Abstract
The incidence of anxiety and depression disorders is increasing worldwide. There is an increasing incidence of hard-to-treat depression with various aspects of origin. Almost 80% of people prefer to use natural remedies and supplements as their primary healthcare solution. Not surprisingly, around one-third of drugs were inspired by nature. Over the past three decades, the use of such remedies has increased significantly. Synthetic antidepressants may cause various negative side effects, whereas herbal medicines are favored because of their ability to relieve symptoms with minimal to no side effects and lower financial burden. This review provides an overview of herbs and biologically active compounds used to treat depression.
1. Introduction
Major depressive disorders (MDDs) were ranked as the 3rd cause of the disease burden in 2008, and it is projected that it will be the leading cause by 2030 [1]. The COVID-19 pandemic has increased the incidence of depression and mood disorders [2]. Depression is diagnosed when an individual experiences persistently low mood, poor concentration, changes in appetite, anhedonia, feelings of guilt or worthlessness, a lack of energy, sleep disturbances, or suicidal thoughts [3,4]. There are similarities in the neurobiological changes that cause depression and neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s diseases [5]. However, depression is often a comorbidity of other illnesses, including Alzheimer’s disease, epilepsy, Parkinson’s disease, migraine, and stroke [6,7].
Depression results from a combination of genetic and environmental factors; however, the exact pathology and cause of the disease are still not well understood [8,9,10]. Epidemiological studies have shown that genetics play a role in up to 80% of cases of depression and bipolar disorder [11]. Epidemiological studies of bipolar disorder show a heritability of 65–80%, while according to the Psychiatric Genomics Consortium and Genome-wide Association Study (GWAS), the heritability based on single-nucleotide polymorphisms is ~20%, indicating several genetic factors that need to be elucidated [12,13]. The highly polygenic architecture of genetic markers of bipolar disorder overlaps with MDD, schizophrenia, and other diseases [14].
Epigenetics plays a special role in depression through short- and long-term gene expression variations that are caused by non-DNA-encoded mechanisms, including DNA methylation or hydroxymethylation, histone modifications, expression of noncoding RNAs, chromatin remodeling, and RNA modification [15].
Another role in the origin and progress of depression is played by the gut microbiome. There is a communication network between the gut and the brain that is bidirectional, dynamic, and complex. Neuropsychiatric, neurodegenerative, or metabolic disorders are also caused by changes in the gut–brain axis. The regulator of this axis is the gut microbiota through metabolic, neuroendocrine pathways. Through histone modification and gene silencing associated with non-coding RNA and DNA methylation, the gut microbiome can modulate diseases. Short-chain fatty acids such as butyrate are also important inhibitors of histone deacetylases. Dysbiosis and epigenetics play an important role in the pathophysiology of depression [16]. The gut–brain axis may also be involved in the development of depression through the metabolism of tryptophan, which has been observed in the hippocampus [17]. The gut–brain axis also has a direct relationship with the HPA active in depression [16]. Depressed patients suffer from a reduced number of Bifidobacterium and Lactobacillus species in the intestinal microbiome, which can lead to inflammation. Probiotic treatment has been shown to be effective [11,18].
In addition to traditional antidepressants, the antidepressant ketamine is also coming to the fore, which accelerates the antidepressant effect of antidepressants by inhibiting the ionotropic glutamate receptor NMDA (N-methyl-D-aspartate) [19,20]. Other innovative therapies involve neurofeedback, a non-invasive intervention that has been increasingly used as a potential treatment for major depressive disorders [21], digital psychotherapy [22], or psychobiotics [23]. However, these approaches need to be validated more deeply and for a longer time.
Research in both preclinical and clinical fields points to several theories related to depression, including monoamines, receptors, the hypothalamus–pituitary axis (HPA), the endocrine system, inflammation, neuroplasticity, mitochondria, neurogenesis, growth factors, circadian rhythms, oxidative stress [24,25], and glutamate [26]. The monoamine hypothesis suggests that depression is caused by a deficiency in neurotransmitters, such as serotonin, dopamine, and noradrenaline [27]. Synthetic antidepressants have been used to treat depression and anxiety since the 1950s [28]. The disadvantages of using synthetic antidepressants include a delayed onset of action, with a time frame of 2–4 weeks, during which serious consequences can occur. Additionally, these drugs often have low efficacy and high toxicity and can cause negative interactions with other medications [24]. First-generation antidepressants, such as tricyclic and monoamine oxidase inhibitors, have largely been replaced by second-generation antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) [29], which are considered safer but also have serious side effects, such as gastrointestinal issues, sexual dysfunction [30], emotional blunting, and weight gain [31,32], and can also increase the risk of suicidal tendencies in some children and teenagers [33]. Synthetic antidepressants have also been linked to various side effects in the treatment of anxiety and neurological diseases [34], and the discontinuation of their use can cause symptoms, such as balance disorders, anxiety, depression, and nausea. Despite the use of antidepressants, 50% of patients receiving monotherapy do not respond effectively, and 20–30% of patients do not respond to a specific antidepressant [35].
Depression is often accompanied by other psychiatric disorders, particularly anxiety, and, to a lesser extent, alcohol or drug abuse. The onset of anxiety usually occurs before depression, whereas the onset of alcohol abuse can occur either before or after depression. This high comorbidity rate highlights the complex nature of depression and the need for a comprehensive treatment approach [4].
Anxiety is caused by excessive stimulation of the neuronal circuit, where the hippocampus and amygdala play a key role in regulating emotions and emotional memory, along with the HPA and sympathetic systems [36,37]. The treatment of anxiety focuses on regulating neurotransmitters, such as serotonin and noradrenaline, as well as the amygdala nuclei, and their interaction with corticotrophin-releasing hormone (CRH) through neuroimaging techniques, such as magnetic resonance imaging (MRI) and tomography [38,39]. The underlying cause of anxiety is an imbalance in neurotransmitters, including γ-aminobutyric acid (GABA), serotonin, noradrenaline, and dopamine. Vigilance is regulated by the locus coeruleus, which is responsible for regulating noradrenaline and is thought to play a role in anxiety, panic, and fear. Forty percent of patients receiving treatment for anxiety also experience an MDD [38].
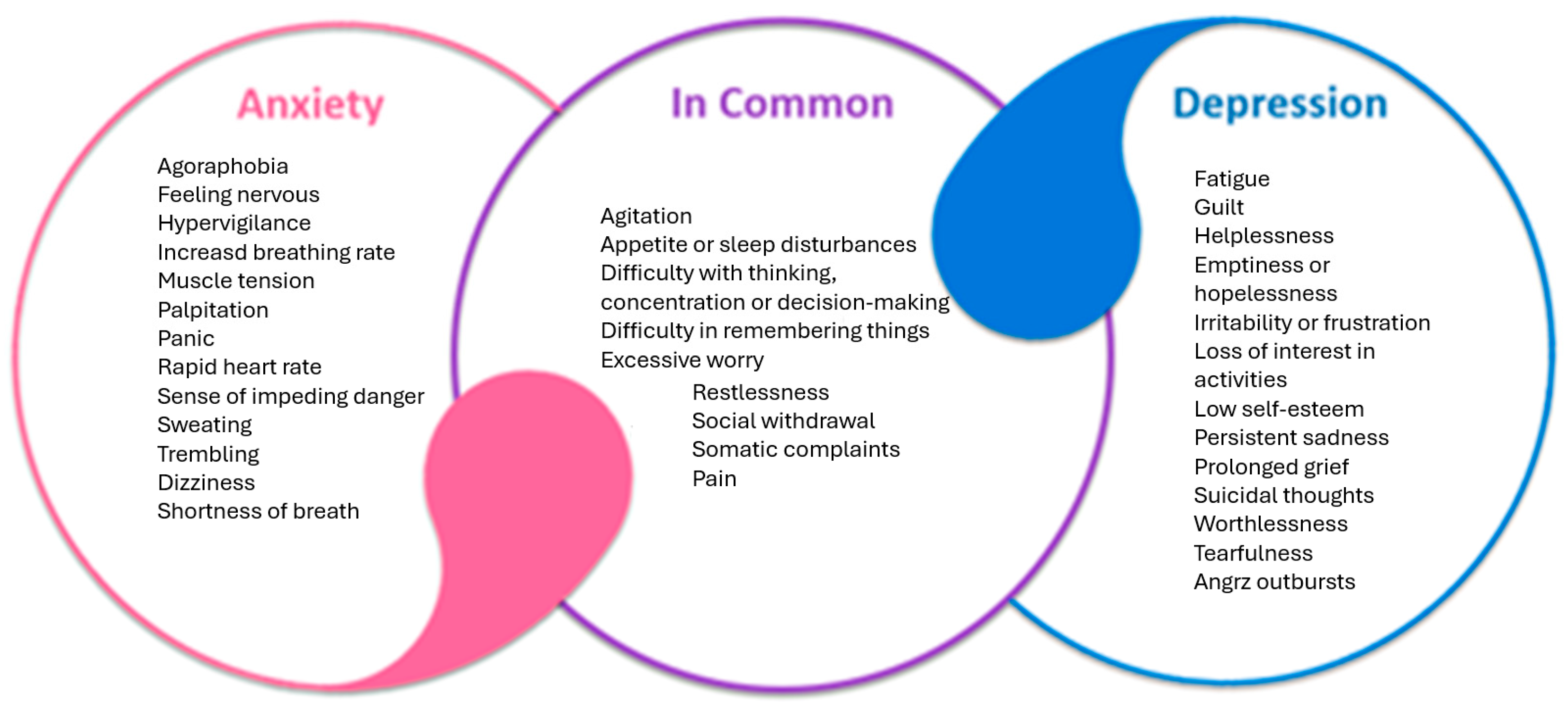
Anxiety and depression are two of the most prevalent psychiatric disorders and often co-occur. In the late 1970s and the 1980s, the distinction between anxiety and depression began to blur, and it was acknowledged that these two disorders had overlapping characteristics (Figure 1). This is supported by biological evidence, such as the dysregulation of the HPA and brain response to serotonergic challenge, which are common in both anxiety and depression [40,41].
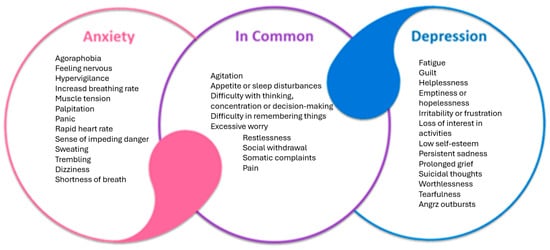
Figure 1.
Common features of depressive and anxiety disorders.
Selection criteria for review: Flavonoids are phenolic substances isolated from a wide range of vascular plants, with over 8000 individual compounds known. To date, more than 4000 different flavonoids have been identified from plant origin. The PubMed and Scopus databases were searched using keywords, such as depression, anxiety, and natural products. The internal criteria were set. We selected 10 plants with described antidepressant activity from the huge number, which may be a limitation of our study. From these, famous ones, such as hypericin or Ginko biloba, were selected, along with others not so well known for affecting depression. All the obtained articles were reviewed, and according to the inclusion and exit criteria, the related articles were selected to write this review article. The Google Scholar database was also searched for reliability.
2. Herbal Versus Modern Medicine
The World Health Organization (WHO) defines herbal medicine as a practice that includes herbs, herbal materials, preparations, and finished products that use parts of plants or other botanical materials as active ingredients [42]. Herbal medicine, also known as phytomedicine or botanical medicine, has a long history and utilizes parts of plants, such as roots, stems, leaves, berries, and flowers, for therapeutic purposes [43]. The WHO states that medicinal plants contain substances that can be used for various therapeutic purposes and serve as precursors for semi-synthesis of chemo-pharmaceuticals [44]. Plant drugs include not only the active components found in plant parts (phytochemicals) but also raw or processed plant materials with added ingredients, such as preservatives, solvents, or dilutions. Over time, some phytochemicals have been shown to provide health benefits to humans [45]. The use of herbal medicines dates back 60,000 years [46], as recorded in Babylon, and each region has its own unique healing practices, philosophies, and systems, such as traditional Chinese medicine [45]; Ayurveda, which is recognized by the Indian government; and Kampo medicine in Japan [47,48]. The WHO defines traditional medicine as a combination of knowledge, skills, and practices based on the beliefs and experiences of various cultures that are used to maintain health and treat physical and mental illnesses [49]. Despite extensive documentation of medicinal plants, their use in culture lacks an understanding of their constituents or function [50].
The WHO has issued guidelines for the proper evaluation of herbal medicines, including the assessment of their quality, stability, safety, and effectiveness. The Food and Drug Administration (FDA) also follows these guidelines to verify plant-based products [45]. However, a safety assessment of herbal medicines can be complex because of the presence of multiple phytochemicals in medicinal plants [51].
On the other hand, the era of modern medicine began at the beginning of the 19th century [52]. Of all pharmaceutical products, 25% are herbal medicines, such as picrotoxin and aspirin [53]. The first drug isolated from plant sources was morphine, a benzylisoquinoline alkaloid derived from the opium poppy (Papaver somniferum), which was approved in 1827 as a pain-reliever [54]. The main advantage of conventional medicine is that the proper dosage is ensured, which is crucial because the dose of a drug determines whether it will have optimal effectiveness, cause toxicity, or have no effect [55]. The approval of a new drug is based on preclinical and clinical studies that provide information on the dose and administration route that produces therapeutic effects without adverse reactions [56]. Precise molecular pathways can be defined for a single molecule in a pill.
3. Herbal Medicine and Herbal Extracts in Depression
It is essential for today’s society to have more effective and less toxic antidepressants with fewer negative interactions with other drugs for the treatment of neurological, psychiatric, and neurodegenerative diseases [57,58,59]. Natural antidepressants may offer a solution to the limitations of synthetic antidepressants [60].
Many people worldwide, especially in developing countries, rely on alternative natural bioactive substances to treat depression because of their potential efficacy and lower financial burden compared to synthetic antidepressants [61,62]. In the past three decades, the use of natural medicines and supplements has increased, with 80% of the population using them as a primary form of healthcare [63,64]. Numerous plant species have been found to be efficacious in treating depression and central nervous system (CNS) diseases [65,66].
Plants contain natural components such as primary and secondary metabolites or other biomacromolecules, each of which plays a specific role in the organism [67]. Pharmacognosy studies the properties of plant-derived substances to discover new medicines, such as aspirin from willow bark [68]. However, the interactions between biologically active substances in plants are complex and influence the overall biological effects of the extract [69]. The idea that a single substance can treat a disease, known as the “silver bullet”, is now considered inadequate in clinical practice [70]. Herbal medicine is often referred to as the “herbal shotgun” because of the presence of many compounds in the extract. Multifactorial diseases are typically treated with a combination of drugs known as polypharmacy [69,70,71]. However, a recent study showed that polypharmacy leads to more adverse drug reactions and does not offer any benefits over monotherapies [72].
Biological effects can also be achieved through polyvalency, in which multiple components work together to produce a therapeutic effect. The multicomponent approach in herbal medicine creates alternative products with multiple targets and minimal side effects, making them suitable candidates for the treatment of CNS diseases [73]. Plants with favorable effects on the nervous system are, for example, genus Gladiolus [62], Hypericum [74], Crocus sativus [75,76], Aloysia [77], Hemerocallis [78], Allium [79], Piper methysticum [80], Lavandula officinalis [81,82,83], Rhodiola rosea [84], Ginkgo biloba [85,86], Salvia officinalis [87,88], Cannabis sativa [89], Convolvulus pluricaulis [90], Echinacea angustifolia [91], and many other plants, such as those used in Chinese medicine [92].
It has been shown that bioactive substances found in plants can have neuroprotective effects in the treatment of mental health conditions, such as depression and neurodegenerative diseases. These substances work through a variety of mechanisms, including the modulation of signaling pathways through different receptors, enzymes, and proteins [6,93,94]. Plant metabolites have been found to bind to neurotransmitter receptors and thus alter the synthesis and function of neurotransmitters [95]; regulate the CNS and endocrine system [96,97,98]; and exert anxiolytic, hypnotic, antidepressant, nootropic, sedative, analgesic [97], tonic, and adaptogenic effects [99]. The interconnected nature of the mechanisms of action in herbal medicine can also help to treat comorbidities, such as resolving anxiety when treating depression or alleviating depression when treating insomnia [100,101]. The effectiveness of herbal medicine is still being determined through “omics” technologies, such as pharmacogenomics, metabolomics, proteomics, and epigenetics [102,103].
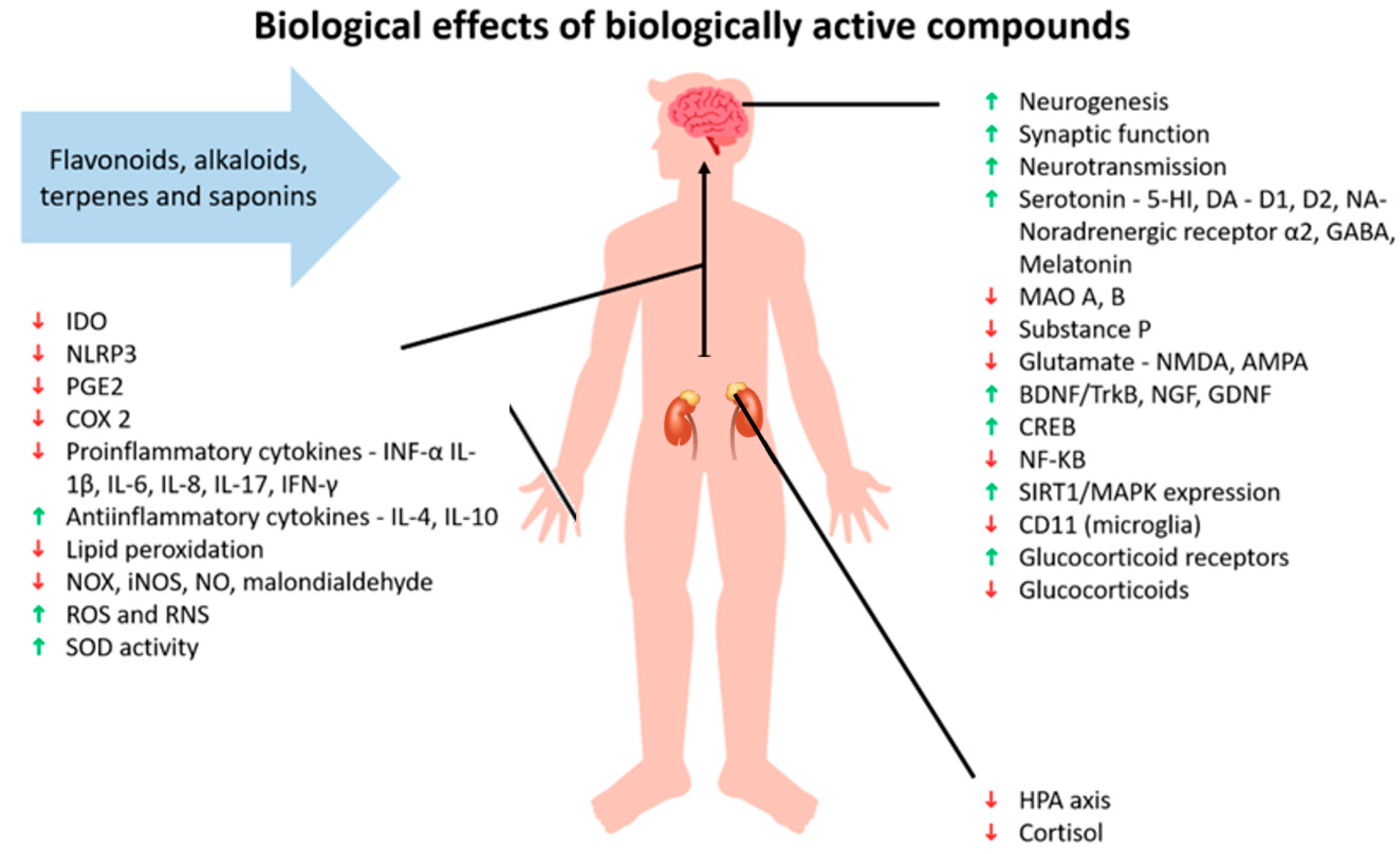
Herbomics involves the use of advanced technologies to study the effects of plants in phytotherapy and can provide clues about the toxicity and effects of given plants [101]. An example is a study that investigated the regulation of gene expression in the hypothalamus of male rats after treatment with imipramine (a tricyclic antidepressant) and Hypericum perforatum for eight weeks. The results showed that both treatments affected genes related to energy production/expenditure, cell structure, neurotransmission, fatty acid metabolism, protein synthesis/degradation, hormones, ion concentration, repetitive DNA sequences, transcription regulation, signal transduction, and synaptic transmission [104] (Figure 2). Another study found that Hypericum perforatum, clomipramine (a synthetic antidepressant), and a mixture of herbs called Xiao-yao-san changed the expression of different proteins that were mainly involved in energy metabolism. Both clomipramine and Hypericum perforatum were found to have similar gene expressions, as demonstrated by the increased expression of two forms of dihydropyrimidinase-related protein 2, which are involved in regeneration, the outgrowth of axons, and heat shock protein (Hsp-70), a neuronal protein-folding protein [105].
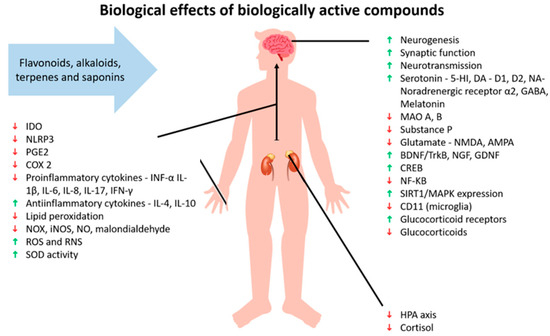
Figure 2.
Effects of biologically active compounds. Abbreviations: indoleam-ine-2,3-dioxygenase (IDO); NLR family pyrin domain containing 3 (NLRP3); prostaglandin E2 (PGE2); cyclooxygenase-2 (COX 2); tumor necrosis factor α (TNF-α); interleukin (IL); oxides of nitrogen (NOX); inducible nitric oxide synthase (iNOS); nitric oxide (NO); reactive oxygen species (ROS); reactive nitrogen species (RNS); superoxide dismutase (SOD); 5-hydroxytryptamine receptor (5-HT); dopamine (DA); dopamine receptor D1; dopamine receptor D2; noradrenaline (NA); γ-aminobutyric acid (GABA); monoamine oxidase (MAO); N-methyl-D-aspartate (NMDA); α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA); brain-derived neurotrophic factor/tropomyosin receptor kinase B (BDNF/TrkB); nerve growth factor (NGF); glial cell line-derived neurotrophic factor (GDNF); cAMP-response element-binding protein (CREB); nuclear factor kappa B (NF-κβ); sirtuin 1/mitogen-activated protein kinase (SIRT1/MAPK); CD11b (microglia marker); and hypothalamus-pituitary axis (HPA). Green arrow indicates support or increase of the individual matter, red arrow indicates decline or decrease of the individual matter.
Antidepressant effects of different plants have been demonstrated. Among them, Hypericum perforatum is a plant native to Asia and Europe [74,106] and is an invasive plant in North and South America, Australia, and South Africa [107]. Among the genus Hypericum, which also includes Hypericum perforatum, there are 450 species [108]. The plant is used in traditional herbal medicines, such as Greek medicine, traditional Chinese medicine, and Islamic traditional medicine [109], for the treatment of many various disorders [109,110,111]. It is used in the form of capsules, tea, tablets, or liquid extract [112].
The main biologically active substances responsible for the antidepressant effect are hyperforin (derivative of phloroglucinol), hypericin (naphthodianthrone) [113], and pseudohypericin (naphthodianthrone) [108]; however, the presence of rutin (a flavonoid) also plays a role in its antidepressant effects [114], as well as quercetin, kaempferol, leteolin [115], quercitrin, or hypeoside [108]. These substances can also prevent the relapse of depression [116]. The therapeutic effect of the plant has been investigated in studies not only with depression [117] but also with anxiety [118], and its anti-inflammatory, antinociceptive, healing effect (wounds) has also been observed [108]. The biologically active substances of Hypericum perforatum influence the levels of neurotransmitters such as norepinephrine, dopamine, and serotonin by the non-selective inhibition of their reabsorption, increasing the levels of 5-hydroxytryptamine 1A receptor (5-HT1A), 5-hydroxytryptamine 1B receptor (5-HT1B), and 5-hydroxytryptamine receptor 2 (5-HT2) and selectively inhibiting monoamine oxidase A (MAO A), monoamine oxidase B (MAO B), glutamate, dopamine, and β receptors in the prefrontal cortex [119,120]. In addition to the neurotransmitters mentioned, GABA and NMDA are affected [121,122,123]. During chronic stress, Hypericum perforatum had an antidepressant effect through the reuptake of serotonin, noradrenaline, and dopamine [124]. An extract of Hypericum perforatum also negatively affected the neuronal uptake of GABA and L-glutamate [125]. In vitro studies have shown that Hypericum perforatum inhibited the binding of [3H]flumazenil to benzodiazepine binding sites of γ-aminobutyric acid type A receptors (GABA-A) in a rat brain [126]. In rats, an increase in acetylcholine levels in the hippocampus and transcription of cyclic AMP-responsive element-binding protein (CREB), decarboxylase (GAD) in the bed nucleus of the stria terminalis, and proopiomelanocortin (POMC) in the pituitary gland were found [127]. In vitro studies with HT-22 cells have shown that Hypericum perforatum reduces lipopolysaccharide-induced inflammation and increases neuronal growth [128]. The extract from Hypericum perforatum also had an effect on the decrease in corticosterone and adrenocorticotropic hormone (ACTH) [129]. A recent study according to Song et al. (2022) reported an antidepressant effect of the aqueous extract of Hypericum perforatum during chronic unpredictable mild stress (CUMS) in rats and mice via the NLR family pyrin domain containing 1 (NLRP1) inflammasome and the CXC motif chemokine ligand/CXC motif chemokine receptor 2/brain-derived neurotrophic factor (CXCL/CXCR2/BDNF) signaling pathway [130]. Clinical studies have found the effect of Hypericum perforatum to be comparable to that of SSRIs and tricyclic antidepressants [131] but better tolerated [132]. However, the combination of Hypericum perforatum and synthetic antidepressants may reduce their efficacy by increasing cytochrome p450 activity [119,133]. Drug interactions with Hypericum perforatum via the modulation of cytochrome p450 may cause adverse effects, such as gastrointestinal symptoms, headache, dizziness, anxiety, fatigue, mania, confusion, neuropathy, alopecia, hypersensitivity, and sedation [108].
The herb has not been approved by the FDA and is considered a dietary supplement [134], even despite the fact that it has proven antidepressant effects in studies [122]. Some of the mechanisms of Hypericum perforatum are found in approved medications for the same complications [127]. Remotiv is a demonstrably commercial product containing Hypericum perforatum extract (referred to as Ze 117), which alleviates stress, nervous tension, and depression and balances emotional stability. The effectiveness of the product has been investigated in preclinical [135,136] and clinical studies [137,138]. Remotiv dry extract tablets at a dose of 250 mg improved short-term spatial and verbal memory in healthy volunteers. The nootropic effect was not observed at the 500 mg dose. Improvement in emotional balance and mood was observed at both doses. The mechanism of action of Remotiv may involve the enhancement of dopaminergic transmission [138]. The modulation of neurotransmitters such as serotonin, dopamine, and noradrenaline has been demonstrated in preclinical studies. Remotiv, when used long term, increases the concentration of neurotransmitters in the synaptic cleft by reducing the presynaptic reuptake of neurotransmitters [139,140,141,142]. One study suggests that Remotiv may reduce alcohol intake and thus may be helpful in the treatment of alcoholism [143]. The relationship of alcohol abuse with depression and anxiety is common [144]. In the treatment of mild-to-moderate MDD, in a systematic review that looked at 35 studies with the efficacy of Hypericum perforatum over a period of 4–12 weeks, the efficacy of Hypericum perforatum was higher than the placebo and similar to conventional antidepressants [145].
Rhodiola rosea (“golden root”) was located and identified by G.V. Krylov in 1961 during the expedition to the Altai Mountains in southern Siberia [146]. Rhodiola rosea is a plant used in traditional medicine in Eastern Europe and Asia [147,148] and in Russia [149]. In traditional medicine, it has been used to increase work productivity; reduce fatigue and impotence; resist symptoms of altitude sickness, anemia, infections, and gastrointestinal diseases; increase fertility; and address influenza, tuberculosis, cancer, scurvy, hemorrhoids, headache, and especially depression [149,150]. Rhodiola rosea contains up to 140 compounds found in underground parts of the plant [149]. It contains components such as herbacetin, rhodiosin belonging to flavonoids, geraniol (monoterpene), tyrosol, salidroside, phenylethanol derivatives belonging to triterpenes, and also rosarin, rosin, rosdirin, and rosavin belonging to phenylpropanoid glycosides [147,148]. When comparing Rhodiola rosea components, a stronger antidepressant effect in in vivo depression models at a dose of 0.26 mg/kg was achieved by rhodioloside/saliroside compared to five components—rosavin, rosin, tyrosol rhodioloside/saliroside, and rosarin. However, the strongest effect occurred with the combination of ingredients, which indicates a synergistic effect. Depending on the dose of 10, 20, or 50 mg of Rhodiola rosea extract in vivo in the forced swim test (FST), there was an increase in swimming time, which demonstrated an antidepressant effect and was higher compared to the administration of Hypericum perforatum and imipramine [151]. Studies in mice have shown that Rhodiola rosea extract can reduce depression-like behavior and increase exploratory behavior in models of CUMS [152]. Plant extracts at a dose of 1.5–6 g/kg have been shown to increase neurogenesis and the proliferation of stem cells in the hippocampus of rats [153]. It also affects the release of various neuropeptides and decreases HPA and G protein-coupled receptor (GPCR) activity [154]. The active ingredient, salidroside, has antidepressant effects in rats, possibly related to the regulation of inflammation and the HPA [155]. In addition, it has anti-inflammatory and neuroprotective effects [156].
Rhodiola rosea is considered a dietary supplement [157] and an adaptogen [158]. In the prevention and treatment of depression, Rhodiola rosea has shown efficacy [159]. The effects of Rhodiola rosea in the treatment of cognitive and psychiatric disorders are the same as those of conventional drugs. However, it does not lead to addiction and does not exceed the body’s ability to respond to and defend against stress, and it also does not prevent the body from returning to a functional state after the drug’s effect has worn off [87]. According to Yu et al. (2019), Rhodiola rosea alleviates depression and anxiety in patients through lipid peroxidation in the mitochondrial respiratory chain and the inhibition of reactive oxygen species (ROS) [160]. Clinical studies have also shown that it can improve mild-to-moderate depression without side effects; modulate neurotransmitters such as norepinephrine, serotonin, and dopamine; and decrease MAO A and MAO B activity [161,162]. In addition to these effects, an interesting finding from one study was that Rhodiola rosea extract at a dose of 500 mg reduced LTD (long-term depression) compared to the placebo, yet it did not affect cortical excitability [163]. Over 6 weeks, the extract from Rhodiola rosea in a dose of 340–680 mg in adult patients with mild and moderate depression had a beneficial effect [164]. In patients with mild anxiety, after 14 days of administration of 200 mg of Rhodiola rosea two times a day, anxiety, stress, depression, and anger were reduced compared to the placebo [165]. Rhodiola rosea does not show toxicity [157,166]. No side effects attributed to the effects of Rhodiola rosea have been reported in clinical studies [167]. In humans, a dose of 680 mg per os of Rhodiola rosea does not cause any serious side effects [164]. Also, in animal studies, Rhodiola rosea did not show acute or chronic toxicity [166,168]. With the exception of an older study in rats at a dose of 3.360 g/kg, it showed low toxicity [167]. Rhodiola rosea extract has shown an antidepressant effect in adults compared to a conventional antidepressant. Rhodiola rosea extract was well tolerated during short-term administration; however, it had no significant effect on mild anxiety [165]. The combined treatment of rhodiola and saffron was studied for mild depression. Oral administration of 154 mg rhodiola and 15 mg saffron twice daily for 6 weeks showed improvement in depression and anxiety in 45 adults [169].
In Chinese medicine, Ginko biloba has been used for more than 5000 years to treat cough, enuresis, and asthma [170]. In addition to terpenoids (diterpenes ginkgolides A, B, C, J, M, N, K, and L) and flavonoids (aromadendrin and 5,7,4 trihydroxy-flavones), Ginkgo biloba also contains polyprenols (di-trans-poly-cis-octadecaprenol), organic acids (ginkgolic acid), and also groups of flavones, flavanol glycosides, and aglycones, such as quercetin, rutin, kaempferol, apigenin, isorhamnetin, myricetin, and luteolin. Terpenoids protect against mitochondrial damage, which may act through two mechanisms: first, through platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) receptor antagonism, as platelet-activating factor (PAF) antagonists reduce neuronal damage and act preventively against ischemia; second, through the interaction of chloride channels [170], as terpenoids block the chloride channel in hippocampal neurons in rats [171]. In addition, terpenoids have vasoregulatory and antioxidant activity, free radical scavenging, and neuroprotective and protective effects against memory and learning disorders. Flavonoids also prevent the formation of ROS and change the expression of antioxidants [170]. Even a higher effect in suppression of free radicals was demonstrated compared to terpenoids [172]. Through the interaction of neuronal receptors, they influence the processes of learning, memory, and cognitive functions, as they cause cerebrovascular blood flow and synaptic plasticity [170]. In addition, they reduce proinflammatory cytokines, such as TNF-α, prostaglandin E2, IL-1β, and NF-kB [173]. Ginkgolic acids, the phenolic compounds isolated from the leaves and seeds of Ginkgo biloba, can cause genotoxic, cytotoxic, mutagenic, and carcinogenic effects [174]. For this reason, they are present in commercial preparations in amounts of <5 ppm. Ginkgo biloba has a beneficial effect on visual acuity, vertigo, atherosclerosis, tinnitus, and psychological and neurological disorders [170]. In addition, it has been shown in vitro that Ginkgo biloba protects neurons from degeneration during ischemic events [175]. Ginkgo biloba has demonstrated antidepressant effects in LH models in rats, where a dose of 150 mg/kg with 24% flavonoids and 6% terpenoids was found to prevent the corticosterone stress response (when administered two weeks before the test), although it did not improve the acquisition of active avoidance [176]. The reduction in depression-like behavior may be due to a reduction in MAO B, NO, and GABA [177].
The commercially available Ginkgo biloba extract EGb-761 balances monoamine activity, which is involved in anxiety and mood disorders [178]. The application of the EGb-761 extract increased the levels of serotonin and 5-hydroxyindoleacetic acid (associated with spatial memory) [179]. Although short-term administration of the EGb-761 extract did not affect serotonin levels, it increased noradrenaline and dopamine in the PFC [180]. In vitro, MAO inhibition was demonstrated by the EGb-761 extract [178] and also an increase in dopamine, noradrenaline, and acetylcholine neurotransmission [181]. The ginkgolide B component of the EGb-761 extract has an antidepressant effect, as it suppresses the increase in corticosteroids through the influence of benzodiazepine receptors [182]. The component ginkgolide B inhibited CRH and arginine vasopressin after per os application to rats for 14 days [183]. The in vitro extract EGb-761 increased testosterone and suppressed prolactin, which increases sexual behavior [184], which is often altered in psychiatric diseases [170]. BDNF is increased after the application of extract EGb-761 [185]. Ginko biloba leaf extracts are used as tablets, oral liquids, or injectable solutions [170]. Orally administered, EGb-761 extract is absorbed in the intestine and metabolized by the flora according to a pharmacokinetic study [186]. The data from the pharmacokinetics revealed terpene trilactones and flavonoids from Ginkgo biloba in the blood and in the brain, indicating that they may cross the blood–brain barrier [173]. Ginkgo biloba is approved in Germany for the treatment of dementia and used in the USA for the treatment of Alzheimer’s disease and dementia [170]. However, based on insufficient and inconsistent evidence, the FDA has not approved the Ginkgo biloba EGb-761 extract preparation for medical use, only as a supplement food product [187].
Crocus sativus, also known as saffron, is grown in hot and dry areas, such as Iran, Tibet, China, and India [188]. It has traditionally been used in Persian medicine to treat depression [189]. In clinical studies, 30 mg/kg of Crocus sativus was found to be equally effective in treating mild-to-moderate depression compared to 20 mg/kg fluoxetine and resulted in a reduction in depressive symptoms after 6 weeks, similar to the effects of 100 mg/kg imipramine and 40 mg/day citalopram (vs. 30 mg/day of Crocus sativus) [190,191,192]. The administration of 50 mg Crocus sativus twice daily for 12 weeks also decreased depression and anxiety [193]. A 6-week clinical study showed that Crocus sativus reduced symptoms of generalized anxiety disorder, whereas sertraline was not effective [194]. The active ingredients responsible for the antidepressant and anxiolytic effects of Crocus sativus include safranal [195] and crocin [196]. Safranal (an aldehyde organic compound and monoterpene belonging to essential oils) and crocin (a carotenoid and monoterpene belonging to essential oils) are involved in modulating neurotransmitters, such as glutamate, GABA, dopamine, noradrenaline, and serotonin [195,197,198,199,200]. Crocin inhibits the activities of cytochrome p450 family 2B (CYP2B), cytochrome p450 family 3A (CYP3A), cytochrome p450 family 2A (CYP2A), and cytochrome p450 family 2 C11 (CYP2C11), whereas safranal increases the activities of CYP3A, CYP2B, and CYP2C1 [201]. As reviewed, clinical studies have evaluated doses ranging from 20 to 400 mg/day of pure saffron. Dosages of up to 1.5 g/day of saffron are thought to be safe; toxic effects have been reported for 5 g doses. However, for mild-to-moderate depression, up to 30 mg/day of saffron extract (stigma or petal) is used [202].
Melissa officinalis, commonly used in Europe for treating the nervous system, has been found to exhibit antidepressant effects. This plant contains flavonoids, which are believed to be responsible for its antidepressant properties. Studies have shown that Melissa officinalis decreases the γ-aminobutyric acid transaminase (GABA-T) levels in the hippocampus [203]. In a double-blind randomized pilot study, Mellisa officinalis (2 g daily) showed a similar effect to fluoxetine in mild-to-moderate depression [204]. It ameliorated the depressive-like behavior of rats in a forced swim test via regulating the serotonergic neurotransmitters [22]. In another study, Mellisa was administered as supplementation for patients with depression, anxiety, and stress disorders. A total of 80 patients were included in the study. Eight-week supplementation with Mellisa capsules (3 g daily) resulted in decreased depression, anxiety, and stress-related symptoms [205]. The current evidence suggests that lemon balm (Mellisa officinalis) may be effective in improving anxiety and depressive symptoms, particularly in the acute setting [21]. The existing research indicates that Mellisa officinalis holds promise as a calming agent exhibiting both anxiolytic and antidepressant properties and can elicit cognitive and sleep-quality enhancement [23].
Flavonoids, unsaturated sterols, and saponins are responsible for the antidepressant effect of Echium amoenum occurring in Europe and the northern part of Iran [206,207]. The effect of flavonoids is comparable to that of SSRIs and tricyclic antidepressants (fluoxetine and imipramine) [208] and is more effective and better tolerated than SSRIs (citalopram) after 8 weeks [209]. Clinical trials and animal models have shown antidepressant, anti-inflammatory, analgesic, and antioxidant effects of Echium amoenum [210,211,212]. After 2 weeks, serotonin and dopamine levels in the cerebrospinal fluid were increased after oral administration of 125 mg/kg Echium amoenum in reserpine-induced depression in male Wistar rats [210]. Fifteen days after the administration of a dose of 5 mg/kg, Echium amoenum improved depression-like behavior (induced by Mn2+) in the FST, immobility time, body weight gain, and sucrose preference and reduced ROS, neurotoxicity, and apoptosis in the hippocampus [213]. In animal studies, the administration of Echium amoenum has been found to increase serotonin and dopamine levels, improve depression-like behavior, and reduce oxidative stress and neurotoxicity in the hippocampus [214,215,216,217].
Curcuma longa, also known as turmeric, is used as an antidepressant in China and India. Clinical trials have shown that curcumin, a component of Curcuma longa, reduced depression symptoms over 8 weeks [218]. Turmeric increases dopamine, BDNF, and serotonin levels [219]; inhibits the NLR family pyrin domain containing 3 (NLRP3), interleukin-1 beta (IL-1β), and nuclear factor kappa-light-chain-enhancer (NF-κB) of activated B cells, MAO A, and MAO B [220]; and causes a decrease in cortisol levels in saliva [221]. In a rodent model of depression, curcumin prevented memory loss, reduced anhedonia, improved abnormal levels of BDNF and extracellular-signal-regulated kinase (ERK), and reduced (ameliorated) autonomic activity [222]. In the FST and elevated plus maze (EPM) tests, curcumin showed antidepressant effects [223].
Humulus lupulus is commonly known as hops and has been found to improve symptoms of depression and anxiety in young people after four weeks of use [224]. This is believed to be due to the presence of various components such as humulone, xanthohumol, resveratrol, and others, which exert anxiolytic effects via the GABA receptor [216]. Hops have also been found to inhibit various P450 enzymes, such as CYP2C9, CYP1A2, CYP2C19, and CYP2C8 [215].
Lichen secondary metabolites and their biological properties have gained scientific attention from the beginning of the 20th century [225]. There are more than 1000 known lichen secondary metabolites so far [226]. All the secondary metabolites of lichens are of fungal origin in the form of crystals on the surface of hyphae and are not adequately soluble in water and are usually isolated from lichens using organic solvents [227]. The most studied lichen secondary metabolites include usnic acid, atranorin (ATR), or gyrophoric acid (GA). GA, as the most abundant lichen secondary metabolite in the Umbilicaria families [228,229], showed significant anxiolytic activity in an EPM test in laboratory animals with depression-like behavior when compared to untreated animals. Moreover, the neurogenesis level was restored to the level of healthy animals [230]. ATR, a dominant lichen secondary metabolite mainly in the families of Cladoniaceae [231], also showed significant anxiolytic activity in the EPM, as it was able to elevate the number of rearings and prolong the time spent in the open arms of the EPM test in treated animals when compared to the untreated depression-like group. The neurogenesis level as well as the level of mature neurons was increased [232].
Many other plants (such as many from Chinese medicine) have shown antidepressant effects; however, because of the limitations of this manuscript, only a select few have been described.
4. Biologically Active Substances with Antidepressant Effect
Plant metabolites with antidepressant effects have diverse chemical structures, including isoprenoids (terpenes), phenolic compounds, and alkaloids [233].
4.1. Flavonoids
Flavonoids (Table 1), a subgroup of secondary metabolites described by the diphenylpropane structure, are found in plants, fruits, and vegetables and have been shown to have health benefits, including the prevention of various diseases [234,235] and treatment of depression [236,237,238]. Specifically, flavonoid compounds are highlighted as robust defenders, addressing oxidative stress and inflammation to avert chronic illnesses [225]. Flavonoids include different classes, such as flavones, anthocyanins, flavonols, flavanols, catechins, flavanones, flavanonols, and chalcones [239]. Unsubstituted flavones and flavanones display the strongest antifungal activity; however, this activity generally decreases with the addition of methyl or hydroxyl groups to the flavone structure, though there are exceptions [226]. Anthocyanins, such as cyanidin-3-O-glucoside chloride, were shown to prevent the development and progression of urethane-induced lung cancer by regulating energy metabolism in mice [227]. The most famous catechin, epigallocatechin gallate (EGCG), may alleviate depression through interactions with gut microbiota and other mechanisms [229]. Moreover, the potential benefits of flavonoids for depression may be attributed to their antioxidant properties [240,241].
St John’s wort is a popular herbal remedy recommended by traditional Chinese medicine (TCM) practitioners and is licensed and widely prescribed for depression in many European countries up to now [228]. For patients with mild-to-moderate depression, St John’s wort has comparable efficacy and safety when compared with SSRIs [74], based on filtered data of FDA-approved and non-approved investigational antidepressive agents used in trials studying major depression [232]. During the analysis of investigational antidepressive agents, several drugs emerged as the most common candidates for study. Among the investigational drugs, several reached the highest phase, phase 4. These drugs include buprenorphine, reboxetine, tianeptine, mianserin, hypericin, and melitracen [232]. Hypericin is an excellent lead molecule since it differs structurally and mechanistically from all known antidepressants. There are hundreds of scientific publications dealing with the effects of hypericin during depression. The molecular mechanisms of action of hypericin include increasing presynaptic efficiency in the hippocampus [43], repairing the dysfunction of gap junctions in depression [242], neuroinflammation attenuation via the NLRP3 pathway [243], and many others.
As a counterpoint to hypericin, myricetin was selected to include in this publication. Myricetin has various pharmacological effects, including antioxidant, antiapoptotic, anti-photoaging, anticancer, antidiabetic, and anti-inflammatory effects [244]. Chronic administration of myricetin 50 mg/kg daily (i.p. 1 h before stress) for 21 days reduced immobility time in the FST and TST, thereby showing reducing depressive-like behavior, improved glutathione peroxidase activity in mice exposed to chronic stress, reduced plasma corticosterone, and normalized BDNF levels in the hippocampus, which may indicate an antioxidant effect [245]. In another study, albino mice and albino rats administered myricetin from Vitis vinifera Linn. at a dose of 10, 30, or 100 mg/kg showed anxiolytic effects in the EPM, open field test (OFT), light/dark apparatus test, and hole board apparatus test and after the administration of lithium 200 mg/kg and meta-chlorophenyl piperazine (MCP). Individual doses of myricetin were administered per os 1 h before the individual tests. Lithium was i.p. 1 h after myricetin administration and MCP 30 min before myricetin administration [246]. Myricetin increased neuronal proliferation, growth, and their survival, especially in the SVZ and SGZ [247]. In the last 5 years, myricetin was studied more for brain injury [248], post-ischemic neurodegeneration [249], or epilepsy [250], rather than depression. One study revealed that myricetin inhibited fear and anxiety-like behavior by HPA regulation and activation of the BDNF-ERK signaling pathway in posttraumatic stress disorder in Sprague-Dawley rats [230].
4.2. Alkaloids
The main sources of alkaloids are plants from the Papaveraceae, Ranunculaceae, Amaryllidaceae, and Solanaceae families [251,252]. These alkaloids are used to treat neurodegenerative diseases and include isoquinoline, oxindole, indole, pyrroindole, aporphine, piperidine, vinca, pyridine, β-carboline, and methylxantheme derivatives [253,254]. Indole, pyridine, and aporphine are agonists of muscarinic and adenosine receptors and antagonists of dopamine, nicotine, and NMDA, respectively. They also inhibit acetylcholinesterase, butyrylcholinesterase, and monooxygenase [253].
Indoles are a class of compounds that consist of carbon-nitrogen rings. They are found in various substances, such as mushroom alkaloids (psilocybin and ergine) and the drug lysergic acid diethylamide, and can affect serotonin activity in the brain [255].

Table 1.
Selected biologically active compounds with antidepressant effects.
Table 1.
Selected biologically active compounds with antidepressant effects.
| Compound | Dose | Model | Activity | Ref. | |
|---|---|---|---|---|---|
| flavonoids | hypericin | 0.1–1 µM | ex vivo hippocampi from newborn Sprague-Dawley rats | enhancing presynaptic efficiency | [55] |
| 0.2 mg/kg | male Sprague-Dawley rats | increase in 5-HT levels in hypothalamus decrease in norepinephrine in hippocampus | [256] | ||
| various | 3808 human patients | comparable response to SSRIs | [74] | ||
| rhodiosin | 2 × 200 mg | 50 human adults | improvement in mental speed improvement in mental resources | [257] | |
| 340 mg | 57 human patients | less antidepressant effect versus sertraline (50 mg) fewer adverse effects, well tolerated | [162] | ||
| myricetin | 50 mg/kg | male C57BL/6 mice | reduction in the immobility time in FST and TST normalization of BDNF levels improvement in glutathione peroxidase activity reduction in corticosterone in plasma | [245] | |
| 10, 30, 100 mg/kg | albino mice and albino rats (strains not listed) | increase in time spent in open arms, number of entries in open arms in EPM increase in rearing in OFT increase in time spent in the lit area, number of transitions in light/dark apparatus increase in head poking in hole board apparatus reduction in lithium-induced head twitches reversal of MCP-induced anxiety | [246] | ||
| luteolin | 10, 30, 40 mg/kg | SPF male mice | ameliorating depressive-like behaviors promoting the Arg-1+ microglial phenotype reducing microglial proinflammatory responses reversing microglial phagocytosis-mediated synapse loss | [258] | |
| terpenes | hyperforin | 2.5, 5 mg/kg | male C57BL/6 J mice | reversed behavior in TST, FST, and splash test increased Zn concentration in hippocampus increased BDNF in hippocampus | [259] |
| 1, 5 mg/kg | B6J mice | activation of the channel via motif at TRPC6 anxiolytic and antidepressant effects (OFT) | [260] | ||
| safranal | 28 mg/day | 128 healthy adults | decrease in negative mood and symptoms related to stress and anxiety | [261] | |
| 50, 200 mg/kg | 30 male Wistar rats | no effect in EPM increase in swimming time in FST increase consumption of sweet solution | [262] | ||
| geraniol | 20, 40 mg/kg | male ICR mice | alleviation the depression-related behaviors of CUMS-exposed mice regulating IL-1β-related neuroinflammation | [263] | |
| alkaloids | berberine | 10, 20 mg/kg | male Wistar rats | increase in serotonin, dopamine, and noradrenaline levels reduction in substance P reduction in lipid peroxide levels decrease immobility time in FST | [264] |
| 50, 100 mg/kg | male ICR mice | decrease in levels of proinflammatory cytokines in hippocampus | [265] | ||
| linalool | 40, 200 mg/kg | male Sprague-Dawley rats | increase in sucrose preference normalization of behavior in OFT decrease motionless time reduction in inflammation in gastrointestinal organs | [266] | |
| huperzine A | 0.05, 0.15 mg/kg | male Sprague-Dawley rats | increase in neurological deficit score increase in cognitive function increase in 5-HT, DA, noradrenaline, and BDNF level in hippocampus and 5-HT and DA in prefrontal cortex | [267] | |
| galantamine | 0.02, 0.2, 2.0 mg/kg | C57BL/6J mice | antidepressant-like effects in FST | [53] |
Abbreviations: 5-hydroxytryptamine receptors (5-HT); selective serotonin reuptake inhibitors (SSRIs); superoxide dismutase (SOD); brain-derived neurotrophic factor (BDNF); elevated plus maze (EPM); metachlorophenylpiperazine (MCP); open field test (OFT); tumor necrosis factor α TNF-α; interleukin-1β (IL-1β); subunit nuclear fac-tor kappa B (NF-κβ p56); forced swim test (FST); inducible nitric oxide synthase (iN-OS); nuclear factor kappa B (NF-κβ); CD11b (microglia marker); transient receptor potential canonical 6 channel (TRPC6); 5-hydroxytryptamine 1A receptor (5-HT1A); cAMP-response element-binding protein (CREB); phosphorylated cAMP-response element-binding protein (p-CREB); dopamine (DA); Bcl-2-associated X protein (Bax); B-cell lymphoma-2 (Bcl-2); spared nerve injury (SNI); and canonical 6 channel (TRPC6).
4.3. Terpenes and Saponins
Natural bioactive substances such as cyclodepsipeptides and diterpenes are secondary metabolites that exhibit neuroprotective effects by interacting with the GABA-A receptor [268]. Additionally, the nervous system can be enhanced by compounds, such as γ-terpinenes, monoterpenes, and β-pinenes [269].
Hyperforin demonstrated its antidepressant effect when administered to mice daily at a dose of 2.5 and 5 mg/kg i.p. for 3 weeks, when it reversed depressive-like behavior induced by CUMS in the TST, FST, or splash test. It also increased the concentration of Zn and BDNF in the hippocampus [259]. The anxiolytic and antidepressant effect of hyperforin was demonstrated by activating TRPC6 via a specific binding motif, which excited hippocampal neurons in mice and significantly reversed anxiety-like behavior in the OFT. TRPC6 deficiency not only causes anxiety and depression but also reduces the excitability of the CA1 region in the hippocampus [260].
The administration of geraniol for 3 weeks at a dose of 20 and 40 mg/kg had antidepressant effects, as it reversed the effects of CUMS in the sucrose preference test, TST, and FST and reversed the increase in IL-1β induced by CUMS, probably through NF-κB and NLRP3 [263].
Safron from Crocus sativus (affron®) administered to 128 adult participants for 4 weeks at a dose of 28 mg/day, but not at a dose of 22 mg/day, reduced negative mood and symptoms related to anxiety and stress with no side effects [261]. In another study, 50 p.o. and 200 mg/kg i.p. of affron® showed anxiolytic and antidepressant effects in the FST and sucrose preference test. In the EPM test, no significant effect was observed [262].
5. Potential Risks of Herbal Medicine
There has been growing interest from both the scientific community and the public regarding the connection between dietary bioactive compounds and human health. Because many beneficial effects of natural compounds on human health have been described, they are studied very intensively. What must be mentioned are some potential risks, such as low bioavailability, drug interactions, the lack of standardization of natural supplements, and many others. There are several factors influencing the solubility and bioavailability of natural compounds, such as their molecular structure, food matrix effects, transporters, pH variations, and gut microbiota metabolism [270]. The bioavailability of these compounds is critical for understanding their potential health benefits [271]. Recently, this challenge tried to be overcome by structural modifications, colloidal systems, and nanotechnology, aiming to increase the absorption and bioavailability of bioactive compounds [272,273].
Another problem may be drug–herb interactions because natural products may interact with drugs by affecting the biological processes that regulate their metabolism and elimination. Until now, little is found in the literature reporting on the subject “herb–drug interaction,” where most of the reports have been reviews [274]. Even despite the recent efforts to encourage the reporting of adverse drug reactions, the number of reports remains relatively low. Therefore, there is a need for global regulatory harmonization of herbs [275].
6. Conclusions
Herbal medicines have been used for thousands of years to treat depression and various diseases. The therapeutic effects of herbal medicines have been proven in the form of monotherapy and complementary therapy for the treatment of mild-to-moderate depression. Many animal models have confirmed the effects (reduction in depressive-like behavior) and neurochemical changes of synthetic antidepressants and plants from herbal medicine. However, these effects have only been demonstrated in a small sample of patients. Despite these facts, it is important that the mechanisms, efficacy, and safety are more clearly demonstrated in both animal and clinical studies.
Conservation, rigorous research grounded in traditional knowledge, stringent quality control, and thorough documentation are crucial in the 21st century to advance the use of herbal medicine.
Author Contributions
Conceptualization, N.U. and T.K.; methodology, N.U. and T.K.; software, N.U.; validation, N.U. and T.K.; formal analysis, N.U., T.K., and T.J.A.; investigation, N.U. and T.K.; resources, N.U. and T.K.; writing—original draft preparation, N.U.; writing—review and editing, N.U., S.M., P.S., and T.K.; visualization, N.U. and T.J.A.; supervision, T.K.; project administration, T.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences, VEGA (1/0648/25).
Acknowledgments
We cordially thank Jan Koval for his enthusiasm and never-ending help with the proofreading.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-HT | 5-hydroxytryptamine |
| 5-HT1A | 5-hydroxytryptamine 1A receptor |
| 5-HT1B | 5-hydroxytryptamine 1B receptor |
| 5-HT2 | 5-hydroxytryptamine receptor 2 |
| ACTH | adrenocorticotropic hormone |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| BDNF | brain-derived neurotrophic factor |
| BDNF/TrkB | brain-derived neurotrophic factor/tropomyosin receptor kinase B |
| CD11b | microglia marker |
| COX 2 | cyclooxygenase-2 |
| CREB | cAMP-response element-binding protein |
| CRH | corticotrophin-releasing hormone |
| CXCL | CXC motif chemokine ligand |
| CXCR2 | CXC motif chemokine receptor 2 |
| CYP2A | cytochrome p450 family 2A |
| CYP2B | cytochrome p450 family 2B |
| CYP2C11 | cytochrome p450, subfamily 2, polypeptide 11 |
| CYP2C9 | Cytochrome p450 family 2 subfamily C member 9 |
| CYP1A2 | cytochrome p450 family 1A2 |
| CYP2C19 | Cytochrome p450 2C19 |
| CYP2C8 | cytochrome p450 family 2 subfamily C member 8 |
| CYP3A | cytochrome p450, family 3, subfamily A |
| D1 | dopamine receptor 1 |
| D2 | dopamine receptor 2 |
| DA | dopamine |
| EPM | elevated plus maze |
| ERK | extracellular-signal-regulated kinase |
| EtOH | ethyl alcohol |
| FDA | Food and Drug Administration |
| FST | forced swim test |
| GABA | γ-aminobutyric acid |
| GABA-A | γ-aminobutyric acid type A receptors |
| GABA-T | γ-aminobutyric acid transaminase |
| GAD | decarboxylase |
| GDNF | glial cell line-derived neurotrophic factor |
| GPCR | G protein-coupled receptor |
| HPA | hypothalamus–pituitary axis |
| IDO | indoleam-ine-2,3-dioxygenase |
| IL | interleukin |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| MAO | monoamine oxidase |
| MAO A | monoamine oxidase A |
| MAO B | monoamine oxidase B |
| MCP | metachlorophenylpiperazine |
| MDDs | major depressive disorders |
| MRI | magnetic resonance imaging |
| NA | noradrenaline |
| NF-kB | nuclear factor kappa B |
| NF-κβ p56 | subunit nuclear factor kappa B |
| NGF | nerve growth factor |
| NLRP1 | NLR family pyrin domain containing 1 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| NOX | oxides of nitrogen |
| OFT | open field test |
| PAF | platelet-activating factor |
| p-CREB | phosphorylated cAMP-response element-binding protein |
| PGE2 | prostaglandin E2 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SIRT1/MAPK | sirtuin 1/mitogen-activated protein kinase |
| SNI | spared nerve injury |
| SOD | superoxide dismutase |
| SSRIs | selective serotonin reuptake inhibitors |
| TNF-α | tumor necrosis factor α |
| TRPC6 | transient receptor potential canonical 6 channel |
| WHO | World Health Organization |
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Leskanicova, A.; Babincak, M.; Mochnacky, F.; Kukelova, D.; Urbanska, N.; Kolesarova, M.; Macekova, D.; Kostolny, J.; Kiskova, T. Sex-dependent differences in stress-induced depression in Wistar rats are accompanied predominantly by changes in phosphatidylcholines and sphingomyelins. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72, 72. [Google Scholar]
- Bains, N.; Abdijadid, S. Major depressive disorder. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2022, 4, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Galts, C.P.; Bettio, L.E.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef]
- Penner, I.-K.; Paul, F. Fatigue as a symptom or comorbidity of neurological diseases. Nat. Rev. Neurol. 2017, 13, 662–675. [Google Scholar] [CrossRef]
- Alshaya, D.S. Genetic and epigenetic factors associated with depression: An updated overview. Saudi J. Biol. Sci. 2022, 29, 103311. [Google Scholar] [CrossRef]
- Belmaker, R.H.; Agam, G. Major depressive disorder. New Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Neuroinflammation, gut-brain axis and depression. Psychiatry Investig. 2019, 17, 2. [Google Scholar] [CrossRef]
- Hara, T.; Owada, Y.; Takata, A. Genetics of bipolar disorder: Insights into its complex architecture and biology from common and rare variants. J. Human Genet. 2023, 68, 183–191. [Google Scholar] [CrossRef]
- Ikeda, M.; Takahashi, A.; Kamatani, Y.; Okahisa, Y.; Kunugi, H.; Mori, N.; Sasaki, T.; Ohmori, T.; Okamoto, Y.; Kawasaki, H. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol. Psychiatry 2018, 23, 639–647. [Google Scholar] [CrossRef]
- Gordovez, F.J.A.; McMahon, F.J. The genetics of bipolar disorder. Mol. Psychiatry 2020, 25, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Mandhare, A.; Tryphena, K.P.; Srivastava, S.; Shaikh, M.F.; Singh, S.B.; Khatri, D.K. Epigenetics in depression and gut-brain axis: A molecular crosstalk. Front. Aging Neurosci. 2022, 14, 1048333. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, W.-t.; Chen, J.-j.; Zhong, Q.; Wu, D.; Niu, L.; Wang, S.; Zeng, Y.; Wang, Y. Tryptophan metabolism as bridge between gut microbiota and brain in chronic social defeat stress-induced depression mice. Front. Cell. Infect. Microbiol. 2023, 13, 1121445. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Mandal, S.; Sinha, V.K.; Goyal, N. Efficacy of ketamine therapy in the treatment of depression. Indian J. Psychiatry 2019, 61, 480–485. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Ghazizadeh, J.; Sadigh-Eteghad, S.; Marx, W.; Fakhari, A.; Hamedeyazdan, S.; Torbati, M.; Taheri-Tarighi, S.; Araj-khodaei, M.; Mirghafourvand, M. The effects of lemon balm (Melissa officinalis L.) on depression and anxiety in clinical trials: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 6690–6705. [Google Scholar] [CrossRef]
- Lin, S.-H.; Chou, M.-L.; Chen, W.-C.; Lai, Y.-S.; Lu, K.-H.; Hao, C.-W.; Sheen, L.-Y. A medicinal herb, Melissa officinalis L. ameliorates depressive-like behavior of rats in the forced swimming test via regulating the serotonergic neurotransmitter. J. Ethnopharmacol. 2015, 175, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Villa, R. The neurobiology of depression: An integrated overview from biological theories to clinical evidence. Mol. Neurobiol. 2017, 54, 4847–4865. [Google Scholar] [CrossRef]
- Kamran, M.; Bibi, F.; Ur Rehman, A.; Morris, D.W. Major Depressive Disorder: Existing Hypotheses about Pathophysiological Mechanisms and New Genetic Findings. Genes 2022, 13, 646. [Google Scholar] [CrossRef]
- Li, Y.F. A hypothesis of monoamine (5-HT)—Glutamate/GABA long neural circuit: Aiming for fast-onset antidepressant discovery. Pharmacol. Ther. 2020, 208, 107494. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Chouinard, G. Chapter 7—The Monoamine Hypothesis of Depression Revisited: Could It Mechanistically Novel Antidepressant Strategies? In Neurobiology of Depression; Quevedo, J., Carvalho, A.F., Zarate, C.A., Eds.; Academic Press: London, UK, 2019; pp. 63–73. [Google Scholar]
- Noel, C. Antidepressants and suicidality: History, the black-box warning, consequences, and current evidence. Ment. Health Clin. 2015, 5, 202–211. [Google Scholar] [CrossRef]
- Latendresse, G.; Elmore, C.; Deneris, A. Selective Serotonin Reuptake Inhibitors as First-Line Antidepressant Therapy for Perinatal Depression. J. Midwifery Women’s Health 2017, 62, 317–328. [Google Scholar] [CrossRef]
- Anagha, K.; Shihabudheen, P.; Uvais, N.A. Side Effect Profiles of Selective Serotonin Reuptake Inhibitors: A Cross-Sectional Study in a Naturalistic Setting. Prim. Care Companion CNS Disord. 2021, 23, 35561. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Zhang, H.; Hong, W.; Zhang, L.; Mi, W.; Qin, J.; He, Y. Reliability and validity of the Chinese version of the Oxford depression questionnaire (ODQ-Chinese). J. Affect. Disord. 2022, 313, 278–282. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. SSRI-induced indifference. Psychiatry 2010, 7, 14. [Google Scholar]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Rickels, K.; Shiovitz, T.M.; Ramey, T.S.; Weaver, J.J.; Knapp, L.E.; Miceli, J.J. Adjunctive therapy with pregabalin in generalized anxiety disorder patients with partial response to SSRI or SNRI treatment. Int. Clin. Psychopharmacol. 2012, 27, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Dresler, T.; Guhn, A.; Tupak, S.V.; Ehlis, A.-C.; Herrmann, M.J.; Fallgatter, A.J.; Deckert, J.; Domschke, K. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. J. Neural Transm. 2013, 120, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [CrossRef]
- Hamati, R. Insights into the Neurobiology of Anxiety and a Potential Target for Pharmacotherapy. J. Neurosci. 2018, 38, 8919–8921. [Google Scholar] [CrossRef]
- Kalin, N.H. The critical relationship between anxiety and depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Levine, J.; Cole, D.P.; Chengappa, K.R. Anxiety disorders and major depression, together or apart. Depress. Anxiety 2001, 14, 94–104. [Google Scholar] [CrossRef]
- World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Kumar, V. Herbal medicines: Overview on regulations in India and South Africa. World J. Pharm. Res. 2017, 6, 690–698. [Google Scholar] [CrossRef]
- World Health Organization. The Promotion and Development of Traditional Medicine: Report of a WHO Meeting [Held in Geneva from 28 November to 2 December 1977]; World Health Organization: Geneva, Switzerland, 1978. [Google Scholar]
- Msomi, N.Z.; Simelane, M.B. Herbal Medicine; InTech: Rijeka, Croatia, 2019; pp. 215–227. [Google Scholar]
- Qazi, M.; Molvi, K. Herbal medicine: A comprehensive review. Int. J. Pharm. Res. 2016, 8, 1–5. [Google Scholar]
- Goyal, M.; Nagori, B.; Sasmal, D. Ayurveda the Ancient Science of Healing: An Insight; IntechOpen: London, UK, 2012. [Google Scholar]
- Watanabe, K.; Matsuura, K.; Gao, P.; Hottenbacher, L.; Tokunaga, H.; Nishimura, K.; Imazu, Y.; Reissenweber, H.; Witt, C.M. Traditional Japanese Kampo medicine: Clinical research between modernity and traditional medicine—The state of research and methodological suggestions for the future. Evidence-Based Complement. Altern. Med. 2011, 2011, 513842. [Google Scholar] [CrossRef] [PubMed]
- Che, C.-T.; George, V.; Ijinu, T.; Pushpangadan, P.; Andrae-Marobela, K. Traditional medicine. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 15–30. [Google Scholar]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Sabharwal, M. Pathophysiology of kidney, gallbladder and urinary stones treatment with herbal and allopathic medicine: A review. Asian Pac. J. Trop. Dis. 2013, 3, 496–504. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Otten, W.; Kanitz, E.; Gräbner, M.; Tuchscherer, A.; Bellmann, O.; Rehfeldt, C.; Metges, C.C. Effects of inadequate maternal dietary protein: Carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet. Res. 2012, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. Multiple cholinesterase inhibitors have antidepressant-like properties in the mouse forced swim test. Behav. Brain Res. 2021, 409, 113323. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Qi, Z. Hypericin prolongs action potential duration in hippocampal neurons by acting on K+ channels. Br. J. Pharmacol. 2010, 159, 1402–1407. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Wang, B.; Xie, L.; Chen, W. New FDA drug approvals for 2024: Synthesis and clinical application. Eur. J. Med. Chem. 2025, 285, 117241. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Mason, B.; Naik, A. Depression: Managing Resistance and Partial Response to Treatment. Am. Fam. Physician 2024, 109, 410–416. [Google Scholar]
- Paganin, W. Multifamily therapy in difficult-to-treat depression: An integrated and promising approach to rethinking clinical strategies. Front. Psychiatry 2024, 15, 1484440. [Google Scholar] [CrossRef]
- Parker, G. A revisionist model for treatment-resistant and difficult-to-treat depression. Aust. N. Z. J. Psychiatry 2024, 58, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, Y.; Lu, M.; Wang, Q. Review of Herbal Medicines for the Treatment of Depression. Nat. Product. Commun. 2022, 17, 1934578X221139082. [Google Scholar] [CrossRef]
- Aquib, M.; Najmi, A.; Akhtar, M. Antidepressant effect of thymoquinone in animal models of depression. Drug Res. 2015, 65, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Matraszek-Gawron, R.; Chwil, M.; Terlecka, P.; Skoczylas, M.M. Recent studies on anti-depressant bioactive substances in selected species from the genera Hemerocallis and Gladiolus: A systematic review. Pharmaceuticals 2019, 12, 172. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Tallón-Ballesteros, A.J. Modern Management Based on Big Data III: Proceedings of MMBD 2022; IOS Press: Amsterdam, The Netherlands, 2022; Volume 352. [Google Scholar]
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Naber, D.; Bullinger, M. Should antidepressants be used in minor depression? Dialogues Clin. Neurosci. 2018, 20, 223–228. [Google Scholar] [CrossRef]
- Simsek, M.; Whitney, K. Examination of Primary and Secondary Metabolites Associated with a Plant-Based Diet and Their Impact on Human Health. Foods 2024, 13, 1020. [Google Scholar] [CrossRef]
- Heinrich, M.; Barnes, J.; Prieto-Garcia, J.; Gibbons, S.; Williamson, E.M. Fundamentals of Pharmacognosy and Phytotherapy E-BOOK; Elsevier Health Sciences: New York, NY, USA, 2017. [Google Scholar]
- Carmona, F.; Pereira, A.M.S. Herbal medicines: Old and new concepts, truths and misunderstandings. Rev. Bras. De Farmacogn. 2013, 23, 379–385. [Google Scholar] [CrossRef]
- Wermuth, C.G. Multitargeted drugs: The end of the ‘one-target-one-disease’ philosophy? Drug Discov. Today 2004, 19, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-K. Antipsychotic Polypharmacy: A Dirty Little Secret or a Fashion? Int. J. Neuropsychopharmacol. 2019, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Stassen, H.H.; Bachmann, S.; Bridler, R.; Cattapan, K.; Herzig, D.; Schneeberger, A.; Seifritz, E. Detailing the effects of polypharmacy in psychiatry: Longitudinal study of 320 patients hospitalized for depression or schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kataria, H.; Mishra, R. Medicinal Plants as Novel Promising Therapeutics for Neuroprotection and Neuroregeneration. In New Age Herbals—Resource, Quality and Pharmacognosy; Springer: Singapore, 2018; pp. 437–453. [Google Scholar]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Shafiee, M.; Arekhi, S.; Omranzadeh, A.; Sahebkar, A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J. Affect. Disord. 2018, 227, 330–337. [Google Scholar] [CrossRef]
- Siddiqui, M.J.; Saleh, M.S.; Basharuddin, S.N.B.; Zamri, S.H.B.; bin Mohd Najib, M.H.; bin Che Ibrahim, M.Z.; Mazha, H.N.B.; Hassan, N.M.; Khatib, A. Saffron (Crocus sativus L.): As an Antidepressant. J. Pharm. Bioallied Sci. 2018, 10, 173–180. [Google Scholar] [CrossRef]
- Costa de Melo, N.; Sánchez-Ortiz, B.L.; dos Santos Sampaio, T.I.; Matias Pereira, A.C.; Pinheiro da Silva Neto, F.L.; Ribeiro da Silva, H.; Alves Soares Cruz, R.; Keita, H.; Soares Pereira, A.M.; Tavares Carvalho, J.C. Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) Moldenke: A study on zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, J.C. Reveals of New Candidate Active Components in Hemerocallis Radix and Its Anti-Depression Action of Mechanism Based on Network Pharmacology Approach. Int. J. Mol. Sci. 2020, 21, 1868. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A. Administration of Allium cepa L. bulb attenuates stress-produced anxiety and depression and improves memory in male mice. Metab. Brain Dis. 2018, 33, 271–281. [Google Scholar] [CrossRef]
- Kuchta, K.; Hladikova, M.; Thomsen, M.; Nahrstedt, A.; Schmidt, M. Kava (Piper methysticum) Extract for the Treatment of Nervous Anxiety, Tension and Restlessness. Drug Res. 2021, 71, 83–93. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Parvin, N.; Assarzadegan, N.; Asghari, S. The effects of lavandula angustifolia mill infusion on depression in patients using citalopram: A comparison study. Iran. Red. Crescent Med. J. 2013, 15, 734. [Google Scholar] [CrossRef]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L. Anxiolytic effect of essential oils and their constituents: A review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Konstantinos, F.; Heun, R. The effects of Rhodiola Rosea supplementation on depression, anxiety and mood–A Systematic Review. Glob. Psychiatry 2020, 3, 72–82. [Google Scholar] [CrossRef]
- Adebayo, O.G.; Ben-Azu, B.; Ajayi, A.M.; Wopara, I.; Aduema, W.; Kolawole, T.A.; Umoren, E.B.; Onyeleonu, I.; Ebo, O.T.; Ajibo, D.N. Gingko biloba abrogate lead-induced neurodegeneration in mice hippocampus: Involvement of NF-κB expression, myeloperoxidase activity and pro-inflammatory mediators. Biol. Trace Elem. Res. 2022, 200, 1736–1749. [Google Scholar] [CrossRef]
- Kumar Singh, S.; Barreto, E.G.; Aliev, G.; Echeverria, V. Ginkgo biloba as an alternative medicine in the treatment of anxiety in dementia and other psychiatric disorders. Curr. Drug Metab. 2017, 18, 112–119. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef]
- Sherman, S.H.; Joshee, N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Act. Plants 2022, 11, 22–38. [Google Scholar]
- Sales, A.J.; Crestani, C.C.; Guimarães, F.S.; Joca, S.R. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 255–261. [Google Scholar] [CrossRef]
- Gupta, G.L.; Fernandes, J. Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed. Pharmacother. 2019, 109, 1698–1708. [Google Scholar] [CrossRef]
- Chuang, H.-W.; Wang, T.-Y.; Huang, C.-C.; Wei, I.H. Echinacoside exhibits antidepressant-like effects through AMPAR–Akt/ERK–mTOR pathway stimulation and BDNF expression in mice. Chin. Med. 2022, 17, 9. [Google Scholar] [CrossRef]
- Mendelson, S.D. Herbal Treatment of Anxiety: Clinical Studies in Western, Chinese and Ayurvedic Traditions; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural compounds and neuroprotection: Mechanisms of action and novel delivery systems. In Vivo 2016, 30, 535–547. [Google Scholar]
- Wang, Z.Y.; Liu, J.Y.; Yang, C.B.; Malampati, S.; Huang, Y.Y.; Li, M.X.; Li, M.; Song, J.X. Neuroprotective natural products for the treatment of Parkinson’s disease by targeting the autophagy–lysosome pathway: A systematic review. Phytother. Res. 2017, 31, 1119–1127. [Google Scholar] [CrossRef]
- Martins, J.; Brijesh, S. Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother. 2018, 104, 343–365. [Google Scholar] [CrossRef]
- Kumar, V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Product. Deriv. 2006, 20, 1023–1035. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: A systematic review. Phytother. Res. 2007, 21, 703–716. [Google Scholar] [CrossRef]
- Spinella, M. The Psychopharmacology of Herbal Medicine: Plant Drugs that Alter Mind, Brain, and Behavior; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Winston, D. Adaptogens: Herbs for Strength, Stamina, and Stress Relief; Simon and Schuster: New York, NY, USA, 2019. [Google Scholar]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Chakraborty, P. Herbal genomics as tools for dissecting new metabolic pathways of unexplored medicinal plants and drug discovery. Biochim. Open 2018, 6, 9–16. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Wong, M.; O’kirwan, F.; Hannestad, J.; Irizarry, K.; Elashoff, D.; Licinio, J. St John’s wort and imipramine-induced gene expression profiles identify cellular functions relevant to antidepressant action and novel pharmacogenetic candidates for the phenotype of antidepressant treatment response. Mol. Psychiatry 2004, 9, 237–251. [Google Scholar] [CrossRef]
- Pennington, K.; Föcking, M.; McManus, C.; Pariante, C.; Dunn, M.; Cotter, D. A proteomic investigation of similarities between conventional and herbal antidepressant treatments. J. Psychopharmacol. 2009, 23, 520–530. [Google Scholar] [CrossRef]
- Popay, I. Hypericum perforatum (St John’s Wort). CABI Compend. 2022, 28268. [Google Scholar] [CrossRef]
- Tingle, J.L.; Cook-Patton, S.C.; Agrawal, A.A. Spillover of a biological control agent (Chrysolina quadrigemina) onto native St. Johnswort (Hypericum punctatum). PeerJ 2016, 4, e1886. [Google Scholar] [CrossRef]
- Suryawanshi, M.V.; Gujarathi, P.P.; Mulla, T.; Bagban, I. Hypericum perforatum: A comprehensive review on pharmacognosy, preclinical studies, putative molecular mechanism, and clinical studies in neurodegenerative diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3803–3818. [Google Scholar] [CrossRef]
- Nobakht, S.Z.; Akaberi, M.; Mohammadpour, A.H.; Moghadam, A.T.; Emami, S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022, 25, 1045. [Google Scholar]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B.S. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Tokgöz, H.B.; Altan, F. Hypericum perforatum L.: A medicinal plant with potential as a curative agent against obesity-associated complications. Mol. Biol. Rep. 2020, 47, 8679–8686. [Google Scholar] [CrossRef]
- Peterson, B.N.H. St John’s Wort. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kholghi, G.; Arjmandi-Rad, S.; Zarrindast, M.-R.; Vaseghi, S. St. John’s wort (Hypericum perforatum) and depression: What happens to the neurotransmitter systems? Naunyn-Schmiedeberg's Arch. Pharmacol. 2022, 395, 629–642. [Google Scholar] [CrossRef]
- Wurglics, M.; Schubert-Zsilavecz, M. Hypericum perforatum: A ‘modern’ herbal antidepressant: Pharmacokinetics of active ingredients. Clin. Pharmacokinet. 2006, 45, 449–468. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef]
- Kasper, S.; Dienel, A.; Kieser, M. Continuation and long-term maintenance treatment with Hypericum extract WS® 5570 after successful acute treatment of mild to moderate depression–rationale and study design. Int. J. Methods Psychiatr. Res. 2004, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Warnick, S.J., Jr.; Mehdi, L.; Kowalkowski, J. Wait-there’s evidence for that? Integrative medicine treatments for major depressive disorder. Int. J. Psychiatry Med. 2021, 56, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Sevastre-Berghian, A.; Toma, V.; Sevastre, B.; Hanganu, D.; Vlase, L.; Benedec, D.; Oniga, I.; Baldea, I.; Olteanu, D.; Moldovan, R. Characterization and biological effects of Hypericum extracts on experimentally-induced-anxiety, oxidative stress and inflammation in rats. J. Physiol. Pharmacol. 2018, 69, 789–800. [Google Scholar]
- Bilia, A.R.; Gallori, S.; Vincieri, F.F. St. John’s wort and depression: Efficacy, safety and tolerability-an update. Life Sci. 2002, 70, 3077–3096. [Google Scholar] [CrossRef]
- Butterweck, V.; Schmidt, M. John’s wort: Role of active compounds for its mechanism of action and efficacy. WMW Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef]
- Di Pierro, F.; Risso, P.; Settembre, R. Role in depression of a multi-fractionated versus a conventional Hypericum perforatum extract. Panminerva Medica 2018, 60, 156–160. [Google Scholar] [CrossRef]
- Furhad, S.B.A. Herbal Supplements. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 5. Complementary and alternative medicine treatments. Can. J. Psychiatry 2016, 61, 576–587. [Google Scholar] [CrossRef]
- Kandilarov, I.K.; Zlatanova, H.I.; Georgieva-Kotetarova, M.T.; Kostadinova, I.I.; Katsarova, M.N.; Dimitrova, S.Z.; Lukanov, L.K.; Sadakov, F. Antidepressant effect and recognition memory improvement of two novel plant extract combinations-antistress I and antistress II on rats subjected to a model of mild chronic stress. Folia Medica 2018, 60, 110–116. [Google Scholar] [CrossRef]
- Vance, K.M.; Ribnicky, D.M.; Hermann, G.E.; Rogers, R.C. St. John’s Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition 2014, 30, S37–S42. [Google Scholar] [CrossRef]
- Baureithel, K.H.; Büter, K.B.; Engesser, A.; Burkard, W.; Schaffner, W. Inhibition of benzodiazepine binding in vitro by amentoflavone, a constituent of various species of Hypericum. Pharm. Acta Helv. 1997, 72, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V. Mechanism of action of St John’s wort in depression: What is known? CNS Drugs 2003, 17, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A.; Schwendler, A.; Hüther, J.; Schwarzbach, H.; Schwarz, A.; Kolb, C.; Abdel-Aziz, H.; Kinscherf, R. Neurotrophic, cytoprotective, and anti-inflammatory effects of St. John’s wort extract on differentiated mouse hippocampal HT-22 neurons. Front. Pharmacol. 2018, 8, 297500. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Lv, Y.; Kelber, O.; Butterweck, V. Mechanism of St. John’s wort extract (STW3-VI) during chronic restraint stress is mediated by the interrelationship of the immune, oxidative defense, and neuroendocrine system. Neuropharmacology 2010, 58, 767–773. [Google Scholar] [CrossRef]
- Song, A.; Wu, Z.; Zhao, W.; Shi, W.; Cheng, R.; Jiang, J.; Ni, Z.; Qu, H.; Qiaolongbatu, X.; Fan, G. The role and mechanism of hyperoside against depression-like behavior in mice via the NLRP1 inflammasome. Medicina 2022, 58, 1749. [Google Scholar] [CrossRef]
- Kasper, S.; Gastpar, M.; Möller, H.-J.; Müller, W.E.; Volz, H.-P.; Dienel, A.; Kieser, M. Better tolerability of St. John’s wort extract WS 5570 compared to treatment with SSRIs: A reanalysis of data from controlled clinical trials in acute major depression. Int. Clin. Psychopharmacol. 2010, 25, 204–213. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Palazzo, M.C.; Oldani, L.; Altamura, A.C. The noradrenergic action in antidepressant treatments: Pharmacological and clinical aspects. CNS Neurosci. Ther. 2011, 17, 723–732. [Google Scholar] [CrossRef]
- Dwyer, A.V.; Whitten, D.L.; Hawrelak, J.A. Herbal medicines, other than St. John’s Wort, in the treatment of depression: A systematic review. Altern. Med. Rev. 2011, 16, 40–50. [Google Scholar]
- Claro, A.E.; Palanza, C.; Mazza, M.; Schuenemann, G.E.U.M.; Rigoni, M.; Pontecorvi, A.; Janiri, L.; Pitocco, D.; Muti, P. Historical use of medicinal plants and future potential from phytotherapy to phitochemicals. Ann. Bot. 2024, 14. [Google Scholar] [CrossRef]
- Crupi, R.; Mazzon, E.; Marino, A.; La Spada, G.; Bramanti, P.; Battaglia, F.; Cuzzocrea, S.; Spina, E. Hypericum perforatum treatment: Effect on behaviour and neurogenesis in a chronic stress model in mice. BMC Complement. Altern. Med. 2011, 11, 7. [Google Scholar] [CrossRef]
- Trofimiuk, E.; Holownia, A.; Braszko, J.J. St. John’s wort may relieve negative effects of stress on spatial working memory by changing synaptic plasticity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Concerto, C.; Boo, H.; Hu, C.; Sandilya, P.; Krish, A.; Chusid, E.; Coira, D.; Aguglia, E.; Battaglia, F. Hypericum perforatum extract modulates cortical plasticity in humans. Psychopharmacology 2018, 235, 145–153. [Google Scholar] [CrossRef]
- Yechiam, E.; Ben-Eliezer, D.; Ashby, N.J.; Bar-Shaked, M. The acute effect of Hypericum perforatum on short-term memory in healthy adults. Psychopharmacology 2019, 236, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Brattström, A. Long-term effects of St. John’s wort (Hypericum perforatum) treatment: A 1-year safety study in mild to moderate depression. Phytomedicine 2009, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Schrader, E.; Meier, B.; Brattström, A. Hypericum treatment of mild–moderate depression in a placebo–controlled study. A prospective, double–blind, randomized, placebo–controlled, multicentre study. Human Psychopharmacol. Clin. Exp. 1998, 13, 163–169. [Google Scholar] [CrossRef]
- Seifritz, E.; Hatzinger, M.; Holsboer-Trachsler, E. Efficacy of Hypericum extract WS® 5570 compared with paroxetine in patients with a moderate major depressive episode–a subgroup analysis. Int. J. Psychiatry Clin. Pract. 2016, 20, 126–132. [Google Scholar] [CrossRef]
- Zahner, C.; Kruttschnitt, E.; Uricher, J.; Lissy, M.; Hirsch, M.; Nicolussi, S.; Krähenbühl, S.; Drewe, J. No clinically relevant interactions of St. John’s wort extract ze 117 low in hyperforin with cytochrome P450 enzymes and P-glycoprotein. Clin. Pharmacol. Ther. 2019, 106, 432–440. [Google Scholar] [CrossRef]
- De Vry, J.; Maurel, S.; Schreiber, R.; De Beun, R.; Jentzsch, K. Comparison of hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur. Neuropsychopharmacol. 1999, 9, 461–468. [Google Scholar] [CrossRef]
- Schuckit, M.A.; Monteiro, M.G. Alcoholism, anxiety and depression. Br. J. Addict. 1988, 83, 1373–1380. [Google Scholar] [CrossRef]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 148. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea. A Phytomedicinal Overv. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Ahmed, F.; Filion, V.; Saleem, A.; Arnason, J.T. Phytochemistry of Rhodiola rosea; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Khokhlova, K.; Zdoryk, O. Authentication of Rhodiola rosea, Rhodiola quadrifida and Rhodiola rosea liquid extract from the Ukrainian market using HPTLC chromatographic profiles. Nat. Prod. Res. 2020, 34, 2842–2846. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.N.; Mhetre, O.S.; Malavdkar, P.R. A Review Article on Rhodiola Rosea: An Adaptogen Having Multiple Benefits. Int. J. Pharmacogn. 2020, 7, 62–69. [Google Scholar]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
- Panossian, A.; Nikoyan, N.; Ohanyan, N.; Hovhannisyan, A.; Abrahamyan, H.; Gabrielyan, E.; Wikman, G. Comparative study of Rhodiola preparations on behavioral despair of rats. Phytomedicine 2008, 15, 84–91. [Google Scholar] [CrossRef]
- Mattioli, L.; Funari, C.; Perfumi, M. Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J. Psychopharmacol. 2009, 23, 130–142. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, Y.; Qu, Z.; Tang, J.; Qin, Y.; Chung, P.; Wong, R.; Hägg, U. The effects of Rhodiola rosea extract on 5-HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine 2009, 16, 830–838. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef]
- Yang, S.-J.; Yu, H.-Y.; Kang, D.-Y.; Ma, Z.-Q.; Qu, R.; Fu, Q.; Ma, S.-P. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014, 124, 451–457. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.; Zhang, J.; Li, H.; Guo, M. Rhodiola rosea: A review in the context of PPPM approach. EPMA J. 2024, 15, 233–259. [Google Scholar] [CrossRef]
- Ishaque, S.; Shamseer, L.; Bukutu, C.; Vohra, S. Rhodiola rosea for physical and mental fatigue: A systematic review. BMC Complement. Altern. Med. 2012, 12, 70. [Google Scholar] [CrossRef]
- Muszyńska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Surowce naturalne mające znaczenie w profilaktyce i wspomagające leczenie depresji. Psychiatr. Pol. 2015, 49, 3. [Google Scholar]
- Yu, H.L.; Zhang, P.P.; Zhang, C.; Zhang, X.; Li, Z.Z.; Li, W.Q.; Fu, A.S. Effects of rhodiola rosea on oxidative stress and negative emotional states in patients with obstructive sleep apnea. J. Clin. Otorhinolaryngol. Head Neck Surg. 2019, 33, 954–957. [Google Scholar] [CrossRef]
- Gao, L.; Wu, C.; Liao, Y.; Wang, J. Antidepressants effects of Rhodiola capsule combined with sertraline for major depressive disorder: A randomized double-blind placebo-controlled clinical trial. J. Affect. Disord. 2020, 265, 99–103. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Zee, J.; Soeller, I.; Li, Q.S.; Rockwell, K.; Amsterdam, J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine 2015, 22, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Concerto, C.; Infortuna, C.; Muscatello, M.R.A.; Bruno, A.; Zoccali, R.; Chusid, E.; Aguglia, E.; Battaglia, F. Exploring the effect of adaptogenic Rhodiola Rosea extract on neuroplasticity in humans. Complement. Ther. Med. 2018, 41, 141–146. [Google Scholar] [CrossRef]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2007, 61, 343–348. [Google Scholar] [CrossRef]
- Cropley, M.; Banks, A.P.; Boyle, J. The effects of Rhodiola rosea L. extract on anxiety, stress, cognition and other mood symptoms. Phytother. Res. 2015, 29, 1934–1939. [Google Scholar] [CrossRef]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef]
- Lekomtseva, Y.; Zhukova, I.; Wacker, A. Rhodiola rosea in subjects with prolonged or chronic fatigue symptoms: Results of an open-label clinical trial. Complement. Med. Res. 2017, 24, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Ruiz, R.M.; Roa-Coria, J.E.; Patiño-Camacho, S.I.; Flores-Murrieta, F.J.; Déciga-Campos, M. Neuropharmacological and Toxicity Evaluations of Ethanol Extract from R hodiola R osea. Drug Dev. Res. 2012, 73, 106–113. [Google Scholar] [CrossRef]
- Bangratz, M.; Ait Abdellah, S.; Berlin, A.; Blondeau, C.; Guilbot, A.; Dubourdeaux, M.; Lemoine, P. A preliminary assessment of a combination of rhodiola and saffron in the management of mild-moderate depression. Neuropsychiatr. Dis. Treat. 2018, 14, 1821–1829. [Google Scholar] [CrossRef]
- Duman, İ.; Eken, Ç.G. Therapeutic Effects of Ginkgo Biloba Extract in Neuropsychiatric Disorders. In Current Research in Health Sciences; Gece Publishing: Ankara, Turkey, 2022; pp. 170–188. [Google Scholar]
- Chatterjee, S.; Kondratskaya, E.; Krishtal, O. Structure-activity studies with Ginkgo biloba extract constituents as receptor-gated chloride channel blockers and modulators. Pharmacopsychiatry 2003, 36, 68–77. [Google Scholar]
- Ramassamy, C.; Longpre, F.; Christen, Y. Ginkgo biloba extract (EGb 761) in Alzheimer’s disease: Is there any evidence? Curr. Alzheimer Res. 2007, 4, 253–262. [Google Scholar] [CrossRef]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, Z.; Xu, J.; Li, Y.; Chen, M. Pharmacological activities of ginkgolic acids in relation to autophagy. Pharmaceuticals 2022, 15, 1469. [Google Scholar] [CrossRef]
- Mdzinarishvili, A.; Kiewert, C.; Kumar, V.; Hillert, M.; Klein, J. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience 2007, 144, 217–222. [Google Scholar] [CrossRef]
- Markus, C.R.; Lammers, J.H. Effects of Ginkgo biloba on corticosterone stress responses after inescapable shock exposure in the rat. Pharmacol. Biochem. Behav. 2003, 76, 487–492. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Shou, S.S.; Lin, Y.X.; Chen, C.C.; Chiang, C.Y.; Yeh, C.Y. Effect of Ginkgo biloba extract on lipopolysaccharide-induced anhedonic depressive-like behavior in male rats. Phytother. Res. 2015, 29, 260–266. [Google Scholar] [CrossRef]
- Fehske, C.J.; Leuner, K.; Müller, W.E. Ginkgo biloba extract (EGb761®) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol. Res. 2009, 60, 68–73. [Google Scholar] [CrossRef]
- Blecharz-Klin, K.; Piechal, A.; Joniec, I.; Pyrzanowska, J.; Widy-Tyszkiewicz, E. Pharmacological and biochemical effects of Ginkgo biloba extract on learning, memory consolidation and motor activity in old rats. Acta Neurobiol. Exp. 2009, 69, 217–231. [Google Scholar] [CrossRef]
- Yoshitake, T.; Yoshitake, S.; Kehr, J. The Ginkgo biloba extract EGb 761® and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol. 2010, 159, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.; Yoshitake, S.; Ijiri, S.; Koch, E.; Nöldner, M.; Yoshitake, T. Ginkgo biloba leaf extract (EGb 761®) and its specific acylated flavonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: Possible implications for the cognitive enhancing properties of EGb 761®. Int. Psychogeriatr. 2012, 24, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Amri, H.; Drieu, K.; Papadopoulos, V. Ex vivo regulation of adrenal cortical cell steroid and protein synthesis, in response to adrenocorticotropic hormone stimulation, by the Ginkgo biloba extract EGb 761 and isolated ginkgolide B. Endocrinology 1997, 138, 5415–5426. [Google Scholar] [CrossRef] [PubMed]
- Marcilhac, A.; Dakine, N.; Bourhim, N.; Guillaume, V.; Grino, M.; Drieu, K.; Oliver, C. Effect of chronic administration of Ginkgo biloba extract or Ginkgolide on the hypothalamic-pituitary-adrenal axis in the rat. Life Sci. 1998, 62, 2329–2340. [Google Scholar] [CrossRef]
- Yeh, K.-Y.; Pu, H.-F.; Kaphle, K.; Lin, S.-F.; Wu, L.-S.; Lin, J.-H.; Tsai, Y.-F. Ginkgo biloba extract enhances male copulatory behavior and reduces serum prolactin levels in rats. Horm. Behav. 2008, 53, 225–231. [Google Scholar] [CrossRef]
- Montes, P.; Ruiz-Sanchez, E.; Rojas, C.; Rojas, P. Ginkgo biloba extract 761: A review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2015, 14, 132–149. [Google Scholar] [CrossRef]
- Kressmann, S.; Müller, W.; Blume, H. Pharmaceutical quality of different Ginkgo biloba brands. J. Pharm. Pharmacol. 2002, 54, 661–669. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, C.-w.; Shi, J.; Lin, Z.-x. Effects of Ginkgo biloba on dementia: An overview of systematic reviews. J. Ethnopharmacol. 2017, 195, 1–9. [Google Scholar] [CrossRef]
- Goel, R.K.; Kaur, D.; Pahwa, P. Assessment of anxiolytic effect of nerolidol in mice. Indian J. Pharmacol. 2016, 48, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, B.H.; Bagheri, R.; Roozbeh, B.; Ashtary-Larky, D.; Gaeini, A.A.; Dutheil, F.; Wong, A. Impact of saffron (Crocus sativus Linn) supplementation and resistance training on markers implicated in depression and happiness levels in untrained young males. Physiol. Behav. 2021, 233, 113352. [Google Scholar] [CrossRef]
- Abe, K.; Saito, H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother. Res. 2000, 14, 149–152. [Google Scholar] [CrossRef]
- Ghajar, A.; Neishabouri, S.M.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A. Crocus sativus L. versus citalopram in the treatment of major depressive disorder with anxious distress: A double-blind, controlled clinical trial. Pharmacopsychiatry 2017, 50, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, E.; Basti, A.A.; Noorbala, A.-A.; Jamshidi, A.-H.; Abbasi, S.H.; Akhondzadeh, S. Crocus sativus L.(petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine 2006, 13, 607–611. [Google Scholar] [CrossRef]
- Mazidi, M.; Shemshian, M.; Mousavi, S.H.; Norouzy, A.; Kermani, T.; Moghiman, T.; Sadeghi, A.; Mokhber, N.; Ghayour-Mobarhan, M.; Ferns, G.A. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med. 2016, 13, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jafarnia, N.; Ghorbani, Z.; Nokhostin, M.; Manayi, A.; Nourimajd, S.; Jahromi, S.R. Effect of saffron (Crocus sativus L.) as an add-on therapy to sertraline in mild to moderate generalized anxiety disorder: A double blind randomized controlled trial. Arch. Neurosci. 2017, 4, e14332. [Google Scholar]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 768–774. [Google Scholar] [CrossRef]
- Xu, G.-L.; Li, G.; Ma, H.-P.; Zhong, H.; Liu, F.; Ao, G.-Z. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J. Agric. Food Chem. 2009, 57, 8325–8330. [Google Scholar] [CrossRef]
- Jalali, F.; Hashemi, S.F. The effect of saffron on depression among recovered consumers of methamphetamine living with HIV/AIDS. Subst. Use Misuse 2018, 53, 1951–1957. [Google Scholar] [CrossRef]
- Lechtenberg, M.; Schepmann, D.; Niehues, M.; Hellenbrand, N.; Wünsch, B.; Hensel, A. Quality and functionality of saffron: Quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and σ1 (sigma-1) receptors. Planta Medica 2008, 74, 764–772. [Google Scholar] [CrossRef]
- Pitsikas, N. Constituents of saffron (Crocus sativus L.) as potential candidates for the treatment of anxiety disorders and schizophrenia. Molecules 2016, 21, 303. [Google Scholar] [CrossRef]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.-H.; Kassaian, S.E.; Tafti, A.-A.N.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014, 155, 216–222. [Google Scholar] [CrossRef]
- Dovrtělová, G.; Nosková, K.; Juřica, J.; Turjap, M.; Zendulka, O. Can bioactive compounds of Crocus sativus L. influence the metabolic activity of selected CYP enzymes in the rat. Physiol. Res. 2015, 644, S453–S458. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. Saffron. Available online: https://www.drugs.com/npp/saffron.html (accessed on 18 February 2025).
- Yoo, D.Y.; Choi, J.H.; Kim, W.; Yoo, K.Y.; Lee, C.H.; Yoon, Y.S.; Won, M.H.; Hwang, I.K. Effects of Melissa officinalis L. (lemon balm) extract on neurogenesis associated with serum corticosterone and GABA in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Araj-Khodaei, M.; Noorbala, A.A.; Yarani, R.; Emadi, F.; Emaratkar, E.; Faghihzadeh, S.; Parsian, Z.; Alijaniha, F.; Kamalinejad, M.; Naseri, M. A double-blind, randomized pilot study for comparison of Melissa officinalis L. and Lavandula angustifolia Mill. with Fluoxetine for the treatment of depression. BMC Complement. Med. Ther. 2020, 20, 207. [Google Scholar] [CrossRef]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Jafari, H.; Mokaberinejad, R.; Raeis-Abdollahib, E. Echium amoenumfrom viewpoint of Avicenna: A brief review. J. Contemp. Med. Sci. Vol. 2018, 4, 187–190. [Google Scholar] [CrossRef]
- Reeves, J.W.; Fisher, A.J.; Newman, M.G.; Granger, D.A. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology 2016, 53, 951–957. [Google Scholar] [CrossRef]
- Abolhassani, M. Antiviral activity of borage (Echium amoenum). Arch. Med. Sci. 2010, 6, 366–369. [Google Scholar] [CrossRef]
- Anushiravani, M.; Manteghi, A.A.; Taghipur, A.; Eslami, M. Comparing effectiveness of a combined herbal drug based on Echium amoenum with Citalopram in the treatment of Major Depressive Disorder. Curr. Drug Discov. Technol. 2019, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Faryadian, S.; Sydmohammadi, A.; Khosravi, A.; Kashiri, M.; Faryadayn, P.; Abasi, N. Aqueous extract of Echlum amoenum elevate CSF serotonin and dopamine level in depression rat. Biomed. Pharmacol. J. 2014, 7, 137–142. [Google Scholar] [CrossRef]
- Nouri, M.; Farajdokht, F.; Torbati, M.; Ranjbar, F.; Hamedyazdan, S.; Araj-Khodaei, M.; Sadigh-Eteghad, S. A close look at echium amoenum processing, neuroactive components, and effects on neuropsychiatric disorders. Galen. Med. J. 2019, 8, e1559. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Navratilova, Z. Bioactivity of Echium amoenum: A mini review. Pharmacology 2019, 20, 14915–14917. [Google Scholar] [CrossRef]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. Physiological and biochemical effects of Echium amoenum extract on Mn2+-imposed Parkinson like disorder in rats. Adv. Pharm. Bull. 2018, 8, 705–713. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Li, M.; Zhang, X.; Lv, C.; Jia, L.; Wang, J.; Lu, J. A comparative study of the main constituents and antidepressant effects of raw and vinegar-baked Bupleuri Radix in rats subjected to chronic unpredictable mild stress. RSC Adv. 2017, 7, 32652–32663. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, X.; Nikolić, D.; Chen, S.-N.; Huang, K.; Li, G.; Pauli, G.F.; van Breemen, R.B. Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 2014, 53, 55–61. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus–a story that begs to be told. A Rev. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar]
- Feng, Y.; Gao, X.; Meng, M.; Xue, H.; Qin, X. Multi-omics reveals the mechanisms of antidepressant-like effects of the low polarity fraction of Bupleuri Radix. J. Ethnopharmacol. 2020, 256, 112806. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Koh, S.S.H.; Chan, H.W.; Ho, C.Y.X. Clinical use of curcumin in depression: A meta-analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, F.N.; Gazal, M.; Bastos, C.R.; Kaster, M.P.; Ghisleni, G. Curcumin in depressive disorders: An overview of potential mechanisms, preclinical and clinical findings. Eur. J. Pharmacol. 2016, 784, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, X.-J.; Liu, P.; Dong, X.-Z.; Mu, L.-H.; Chen, Y.-B.; Liu, M.-Y.; Yu, B.-Y. Antidepressant and neuroprotective effect of the Chinese herb kaixinsan against lentiviral shRNA knockdown brain-derived neurotrophic factor-induced injury in vitro and in vivo. Neuropsychobiology 2014, 69, 129–139. [Google Scholar] [CrossRef]
- da Silva Marques, J.G.; Antunes, F.T.T.; da Silva Brum, L.F.; Pedron, C.; de Oliveira, I.B.; Ferraz, A.d.B.F.; Martins, M.I.M.; Dallegrave, E.; de Souza, A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021, 399, 113002. [Google Scholar] [CrossRef]
- Kyrou, I.; Christou, A.; Panagiotakos, D.; Stefanaki, C.; Skenderi, K.; Katsana, K.; Tsigos, C. Effects of a hops (Humulus lupulus L.) dry extract supplement on self-reported depression, anxiety and stress levels in apparently healthy young adults: A randomized, placebo-controlled, double-blind, crossover pilot study. Hormones 2017, 16, 171–180. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini-Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef]
- Bačkor, M.; Goga, M.; Ručová, D.; Urminská, D.; Bačkorová, M.; Klejdus, B. Allelopathic effects of three lichen secondary metabolites on cultures of aposymbiotically grown lichen photobionts and free-living alga Scenedesmus quadricauda. South. Afr. J. Bot. 2023, 162, 688–693. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Kosanić, M.; Ranković, B. Lichen Secondary Metabolites as Potential Antibiotic Agents. In Lichen Secondary Metabolites Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 81–104. [Google Scholar]
- Sovrlic, M.; Manojlovic, N.; Kosanic, M.; Kočović, A.; Tomović, J.; Vasiljević, P. Investigation of phytochemical composition and in vitro antioxidant potential of different extracts and gyrophoric acid derived from the lichen Umbilicaria grisea growing in Serbia. Farmacia 2024, 72, 1369–1375. [Google Scholar] [CrossRef]
- Tomović, J.; Kočović, A.; Anđić, M.; Bradić, J.; Zubić, N.; Jakovljević, V.; Sovrlić, M.; Vasiljević, P.; Manojlović, N. Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa. Sci. Pharm. 2024, 92, 27. [Google Scholar] [CrossRef]
- Urbanska, N.; Karasova, M.; Jendzelovska, Z.; Majerník, M.; Kolesarova, M.; Kecsey, D.; Jendzelovsky, R.; Bohus, P.; Kiskova, T. Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats. Int. J. Mol. Sci. 2024, 25, 11840. [Google Scholar] [CrossRef]
- Dimitrijević, I.; Mitić, V.; Stankov Jovanović, V.; Stanković, M.; Zlatanović, I.; Stojanović, G. Cladonia rangiformis Acetone Extract— New Insight into the Chemical Composition and Biological Activity. Nat. Prod. Commun. 2023, 18, 1934578X231212159. [Google Scholar] [CrossRef]
- Urbanska, N.; Simko, P.; Leskanicova, A.; Karasova, M.; Jendzelovska, Z.; Jendzelovsky, R.; Rucova, D.; Kolesarova, M.; Goga, M.; Backor, M.; et al. Atranorin, a Secondary Metabolite of Lichens, Exhibited Anxiolytic/Antidepressant Activity in Wistar Rats. Life 2022, 12, 1850. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Farahani, M.S.; Rahimi, R. Phytochemical constituents as future antidepressants: A comprehensive review. Rev. Neurosci. 2015, 26, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.V. Special Issue “flavonoids and their disease prevention and treatment potential”: Recent advances and future perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef]
- Dereli, F.T.G.; Ilhan, M.; Akkol, E.K. Identification of the main active antidepressant constituents in a traditional Turkish medicinal plant, Centaurea kurdica Reichardt. J. Ethnopharmacol. 2020, 249, 112373. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Gürağaç Dereli, F.T.; Ilhan, M. Assessment of antidepressant effect of the aerial parts of micromeria myrtifolia Boiss. & Hohen Mice. Molecules 2019, 24, 1869. [Google Scholar] [CrossRef]
- Park, S.-J.; Jaiswal, V.; Lee, H.-J. Dietary Intake of Flavonoids and Carotenoids Is Associated with Anti-Depressive Symptoms: Epidemiological Study and In Silico—Mechanism Analysis. Antioxidants 2021, 11, 53. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Dereli, F.T.G.; Ilhan, M.; Akkol, E.K. New drug discovery from medicinal plants and phytoconstituents for depressive disorders. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2019, 18, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant flavonoids and their relationship with oxidative stress. Oxidative Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Lai, H.; Sun, Y.; Yan, X.; Ai, Q.; Lin, M.; Yang, S.; Yang, Y.; Chu, S.; et al. Novel antidepressant mechanism of hypericin: Role of connexin 43-based gap junctions. Biomed. Pharmacother. 2023, 167, 115545. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, Y.; Han, X.; Zhu, Y.; Li, X.; Zhang, Y.; Lu, Y. The protective effect of hypericin on postpartum depression rat model by inhibiting the NLRP3 inflammasome activation and regulating glucocorticoid metabolism. Int. Immunopharmacol. 2022, 105, 108560. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, G.; Cui, L.; Wang, Q. Myricetin attenuates depressant-like behavior in mice subjected to repeated restraint stress. Int. J. Mol. Sci. 2015, 16, 28377–28385. [Google Scholar] [CrossRef]
- Mohan, M.; Jadhav, S.S.; Kasture, V.S.; Kasture, S.B. Effect of myricetin on behavioral paradigms of anxiety. Pharm. Biol. 2009, 47, 927–931. [Google Scholar] [CrossRef]
- Meyer, E.; Mori, M.A.; Campos, A.C.; Andreatini, R.; Guimarães, F.S.; Milani, H.; de Oliveira, R.M.W. Myricitrin induces antidepressant-like effects and facilitates adult neurogenesis in mice. Behav. Brain Res. 2017, 316, 59–65. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, S.; Zhu, X.; Jiang, Y.; Lu, Z.; Gong, P. Myricetin suppresses traumatic brain injury-induced inflammatory response via EGFR/AKT/STAT pathway. Sci. Rep. 2023, 13, 22764. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Myricetin as a Promising Molecule for the Treatment of Post-Ischemic Brain Neurodegeneration. Nutrients 2021, 13, 342. [Google Scholar] [CrossRef]
- Demyashkin, G.; Blinova, E.; Grigoryan, M.; Parshenkov, M.; Skovorodko, P.; Ius, V.; Lebed, A.; Shegay, P.; Kaprin, A. Neuroprotective Effects of Myricetin on PTZ-Induced Seizures in Mice: Evaluation of Oxidation, Neuroinflammation and Metabolism, and Apoptosis in the Hippocampus. Curr. Issues Mol. Biol. 2024, 46, 8914–8944. [Google Scholar] [CrossRef] [PubMed]
- Bayazeid, O.; Nemutlu, E.; Eylem, C.C.; İlhan, M.; Küpeli-Akkol, E.; Karahan, H.; Kelicen-Uğur, P.; Ersoz, T.; Yalçın, F.N. Neuroactivity of the naturally occurring aporphine alkaloid, roemerine. Nat. Prod. Res. 2021, 35, 6147–6152. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, S.; Girdhar, A.; Verma, S.K.; Lather, V.; Pandita, D. Plant derived alkaloids in major neurodegenerative diseases: From animal models to clinical trials. J. Ayurvedic Herb. Med. 2015, 1, 91–100. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Ng, Y.P.; Or, T.C.T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Eom, S.; Jung, W.; Lee, J.; Yeom, H.D.; Lee, S.; Kim, C.; Park, H.-D.; Lee, J.H. Differential Regulation of Human Serotonin Receptor Type 3A by Chanoclavine and Ergonovine. Molecules 2021, 26, 1211. [Google Scholar] [CrossRef]
- Butterweck, V.; Böckers, T.; Korte, B.; Wittkowski, W.; Winterhoff, H. Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res. 2002, 930, 21–29. [Google Scholar] [CrossRef]
- Koop, T.; Dienel, A.; Heldmann, M.; Münte, T.F. Effects of a Rhodiola rosea extract on mental resource allocation and attention: An event-related potential dual task study. Phytother. Res. 2020, 34, 3287–3297. [Google Scholar] [CrossRef]
- Yuan, N.-j.; Zhu, W.-j.; Ma, Q.-y.; Huang, M.-y.; Huo, R.-r.; She, K.-j.; Pan, J.-p.; Wang, J.-g.; Chen, J.-x. Luteolin ameliorates chronic stress-induced depressive-like behaviors in mice by promoting the Arginase-1+ microglial phenotype via a PPARγ-dependent mechanism. Acta Pharmacol. Sin. 2024, 46, 575–591. [Google Scholar] [CrossRef]
- Szewczyk, B.; Pochwat, B.; Muszyńska, B.; Opoka, W.; Krakowska, A.; Rafało-Ulińska, A.; Friedland, K.; Nowak, G. Antidepressant-like activity of hyperforin and changes in BDNF and zinc levels in mice exposed to chronic unpredictable mild stress. Behav. Brain Res. 2019, 372, 112045. [Google Scholar] [CrossRef]
- El Hamdaoui, Y.; Zheng, F.; Fritz, N.; Ye, L.; Tran, M.A.; Schwickert, K.; Schirmeister, T.; Braeuning, A.; Lichtenstein, D.; Hellmich, U.A. Analysis of hyperforin (St. John’s wort) action at TRPC6 channel leads to the development of a new class of antidepressant drugs. Mol. Psychiatry 2022, 27, 5070–5085. [Google Scholar] [CrossRef] [PubMed]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Ballesta, A.; Martin, R.; Gomez de Heras, R. Antianhedonic and antidepressant effects of affron®, a standardized saffron (Crocus sativus L.) extract. Molecules 2020, 25, 3207. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Xue, J.-S.; Li, H.-Y.; Ma, Z.-Q.; Fu, Q.; Qu, R.; Ma, S.-P. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol. Behav. 2015, 152, 264–271. [Google Scholar] [CrossRef]
- Arora, V.; Chopra, K. Possible involvement of oxido-nitrosative stress induced neuro-inflammatory cascade and monoaminergic pathway: Underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J. Affect. Disord. 2013, 151, 1041–1052. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Niu, L.; Wang, L.-L.; Bai, L.; Fang, X.-Y.; Li, Y.-C.; Yi, L.-T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017, 134, 220–227. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, H.; Zhang, L.; Fu, F. Administration of Huperzine A exerts antidepressant-like activity in a rat model of post-stroke depression. Pharmacol. Biochem. Behav. 2017, 158, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Nechifor, A.; Dima, S.; Li, Y.; Jafari, S.M. Oral bioavailability of bioactive compounds; modulating factors, in vitro analysis methods, and enhancing strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 8501–8539. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Conte-Junior, C.A. Dietary Bioactive Compounds and Human Health: The Role of Bioavailability. Nutrients 2024, 17, 48. [Google Scholar] [CrossRef]
- Meena, L.; Gowda, N.A.N.; Sunil, C.K.; Rawson, A.; Janghu, S. Effect of ultrasonication on food bioactive compounds and their bio-accessibility: A review. J. Food Compos. Anal. 2024, 126, 105899. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 381–406. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Ventura-Martínez, R.; Silveira, D.; Déciga-Campos, M. Editorial: Pharmacological interaction between drugs and medicinal plants, Volume II. Front. Pharmacol. 2024, 15, 1372366. [Google Scholar] [CrossRef]
- Choudhury, A.; Singh, P.A.; Bajwa, N.; Dash, S.; Bisht, P. Pharmacovigilance of herbal medicines: Concerns and future prospects. J. Ethnopharmacol. 2023, 309, 116383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).