The Potential of Selected Plants and Their Biologically Active Molecules in the Treatment of Depression and Anxiety Disorders
Abstract
:1. Introduction
2. Herbal Versus Modern Medicine
3. Herbal Medicine and Herbal Extracts in Depression
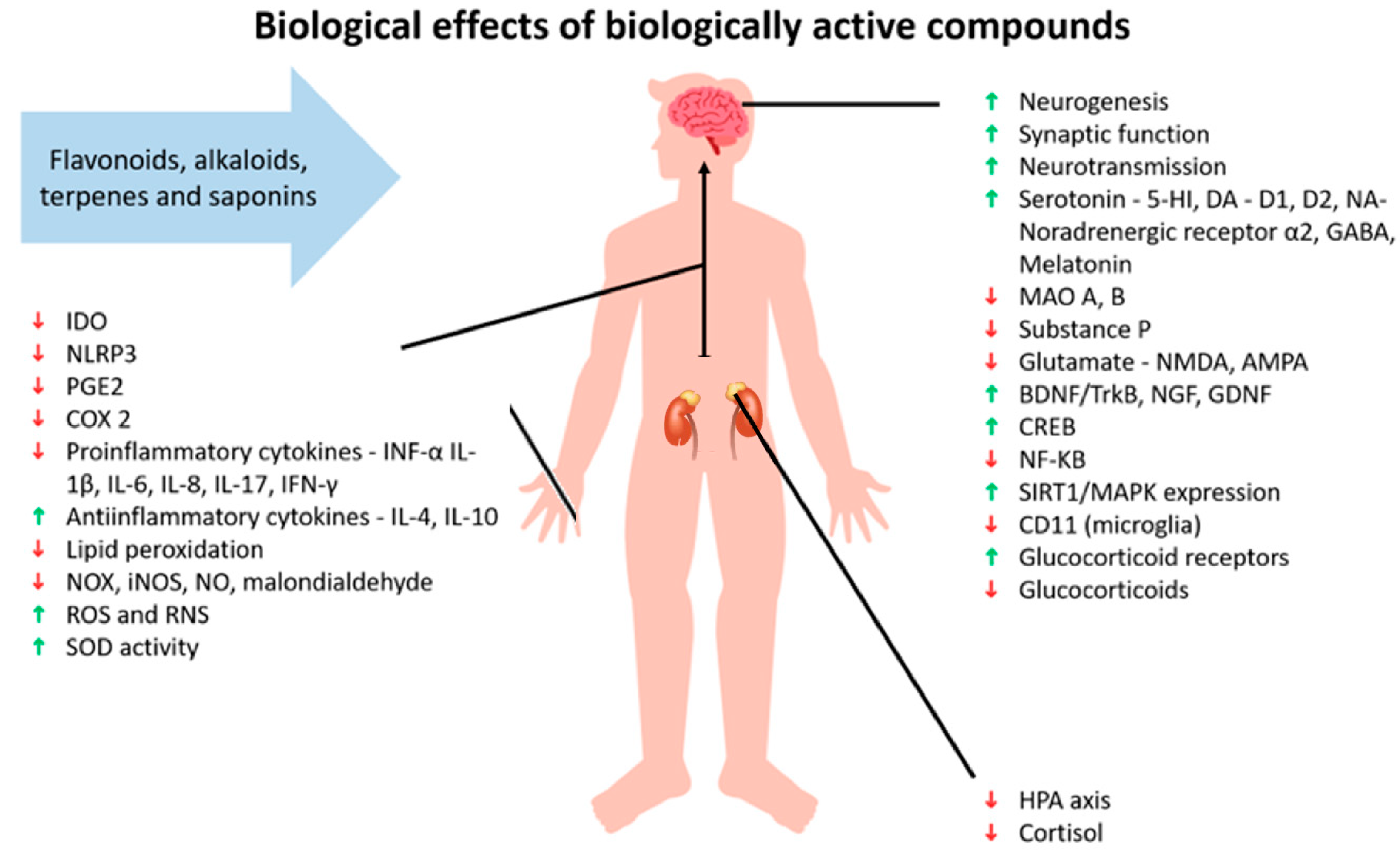
4. Biologically Active Substances with Antidepressant Effect
4.1. Flavonoids
4.2. Alkaloids
| Compound | Dose | Model | Activity | Ref. | |
|---|---|---|---|---|---|
| flavonoids | hypericin | 0.1–1 µM | ex vivo hippocampi from newborn Sprague-Dawley rats | enhancing presynaptic efficiency | [55] |
| 0.2 mg/kg | male Sprague-Dawley rats | increase in 5-HT levels in hypothalamus decrease in norepinephrine in hippocampus | [256] | ||
| various | 3808 human patients | comparable response to SSRIs | [74] | ||
| rhodiosin | 2 × 200 mg | 50 human adults | improvement in mental speed improvement in mental resources | [257] | |
| 340 mg | 57 human patients | less antidepressant effect versus sertraline (50 mg) fewer adverse effects, well tolerated | [162] | ||
| myricetin | 50 mg/kg | male C57BL/6 mice | reduction in the immobility time in FST and TST normalization of BDNF levels improvement in glutathione peroxidase activity reduction in corticosterone in plasma | [245] | |
| 10, 30, 100 mg/kg | albino mice and albino rats (strains not listed) | increase in time spent in open arms, number of entries in open arms in EPM increase in rearing in OFT increase in time spent in the lit area, number of transitions in light/dark apparatus increase in head poking in hole board apparatus reduction in lithium-induced head twitches reversal of MCP-induced anxiety | [246] | ||
| luteolin | 10, 30, 40 mg/kg | SPF male mice | ameliorating depressive-like behaviors promoting the Arg-1+ microglial phenotype reducing microglial proinflammatory responses reversing microglial phagocytosis-mediated synapse loss | [258] | |
| terpenes | hyperforin | 2.5, 5 mg/kg | male C57BL/6 J mice | reversed behavior in TST, FST, and splash test increased Zn concentration in hippocampus increased BDNF in hippocampus | [259] |
| 1, 5 mg/kg | B6J mice | activation of the channel via motif at TRPC6 anxiolytic and antidepressant effects (OFT) | [260] | ||
| safranal | 28 mg/day | 128 healthy adults | decrease in negative mood and symptoms related to stress and anxiety | [261] | |
| 50, 200 mg/kg | 30 male Wistar rats | no effect in EPM increase in swimming time in FST increase consumption of sweet solution | [262] | ||
| geraniol | 20, 40 mg/kg | male ICR mice | alleviation the depression-related behaviors of CUMS-exposed mice regulating IL-1β-related neuroinflammation | [263] | |
| alkaloids | berberine | 10, 20 mg/kg | male Wistar rats | increase in serotonin, dopamine, and noradrenaline levels reduction in substance P reduction in lipid peroxide levels decrease immobility time in FST | [264] |
| 50, 100 mg/kg | male ICR mice | decrease in levels of proinflammatory cytokines in hippocampus | [265] | ||
| linalool | 40, 200 mg/kg | male Sprague-Dawley rats | increase in sucrose preference normalization of behavior in OFT decrease motionless time reduction in inflammation in gastrointestinal organs | [266] | |
| huperzine A | 0.05, 0.15 mg/kg | male Sprague-Dawley rats | increase in neurological deficit score increase in cognitive function increase in 5-HT, DA, noradrenaline, and BDNF level in hippocampus and 5-HT and DA in prefrontal cortex | [267] | |
| galantamine | 0.02, 0.2, 2.0 mg/kg | C57BL/6J mice | antidepressant-like effects in FST | [53] |
4.3. Terpenes and Saponins
5. Potential Risks of Herbal Medicine
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| 5-HT1A | 5-hydroxytryptamine 1A receptor |
| 5-HT1B | 5-hydroxytryptamine 1B receptor |
| 5-HT2 | 5-hydroxytryptamine receptor 2 |
| ACTH | adrenocorticotropic hormone |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| BDNF | brain-derived neurotrophic factor |
| BDNF/TrkB | brain-derived neurotrophic factor/tropomyosin receptor kinase B |
| CD11b | microglia marker |
| COX 2 | cyclooxygenase-2 |
| CREB | cAMP-response element-binding protein |
| CRH | corticotrophin-releasing hormone |
| CXCL | CXC motif chemokine ligand |
| CXCR2 | CXC motif chemokine receptor 2 |
| CYP2A | cytochrome p450 family 2A |
| CYP2B | cytochrome p450 family 2B |
| CYP2C11 | cytochrome p450, subfamily 2, polypeptide 11 |
| CYP2C9 | Cytochrome p450 family 2 subfamily C member 9 |
| CYP1A2 | cytochrome p450 family 1A2 |
| CYP2C19 | Cytochrome p450 2C19 |
| CYP2C8 | cytochrome p450 family 2 subfamily C member 8 |
| CYP3A | cytochrome p450, family 3, subfamily A |
| D1 | dopamine receptor 1 |
| D2 | dopamine receptor 2 |
| DA | dopamine |
| EPM | elevated plus maze |
| ERK | extracellular-signal-regulated kinase |
| EtOH | ethyl alcohol |
| FDA | Food and Drug Administration |
| FST | forced swim test |
| GABA | γ-aminobutyric acid |
| GABA-A | γ-aminobutyric acid type A receptors |
| GABA-T | γ-aminobutyric acid transaminase |
| GAD | decarboxylase |
| GDNF | glial cell line-derived neurotrophic factor |
| GPCR | G protein-coupled receptor |
| HPA | hypothalamus–pituitary axis |
| IDO | indoleam-ine-2,3-dioxygenase |
| IL | interleukin |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| MAO | monoamine oxidase |
| MAO A | monoamine oxidase A |
| MAO B | monoamine oxidase B |
| MCP | metachlorophenylpiperazine |
| MDDs | major depressive disorders |
| MRI | magnetic resonance imaging |
| NA | noradrenaline |
| NF-kB | nuclear factor kappa B |
| NF-κβ p56 | subunit nuclear factor kappa B |
| NGF | nerve growth factor |
| NLRP1 | NLR family pyrin domain containing 1 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| NOX | oxides of nitrogen |
| OFT | open field test |
| PAF | platelet-activating factor |
| p-CREB | phosphorylated cAMP-response element-binding protein |
| PGE2 | prostaglandin E2 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SIRT1/MAPK | sirtuin 1/mitogen-activated protein kinase |
| SNI | spared nerve injury |
| SOD | superoxide dismutase |
| SSRIs | selective serotonin reuptake inhibitors |
| TNF-α | tumor necrosis factor α |
| TRPC6 | transient receptor potential canonical 6 channel |
| WHO | World Health Organization |
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Leskanicova, A.; Babincak, M.; Mochnacky, F.; Kukelova, D.; Urbanska, N.; Kolesarova, M.; Macekova, D.; Kostolny, J.; Kiskova, T. Sex-dependent differences in stress-induced depression in Wistar rats are accompanied predominantly by changes in phosphatidylcholines and sphingomyelins. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72, 72. [Google Scholar]
- Bains, N.; Abdijadid, S. Major depressive disorder. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2022, 4, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Galts, C.P.; Bettio, L.E.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef]
- Penner, I.-K.; Paul, F. Fatigue as a symptom or comorbidity of neurological diseases. Nat. Rev. Neurol. 2017, 13, 662–675. [Google Scholar] [CrossRef]
- Alshaya, D.S. Genetic and epigenetic factors associated with depression: An updated overview. Saudi J. Biol. Sci. 2022, 29, 103311. [Google Scholar] [CrossRef]
- Belmaker, R.H.; Agam, G. Major depressive disorder. New Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Neuroinflammation, gut-brain axis and depression. Psychiatry Investig. 2019, 17, 2. [Google Scholar] [CrossRef]
- Hara, T.; Owada, Y.; Takata, A. Genetics of bipolar disorder: Insights into its complex architecture and biology from common and rare variants. J. Human Genet. 2023, 68, 183–191. [Google Scholar] [CrossRef]
- Ikeda, M.; Takahashi, A.; Kamatani, Y.; Okahisa, Y.; Kunugi, H.; Mori, N.; Sasaki, T.; Ohmori, T.; Okamoto, Y.; Kawasaki, H. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol. Psychiatry 2018, 23, 639–647. [Google Scholar] [CrossRef]
- Gordovez, F.J.A.; McMahon, F.J. The genetics of bipolar disorder. Mol. Psychiatry 2020, 25, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Mandhare, A.; Tryphena, K.P.; Srivastava, S.; Shaikh, M.F.; Singh, S.B.; Khatri, D.K. Epigenetics in depression and gut-brain axis: A molecular crosstalk. Front. Aging Neurosci. 2022, 14, 1048333. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, W.-t.; Chen, J.-j.; Zhong, Q.; Wu, D.; Niu, L.; Wang, S.; Zeng, Y.; Wang, Y. Tryptophan metabolism as bridge between gut microbiota and brain in chronic social defeat stress-induced depression mice. Front. Cell. Infect. Microbiol. 2023, 13, 1121445. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Mandal, S.; Sinha, V.K.; Goyal, N. Efficacy of ketamine therapy in the treatment of depression. Indian J. Psychiatry 2019, 61, 480–485. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Ghazizadeh, J.; Sadigh-Eteghad, S.; Marx, W.; Fakhari, A.; Hamedeyazdan, S.; Torbati, M.; Taheri-Tarighi, S.; Araj-khodaei, M.; Mirghafourvand, M. The effects of lemon balm (Melissa officinalis L.) on depression and anxiety in clinical trials: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 6690–6705. [Google Scholar] [CrossRef]
- Lin, S.-H.; Chou, M.-L.; Chen, W.-C.; Lai, Y.-S.; Lu, K.-H.; Hao, C.-W.; Sheen, L.-Y. A medicinal herb, Melissa officinalis L. ameliorates depressive-like behavior of rats in the forced swimming test via regulating the serotonergic neurotransmitter. J. Ethnopharmacol. 2015, 175, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Villa, R. The neurobiology of depression: An integrated overview from biological theories to clinical evidence. Mol. Neurobiol. 2017, 54, 4847–4865. [Google Scholar] [CrossRef]
- Kamran, M.; Bibi, F.; Ur Rehman, A.; Morris, D.W. Major Depressive Disorder: Existing Hypotheses about Pathophysiological Mechanisms and New Genetic Findings. Genes 2022, 13, 646. [Google Scholar] [CrossRef]
- Li, Y.F. A hypothesis of monoamine (5-HT)—Glutamate/GABA long neural circuit: Aiming for fast-onset antidepressant discovery. Pharmacol. Ther. 2020, 208, 107494. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Chouinard, G. Chapter 7—The Monoamine Hypothesis of Depression Revisited: Could It Mechanistically Novel Antidepressant Strategies? In Neurobiology of Depression; Quevedo, J., Carvalho, A.F., Zarate, C.A., Eds.; Academic Press: London, UK, 2019; pp. 63–73. [Google Scholar]
- Noel, C. Antidepressants and suicidality: History, the black-box warning, consequences, and current evidence. Ment. Health Clin. 2015, 5, 202–211. [Google Scholar] [CrossRef]
- Latendresse, G.; Elmore, C.; Deneris, A. Selective Serotonin Reuptake Inhibitors as First-Line Antidepressant Therapy for Perinatal Depression. J. Midwifery Women’s Health 2017, 62, 317–328. [Google Scholar] [CrossRef]
- Anagha, K.; Shihabudheen, P.; Uvais, N.A. Side Effect Profiles of Selective Serotonin Reuptake Inhibitors: A Cross-Sectional Study in a Naturalistic Setting. Prim. Care Companion CNS Disord. 2021, 23, 35561. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Zhang, H.; Hong, W.; Zhang, L.; Mi, W.; Qin, J.; He, Y. Reliability and validity of the Chinese version of the Oxford depression questionnaire (ODQ-Chinese). J. Affect. Disord. 2022, 313, 278–282. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. SSRI-induced indifference. Psychiatry 2010, 7, 14. [Google Scholar]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Rickels, K.; Shiovitz, T.M.; Ramey, T.S.; Weaver, J.J.; Knapp, L.E.; Miceli, J.J. Adjunctive therapy with pregabalin in generalized anxiety disorder patients with partial response to SSRI or SNRI treatment. Int. Clin. Psychopharmacol. 2012, 27, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Dresler, T.; Guhn, A.; Tupak, S.V.; Ehlis, A.-C.; Herrmann, M.J.; Fallgatter, A.J.; Deckert, J.; Domschke, K. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. J. Neural Transm. 2013, 120, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [CrossRef]
- Hamati, R. Insights into the Neurobiology of Anxiety and a Potential Target for Pharmacotherapy. J. Neurosci. 2018, 38, 8919–8921. [Google Scholar] [CrossRef]
- Kalin, N.H. The critical relationship between anxiety and depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Levine, J.; Cole, D.P.; Chengappa, K.R. Anxiety disorders and major depression, together or apart. Depress. Anxiety 2001, 14, 94–104. [Google Scholar] [CrossRef]
- World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Kumar, V. Herbal medicines: Overview on regulations in India and South Africa. World J. Pharm. Res. 2017, 6, 690–698. [Google Scholar] [CrossRef]
- World Health Organization. The Promotion and Development of Traditional Medicine: Report of a WHO Meeting [Held in Geneva from 28 November to 2 December 1977]; World Health Organization: Geneva, Switzerland, 1978. [Google Scholar]
- Msomi, N.Z.; Simelane, M.B. Herbal Medicine; InTech: Rijeka, Croatia, 2019; pp. 215–227. [Google Scholar]
- Qazi, M.; Molvi, K. Herbal medicine: A comprehensive review. Int. J. Pharm. Res. 2016, 8, 1–5. [Google Scholar]
- Goyal, M.; Nagori, B.; Sasmal, D. Ayurveda the Ancient Science of Healing: An Insight; IntechOpen: London, UK, 2012. [Google Scholar]
- Watanabe, K.; Matsuura, K.; Gao, P.; Hottenbacher, L.; Tokunaga, H.; Nishimura, K.; Imazu, Y.; Reissenweber, H.; Witt, C.M. Traditional Japanese Kampo medicine: Clinical research between modernity and traditional medicine—The state of research and methodological suggestions for the future. Evidence-Based Complement. Altern. Med. 2011, 2011, 513842. [Google Scholar] [CrossRef] [PubMed]
- Che, C.-T.; George, V.; Ijinu, T.; Pushpangadan, P.; Andrae-Marobela, K. Traditional medicine. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 15–30. [Google Scholar]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Sabharwal, M. Pathophysiology of kidney, gallbladder and urinary stones treatment with herbal and allopathic medicine: A review. Asian Pac. J. Trop. Dis. 2013, 3, 496–504. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Otten, W.; Kanitz, E.; Gräbner, M.; Tuchscherer, A.; Bellmann, O.; Rehfeldt, C.; Metges, C.C. Effects of inadequate maternal dietary protein: Carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet. Res. 2012, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. Multiple cholinesterase inhibitors have antidepressant-like properties in the mouse forced swim test. Behav. Brain Res. 2021, 409, 113323. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Qi, Z. Hypericin prolongs action potential duration in hippocampal neurons by acting on K+ channels. Br. J. Pharmacol. 2010, 159, 1402–1407. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Wang, B.; Xie, L.; Chen, W. New FDA drug approvals for 2024: Synthesis and clinical application. Eur. J. Med. Chem. 2025, 285, 117241. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Mason, B.; Naik, A. Depression: Managing Resistance and Partial Response to Treatment. Am. Fam. Physician 2024, 109, 410–416. [Google Scholar]
- Paganin, W. Multifamily therapy in difficult-to-treat depression: An integrated and promising approach to rethinking clinical strategies. Front. Psychiatry 2024, 15, 1484440. [Google Scholar] [CrossRef]
- Parker, G. A revisionist model for treatment-resistant and difficult-to-treat depression. Aust. N. Z. J. Psychiatry 2024, 58, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, Y.; Lu, M.; Wang, Q. Review of Herbal Medicines for the Treatment of Depression. Nat. Product. Commun. 2022, 17, 1934578X221139082. [Google Scholar] [CrossRef]
- Aquib, M.; Najmi, A.; Akhtar, M. Antidepressant effect of thymoquinone in animal models of depression. Drug Res. 2015, 65, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Matraszek-Gawron, R.; Chwil, M.; Terlecka, P.; Skoczylas, M.M. Recent studies on anti-depressant bioactive substances in selected species from the genera Hemerocallis and Gladiolus: A systematic review. Pharmaceuticals 2019, 12, 172. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Tallón-Ballesteros, A.J. Modern Management Based on Big Data III: Proceedings of MMBD 2022; IOS Press: Amsterdam, The Netherlands, 2022; Volume 352. [Google Scholar]
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Naber, D.; Bullinger, M. Should antidepressants be used in minor depression? Dialogues Clin. Neurosci. 2018, 20, 223–228. [Google Scholar] [CrossRef]
- Simsek, M.; Whitney, K. Examination of Primary and Secondary Metabolites Associated with a Plant-Based Diet and Their Impact on Human Health. Foods 2024, 13, 1020. [Google Scholar] [CrossRef]
- Heinrich, M.; Barnes, J.; Prieto-Garcia, J.; Gibbons, S.; Williamson, E.M. Fundamentals of Pharmacognosy and Phytotherapy E-BOOK; Elsevier Health Sciences: New York, NY, USA, 2017. [Google Scholar]
- Carmona, F.; Pereira, A.M.S. Herbal medicines: Old and new concepts, truths and misunderstandings. Rev. Bras. De Farmacogn. 2013, 23, 379–385. [Google Scholar] [CrossRef]
- Wermuth, C.G. Multitargeted drugs: The end of the ‘one-target-one-disease’ philosophy? Drug Discov. Today 2004, 19, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-K. Antipsychotic Polypharmacy: A Dirty Little Secret or a Fashion? Int. J. Neuropsychopharmacol. 2019, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Stassen, H.H.; Bachmann, S.; Bridler, R.; Cattapan, K.; Herzig, D.; Schneeberger, A.; Seifritz, E. Detailing the effects of polypharmacy in psychiatry: Longitudinal study of 320 patients hospitalized for depression or schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kataria, H.; Mishra, R. Medicinal Plants as Novel Promising Therapeutics for Neuroprotection and Neuroregeneration. In New Age Herbals—Resource, Quality and Pharmacognosy; Springer: Singapore, 2018; pp. 437–453. [Google Scholar]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Shafiee, M.; Arekhi, S.; Omranzadeh, A.; Sahebkar, A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J. Affect. Disord. 2018, 227, 330–337. [Google Scholar] [CrossRef]
- Siddiqui, M.J.; Saleh, M.S.; Basharuddin, S.N.B.; Zamri, S.H.B.; bin Mohd Najib, M.H.; bin Che Ibrahim, M.Z.; Mazha, H.N.B.; Hassan, N.M.; Khatib, A. Saffron (Crocus sativus L.): As an Antidepressant. J. Pharm. Bioallied Sci. 2018, 10, 173–180. [Google Scholar] [CrossRef]
- Costa de Melo, N.; Sánchez-Ortiz, B.L.; dos Santos Sampaio, T.I.; Matias Pereira, A.C.; Pinheiro da Silva Neto, F.L.; Ribeiro da Silva, H.; Alves Soares Cruz, R.; Keita, H.; Soares Pereira, A.M.; Tavares Carvalho, J.C. Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) Moldenke: A study on zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, J.C. Reveals of New Candidate Active Components in Hemerocallis Radix and Its Anti-Depression Action of Mechanism Based on Network Pharmacology Approach. Int. J. Mol. Sci. 2020, 21, 1868. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A. Administration of Allium cepa L. bulb attenuates stress-produced anxiety and depression and improves memory in male mice. Metab. Brain Dis. 2018, 33, 271–281. [Google Scholar] [CrossRef]
- Kuchta, K.; Hladikova, M.; Thomsen, M.; Nahrstedt, A.; Schmidt, M. Kava (Piper methysticum) Extract for the Treatment of Nervous Anxiety, Tension and Restlessness. Drug Res. 2021, 71, 83–93. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Parvin, N.; Assarzadegan, N.; Asghari, S. The effects of lavandula angustifolia mill infusion on depression in patients using citalopram: A comparison study. Iran. Red. Crescent Med. J. 2013, 15, 734. [Google Scholar] [CrossRef]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L. Anxiolytic effect of essential oils and their constituents: A review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Konstantinos, F.; Heun, R. The effects of Rhodiola Rosea supplementation on depression, anxiety and mood–A Systematic Review. Glob. Psychiatry 2020, 3, 72–82. [Google Scholar] [CrossRef]
- Adebayo, O.G.; Ben-Azu, B.; Ajayi, A.M.; Wopara, I.; Aduema, W.; Kolawole, T.A.; Umoren, E.B.; Onyeleonu, I.; Ebo, O.T.; Ajibo, D.N. Gingko biloba abrogate lead-induced neurodegeneration in mice hippocampus: Involvement of NF-κB expression, myeloperoxidase activity and pro-inflammatory mediators. Biol. Trace Elem. Res. 2022, 200, 1736–1749. [Google Scholar] [CrossRef]
- Kumar Singh, S.; Barreto, E.G.; Aliev, G.; Echeverria, V. Ginkgo biloba as an alternative medicine in the treatment of anxiety in dementia and other psychiatric disorders. Curr. Drug Metab. 2017, 18, 112–119. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef]
- Sherman, S.H.; Joshee, N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Act. Plants 2022, 11, 22–38. [Google Scholar]
- Sales, A.J.; Crestani, C.C.; Guimarães, F.S.; Joca, S.R. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 255–261. [Google Scholar] [CrossRef]
- Gupta, G.L.; Fernandes, J. Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed. Pharmacother. 2019, 109, 1698–1708. [Google Scholar] [CrossRef]
- Chuang, H.-W.; Wang, T.-Y.; Huang, C.-C.; Wei, I.H. Echinacoside exhibits antidepressant-like effects through AMPAR–Akt/ERK–mTOR pathway stimulation and BDNF expression in mice. Chin. Med. 2022, 17, 9. [Google Scholar] [CrossRef]
- Mendelson, S.D. Herbal Treatment of Anxiety: Clinical Studies in Western, Chinese and Ayurvedic Traditions; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural compounds and neuroprotection: Mechanisms of action and novel delivery systems. In Vivo 2016, 30, 535–547. [Google Scholar]
- Wang, Z.Y.; Liu, J.Y.; Yang, C.B.; Malampati, S.; Huang, Y.Y.; Li, M.X.; Li, M.; Song, J.X. Neuroprotective natural products for the treatment of Parkinson’s disease by targeting the autophagy–lysosome pathway: A systematic review. Phytother. Res. 2017, 31, 1119–1127. [Google Scholar] [CrossRef]
- Martins, J.; Brijesh, S. Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother. 2018, 104, 343–365. [Google Scholar] [CrossRef]
- Kumar, V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Product. Deriv. 2006, 20, 1023–1035. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: A systematic review. Phytother. Res. 2007, 21, 703–716. [Google Scholar] [CrossRef]
- Spinella, M. The Psychopharmacology of Herbal Medicine: Plant Drugs that Alter Mind, Brain, and Behavior; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Winston, D. Adaptogens: Herbs for Strength, Stamina, and Stress Relief; Simon and Schuster: New York, NY, USA, 2019. [Google Scholar]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Chakraborty, P. Herbal genomics as tools for dissecting new metabolic pathways of unexplored medicinal plants and drug discovery. Biochim. Open 2018, 6, 9–16. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Wong, M.; O’kirwan, F.; Hannestad, J.; Irizarry, K.; Elashoff, D.; Licinio, J. St John’s wort and imipramine-induced gene expression profiles identify cellular functions relevant to antidepressant action and novel pharmacogenetic candidates for the phenotype of antidepressant treatment response. Mol. Psychiatry 2004, 9, 237–251. [Google Scholar] [CrossRef]
- Pennington, K.; Föcking, M.; McManus, C.; Pariante, C.; Dunn, M.; Cotter, D. A proteomic investigation of similarities between conventional and herbal antidepressant treatments. J. Psychopharmacol. 2009, 23, 520–530. [Google Scholar] [CrossRef]
- Popay, I. Hypericum perforatum (St John’s Wort). CABI Compend. 2022, 28268. [Google Scholar] [CrossRef]
- Tingle, J.L.; Cook-Patton, S.C.; Agrawal, A.A. Spillover of a biological control agent (Chrysolina quadrigemina) onto native St. Johnswort (Hypericum punctatum). PeerJ 2016, 4, e1886. [Google Scholar] [CrossRef]
- Suryawanshi, M.V.; Gujarathi, P.P.; Mulla, T.; Bagban, I. Hypericum perforatum: A comprehensive review on pharmacognosy, preclinical studies, putative molecular mechanism, and clinical studies in neurodegenerative diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3803–3818. [Google Scholar] [CrossRef]
- Nobakht, S.Z.; Akaberi, M.; Mohammadpour, A.H.; Moghadam, A.T.; Emami, S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022, 25, 1045. [Google Scholar]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B.S. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Tokgöz, H.B.; Altan, F. Hypericum perforatum L.: A medicinal plant with potential as a curative agent against obesity-associated complications. Mol. Biol. Rep. 2020, 47, 8679–8686. [Google Scholar] [CrossRef]
- Peterson, B.N.H. St John’s Wort. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kholghi, G.; Arjmandi-Rad, S.; Zarrindast, M.-R.; Vaseghi, S. St. John’s wort (Hypericum perforatum) and depression: What happens to the neurotransmitter systems? Naunyn-Schmiedeberg's Arch. Pharmacol. 2022, 395, 629–642. [Google Scholar] [CrossRef]
- Wurglics, M.; Schubert-Zsilavecz, M. Hypericum perforatum: A ‘modern’ herbal antidepressant: Pharmacokinetics of active ingredients. Clin. Pharmacokinet. 2006, 45, 449–468. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef]
- Kasper, S.; Dienel, A.; Kieser, M. Continuation and long-term maintenance treatment with Hypericum extract WS® 5570 after successful acute treatment of mild to moderate depression–rationale and study design. Int. J. Methods Psychiatr. Res. 2004, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Warnick, S.J., Jr.; Mehdi, L.; Kowalkowski, J. Wait-there’s evidence for that? Integrative medicine treatments for major depressive disorder. Int. J. Psychiatry Med. 2021, 56, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Sevastre-Berghian, A.; Toma, V.; Sevastre, B.; Hanganu, D.; Vlase, L.; Benedec, D.; Oniga, I.; Baldea, I.; Olteanu, D.; Moldovan, R. Characterization and biological effects of Hypericum extracts on experimentally-induced-anxiety, oxidative stress and inflammation in rats. J. Physiol. Pharmacol. 2018, 69, 789–800. [Google Scholar]
- Bilia, A.R.; Gallori, S.; Vincieri, F.F. St. John’s wort and depression: Efficacy, safety and tolerability-an update. Life Sci. 2002, 70, 3077–3096. [Google Scholar] [CrossRef]
- Butterweck, V.; Schmidt, M. John’s wort: Role of active compounds for its mechanism of action and efficacy. WMW Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef]
- Di Pierro, F.; Risso, P.; Settembre, R. Role in depression of a multi-fractionated versus a conventional Hypericum perforatum extract. Panminerva Medica 2018, 60, 156–160. [Google Scholar] [CrossRef]
- Furhad, S.B.A. Herbal Supplements. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 5. Complementary and alternative medicine treatments. Can. J. Psychiatry 2016, 61, 576–587. [Google Scholar] [CrossRef]
- Kandilarov, I.K.; Zlatanova, H.I.; Georgieva-Kotetarova, M.T.; Kostadinova, I.I.; Katsarova, M.N.; Dimitrova, S.Z.; Lukanov, L.K.; Sadakov, F. Antidepressant effect and recognition memory improvement of two novel plant extract combinations-antistress I and antistress II on rats subjected to a model of mild chronic stress. Folia Medica 2018, 60, 110–116. [Google Scholar] [CrossRef]
- Vance, K.M.; Ribnicky, D.M.; Hermann, G.E.; Rogers, R.C. St. John’s Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition 2014, 30, S37–S42. [Google Scholar] [CrossRef]
- Baureithel, K.H.; Büter, K.B.; Engesser, A.; Burkard, W.; Schaffner, W. Inhibition of benzodiazepine binding in vitro by amentoflavone, a constituent of various species of Hypericum. Pharm. Acta Helv. 1997, 72, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V. Mechanism of action of St John’s wort in depression: What is known? CNS Drugs 2003, 17, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A.; Schwendler, A.; Hüther, J.; Schwarzbach, H.; Schwarz, A.; Kolb, C.; Abdel-Aziz, H.; Kinscherf, R. Neurotrophic, cytoprotective, and anti-inflammatory effects of St. John’s wort extract on differentiated mouse hippocampal HT-22 neurons. Front. Pharmacol. 2018, 8, 297500. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Lv, Y.; Kelber, O.; Butterweck, V. Mechanism of St. John’s wort extract (STW3-VI) during chronic restraint stress is mediated by the interrelationship of the immune, oxidative defense, and neuroendocrine system. Neuropharmacology 2010, 58, 767–773. [Google Scholar] [CrossRef]
- Song, A.; Wu, Z.; Zhao, W.; Shi, W.; Cheng, R.; Jiang, J.; Ni, Z.; Qu, H.; Qiaolongbatu, X.; Fan, G. The role and mechanism of hyperoside against depression-like behavior in mice via the NLRP1 inflammasome. Medicina 2022, 58, 1749. [Google Scholar] [CrossRef]
- Kasper, S.; Gastpar, M.; Möller, H.-J.; Müller, W.E.; Volz, H.-P.; Dienel, A.; Kieser, M. Better tolerability of St. John’s wort extract WS 5570 compared to treatment with SSRIs: A reanalysis of data from controlled clinical trials in acute major depression. Int. Clin. Psychopharmacol. 2010, 25, 204–213. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Palazzo, M.C.; Oldani, L.; Altamura, A.C. The noradrenergic action in antidepressant treatments: Pharmacological and clinical aspects. CNS Neurosci. Ther. 2011, 17, 723–732. [Google Scholar] [CrossRef]
- Dwyer, A.V.; Whitten, D.L.; Hawrelak, J.A. Herbal medicines, other than St. John’s Wort, in the treatment of depression: A systematic review. Altern. Med. Rev. 2011, 16, 40–50. [Google Scholar]
- Claro, A.E.; Palanza, C.; Mazza, M.; Schuenemann, G.E.U.M.; Rigoni, M.; Pontecorvi, A.; Janiri, L.; Pitocco, D.; Muti, P. Historical use of medicinal plants and future potential from phytotherapy to phitochemicals. Ann. Bot. 2024, 14. [Google Scholar] [CrossRef]
- Crupi, R.; Mazzon, E.; Marino, A.; La Spada, G.; Bramanti, P.; Battaglia, F.; Cuzzocrea, S.; Spina, E. Hypericum perforatum treatment: Effect on behaviour and neurogenesis in a chronic stress model in mice. BMC Complement. Altern. Med. 2011, 11, 7. [Google Scholar] [CrossRef]
- Trofimiuk, E.; Holownia, A.; Braszko, J.J. St. John’s wort may relieve negative effects of stress on spatial working memory by changing synaptic plasticity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Concerto, C.; Boo, H.; Hu, C.; Sandilya, P.; Krish, A.; Chusid, E.; Coira, D.; Aguglia, E.; Battaglia, F. Hypericum perforatum extract modulates cortical plasticity in humans. Psychopharmacology 2018, 235, 145–153. [Google Scholar] [CrossRef]
- Yechiam, E.; Ben-Eliezer, D.; Ashby, N.J.; Bar-Shaked, M. The acute effect of Hypericum perforatum on short-term memory in healthy adults. Psychopharmacology 2019, 236, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Brattström, A. Long-term effects of St. John’s wort (Hypericum perforatum) treatment: A 1-year safety study in mild to moderate depression. Phytomedicine 2009, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Schrader, E.; Meier, B.; Brattström, A. Hypericum treatment of mild–moderate depression in a placebo–controlled study. A prospective, double–blind, randomized, placebo–controlled, multicentre study. Human Psychopharmacol. Clin. Exp. 1998, 13, 163–169. [Google Scholar] [CrossRef]
- Seifritz, E.; Hatzinger, M.; Holsboer-Trachsler, E. Efficacy of Hypericum extract WS® 5570 compared with paroxetine in patients with a moderate major depressive episode–a subgroup analysis. Int. J. Psychiatry Clin. Pract. 2016, 20, 126–132. [Google Scholar] [CrossRef]
- Zahner, C.; Kruttschnitt, E.; Uricher, J.; Lissy, M.; Hirsch, M.; Nicolussi, S.; Krähenbühl, S.; Drewe, J. No clinically relevant interactions of St. John’s wort extract ze 117 low in hyperforin with cytochrome P450 enzymes and P-glycoprotein. Clin. Pharmacol. Ther. 2019, 106, 432–440. [Google Scholar] [CrossRef]
- De Vry, J.; Maurel, S.; Schreiber, R.; De Beun, R.; Jentzsch, K. Comparison of hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur. Neuropsychopharmacol. 1999, 9, 461–468. [Google Scholar] [CrossRef]
- Schuckit, M.A.; Monteiro, M.G. Alcoholism, anxiety and depression. Br. J. Addict. 1988, 83, 1373–1380. [Google Scholar] [CrossRef]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 148. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea. A Phytomedicinal Overv. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Ahmed, F.; Filion, V.; Saleem, A.; Arnason, J.T. Phytochemistry of Rhodiola rosea; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Khokhlova, K.; Zdoryk, O. Authentication of Rhodiola rosea, Rhodiola quadrifida and Rhodiola rosea liquid extract from the Ukrainian market using HPTLC chromatographic profiles. Nat. Prod. Res. 2020, 34, 2842–2846. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.N.; Mhetre, O.S.; Malavdkar, P.R. A Review Article on Rhodiola Rosea: An Adaptogen Having Multiple Benefits. Int. J. Pharmacogn. 2020, 7, 62–69. [Google Scholar]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
- Panossian, A.; Nikoyan, N.; Ohanyan, N.; Hovhannisyan, A.; Abrahamyan, H.; Gabrielyan, E.; Wikman, G. Comparative study of Rhodiola preparations on behavioral despair of rats. Phytomedicine 2008, 15, 84–91. [Google Scholar] [CrossRef]
- Mattioli, L.; Funari, C.; Perfumi, M. Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J. Psychopharmacol. 2009, 23, 130–142. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, Y.; Qu, Z.; Tang, J.; Qin, Y.; Chung, P.; Wong, R.; Hägg, U. The effects of Rhodiola rosea extract on 5-HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine 2009, 16, 830–838. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef]
- Yang, S.-J.; Yu, H.-Y.; Kang, D.-Y.; Ma, Z.-Q.; Qu, R.; Fu, Q.; Ma, S.-P. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014, 124, 451–457. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.; Zhang, J.; Li, H.; Guo, M. Rhodiola rosea: A review in the context of PPPM approach. EPMA J. 2024, 15, 233–259. [Google Scholar] [CrossRef]
- Ishaque, S.; Shamseer, L.; Bukutu, C.; Vohra, S. Rhodiola rosea for physical and mental fatigue: A systematic review. BMC Complement. Altern. Med. 2012, 12, 70. [Google Scholar] [CrossRef]
- Muszyńska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Surowce naturalne mające znaczenie w profilaktyce i wspomagające leczenie depresji. Psychiatr. Pol. 2015, 49, 3. [Google Scholar]
- Yu, H.L.; Zhang, P.P.; Zhang, C.; Zhang, X.; Li, Z.Z.; Li, W.Q.; Fu, A.S. Effects of rhodiola rosea on oxidative stress and negative emotional states in patients with obstructive sleep apnea. J. Clin. Otorhinolaryngol. Head Neck Surg. 2019, 33, 954–957. [Google Scholar] [CrossRef]
- Gao, L.; Wu, C.; Liao, Y.; Wang, J. Antidepressants effects of Rhodiola capsule combined with sertraline for major depressive disorder: A randomized double-blind placebo-controlled clinical trial. J. Affect. Disord. 2020, 265, 99–103. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Zee, J.; Soeller, I.; Li, Q.S.; Rockwell, K.; Amsterdam, J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine 2015, 22, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Concerto, C.; Infortuna, C.; Muscatello, M.R.A.; Bruno, A.; Zoccali, R.; Chusid, E.; Aguglia, E.; Battaglia, F. Exploring the effect of adaptogenic Rhodiola Rosea extract on neuroplasticity in humans. Complement. Ther. Med. 2018, 41, 141–146. [Google Scholar] [CrossRef]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2007, 61, 343–348. [Google Scholar] [CrossRef]
- Cropley, M.; Banks, A.P.; Boyle, J. The effects of Rhodiola rosea L. extract on anxiety, stress, cognition and other mood symptoms. Phytother. Res. 2015, 29, 1934–1939. [Google Scholar] [CrossRef]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef]
- Lekomtseva, Y.; Zhukova, I.; Wacker, A. Rhodiola rosea in subjects with prolonged or chronic fatigue symptoms: Results of an open-label clinical trial. Complement. Med. Res. 2017, 24, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Ruiz, R.M.; Roa-Coria, J.E.; Patiño-Camacho, S.I.; Flores-Murrieta, F.J.; Déciga-Campos, M. Neuropharmacological and Toxicity Evaluations of Ethanol Extract from R hodiola R osea. Drug Dev. Res. 2012, 73, 106–113. [Google Scholar] [CrossRef]
- Bangratz, M.; Ait Abdellah, S.; Berlin, A.; Blondeau, C.; Guilbot, A.; Dubourdeaux, M.; Lemoine, P. A preliminary assessment of a combination of rhodiola and saffron in the management of mild-moderate depression. Neuropsychiatr. Dis. Treat. 2018, 14, 1821–1829. [Google Scholar] [CrossRef]
- Duman, İ.; Eken, Ç.G. Therapeutic Effects of Ginkgo Biloba Extract in Neuropsychiatric Disorders. In Current Research in Health Sciences; Gece Publishing: Ankara, Turkey, 2022; pp. 170–188. [Google Scholar]
- Chatterjee, S.; Kondratskaya, E.; Krishtal, O. Structure-activity studies with Ginkgo biloba extract constituents as receptor-gated chloride channel blockers and modulators. Pharmacopsychiatry 2003, 36, 68–77. [Google Scholar]
- Ramassamy, C.; Longpre, F.; Christen, Y. Ginkgo biloba extract (EGb 761) in Alzheimer’s disease: Is there any evidence? Curr. Alzheimer Res. 2007, 4, 253–262. [Google Scholar] [CrossRef]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, Z.; Xu, J.; Li, Y.; Chen, M. Pharmacological activities of ginkgolic acids in relation to autophagy. Pharmaceuticals 2022, 15, 1469. [Google Scholar] [CrossRef]
- Mdzinarishvili, A.; Kiewert, C.; Kumar, V.; Hillert, M.; Klein, J. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience 2007, 144, 217–222. [Google Scholar] [CrossRef]
- Markus, C.R.; Lammers, J.H. Effects of Ginkgo biloba on corticosterone stress responses after inescapable shock exposure in the rat. Pharmacol. Biochem. Behav. 2003, 76, 487–492. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Shou, S.S.; Lin, Y.X.; Chen, C.C.; Chiang, C.Y.; Yeh, C.Y. Effect of Ginkgo biloba extract on lipopolysaccharide-induced anhedonic depressive-like behavior in male rats. Phytother. Res. 2015, 29, 260–266. [Google Scholar] [CrossRef]
- Fehske, C.J.; Leuner, K.; Müller, W.E. Ginkgo biloba extract (EGb761®) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol. Res. 2009, 60, 68–73. [Google Scholar] [CrossRef]
- Blecharz-Klin, K.; Piechal, A.; Joniec, I.; Pyrzanowska, J.; Widy-Tyszkiewicz, E. Pharmacological and biochemical effects of Ginkgo biloba extract on learning, memory consolidation and motor activity in old rats. Acta Neurobiol. Exp. 2009, 69, 217–231. [Google Scholar] [CrossRef]
- Yoshitake, T.; Yoshitake, S.; Kehr, J. The Ginkgo biloba extract EGb 761® and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol. 2010, 159, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.; Yoshitake, S.; Ijiri, S.; Koch, E.; Nöldner, M.; Yoshitake, T. Ginkgo biloba leaf extract (EGb 761®) and its specific acylated flavonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: Possible implications for the cognitive enhancing properties of EGb 761®. Int. Psychogeriatr. 2012, 24, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Amri, H.; Drieu, K.; Papadopoulos, V. Ex vivo regulation of adrenal cortical cell steroid and protein synthesis, in response to adrenocorticotropic hormone stimulation, by the Ginkgo biloba extract EGb 761 and isolated ginkgolide B. Endocrinology 1997, 138, 5415–5426. [Google Scholar] [CrossRef] [PubMed]
- Marcilhac, A.; Dakine, N.; Bourhim, N.; Guillaume, V.; Grino, M.; Drieu, K.; Oliver, C. Effect of chronic administration of Ginkgo biloba extract or Ginkgolide on the hypothalamic-pituitary-adrenal axis in the rat. Life Sci. 1998, 62, 2329–2340. [Google Scholar] [CrossRef]
- Yeh, K.-Y.; Pu, H.-F.; Kaphle, K.; Lin, S.-F.; Wu, L.-S.; Lin, J.-H.; Tsai, Y.-F. Ginkgo biloba extract enhances male copulatory behavior and reduces serum prolactin levels in rats. Horm. Behav. 2008, 53, 225–231. [Google Scholar] [CrossRef]
- Montes, P.; Ruiz-Sanchez, E.; Rojas, C.; Rojas, P. Ginkgo biloba extract 761: A review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2015, 14, 132–149. [Google Scholar] [CrossRef]
- Kressmann, S.; Müller, W.; Blume, H. Pharmaceutical quality of different Ginkgo biloba brands. J. Pharm. Pharmacol. 2002, 54, 661–669. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, C.-w.; Shi, J.; Lin, Z.-x. Effects of Ginkgo biloba on dementia: An overview of systematic reviews. J. Ethnopharmacol. 2017, 195, 1–9. [Google Scholar] [CrossRef]
- Goel, R.K.; Kaur, D.; Pahwa, P. Assessment of anxiolytic effect of nerolidol in mice. Indian J. Pharmacol. 2016, 48, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, B.H.; Bagheri, R.; Roozbeh, B.; Ashtary-Larky, D.; Gaeini, A.A.; Dutheil, F.; Wong, A. Impact of saffron (Crocus sativus Linn) supplementation and resistance training on markers implicated in depression and happiness levels in untrained young males. Physiol. Behav. 2021, 233, 113352. [Google Scholar] [CrossRef]
- Abe, K.; Saito, H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother. Res. 2000, 14, 149–152. [Google Scholar] [CrossRef]
- Ghajar, A.; Neishabouri, S.M.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A. Crocus sativus L. versus citalopram in the treatment of major depressive disorder with anxious distress: A double-blind, controlled clinical trial. Pharmacopsychiatry 2017, 50, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, E.; Basti, A.A.; Noorbala, A.-A.; Jamshidi, A.-H.; Abbasi, S.H.; Akhondzadeh, S. Crocus sativus L.(petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine 2006, 13, 607–611. [Google Scholar] [CrossRef]
- Mazidi, M.; Shemshian, M.; Mousavi, S.H.; Norouzy, A.; Kermani, T.; Moghiman, T.; Sadeghi, A.; Mokhber, N.; Ghayour-Mobarhan, M.; Ferns, G.A. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med. 2016, 13, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jafarnia, N.; Ghorbani, Z.; Nokhostin, M.; Manayi, A.; Nourimajd, S.; Jahromi, S.R. Effect of saffron (Crocus sativus L.) as an add-on therapy to sertraline in mild to moderate generalized anxiety disorder: A double blind randomized controlled trial. Arch. Neurosci. 2017, 4, e14332. [Google Scholar]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 768–774. [Google Scholar] [CrossRef]
- Xu, G.-L.; Li, G.; Ma, H.-P.; Zhong, H.; Liu, F.; Ao, G.-Z. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J. Agric. Food Chem. 2009, 57, 8325–8330. [Google Scholar] [CrossRef]
- Jalali, F.; Hashemi, S.F. The effect of saffron on depression among recovered consumers of methamphetamine living with HIV/AIDS. Subst. Use Misuse 2018, 53, 1951–1957. [Google Scholar] [CrossRef]
- Lechtenberg, M.; Schepmann, D.; Niehues, M.; Hellenbrand, N.; Wünsch, B.; Hensel, A. Quality and functionality of saffron: Quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and σ1 (sigma-1) receptors. Planta Medica 2008, 74, 764–772. [Google Scholar] [CrossRef]
- Pitsikas, N. Constituents of saffron (Crocus sativus L.) as potential candidates for the treatment of anxiety disorders and schizophrenia. Molecules 2016, 21, 303. [Google Scholar] [CrossRef]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.-H.; Kassaian, S.E.; Tafti, A.-A.N.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014, 155, 216–222. [Google Scholar] [CrossRef]
- Dovrtělová, G.; Nosková, K.; Juřica, J.; Turjap, M.; Zendulka, O. Can bioactive compounds of Crocus sativus L. influence the metabolic activity of selected CYP enzymes in the rat. Physiol. Res. 2015, 644, S453–S458. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. Saffron. Available online: https://www.drugs.com/npp/saffron.html (accessed on 18 February 2025).
- Yoo, D.Y.; Choi, J.H.; Kim, W.; Yoo, K.Y.; Lee, C.H.; Yoon, Y.S.; Won, M.H.; Hwang, I.K. Effects of Melissa officinalis L. (lemon balm) extract on neurogenesis associated with serum corticosterone and GABA in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Araj-Khodaei, M.; Noorbala, A.A.; Yarani, R.; Emadi, F.; Emaratkar, E.; Faghihzadeh, S.; Parsian, Z.; Alijaniha, F.; Kamalinejad, M.; Naseri, M. A double-blind, randomized pilot study for comparison of Melissa officinalis L. and Lavandula angustifolia Mill. with Fluoxetine for the treatment of depression. BMC Complement. Med. Ther. 2020, 20, 207. [Google Scholar] [CrossRef]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Jafari, H.; Mokaberinejad, R.; Raeis-Abdollahib, E. Echium amoenumfrom viewpoint of Avicenna: A brief review. J. Contemp. Med. Sci. Vol. 2018, 4, 187–190. [Google Scholar] [CrossRef]
- Reeves, J.W.; Fisher, A.J.; Newman, M.G.; Granger, D.A. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology 2016, 53, 951–957. [Google Scholar] [CrossRef]
- Abolhassani, M. Antiviral activity of borage (Echium amoenum). Arch. Med. Sci. 2010, 6, 366–369. [Google Scholar] [CrossRef]
- Anushiravani, M.; Manteghi, A.A.; Taghipur, A.; Eslami, M. Comparing effectiveness of a combined herbal drug based on Echium amoenum with Citalopram in the treatment of Major Depressive Disorder. Curr. Drug Discov. Technol. 2019, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Faryadian, S.; Sydmohammadi, A.; Khosravi, A.; Kashiri, M.; Faryadayn, P.; Abasi, N. Aqueous extract of Echlum amoenum elevate CSF serotonin and dopamine level in depression rat. Biomed. Pharmacol. J. 2014, 7, 137–142. [Google Scholar] [CrossRef]
- Nouri, M.; Farajdokht, F.; Torbati, M.; Ranjbar, F.; Hamedyazdan, S.; Araj-Khodaei, M.; Sadigh-Eteghad, S. A close look at echium amoenum processing, neuroactive components, and effects on neuropsychiatric disorders. Galen. Med. J. 2019, 8, e1559. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Navratilova, Z. Bioactivity of Echium amoenum: A mini review. Pharmacology 2019, 20, 14915–14917. [Google Scholar] [CrossRef]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. Physiological and biochemical effects of Echium amoenum extract on Mn2+-imposed Parkinson like disorder in rats. Adv. Pharm. Bull. 2018, 8, 705–713. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Li, M.; Zhang, X.; Lv, C.; Jia, L.; Wang, J.; Lu, J. A comparative study of the main constituents and antidepressant effects of raw and vinegar-baked Bupleuri Radix in rats subjected to chronic unpredictable mild stress. RSC Adv. 2017, 7, 32652–32663. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, X.; Nikolić, D.; Chen, S.-N.; Huang, K.; Li, G.; Pauli, G.F.; van Breemen, R.B. Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 2014, 53, 55–61. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus–a story that begs to be told. A Rev. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar]
- Feng, Y.; Gao, X.; Meng, M.; Xue, H.; Qin, X. Multi-omics reveals the mechanisms of antidepressant-like effects of the low polarity fraction of Bupleuri Radix. J. Ethnopharmacol. 2020, 256, 112806. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Koh, S.S.H.; Chan, H.W.; Ho, C.Y.X. Clinical use of curcumin in depression: A meta-analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, F.N.; Gazal, M.; Bastos, C.R.; Kaster, M.P.; Ghisleni, G. Curcumin in depressive disorders: An overview of potential mechanisms, preclinical and clinical findings. Eur. J. Pharmacol. 2016, 784, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, X.-J.; Liu, P.; Dong, X.-Z.; Mu, L.-H.; Chen, Y.-B.; Liu, M.-Y.; Yu, B.-Y. Antidepressant and neuroprotective effect of the Chinese herb kaixinsan against lentiviral shRNA knockdown brain-derived neurotrophic factor-induced injury in vitro and in vivo. Neuropsychobiology 2014, 69, 129–139. [Google Scholar] [CrossRef]
- da Silva Marques, J.G.; Antunes, F.T.T.; da Silva Brum, L.F.; Pedron, C.; de Oliveira, I.B.; Ferraz, A.d.B.F.; Martins, M.I.M.; Dallegrave, E.; de Souza, A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021, 399, 113002. [Google Scholar] [CrossRef]
- Kyrou, I.; Christou, A.; Panagiotakos, D.; Stefanaki, C.; Skenderi, K.; Katsana, K.; Tsigos, C. Effects of a hops (Humulus lupulus L.) dry extract supplement on self-reported depression, anxiety and stress levels in apparently healthy young adults: A randomized, placebo-controlled, double-blind, crossover pilot study. Hormones 2017, 16, 171–180. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini-Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef]
- Bačkor, M.; Goga, M.; Ručová, D.; Urminská, D.; Bačkorová, M.; Klejdus, B. Allelopathic effects of three lichen secondary metabolites on cultures of aposymbiotically grown lichen photobionts and free-living alga Scenedesmus quadricauda. South. Afr. J. Bot. 2023, 162, 688–693. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Kosanić, M.; Ranković, B. Lichen Secondary Metabolites as Potential Antibiotic Agents. In Lichen Secondary Metabolites Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 81–104. [Google Scholar]
- Sovrlic, M.; Manojlovic, N.; Kosanic, M.; Kočović, A.; Tomović, J.; Vasiljević, P. Investigation of phytochemical composition and in vitro antioxidant potential of different extracts and gyrophoric acid derived from the lichen Umbilicaria grisea growing in Serbia. Farmacia 2024, 72, 1369–1375. [Google Scholar] [CrossRef]
- Tomović, J.; Kočović, A.; Anđić, M.; Bradić, J.; Zubić, N.; Jakovljević, V.; Sovrlić, M.; Vasiljević, P.; Manojlović, N. Bioactive Components Analysis and Pharmacological Properties of Extracts and Metabolites of Lichen Umbilicaria crustulosa. Sci. Pharm. 2024, 92, 27. [Google Scholar] [CrossRef]
- Urbanska, N.; Karasova, M.; Jendzelovska, Z.; Majerník, M.; Kolesarova, M.; Kecsey, D.; Jendzelovsky, R.; Bohus, P.; Kiskova, T. Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats. Int. J. Mol. Sci. 2024, 25, 11840. [Google Scholar] [CrossRef]
- Dimitrijević, I.; Mitić, V.; Stankov Jovanović, V.; Stanković, M.; Zlatanović, I.; Stojanović, G. Cladonia rangiformis Acetone Extract— New Insight into the Chemical Composition and Biological Activity. Nat. Prod. Commun. 2023, 18, 1934578X231212159. [Google Scholar] [CrossRef]
- Urbanska, N.; Simko, P.; Leskanicova, A.; Karasova, M.; Jendzelovska, Z.; Jendzelovsky, R.; Rucova, D.; Kolesarova, M.; Goga, M.; Backor, M.; et al. Atranorin, a Secondary Metabolite of Lichens, Exhibited Anxiolytic/Antidepressant Activity in Wistar Rats. Life 2022, 12, 1850. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Farahani, M.S.; Rahimi, R. Phytochemical constituents as future antidepressants: A comprehensive review. Rev. Neurosci. 2015, 26, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.V. Special Issue “flavonoids and their disease prevention and treatment potential”: Recent advances and future perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef]
- Dereli, F.T.G.; Ilhan, M.; Akkol, E.K. Identification of the main active antidepressant constituents in a traditional Turkish medicinal plant, Centaurea kurdica Reichardt. J. Ethnopharmacol. 2020, 249, 112373. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Gürağaç Dereli, F.T.; Ilhan, M. Assessment of antidepressant effect of the aerial parts of micromeria myrtifolia Boiss. & Hohen Mice. Molecules 2019, 24, 1869. [Google Scholar] [CrossRef]
- Park, S.-J.; Jaiswal, V.; Lee, H.-J. Dietary Intake of Flavonoids and Carotenoids Is Associated with Anti-Depressive Symptoms: Epidemiological Study and In Silico—Mechanism Analysis. Antioxidants 2021, 11, 53. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Dereli, F.T.G.; Ilhan, M.; Akkol, E.K. New drug discovery from medicinal plants and phytoconstituents for depressive disorders. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2019, 18, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant flavonoids and their relationship with oxidative stress. Oxidative Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Lai, H.; Sun, Y.; Yan, X.; Ai, Q.; Lin, M.; Yang, S.; Yang, Y.; Chu, S.; et al. Novel antidepressant mechanism of hypericin: Role of connexin 43-based gap junctions. Biomed. Pharmacother. 2023, 167, 115545. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, Y.; Han, X.; Zhu, Y.; Li, X.; Zhang, Y.; Lu, Y. The protective effect of hypericin on postpartum depression rat model by inhibiting the NLRP3 inflammasome activation and regulating glucocorticoid metabolism. Int. Immunopharmacol. 2022, 105, 108560. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, G.; Cui, L.; Wang, Q. Myricetin attenuates depressant-like behavior in mice subjected to repeated restraint stress. Int. J. Mol. Sci. 2015, 16, 28377–28385. [Google Scholar] [CrossRef]
- Mohan, M.; Jadhav, S.S.; Kasture, V.S.; Kasture, S.B. Effect of myricetin on behavioral paradigms of anxiety. Pharm. Biol. 2009, 47, 927–931. [Google Scholar] [CrossRef]
- Meyer, E.; Mori, M.A.; Campos, A.C.; Andreatini, R.; Guimarães, F.S.; Milani, H.; de Oliveira, R.M.W. Myricitrin induces antidepressant-like effects and facilitates adult neurogenesis in mice. Behav. Brain Res. 2017, 316, 59–65. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, S.; Zhu, X.; Jiang, Y.; Lu, Z.; Gong, P. Myricetin suppresses traumatic brain injury-induced inflammatory response via EGFR/AKT/STAT pathway. Sci. Rep. 2023, 13, 22764. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Myricetin as a Promising Molecule for the Treatment of Post-Ischemic Brain Neurodegeneration. Nutrients 2021, 13, 342. [Google Scholar] [CrossRef]
- Demyashkin, G.; Blinova, E.; Grigoryan, M.; Parshenkov, M.; Skovorodko, P.; Ius, V.; Lebed, A.; Shegay, P.; Kaprin, A. Neuroprotective Effects of Myricetin on PTZ-Induced Seizures in Mice: Evaluation of Oxidation, Neuroinflammation and Metabolism, and Apoptosis in the Hippocampus. Curr. Issues Mol. Biol. 2024, 46, 8914–8944. [Google Scholar] [CrossRef] [PubMed]
- Bayazeid, O.; Nemutlu, E.; Eylem, C.C.; İlhan, M.; Küpeli-Akkol, E.; Karahan, H.; Kelicen-Uğur, P.; Ersoz, T.; Yalçın, F.N. Neuroactivity of the naturally occurring aporphine alkaloid, roemerine. Nat. Prod. Res. 2021, 35, 6147–6152. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, S.; Girdhar, A.; Verma, S.K.; Lather, V.; Pandita, D. Plant derived alkaloids in major neurodegenerative diseases: From animal models to clinical trials. J. Ayurvedic Herb. Med. 2015, 1, 91–100. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Ng, Y.P.; Or, T.C.T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Eom, S.; Jung, W.; Lee, J.; Yeom, H.D.; Lee, S.; Kim, C.; Park, H.-D.; Lee, J.H. Differential Regulation of Human Serotonin Receptor Type 3A by Chanoclavine and Ergonovine. Molecules 2021, 26, 1211. [Google Scholar] [CrossRef]
- Butterweck, V.; Böckers, T.; Korte, B.; Wittkowski, W.; Winterhoff, H. Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res. 2002, 930, 21–29. [Google Scholar] [CrossRef]
- Koop, T.; Dienel, A.; Heldmann, M.; Münte, T.F. Effects of a Rhodiola rosea extract on mental resource allocation and attention: An event-related potential dual task study. Phytother. Res. 2020, 34, 3287–3297. [Google Scholar] [CrossRef]
- Yuan, N.-j.; Zhu, W.-j.; Ma, Q.-y.; Huang, M.-y.; Huo, R.-r.; She, K.-j.; Pan, J.-p.; Wang, J.-g.; Chen, J.-x. Luteolin ameliorates chronic stress-induced depressive-like behaviors in mice by promoting the Arginase-1+ microglial phenotype via a PPARγ-dependent mechanism. Acta Pharmacol. Sin. 2024, 46, 575–591. [Google Scholar] [CrossRef]
- Szewczyk, B.; Pochwat, B.; Muszyńska, B.; Opoka, W.; Krakowska, A.; Rafało-Ulińska, A.; Friedland, K.; Nowak, G. Antidepressant-like activity of hyperforin and changes in BDNF and zinc levels in mice exposed to chronic unpredictable mild stress. Behav. Brain Res. 2019, 372, 112045. [Google Scholar] [CrossRef]
- El Hamdaoui, Y.; Zheng, F.; Fritz, N.; Ye, L.; Tran, M.A.; Schwickert, K.; Schirmeister, T.; Braeuning, A.; Lichtenstein, D.; Hellmich, U.A. Analysis of hyperforin (St. John’s wort) action at TRPC6 channel leads to the development of a new class of antidepressant drugs. Mol. Psychiatry 2022, 27, 5070–5085. [Google Scholar] [CrossRef] [PubMed]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Ballesta, A.; Martin, R.; Gomez de Heras, R. Antianhedonic and antidepressant effects of affron®, a standardized saffron (Crocus sativus L.) extract. Molecules 2020, 25, 3207. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Xue, J.-S.; Li, H.-Y.; Ma, Z.-Q.; Fu, Q.; Qu, R.; Ma, S.-P. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol. Behav. 2015, 152, 264–271. [Google Scholar] [CrossRef]
- Arora, V.; Chopra, K. Possible involvement of oxido-nitrosative stress induced neuro-inflammatory cascade and monoaminergic pathway: Underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J. Affect. Disord. 2013, 151, 1041–1052. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Niu, L.; Wang, L.-L.; Bai, L.; Fang, X.-Y.; Li, Y.-C.; Yi, L.-T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017, 134, 220–227. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, H.; Zhang, L.; Fu, F. Administration of Huperzine A exerts antidepressant-like activity in a rat model of post-stroke depression. Pharmacol. Biochem. Behav. 2017, 158, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Nechifor, A.; Dima, S.; Li, Y.; Jafari, S.M. Oral bioavailability of bioactive compounds; modulating factors, in vitro analysis methods, and enhancing strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 8501–8539. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Conte-Junior, C.A. Dietary Bioactive Compounds and Human Health: The Role of Bioavailability. Nutrients 2024, 17, 48. [Google Scholar] [CrossRef]
- Meena, L.; Gowda, N.A.N.; Sunil, C.K.; Rawson, A.; Janghu, S. Effect of ultrasonication on food bioactive compounds and their bio-accessibility: A review. J. Food Compos. Anal. 2024, 126, 105899. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 381–406. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Ventura-Martínez, R.; Silveira, D.; Déciga-Campos, M. Editorial: Pharmacological interaction between drugs and medicinal plants, Volume II. Front. Pharmacol. 2024, 15, 1372366. [Google Scholar] [CrossRef]
- Choudhury, A.; Singh, P.A.; Bajwa, N.; Dash, S.; Bisht, P. Pharmacovigilance of herbal medicines: Concerns and future prospects. J. Ethnopharmacol. 2023, 309, 116383. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanska, N.; Ashaolu, T.J.; Mattova, S.; Simko, P.; Kiskova, T. The Potential of Selected Plants and Their Biologically Active Molecules in the Treatment of Depression and Anxiety Disorders. Int. J. Mol. Sci. 2025, 26, 2368. https://doi.org/10.3390/ijms26052368
Urbanska N, Ashaolu TJ, Mattova S, Simko P, Kiskova T. The Potential of Selected Plants and Their Biologically Active Molecules in the Treatment of Depression and Anxiety Disorders. International Journal of Molecular Sciences. 2025; 26(5):2368. https://doi.org/10.3390/ijms26052368
Chicago/Turabian StyleUrbanska, Nicol, Tolulope Joshua Ashaolu, Simona Mattova, Patrik Simko, and Terezia Kiskova. 2025. "The Potential of Selected Plants and Their Biologically Active Molecules in the Treatment of Depression and Anxiety Disorders" International Journal of Molecular Sciences 26, no. 5: 2368. https://doi.org/10.3390/ijms26052368
APA StyleUrbanska, N., Ashaolu, T. J., Mattova, S., Simko, P., & Kiskova, T. (2025). The Potential of Selected Plants and Their Biologically Active Molecules in the Treatment of Depression and Anxiety Disorders. International Journal of Molecular Sciences, 26(5), 2368. https://doi.org/10.3390/ijms26052368






