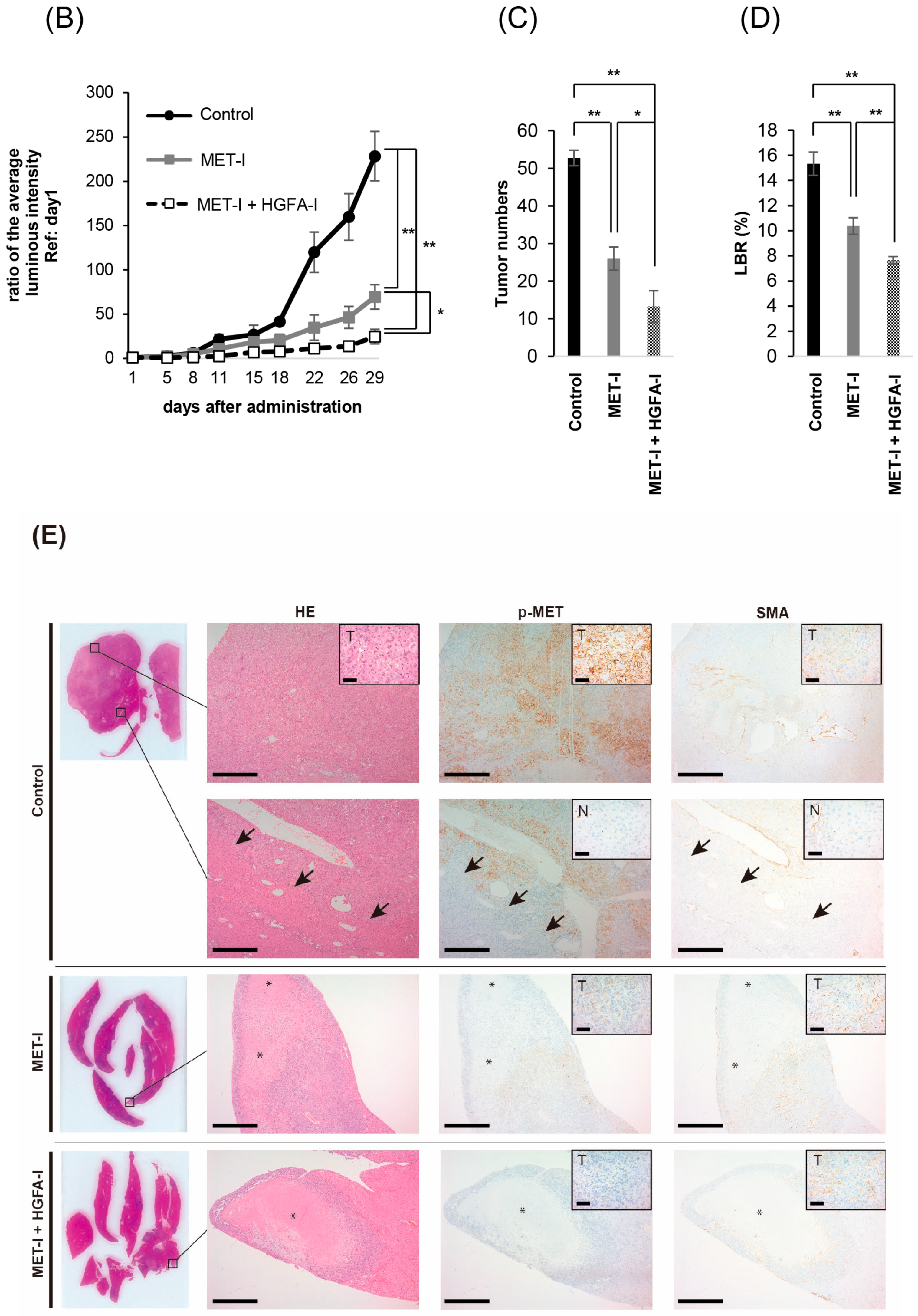
2.4. Effect of Inhibition of MET and HGF-Activation in Liver Metastasis In Vivo
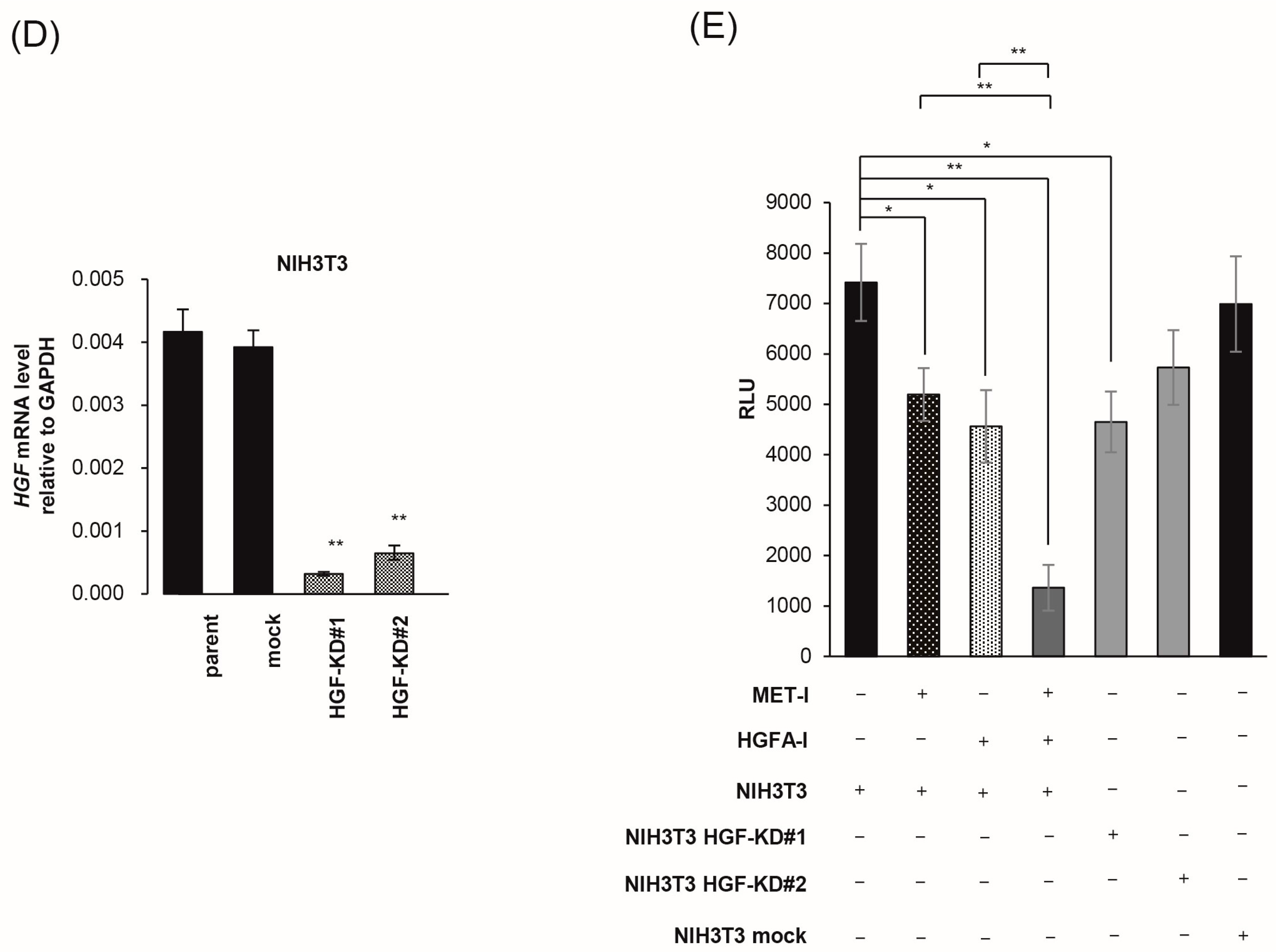
2.5. Inhibition of Fibroblast-Derived HGF: Analysis by Co-Culture with Mouse Fibroblast Cell, NIH3T3
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Establishment of Mouse CRPC Cell Line (CRTC2)
4.3. Real-Time Quantitative PCR (RT-qPCR)
4.4. Microarray Analysis of Expression Molecules in CRTC2 and the Liver Metastasis
4.5. Antibodies and Reagents
4.6. Protein Extraction and Immunoblot Analysis
4.7. Proliferation Assay of Dual Inhibition of MET and HGF-Activation for CRTC2 Cells
4.8. Transfection
4.9. Co-Culture
4.10. Animal Experiments
4.11. Immunohistochemistry
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRPC | Castration-resistant prostate cancer |
| HGF | Hepatocyte growth factor |
| HGFAC | Hepatocyte growth factor activator coding gene |
| HPN | Hepsin coding gene |
| MET | HGF receptor |
| CAF | Cancer associated fibroblast |
| TKI | Tyrosine kinase inhibitor |
| NSCLC | Non-small cell lung cancer |
| CCA | Cholangiocarcinoma |
| SMA | Smooth muscle actin |
References
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Shimada, T.; Kano, H.; Kadomoto, S.; Makino, T.; Naito, R.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Androgen receptor signaling-targeted therapy and taxane chemotherapy induce visceral metastasis in castration-resistant prostate cancer. Prostate 2021, 81, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cetin, K.; Beebe-Dimmer, J.L.; Fryzek, J.P.; Markus, R.; Carducci, M.A. Recent time trends in the epidemiology of stage IV prostate cancer in the United States: Analysis of data from the Surveillance, Epidemiology, and End Results Program. Urology 2010, 75, 1396–1404. [Google Scholar] [CrossRef][Green Version]
- Fujimoto, H.; Nakanishi, H.; Miki, T.; Kubota, Y.; Takahashi, S.; Suzuki, K.; Kanayama, H.; Mikami, K.; Homma, Y. Oncological outcomes of the prostate cancer patients registered in 2004: Report from the Cancer Registration Committee of the JUA. Int. J. Urol. 2011, 18, 876–881. [Google Scholar] [CrossRef]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Shirotake, S.; Umezawa, Y.; Okabe, T.; Kaneko, G.; Kanao, K.; Nishimoto, K.; Oyama, M. A case of castration-resistant prostate cancer with liver metastases achieved a complete response by docetaxel chemotherapy. Transl. Androl. Urol. 2020, 9, 819–823. [Google Scholar] [CrossRef]
- Bray, A.W.; Duan, R.; Malalur, P.; Drusbosky, L.M.; Gourdin, T.S.; Hill, E.G.; Lilly, M.B. Elevated serum CEA is associated with liver metastasis and distinctive circulating tumor DNA alterations in patients with castration-resistant prostate cancer. Prostate 2022, 82, 1264–1272. [Google Scholar] [CrossRef]
- Mukai, S.; Yamasaki, K.; Fujii, M.; Nagai, T.; Terada, N.; Kataoka, H.; Kamoto, T. Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. Int. J. Mol. Sci. 2020, 21, 2663. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kataoka, H. Mechanisms of hepatocyte growth factor activation in cancer tissues. Cancers 2014, 6, 1890–1904. [Google Scholar] [CrossRef]
- Kataoka, H.; Kawaguchi, M.; Fukushima, T.; Shimomura, T. Hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2): Emerging key players in epithelial integrity and cancer. Pathol. Int. 2018, 68, 145–158. [Google Scholar] [CrossRef]
- Owusu, B.Y.; Thomas, S.; Venukadasula, P.; Han, Z.; Janetka, J.W.; Galemmo Jr, R.A.; Klampfer, L. Targeting the tumor-promoting microenvironment in MET-amplified NSCLC cells with a novel inhibitor of pro-HGF activation. Oncotarget 2017, 8, 63014–63025. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Giovannetti, E.; Peters, G.J.; Firuzi, O. Combination of HGF/MET-targeting agents and other therapeutic strategies in cancer. Crit. Rev. Oncol. Hematol. 2021, 160, 103234. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Mizuuchi, H.; Maehara, Y.; Mitsudomi, T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation—Diversity, ductility, and destiny. Cancer Metastasis Rev. 2012, 31, 807–814. [Google Scholar] [CrossRef]
- Owusu, B.Y.; Galemmo, R.; Janetka, J.; Klampfer, L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment. Cancers 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, S.; Cazzanti, M.; Bertotti, A.; Rideout, W.M., 3rd; Han, M.; Gyuris, J.; Perera, T.; Comoglio, P.M.; Trusolino, L.; Michieli, P. Microenvironment-derived HGF overcomes genetically determined sensitivity to anti-MET drugs. Cancer Res. 2014, 74, 6598–6609. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Ilyas, S.I.; Affo, S.; Goyal, L.; Lamarca, A.; Sapisochi, G.; Yang, J.D.; Gores, G.J. Cholangiocarcinoma—Novel biological insights and therapeutic strategies. Nat. Rev. Clin. Oncol. 2023, 20, 470–486. [Google Scholar] [CrossRef]
- Affo, S.; Filliol, A.; Gores, G.J.; Schwabe, R.F. Fibroblasts in liver cancer: Functions and therapeutic translation. Lancet Gastroenterol. Hepatol. 2023, 8, 748–759. [Google Scholar] [CrossRef]
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.K.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882. [Google Scholar] [CrossRef]
- Jeet, V.; Ow, K.; Doherty, E.; Curley, B.; Russell, P.J.; Khatri, A. Broadening of transgenic adenocarcinoma of the mouse prostate (TRAMP) model to represent late stage androgen depletion independent cancer. Prostate 2008, 68, 548–562. [Google Scholar] [CrossRef]
- Simons, B.W.; Dalrymple, S.; Rosen, M.; Zheng, L.; Brennen, W.N. A hemi-spleen injection model of liver metastasis for prostate cancer. Prostate 2020, 80, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancerassociated fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Janetka, W.J.; Benson, M.R. Extracellular Targeting of Cell Signaling in Cancer; Strategies Directed at MET and RON Receptor Tyrosine Kinase Pathways; Wiley: Hoboken, NJ, USA, 2018; pp. 1–154. [Google Scholar]
- Knudsen, B.S.; Gmyrek, G.A.; Inra, J.; Scherr, D.S.; Vaughan, E.D.; Nanus, D.M.; Kattan, M.W.; Gerald, W.L.; Vande Woude, G.F. High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002, 60, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, E.I.; Kolijn, K.; De Herdt, M.J.; van der Steen, B.; Hoogland, A.M.; Sleddens, H.F.; Looijenga, L.H.; van Leenders, G.J. MET expression during prostate cancer progression. Oncotarget 2016, 7, 31029–31036. [Google Scholar] [CrossRef]
- Nakashiro, K.; Hara, S.; Shinohara, Y.; Oyasu, M.; Kawamata, H.; Shintani, S.; Hamakawa, H.; Oyasu, R. Phenotypic switch from paracrine to autocrine role of hepatocyte growth factor in an androgen-independent human prostatic carcinoma cell line, CWR22R. Am. J. Pathol. 2004, 165, 533–540. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Chowdhury, S.; McGregor, B.; Merseburger, A.S.; Oudard, S.; Saad, F.; Soares, A.; Benzaghou, F.; et al. A phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer. Future Oncol. 2022, 18, 1185–1198. [Google Scholar] [CrossRef]
- Sugie, S.; Mukai, S.; Yamasaki, K.; Kamibeppu, T.; Tsukino, H.; Kamoto, T. Plasma macrophage-stimulating protein and hepatocyte growth factor levels are associated with prostate cancer progression. Hum. Cell 2016, 29, 22–29. [Google Scholar] [CrossRef]
- Nagakawa, O.; Yamagishi, T.; Fujiuchi, Y.; Junicho, A.; Akashi, T.; Nagaike, K.; Fuse, H. Serum hepatocyte growth factor activator (HGFA) in benign prostatic hyperplasia and prostate cancer. Eur. Urol. 2005, 48, 686–690. [Google Scholar] [CrossRef]
- Yao, J.F.; Li, X.J.; Yan, L.K.; He, S.; Zheng, J.B.; Wang, X.R.; Zhou, P.H.; Zhang, L.; Wei, G.B.; Sun, X.J. Role of HGF/c-Met in the treatment of colorectal cancer with liver metastasis. J. Biochem. Mol. Toxicol. 2019, 33, e22316. [Google Scholar] [CrossRef]
- Amemiya, H.; Kono, K.; Itakura, J.; Tang, R.F.; Takahashi, A.; An, F.Q.; Kamei, S.; Iizuka, H.; Fujii, H.; Matsumoto, Y. c-Met expression in gastric cancer with liver metastasis. Oncology 2002, 63, 286–296. [Google Scholar] [CrossRef]
- Elliott, V.A.; Rychahou, P.; Zaytseva, Y.Y.; Evers, B.M. Activation of c-Met and upregulation of CD44 expression are associated with the metastatic phenotype in the colorectal cancer liver metastasis model. PLoS ONE 2014, 9, e97432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Matsumoto, K.; Taniura, N.; Tomioka, D.; Nakamura, T. Hepatic gene expression of NK4, an HGF-antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther. 2004, 11, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Owusu, B.Y.; Bansal, N.; Venukadasula, P.K.; Ross, L.J.; Messick, T.E.; Goel, S.; Galemmo, R.A.; Klampfer, L. Inhibition of pro-HGF activation by SRI31215, a novel approach to block oncogenic HGF/MET signaling. Oncotarget 2016, 7, 29492–29506. [Google Scholar] [CrossRef]
- Basilico, C.; Modica, C.; Maione, F.; Vigna, E.; Comoglio, P.M. Targeting the MET oncogene by concomitant inhibition of receptor and ligand via an antibody-“decoy” strategy. Int. J. Cancer 2018, 143, 1774–1785. [Google Scholar] [CrossRef]
- So, S.; Park, Y.; Kang, S.S.; Han, J.; Sunwoo, J.H.; Lee, W.; Kim, J.; Ye, E.A.; Kim, J.Y.; Tchah, H.; et al. Therapeutic Potency of Induced Pluripotent Stem-Cell-Derived Corneal Endothelial-like Cells for Corneal Endothelial Dysfunction. Int. J. Mol. Sci. 2022, 24, 701. [Google Scholar] [CrossRef]
- Venukadasula, P.K.M.O.B.; Bansal, N.; Ross, L.J.; Hobrath, J.V.; Bao, D.; Truss, J.W.; Stackhouse, M.; Messick, T.E.; Klampfer, L.; Galemmo, R.A. Design and Synthesis of Nonpeptide Inhibitors of Hepatocyte Growth Factor Activation. ACS Med. Chem. Lett. 2016, 7, 177–181. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, S.; Iwano, S.; Akioka, T.; Kuchimaru, T.; Kawaguchi, M.; Fukushima, T.; Sato, Y.; Kataoka, H.; Kamoto, T.; Mukai, S.; et al. Combined Therapy Targeting MET and Pro-HGF Activation Shows Significant Therapeutic Effect Against Liver Metastasis of CRPC. Int. J. Mol. Sci. 2025, 26, 2308. https://doi.org/10.3390/ijms26052308
Kimura S, Iwano S, Akioka T, Kuchimaru T, Kawaguchi M, Fukushima T, Sato Y, Kataoka H, Kamoto T, Mukai S, et al. Combined Therapy Targeting MET and Pro-HGF Activation Shows Significant Therapeutic Effect Against Liver Metastasis of CRPC. International Journal of Molecular Sciences. 2025; 26(5):2308. https://doi.org/10.3390/ijms26052308
Chicago/Turabian StyleKimura, Shoichi, Satoshi Iwano, Takahiro Akioka, Takahiro Kuchimaru, Makiko Kawaguchi, Tsuyoshi Fukushima, Yuichiro Sato, Hiroaki Kataoka, Toshiyuki Kamoto, Shoichiro Mukai, and et al. 2025. "Combined Therapy Targeting MET and Pro-HGF Activation Shows Significant Therapeutic Effect Against Liver Metastasis of CRPC" International Journal of Molecular Sciences 26, no. 5: 2308. https://doi.org/10.3390/ijms26052308
APA StyleKimura, S., Iwano, S., Akioka, T., Kuchimaru, T., Kawaguchi, M., Fukushima, T., Sato, Y., Kataoka, H., Kamoto, T., Mukai, S., & Sawada, A. (2025). Combined Therapy Targeting MET and Pro-HGF Activation Shows Significant Therapeutic Effect Against Liver Metastasis of CRPC. International Journal of Molecular Sciences, 26(5), 2308. https://doi.org/10.3390/ijms26052308






