Panduratin A from Boesenbergia rotunda Effectively Inhibits EGFR/STAT3/Akt Signaling Pathways, Inducing Apoptosis in NSCLC Cells with Wild-Type and T790M Mutations in EGFR
Abstract
1. Introduction
2. Results
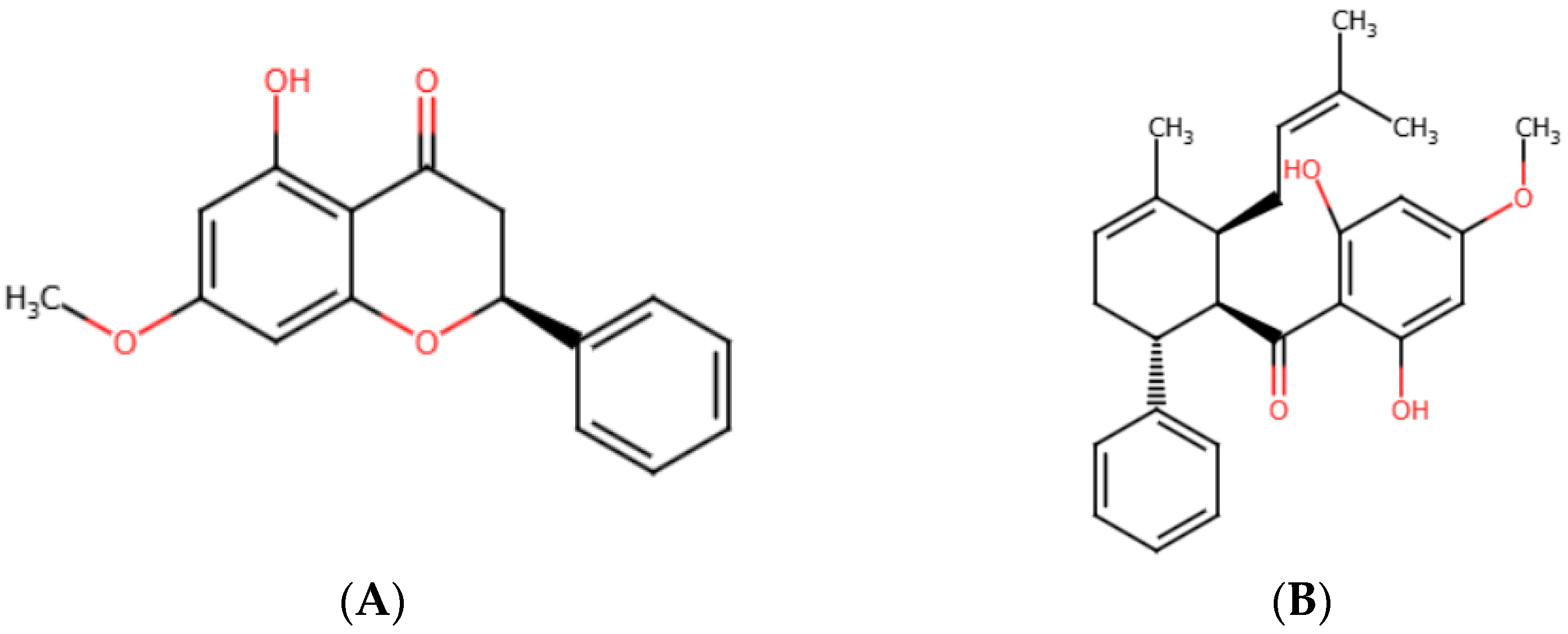
2.1. In Vitro Cytotoxicity Screening of Pinostrobin and Panduratin A Against NSCLC Cell Lines
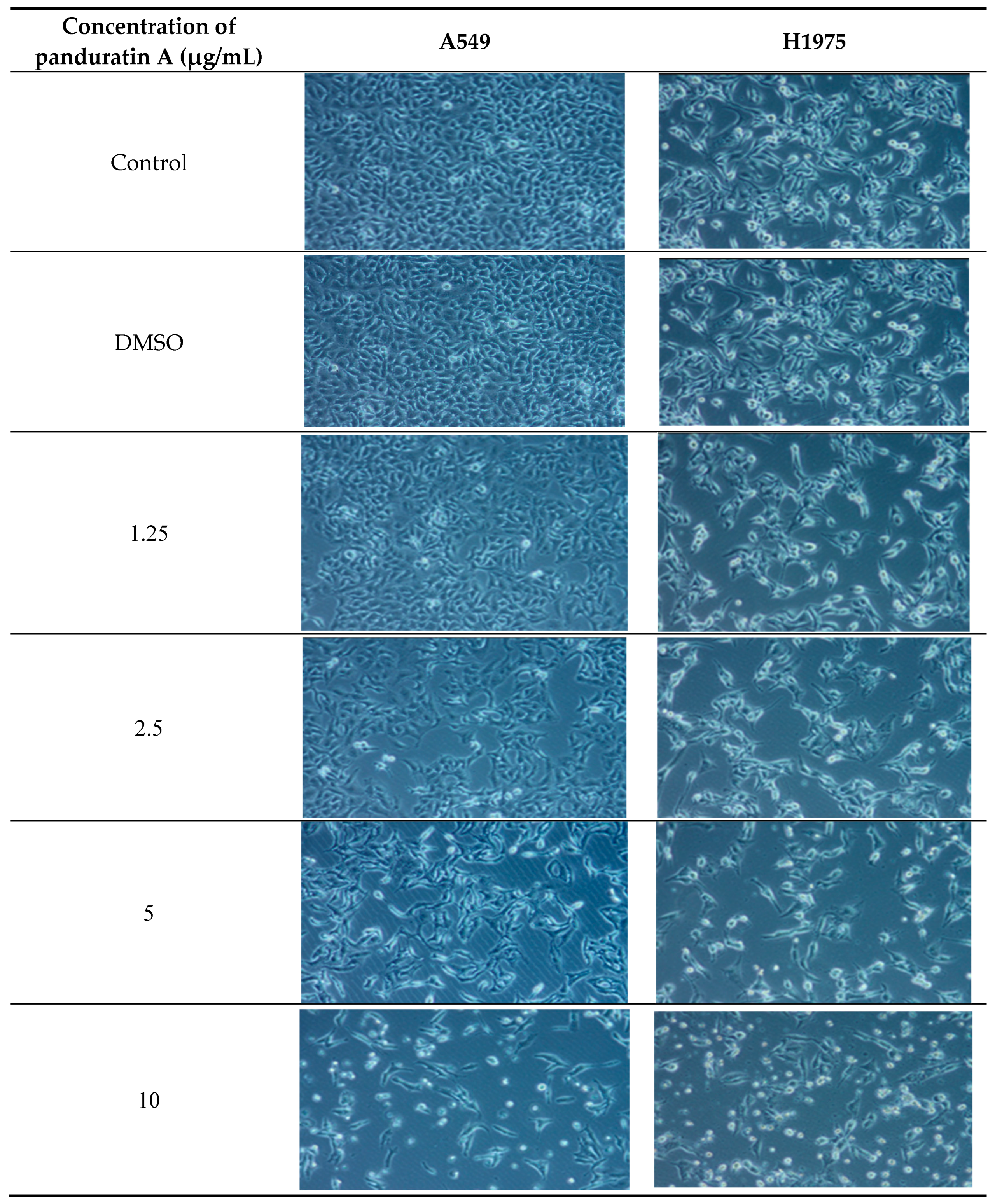
2.2. Panduratin A Induces Apoptosis in A549 and H1975 Cell Lines
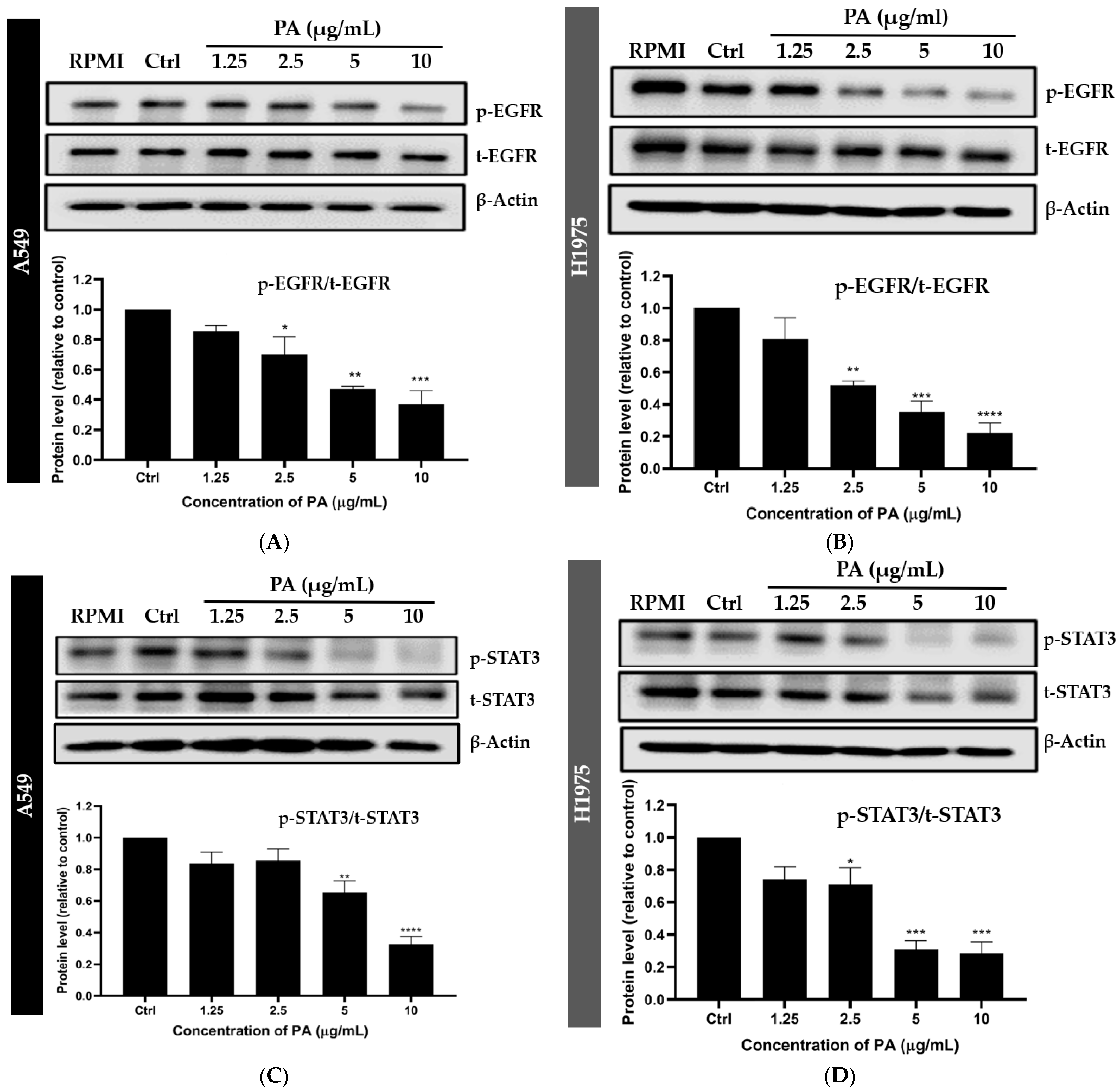
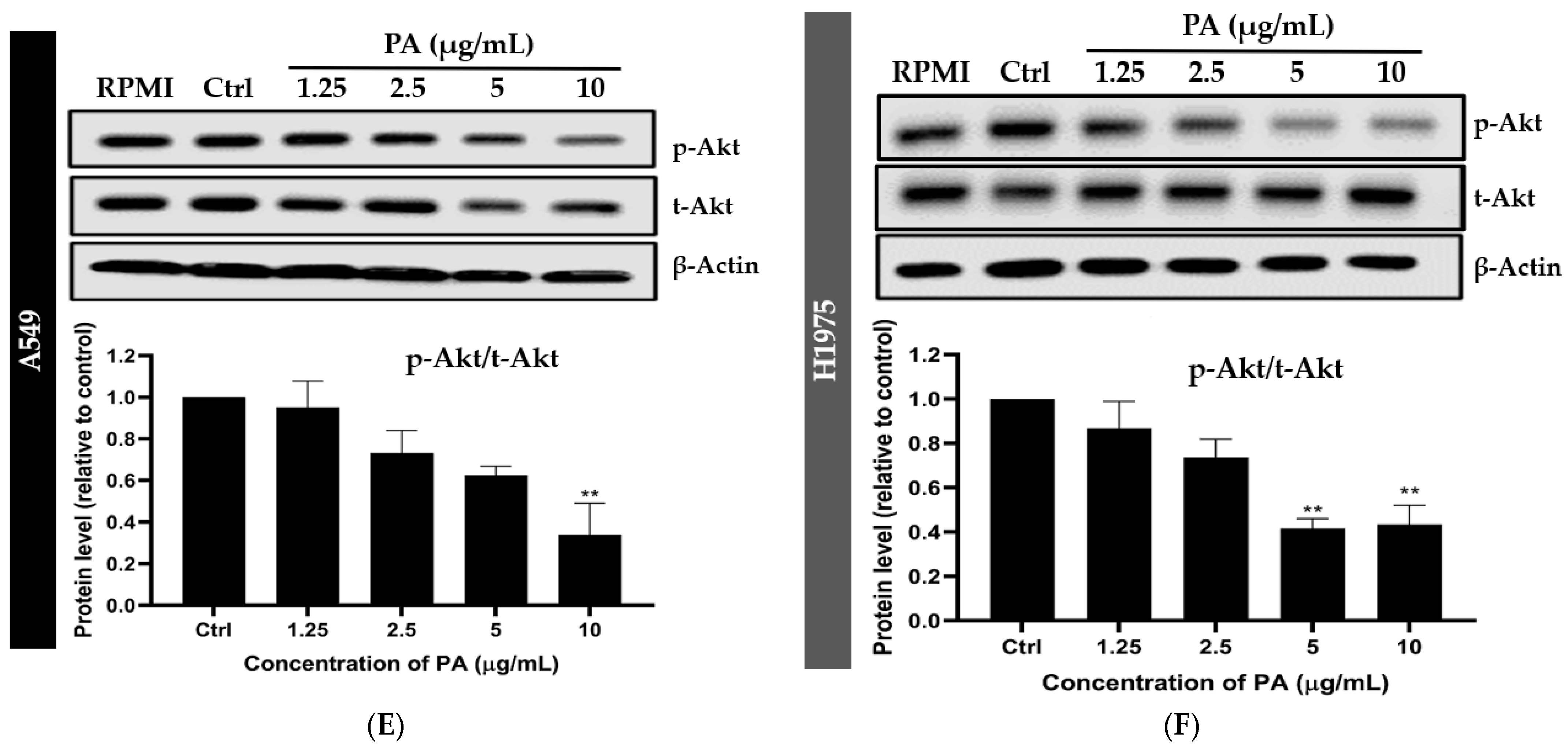
2.3. Panduratin A Inhibits EGFR, STAT3, and Akt Signaling Pathways in A549 and H1975 NSCLC Cell Lines
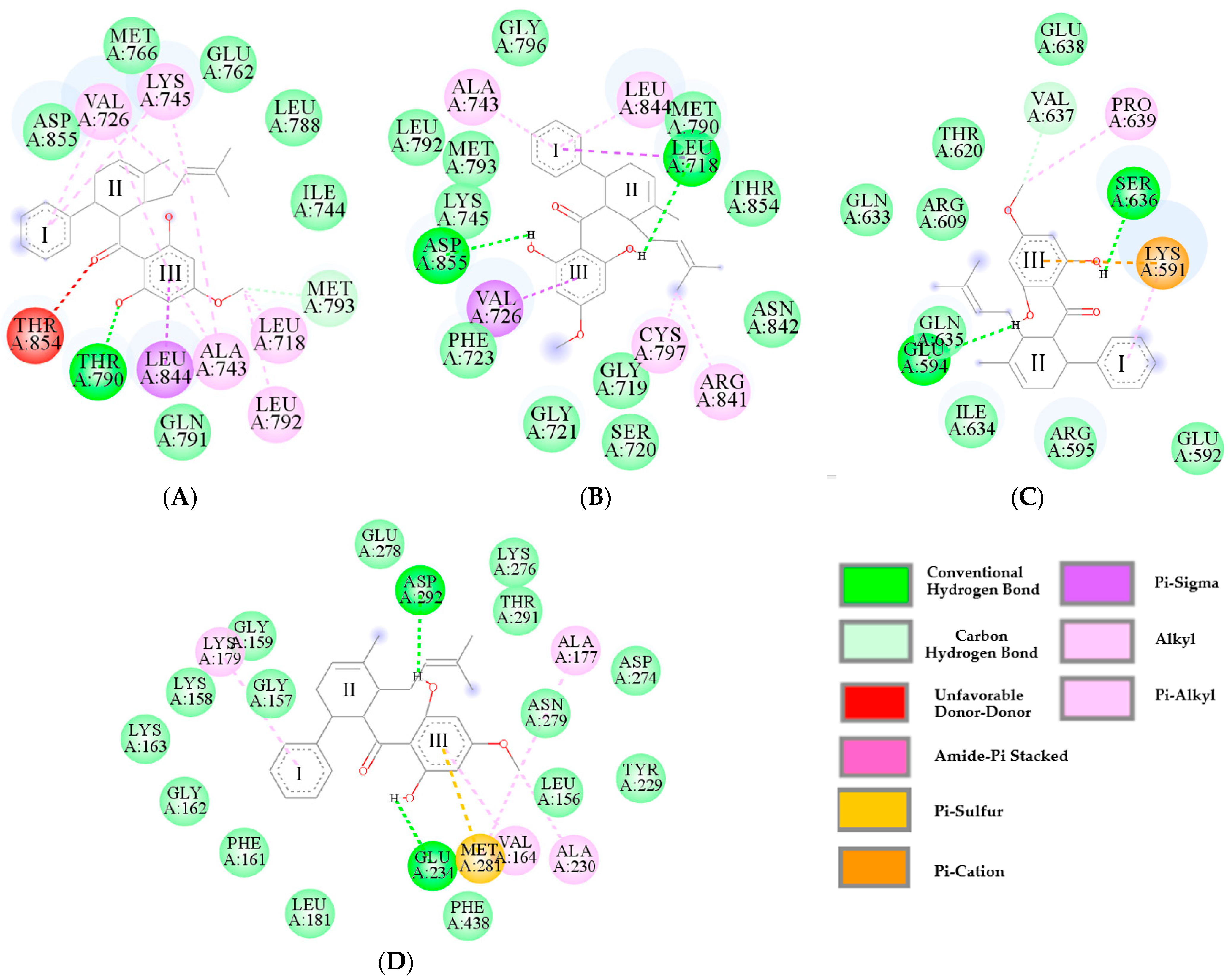
2.4. Predictive Binding Affinity of Panduratin A Against EGFR, STAT3, and Akt Signaling Proteins
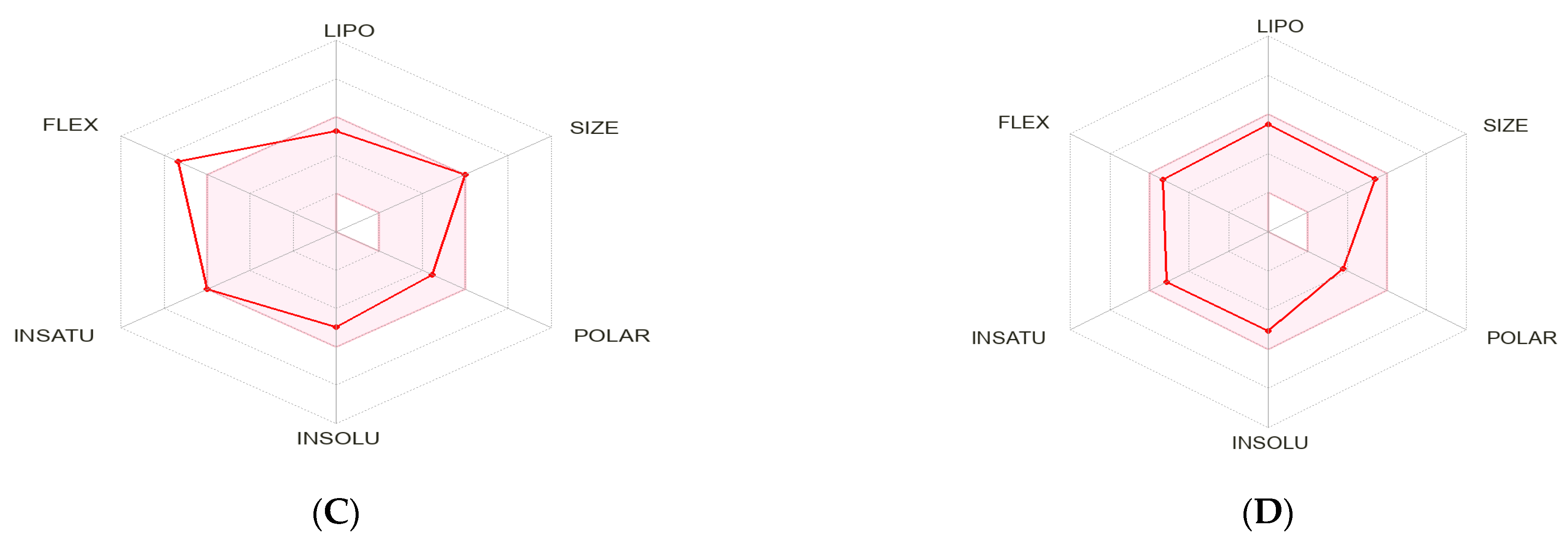
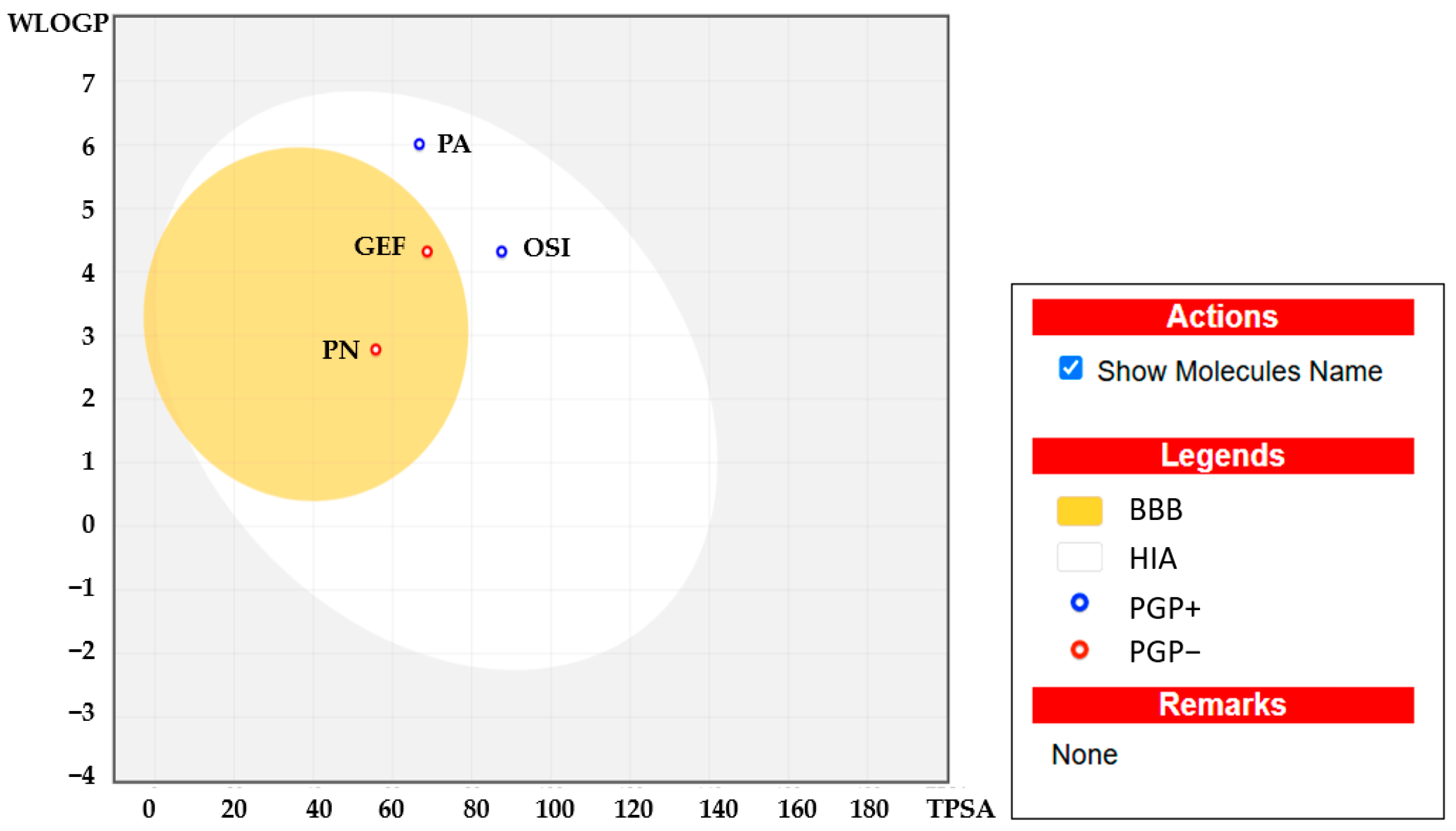
2.5. Pharmacokinetic Characteristics and ADMET Prediction
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Antibodies
4.2. Cell Lines and Culture
4.3. Cell Viability Assay
4.4. Western Blotting
4.5. Flow Cytometric Evaluation of Apoptosis
4.6. Molecular Docking
4.7. Assessment of Drug-Likeness and In Silico ADMET Prediction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdmetSAR | Admet structure activity relationship |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ANOVA | One-way analysis of variance |
| ALA | Alanine |
| ARG | Arginine |
| ASP | Aspartic acid |
| BBB | Blood–brain barrier |
| BSA | Bovine serum albumin |
| CPC | Centrifugal partition chromatography |
| CYS | Cysteine |
| DMSO | Dimethyl sulfoxide |
| EGFR TKIs | Epidermal growth factor receptor tyrosine kinase inhibitors |
| ERK | Extracellular signal-regulated kinase |
| FLEX | Flexibility |
| GEF | Gefitinib |
| GLU | Glutamic acid |
| ΔG | Binding free energy |
| HIA | High intestinal absorption |
| IC50 | Half-maximal inhibitory concentration |
| INSATU | Saturation |
| INSOLU | Solubility |
| JAK | Janus kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| Ki | Inhibition constant |
| LEU | Leucine |
| LIPO | Lipophilicity |
| LYS | Lysine |
| MEK | Mitogen-activated protein kinase |
| MET | Methionine |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| mTOR | Mammalian target of rapamycin |
| NSCLC | Non-small cell lung cancer |
| OSI | Osimertinib |
| PA | Panduratin A |
| PDB | Protein Data Bank |
| PI | Propidium iodide |
| PI3K | Phosphoinositide 3-kinase |
| PN | Pinostrobin |
| POLAR | Polarity |
| PRO | Proline |
| Raf | Rapidly accelerated fibrosarcoma kinase |
| Ras | Rat sarcoma virus protein |
| RMSD | Root mean square deviation |
| SCLC | Small cell lung cancer |
| SER | Serine |
| SI | Selectivity index |
| SMILES | Simplified Molecular Input Line Entry System |
| STAT3 | Signal transducer and activator of transcription 3 |
| THR | Threonine |
| VAL | Valine |
References
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef]
- Mahalapbutr, P.; Wonganan, P.; Chavasiri, W.; Rungrotmongkol, T. Butoxy Mansonone G inhibits STAT3 and Akt signaling pathways in non-small cell lung cancers: Combined experimental and theoretical investigations. Cancers 2019, 11, 437. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Vasudevan, S.; Sharma, S.K.; Panigrahi, M.; Suryavanshi, M.; Saifi, M.; Batra, U. Biomarker testing for advanced lung cancer by next-generation sequencing; a valid method to achieve a comprehensive glimpse at mutational landscape. Appl. Cancer Res. 2020, 40, 4. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Yang, J.-H.; Mok, T.; Loong, H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 2018, 29, i3–i9. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Li, Q.; Xu, X.; Li, X.; You, X.; Yu, Z. Genetic profile of non-small cell lung cancer (NSCLC): A hospital-based survey in Jinhua. Mol. Genet. Genom. Med. 2020, 8, e1398. [Google Scholar] [CrossRef]
- Leduc, C.; Merlio, J.; Besse, B.; Blons, H.; Debieuvre, D.; Bringuier, P.; Monnet, I.; Rouquette, I.; Fraboulet-Moreau, S.; Lemoine, A. Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: Results of the nationwide French Cooperative Thoracic Intergroup (IFCT) program. Ann. Oncol. 2017, 28, 2715–2724. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Xia, B.; Chen, E.; Zhao, Q.; Zhang, X.; Chen, X.; Chen, X.; Ma, S. Epidermal growth factor receptor (EGFR) T790M mutation identified in plasma indicates failure sites and predicts clinical prognosis in non-small cell lung cancer progression during first-generation tyrosine kinase inhibitor therapy: A prospective observational study. Cancer Commun. 2018, 38, 28. [Google Scholar]
- Dutta, P.; Sabri, N.; Li, J.; Li, W.X. Role of STAT3 in lung cancer. Jak-Stat 2014, 3, e999503. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Lao, X.Q.; Ho, K.-F.; Goggins, W.B.; Tse, S.L. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef]
- Philip, A.; Samuel, B.A. Advanced drug delivery systems in lung cancer. In Advanced Drug Delivery Systems in the Management of Cancer; Elsevier: Amsterdam, The Netherlands, 2021; pp. 101–106. [Google Scholar]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Marinato, G.M.; Mulargiu, C.; Marino, M.; Pasello, G.; Guarneri, V.; Bonanno, L. The study of primary and acquired resistance to first-line osimertinib to improve the outcome of EGFR-mutated advanced Non-small cell lung cancer patients: The challenge is open for new therapeutic strategies. Crit. Rev. Oncol./Hematol. 2024, 196, 104295. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.-C.; Huang, C.-H.; Ju, J.-S.; Chiu, T.-H.; Tung, P.-H.; Wang, C.-C.; Liu, C.-Y.; Chung, F.-T.; Fang, Y.-F.; Guo, Y.-K. First-or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211035710. [Google Scholar] [CrossRef]
- Hadni, H.; Elhallaouia, M. In silico design of EGFRL858R/T790M/C797S inhibitors via 3D-QSAR, molecular docking, ADMET properties and molecular dynamics simulations. Heliyon 2022, 8, e11537. [Google Scholar] [CrossRef]
- Li, W.Q.; Li, L.Y.; Chai, J.; Cui, J.W. Cost-effectiveness analysis of first-line treatments for advanced epidermal growth factor receptor-mutant non-small cell lung cancer patients. Cancer Med. 2021, 10, 1964–1974. [Google Scholar] [CrossRef]
- Aguiar, P.N.; Haaland, B.; Park, W.; San Tan, P.; Del Giglio, A.; de Lima Lopes, G. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non–small cell lung cancer. JAMA Oncol. 2018, 4, 1080–1084. [Google Scholar] [CrossRef]
- Huo, G.; Song, Y.; Liu, W.; Cao, X.; Chen, P. Osimertinib in the treatment of resected EGFR-mutated non-small cell lung cancer: A cost-effectiveness analysis in the United States. Front. Pharmacol. 2024, 15, 1300183. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Segal, E.M. Osimertinib: A Novel Therapeutic Option for Overcoming T790M Mutations in Non–Small Cell Lung Cancer. J. Adv. Pract. Oncol. 2017, 8, 196. [Google Scholar]
- Yang, T.; Zhang, W.; Cao, S.; Sun, S.; Cai, X.; Xu, L.; Li, P.; Zheng, Z.; Li, S. Discovery of highly potent and selective EGFRT790M/L858R TKIs against NSCLC based on molecular dynamic simulation. Eur. J. Med. Chem. 2022, 228, 113984. [Google Scholar] [CrossRef]
- Khaddour, K.; Jonna, S.; Deneka, A.; Patel, J.D.; Abazeed, M.E.; Golemis, E.; Borghaei, H.; Boumber, Y. Targeting the epidermal growth factor receptor in EGFR-mutated lung cancer: Current and emerging therapies. Cancers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Hasan, G.M.; Eldin, S.M.; Adnan, M.; Riaz, M.B.; Islam, A.; Khan, I.; Hassan, M.I. Investigating regulated signaling pathways in therapeutic targeting of non-small cell lung carcinoma. Biomed. Pharmacother. 2023, 161, 114452. [Google Scholar] [CrossRef]
- Abourehab, M.A.; Alqahtani, A.M.; Youssif, B.G.; Gouda, A.M. Globally approved EGFR inhibitors: Insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Moghaddam, S.J. KRAS-mutant lung cancer: Targeting molecular and immunologic pathways, therapeutic advantages and restrictions. Cells 2023, 12, 749. [Google Scholar] [CrossRef]
- Santarpia, M.; Liguori, A.; Karachaliou, N.; Gonzalez-Cao, M.; Daffinà, M.G.; D’Aveni, A.; Marabello, G.; Altavilla, G.; Rosell, R. Osimertinib in the treatment of non-small-cell lung cancer: Design, development and place in therapy. Lung Cancer Targets Ther. 2017, 8, 109–125. [Google Scholar] [CrossRef]
- Samee, W.; Eiamart, W.; Tadtong, S.; Chittasupho, C. Simultaneous Determination of Stilbenes, Flavones, Coumestans, Anthraquinones, and Chalcones in Ethanolic Extract of Pet-Sang-Kard Mixed Herbal Remedy Using HPLC-PDA Analysis. Molecules 2025, 30, 222. [Google Scholar] [CrossRef]
- Bailly, C. Toward the use of Boesenbergia rotunda extracts and the chalcone panduratin A to treat periodontitis. J. Oral Biosci. 2022, 64, 183–192. [Google Scholar] [CrossRef]
- Cheah, S.C.; Appleton, D.R.; Lee, S.T.; Lam, M.L.; Hadi, A.H.; Mustafa, M.R. Panduratin A inhibits the growth of A549 cells through induction of apoptosis and inhibition of NF-kappaB translocation. Molecules 2011, 16, 2583–2598. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Ahmad, A.; Khan, M.S.; Shahanawaz, S.; Ahmad, S.; Ansari, I.A. Pinostrobin suppresses the proliferation of lung carcinoma cells by abrogating the cell cycle progression through the inhibition of Notch signaling pathway. S. Afr. J. Bot. 2022, 151, 614–622. [Google Scholar] [CrossRef]
- Lai, S.-L.; Mustafa, M.R.; Wong, P.-F. Panduratin A induces protective autophagy in melanoma via the AMPK and mTOR pathway. Phytomedicine 2018, 42, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cao, Y.; Zhou, P.; Gui, S.; Wu, X.; Xia, Y.; Tu, J. Panduratin A inhibits cell proliferation by inducing G0/G1 phase cell cycle arrest and induces apoptosis in breast cancer cells. Biomol. Ther. 2018, 26, 328–334. [Google Scholar] [CrossRef]
- Pan, C.; Duan, H.; Wu, Y.; Zhu, C.; Yi, C.; Duan, Y.; Lu, D.; Guo, C.; Wu, D.; Wang, Y. Inhibition of DNA-PK by gefitinib causes synergism between gefitinib and cisplatin in NSCLC. Int. J. Oncol. 2020, 57, 939–955. [Google Scholar] [CrossRef]
- Onyancha, J.M.; Gikonyo, N.K.; Wachira, S.W.; Mwitari, P.G.; Gicheru, M.M. Anticancer activities and safety evaluation of selected Kenyan plant extracts against breast cancer cell lines. Acad. J. 2017, 10, 21–26. [Google Scholar]
- Widiandani, T.; Tandian, T.; Zufar, B.D.; Suryadi, A.; Purwanto, B.T.; Hardjono, S. In vitro study of pinostrobin propionate and pinostrobin butyrate: Cytotoxic activity against breast cancer cell T47D and its selectivity index. J. Public Health Afr. 2023, 14, 2516. [Google Scholar] [CrossRef]
- González, A.S.; Soto Tellini, V.H.; Benjumea Gutiérrez, D.M. Study of the dermal anti-inflammatory, antioxidant, and analgesic activity of pinostrobin. Heliyon 2022, 8, e10413. [Google Scholar] [CrossRef]
- Tozuka, T.; Noro, R.; Yoshida, K.; Takahashi, S.; Hirao, M.; Matsuda, K.; Kato, Y.; Nakamichi, S.; Takeuchi, S.; Matsumoto, M. Phosphoproteomic Analysis Identified Mutual Phosphorylation of FAK and Src as a Mechanism of Osimertinib Resistance in EGFR-Mutant Lung Cancer. JTO Clin. Res. Rep. 2024, 5, 100668. [Google Scholar] [CrossRef]
- Vaid, A.K.; Gupta, A.; Momi, G. Overall survival in stage IV EGFR mutation-positive NSCLC: Comparing first-, second-and third-generation EGFR-TKIs. Int. J. Oncol. 2021, 58, 171–184. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Lim, K.-H.; Chiang, Y.-W.; Sie, Z.-L.; Chang, J.; Ho, A.-S.; Cheng, C.-C. STAT3 induces G9a to exacerbate HER3 expression for the survival of epidermal growth factor receptor-tyrosine kinase inhibitors in lung cancers. BMC Cancer 2019, 19, 959. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Armstrong, E.A.; Benavente, S.; Chinnaiyan, P.; Harari, P.M. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR) combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004, 64, 5355–5362. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, R.; Peng, S.; Chen, L.; Li, Q.; Wang, W. Hepatocyte growth factor reduces sensitivity to the epidermal growth factor receptor-tyrosine kinase inhibitor, gefitinib, in lung adenocarcinoma cells harboring wild-type EGFR. Oncotarget 2016, 7, 16273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheah, S.-C.; Lai, S.-L.; Lee, S.-T.; Hadi, A.H.A.; Mustafa, M.R. Panduratin A, a possible inhibitor in metastasized A549 cells through inhibition of NF-kappa B translocation and chemoinvasion. Molecules 2013, 18, 8764–8778. [Google Scholar] [CrossRef]
- Zou, M.; Xia, S.; Zhuang, L.; Han, N.; Chu, Q.; Chao, T.; Peng, P.; Chen, Y.; Gui, Q.; Yu, S. Knockdown of the Bcl-2 gene increases sensitivity to EGFR tyrosine kinase inhibitors in the H1975 lung cancer cell line harboring T790M mutation. Int. J. Oncol. 2013, 42, 2094–2102. [Google Scholar] [CrossRef][Green Version]
- Ciaramella, V.; Della Corte, C.; Barra, G.; Di Liello, R.; Viscardi, G.; Orditura, M.; Martinelli, E.; Ciardiello, F.; Morgillo, F. Acquired resistance mechanism of osimertinib targeting EGFR in human lung cancer. Ann. Oncol. 2018, 29, viii677–viii678. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Li, S.; Wang, T.; Chen, L.; Li, H.; Ding, Q.; Li, X.; Zhu, S.; Tang, X. Cytochalasin H enhances sensitivity to gefitinib in non-small-cell lung cancer cells through inhibiting EGFR activation and PD-L1 expression. Sci. Rep. 2024, 14, 25276. [Google Scholar] [CrossRef]
- Bracht, J.W.P.; Karachaliou, N.; Berenguer, J.; Pedraz-Valdunciel, C.; Filipska, M.; Codony-Servat, C.; Codony-Servat, J.; Rosell, R. Osimertinib and pterostilbene in EGFR-mutation-positive non-small cell lung cancer (NSCLC). Int. J. Biol. Sci. 2019, 15, 2607. [Google Scholar] [CrossRef]
- Gurung, A.B.; Ali, M.A.; Al-Hemaid, F.; El-Zaidy, M.; Lee, J. In silico analyses of major active constituents of fingerroot (Boesenbergia rotunda) unveils inhibitory activities against SARS-CoV-2 main protease enzyme. Saudi J. Biol. Sci. 2022, 29, 65–74. [Google Scholar] [CrossRef]
- Sujana, D.; Sumiwi, S.; Saptarini, N.; Levita, J. ADMET prediction and molecular docking simulation of phytoconstituents in Boesenbergia rotunda rhizome with the effector caspases to understand their protective effects. Rasayan J. Chem. 2022, 15, 2401. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Pujari, I.; Sengupta, R.; Babu, V.S. Docking and ADMET studies for investigating the anticancer potency of Moscatilin on APC10/DOC1 and PKM2 against five clinical drugs. J. Genet. Eng. Biotechnol. 2021, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Nhlapho, S.; Nyathi, M.H.L.; Ngwenya, B.L.; Dube, T.; Telukdarie, A.; Munien, I.; Vermeulen, A.; Chude-Okonkwo, U.A. Druggability of Pharmaceutical Compounds Using Lipinski Rules with Machine Learning. Sci. Pharm. 2024, 3, 177–192. [Google Scholar] [CrossRef]
- Kurnia, D.; Putri, S.A.; Tumilaar, S.G.; Zainuddin, A.; Dharsono, H.D.A.; Amin, M.F. In silico study of antiviral activity of polyphenol compounds from Ocimum basilicum by molecular docking, ADMET, and drug-likeness analysis. Adv. Appl. Bioinform. Chem. 2023, 16, 37–47. [Google Scholar] [CrossRef]
- Stojanov, S.J.; Podolski-Renic, A.; Dinić, J.; Dragoj, M.; Jovanović, M.; Stepanović, A.; Lupšić, E.; Bajović, R.; Glumac, S.; Marić, D. 69P Osimertinib is selective against NSCLC cells and modulates the multidrug-resistant phenotype in patient-derived cell cultures and co-cultures of NSCLC cells and fibroblasts. ESMO Open 2023, 8, 100927. [Google Scholar] [CrossRef]
- Passaro, A.; Guerini-Rocco, E.; Pochesci, A.; Vacirca, D.; Spitaleri, G.; Catania, C.M.; Rappa, A.; Barberis, M.; de Marinis, F. Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment. Pharmacol. Res. 2017, 117, 406–415. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Minko, T. Development of liposomal vesicles for osimertinib delivery to egfr mutation—Positive lung cancer cells. Pharmaceutics 2020, 12, 939. [Google Scholar] [CrossRef]
- Li, Y.; Song, Z.; Jin, Y.; Tang, Z.; Kang, J.; Ma, X. Novel selective and potent EGFR inhibitor that overcomes T790M-Mediated resistance in non-small cell lung cancer. Molecules 2016, 21, 1462. [Google Scholar] [CrossRef]
- Sun, S.; Kim, M.J.; Omar, A.M.; Phan, N.D.; Awale, S. (+)-Panduratin A induces PANC-1 human pancreatic cancer cell death preferentially under nutrient starvation by inhibiting PI3K/Akt/mTOR/autophagy signaling pathway. Phytomed. Plus 2021, 1, 100101. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Belka, M.; Papierska, K. Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones. Cancers 2024, 16, 1092. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Yu, X.; Liu, W.; Zhou, L.; Li, W. Licochalcone A inhibits EGFR signalling and translationally suppresses survivin expression in human cancer cells. J. Cell. Mol. Med. 2021, 25, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Eiamart, W.; Wittayalertpanya, S.; Tadtong, S.; Samee, W. Efficient Simultaneous Isolation of Pinostrobin and Panduratin A from Boesenbergia rotunda Using Centrifugal Partition Chromatography. Molecules 2024, 29, 5186. [Google Scholar] [CrossRef] [PubMed]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol modulation of apoptosis and cell cycle response to cisplatin in head and neck cancer cell lines. Int. J. Mol. Sci. 2021, 22, 6322. [Google Scholar] [CrossRef] [PubMed]
- Heppner, D.E.; Wittlinger, F.; Beyett, T.S.; Shaurova, T.; Urul, D.A.; Buckley, B.; Pham, C.D.; Schaeffner, I.K.; Yang, B.; Ogboo, B.C. Structural basis for inhibition of mutant EGFR with lazertinib (YH25448). ACS Med. Chem. Lett. 2022, 13, 1856–1863. [Google Scholar] [CrossRef]
- Arora, P.; Kumar, S.; Bansal, S.; Sharma, P. Synthesis, In vitro Cytotoxicity, Molecular docking of Few Quinazolinone Incorporated Naphthyl Chalcones: As Potential Dual Targeting Anticancer Agents to Treat Lung Cancer and Colorectal Cancer. Rasayan J. Chem. 2023, 16, 231–245. [Google Scholar] [CrossRef]




| Compounds | IC50 (µg/mL) | Selectivity Index | |||
|---|---|---|---|---|---|
| A549 | H1975 | MRC5 | A549 | H1975 | |
| Panduratin A | 6.030 ± 0.21 | 5.578 ± 0.15 | 12.963 ± 0.36 | 2.15 | 2.32 |
| Pinostrobin | >100, due to the compound’s poor solubility in RPMI medium | _ | _ | _ | |
| Gefitinib | >40 | >40 | _ | _ | _ |
| Osimertinib | >40 | 9.466 ± 0.307 | _ | _ | _ |
| Compounds | Protein Targets | |||||||
|---|---|---|---|---|---|---|---|---|
| EGFRWT (7UKV) | EGFRT790M (5Y9T) | STAT3 (1BG1) | Akt (4GV1) | |||||
| Ki (µM) | ΔGbind Score (kcal/mol) | Ki (µM) | ΔGbind Score (kcal/mol) | Ki (µM) | ΔGbind Score (kcal/mol) | Ki (µM) | ΔGbind Score (kcal/mol) | |
| Panduratin A | 4.16 | −7.34 | 8.68 | −6.91 | 3.97 | −7.37 | 1.23 | −8.06 |
| Pinostrobin | 14.49 | −6.60 | 36.94 | −6.05 | 28.83 | −6.19 | 1.74 | −7.86 |
| Gefitinib | 6.98 | −7.03 | 11.74 | −6.73 | 11.35 | −6.75 | 3.13 | −7.51 |
| Osimertinib | 2.08 | −7.75 | 3.38 | −7.46 | 6.26 | −7.10 | 0.439 | −8.57 |
| RMSD (redocked) | 1.894 | 1.846 | 1.350 | 1.339 | ||||
| Functional Groups | Interacting Amino Acids | |||
|---|---|---|---|---|
| EGFRWT (7UKV) | EGFRT790M (5Y9T) | STAT3 (1BG1) | Akt (4GV1) | |
| Aromatic (Ring I) | VAL726, LYS745 | LEU844, ALA743, LEU718 | LYS591 | LYS179 |
| Methyl cyclohexane (Ring II) | - | CYS797, ARG841 | - | - |
| 2-methyl-2-butene | LYS745, VAL76, ALA743 | - | - | - |
| Carbonyl | THR854 | - | - | - |
| Aromatic (Ring III) | LEU844, ALA743, VAL726 | VAL726 | LYS591 | MET281, VAL164 |
| Methoxy (Ring III) | LEU718, LEU792, MET793 | - | VAL637 PRO639 | ALA230, MET281, ALA177 |
| Hydroxyl (Ring III) (Hydrogen bond interactions) | THR790 | ASP855, LEU718 | GLU594, SER636 | ASP292, GLU234 |
| Compounds | MW (Da) | HBD | HBA | WLogP | TPSA | RB | MR | Lipinski’s Rule of Five | Veber’s Rule | HIA (%) | BBB | Toxicity/ Carcinogenicity/ Mutagenicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panduratin A | 406.51 | 2 | 4 | 6.01 | 66.76 | 6 | 121.48 | Yes (4/5) | Yes | High 96.9% | No | No |
| Pinostrobin | 270.28 | 1 | 4 | 2.78 | 55.76 | 2 | 74.02 | Yes (5/5) | Yes | High 99.2% | Yes | No |
| Gefitinib | 499.61 | 2 | 5 | 4.32 | 87.55 | 11 | 150.43 | Yes (5/5) | No | High 94.9% | No | No |
| Osimertinib | 446.90 | 1 | 7 | 4.32 | 68.74 | 8 | 121.66 | Yes (5/5) | Yes | High 98.5% | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiamart, W.; Wonganan, P.; Tadtong, S.; Samee, W. Panduratin A from Boesenbergia rotunda Effectively Inhibits EGFR/STAT3/Akt Signaling Pathways, Inducing Apoptosis in NSCLC Cells with Wild-Type and T790M Mutations in EGFR. Int. J. Mol. Sci. 2025, 26, 2350. https://doi.org/10.3390/ijms26052350
Eiamart W, Wonganan P, Tadtong S, Samee W. Panduratin A from Boesenbergia rotunda Effectively Inhibits EGFR/STAT3/Akt Signaling Pathways, Inducing Apoptosis in NSCLC Cells with Wild-Type and T790M Mutations in EGFR. International Journal of Molecular Sciences. 2025; 26(5):2350. https://doi.org/10.3390/ijms26052350
Chicago/Turabian StyleEiamart, Wanna, Piyanuch Wonganan, Sarin Tadtong, and Weerasak Samee. 2025. "Panduratin A from Boesenbergia rotunda Effectively Inhibits EGFR/STAT3/Akt Signaling Pathways, Inducing Apoptosis in NSCLC Cells with Wild-Type and T790M Mutations in EGFR" International Journal of Molecular Sciences 26, no. 5: 2350. https://doi.org/10.3390/ijms26052350
APA StyleEiamart, W., Wonganan, P., Tadtong, S., & Samee, W. (2025). Panduratin A from Boesenbergia rotunda Effectively Inhibits EGFR/STAT3/Akt Signaling Pathways, Inducing Apoptosis in NSCLC Cells with Wild-Type and T790M Mutations in EGFR. International Journal of Molecular Sciences, 26(5), 2350. https://doi.org/10.3390/ijms26052350







