Comparison of Regulatory T-Cell Subpopulations in Antithymocytic Globulin Versus Post-Transplant Cyclophosphamide for Preventing Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation—A Retrospective Study
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
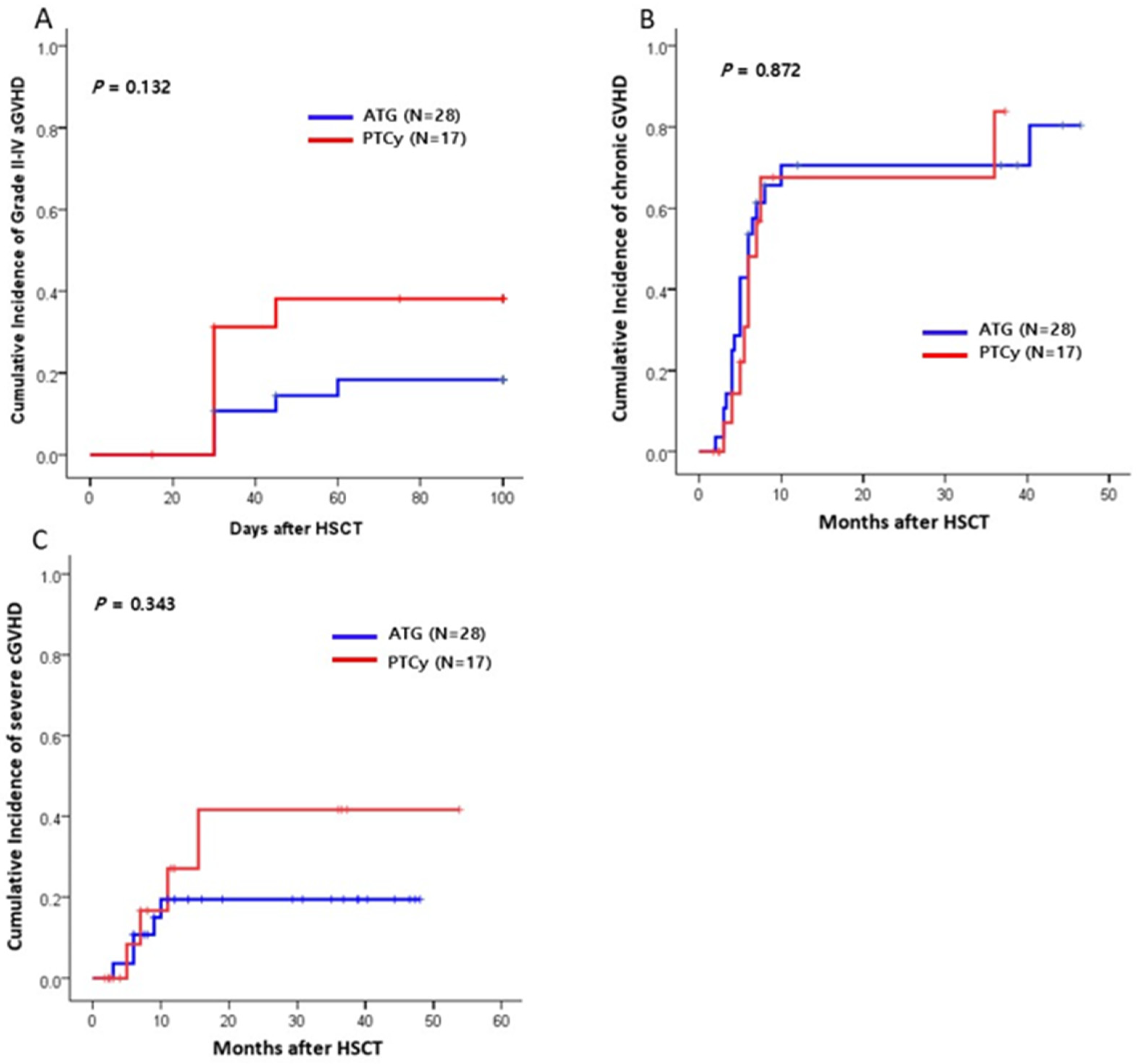
2.2. GVHD
2.3. Survival Outcomes
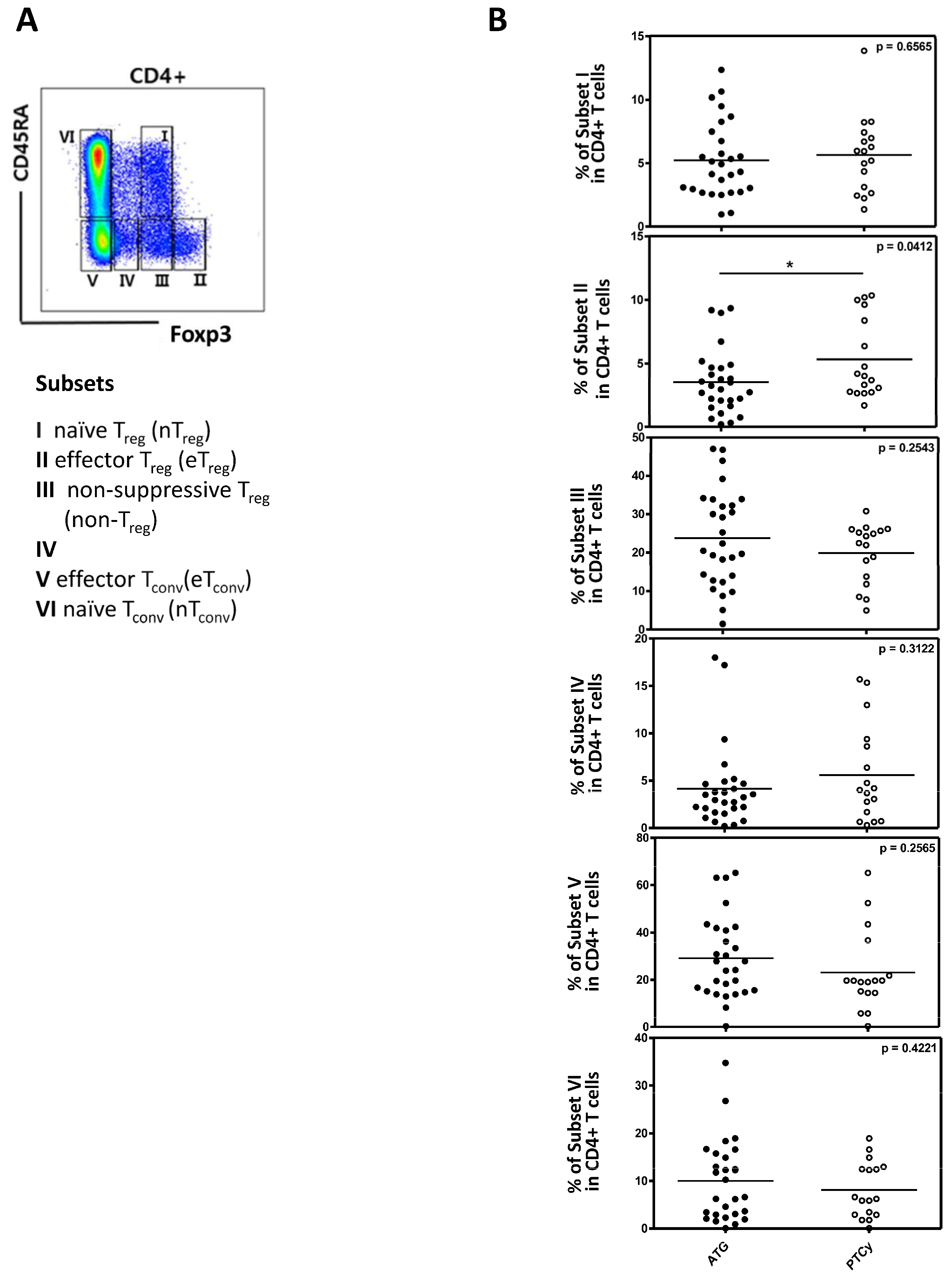
2.4. Regulatory T Cells and Their Subpopulations
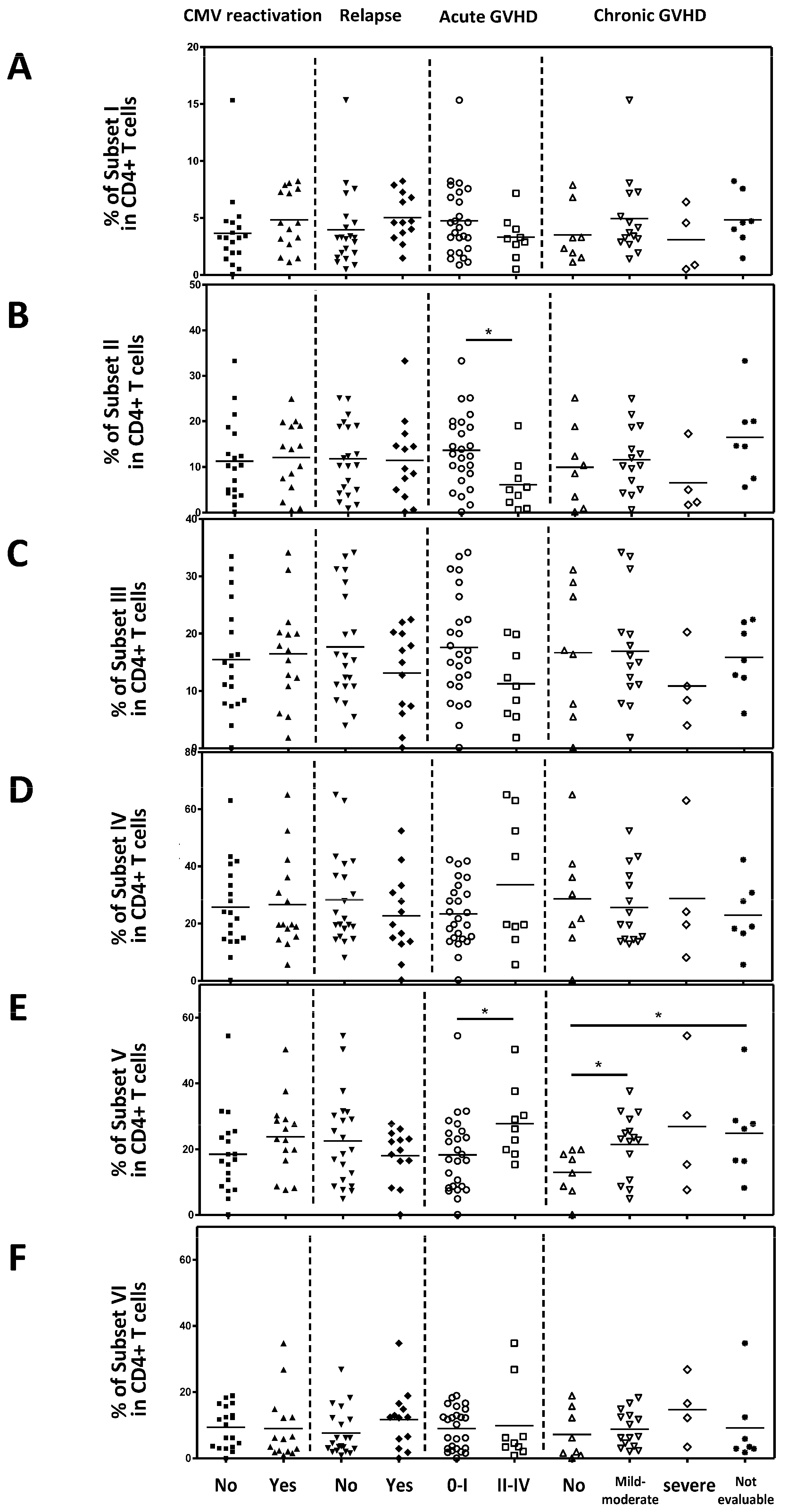
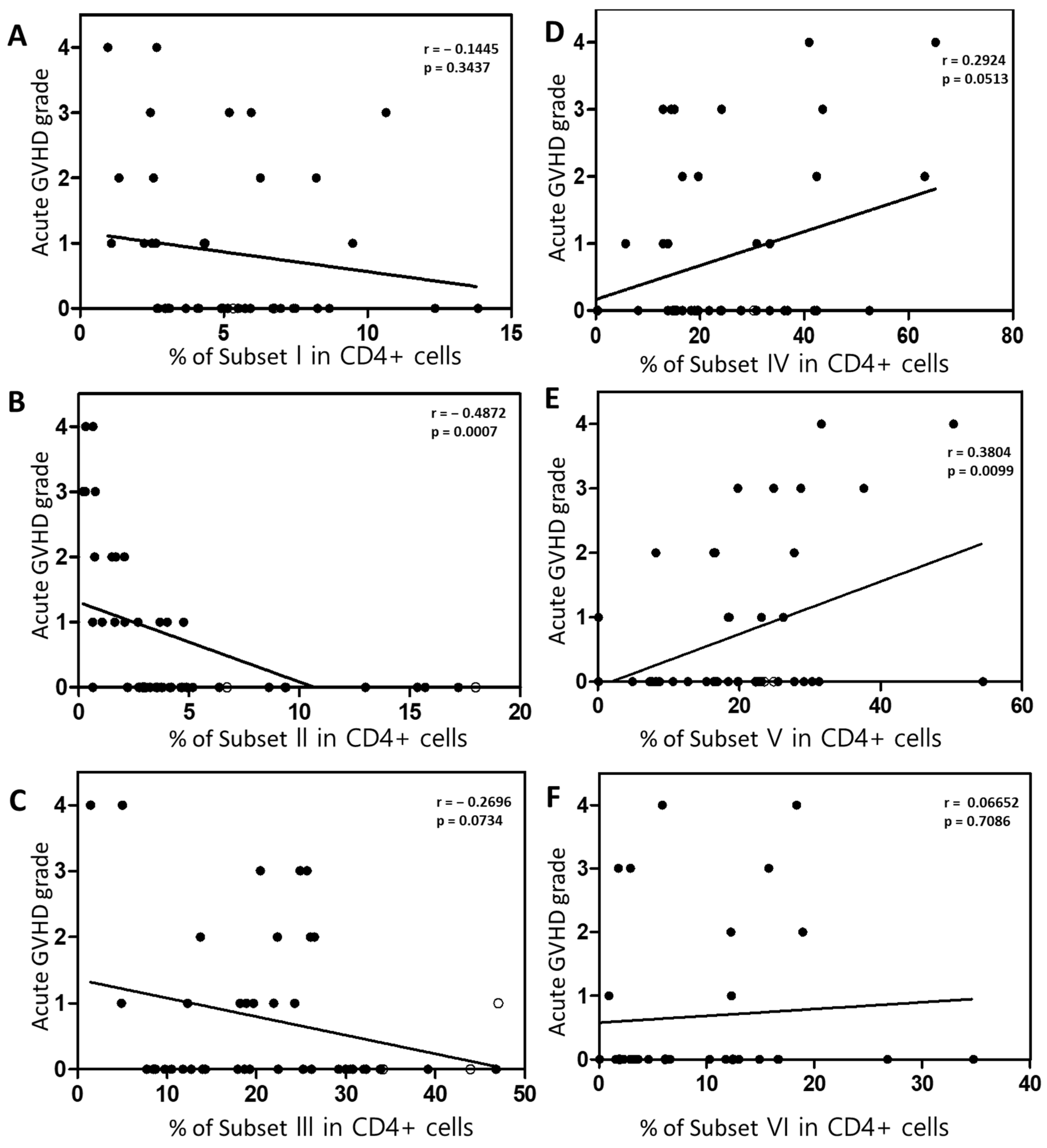
2.5. The Association Between Active Treg Cells and Clinical Outcomes
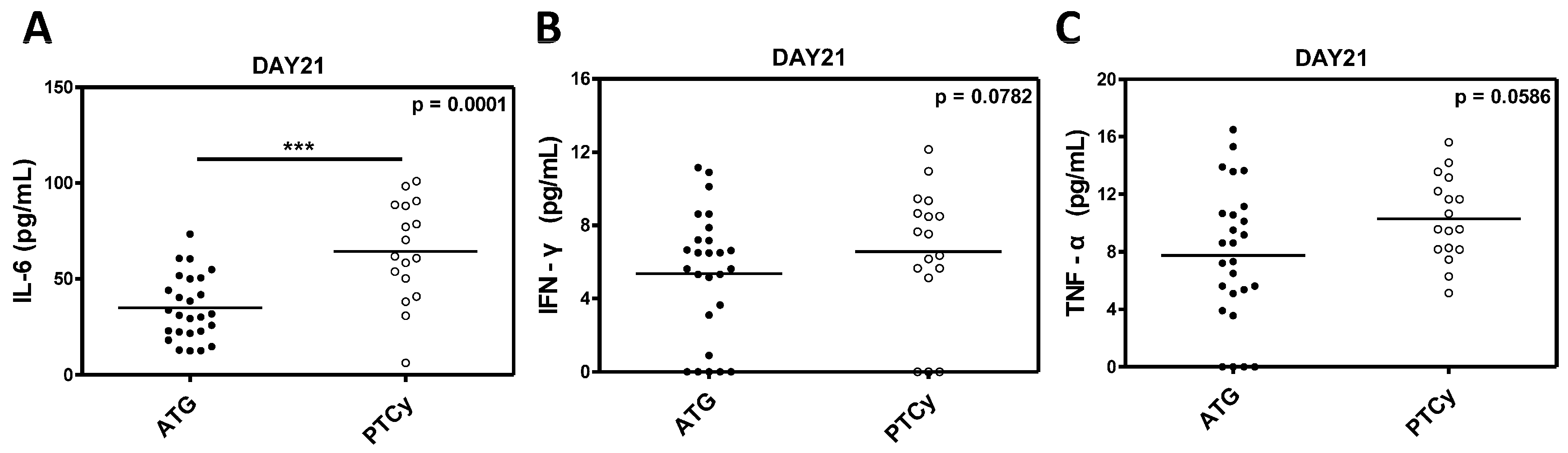
2.6. Inflammatory Cytokine Levels
3. Discussion
4. Materials and Methods
4.1. Patients and Treatments
4.2. Clinical Outcomes
4.3. Assessment of Regulatory T Cells Subpopulation and Cytokine
4.4. Statistical Analysis
4.5. Ethics Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gooley, T.A.; Chien, J.W.; Pergam, S.A.; Hingorani, S.; Sorror, M.L.; Boeckh, M.; Martin, P.J.; Sandmaier, B.M.; Marr, K.A.; Appelbaum, F.R.; et al. Reduced Mortality after Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and Management of Graft Versus Host Disease after Stem-Cell Transplantation for Haematological Malignancies: Updated Consensus Recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Solano, C.; Wolschke, C.; Bandini, G.; Patriarca, F.; Pini, M.; Nagler, A.; Selleri, C.; Risitano, A.; Messina, G.; et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2016, 374, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Rimando, J.; McCurdy, S.R.; Luznik, L. How I Prevent GVHD in High-Risk Patients: Posttransplant Cyclophosphamide and Beyond. Blood 2023, 141, 49–59. [Google Scholar] [CrossRef]
- Mohty, M. Mechanisms of Action of Antithymocyte Globulin: T-Cell Depletion and Beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F. Antithymocyte Globulin for Graft-Versus-Host Disease Prophylaxis after Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3993–3995. [Google Scholar] [CrossRef]
- Luznik, L.; Fuchs, E.J. High-Dose, Post-Transplantation Cyclophosphamide to Promote Graft-Host Tolerance after Allogeneic Hematopoietic Stem Cell Transplantation. Immunol. Res. 2010, 47, 65–77. [Google Scholar] [CrossRef]
- Bashey, A.; Zhang, X.; Sizemore, C.A.; Manion, K.; Brown, S.; Holland, H.K.; Morris, L.E.; Solomon, S.R. T-Cell-Replete HLA-Haploidentical Hematopoietic Transplantation for Hematologic Malignancies Using Post-Transplantation Cyclophosphamide Results in Outcomes Equivalent to Those of Contemporaneous HLA-Matched Related and Unrelated Donor Transplantation. J. Clin. Oncol. 2013, 31, 1310–1316. [Google Scholar] [CrossRef]
- McCurdy, S.R.; Kasamon, Y.L.; Kanakry, C.G.; Bolaños-Meade, J.; Tsai, H.L.; Showel, M.M.; Kanakry, J.A.; Symons, H.J.; Gojo, I.; Smith, B.D.; et al. Comparable Composite Endpoints After HLA-Matched and HLA-Haploidentical Transplantation with Post-Transplantation Cyclophosphamide. Haematologica 2017, 102, 391–400. [Google Scholar] [CrossRef]
- Baron, F.; Labopin, M.; Tischer, J.; Ciceri, F.; Raiola, A.M.; Blaise, D.; Sica, S.; Vydra, J.; Fanin, R.; Diez-Martin, J.L.; et al. Comparison of HLA-Mismatched Unrelated Donor Transplantation with Post-Transplant Cyclophosphamide versus HLA-Haploidentical Transplantation in Patients with Active Acute Myeloid Leukemia. Bone Marrow Transplant. 2022, 57, 1657–1663. [Google Scholar] [CrossRef]
- Nagler, A.; Kanate, A.S.; Labopin, M.; Ciceri, F.; Angelucci, E.; Koc, Y.; Gülbas, Z.; Arcese, W.; Tischer, J.; Pioltelli, P.; et al. Post-Transplant Cyclophosphamide versus Anti-Thymocyte Globulin for Graft-Versus-Host Disease Prevention in Haploidentical Transplantation for Adult Acute Lymphoblastic Leukemia. Haematologica 2021, 106, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Matsuoka, K.I. Harnessing Treg Homeostasis to Optimize Posttransplant Immunity: Current Concepts and Future Perspectives. Front. Immunol. 2021, 12, 713358. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kim, H.T.; McDonough, S.; Bascug, G.; Warshauer, B.; Koreth, J.; Cutler, C.; Ho, V.T.; Alyea, E.P.; Antin, J.H.; et al. Altered Regulatory T Cell Homeostasis in Patients with CD4+ Lymphopenia Following Allogeneic Hematopoietic Stem Cell Transplantation. J. Clin. Investig. 2010, 120, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Meguri, Y.; Asano, T.; Yoshioka, T.; Iwamoto, M.; Ikegawa, S.; Sugiura, H.; Kishi, Y.; Nakamura, M.; Sando, Y.; Kondo, T.; et al. Responses of Regulatory and Effector T-Cells to Low-Dose Interleukin-2 Differ Depending on the Immune Environment after Allogeneic Stem Cell Transplantation. Front. Immunol. 2022, 13, 891925. [Google Scholar] [CrossRef]
- Kennedy-Nasser, A.A.; Ku, S.; Castillo-Caro, P.; Hazrat, Y.; Wu, M.F.; Liu, H.; Melenhorst, J.; Barrett, A.J.; Ito, S.; Foster, A.; et al. Ultra low-dose IL-2 for GVHD Prophylaxis After Allogeneic Hematopoietic Stem Cell Transplantation Mediates Expansion of Regulatory T Cells Without Diminishing Antiviral and Antileukemic Activity. Clin. Cancer Res. 2014, 20, 2215–2225. [Google Scholar] [CrossRef]
- Matsuoka, K.I. Low-Dose Interleukin-2 as a Modulator of Treg Homeostasis After HSCT: Current Understanding and Future Perspectives. Int. J. Hematol. 2018, 107, 130–137. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Zeiser, R.; Dasilva, D.L.; Chang, D.S.; Beilhack, A.; Contag, C.H.; Negrin, R.S. In Vivo Dynamics of Regulatory T-Cell Trafficking and Survival Predict Effective Strategies to Control Graft-Versus-Host Disease Following Allogeneic Transplantation. Blood 2007, 109, 2649–2656. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shashidhar, S.; Chang, D.S.; Ho, L.; Kambham, N.; Bachmann, M.; Brown, J.M.; Negrin, R.S. The Impact of Regulatory T Cells on T-Cell Immunity Following Hematopoietic Cell Transplantation. Blood 2008, 111, 945–953. [Google Scholar] [CrossRef]
- Landwehr-Kenzel, S.; Müller-Jensen, L.; Kuehl, J.S.; Abou-El-Enein, M.; Hoffmann, H.; Muench, S.; Kaiser, D.; Roemhild, A.; von Bernuth, H.; Voeller, M.; et al. Adoptive Transfer of Ex Vivo Expanded Regulatory T Cells Improves Immune Cell Engraftment and Therapy-Refractory Chronic GvHD. Mol. Ther. 2022, 30, 2298–2314. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Cuadrado, E.; van den Biggelaar, M.; de Kivit, S.; Chen, Y.Y.; Slot, M.; Doubal, I.; Meijer, A.; van Lier, R.A.W.; Borst, J.; Amsen, D. Proteomic Analyses of Human Regulatory T Cells Reveal Adaptations in Signaling Pathways that Protect Cellular Identity. Immunity 2018, 48, 1046–1059.e6. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Chae, J.N.; Kim, S.H.; Ha, J.S. Subpopulations of Regulatory T Cells in Rheumatoid Arthritis, Systemic Lupus Erythematosus, and Behcet’s Disease. J. Korean Med. Sci. 2012, 27, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T Cells in Autoimmune Disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef]
- Go, E.; Yoo, S.J.; Choi, S.; Sun, P.; Jung, M.K.; Kwon, S.; Heo, B.Y.; Kim, Y.; Kang, J.G.; Kim, J.; et al. Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased T(reg) CD25 Expression and Reduced Frequency of Effector T(reg) Subpopulation. Cells 2021, 10, 801. [Google Scholar] [CrossRef]
- Koh, J.S.; Lee, M.W.; Pham, T.T.D.; Heo, B.Y.; Choi, S.; Lee, S.W.; Seo, W.; Kang, S.; Lee, S.B.; Kim, C.H.; et al. Post-Transplant Cyclophosphamide Plus Anti-Thymocyte Globulin Decreased Serum IL-6 Levels When Compared with Post-Transplant Cyclophosphamide Alone After Haploidentical Hematopoietic Stem Cell Transplantation. Blood Res. 2025, 60, 5. [Google Scholar] [CrossRef]
- Abboud, R.; Keller, J.; Slade, M.; DiPersio, J.F.; Westervelt, P.; Rettig, M.P.; Meier, S.; Fehniger, T.A.; Abboud, C.N.; Uy, G.L.; et al. Severe Cytokine-Release Syndrome after T Cell-Replete Peripheral Blood Haploidentical Donor Transplantation Is Associated with Poor Survival and Anti-IL-6 Therapy Is Safe and Well Tolerated. Biol. Blood Marrow Transplant. 2016, 22, 1851–1860. [Google Scholar] [CrossRef]
- Nishimoto, M.; Hirose, A.; Koh, H.; Nakamae, M.; Nanno, S.; Okamura, H.; Nakane, T.; Nakashima, Y.; Hino, M.; Nakamae, H. Clinical Impacts of Using Serum IL-6 Level as an Indicator of Cytokine Release Syndrome after HLA-Haploidentical Transplantation with Post-Transplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2019, 25, 2061–2069. [Google Scholar] [CrossRef]
- Korn, T.; Hiltensperger, M. Role of IL-6 in the Commitment of T Cell Subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Fleskens, V.; Fu, J.; Brenkman, A.B.; Bekker, C.P.; Pals, C.E.; Meerding, J.; Berkers, C.R.; Barbi, J.; Gröne, A.; et al. Stabilization of the Transcription Factor Foxp3 by the Deubiquitinase USP7 Increases Treg-Cell-Suppressive Capacity. Immunity 2013, 39, 259–271. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity due to CAR T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Lorentino, F.; Nitti, R.; Lupo Stanghellini, M.T.; Giglio, F.; Clerici, D.; Xue, E.; Lazzari, L.; Piemontese, S.; Mastaglio, S.; et al. Interleukin-6 as Biomarker for Acute GvHD and Survival After Allogeneic Transplant with Post-transplant Cyclophosphamide. Front. Immunol. 2019, 10, 2319. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, A.; Sun, Y.; Labopin, M.; Bacigalupo, A.; Lorentino, F.; Arcese, W.; Santarone, S.; Gülbas, Z.; Blaise, D.; Messina, G.; et al. Post-Transplant Cyclophosphamide versus Anti-Thymocyte Globulin as Graft- Versus-Host Disease Prophylaxis in Haploidentical Transplant. Haematologica 2017, 102, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, J.; Hu, J.; Lin, L.; Xu, Y. Post-transplant Cyclophosphamide Versus Antithymocyte Globulin in Allogeneic Hematopoietic Cell Transplantation: A Meta-Analysis. Ann. Hematol. 2021, 100, 529–540. [Google Scholar] [CrossRef]
- Rambaldi, B.; Kim, H.T.; Reynolds, C.; Chamling Rai, S.; Arihara, Y.; Kubo, T.; Buon, L.; Gooptu, M.; Koreth, J.; Cutler, C.; et al. Impaired T- and NK-Cell Reconstitution after Haploidentical HCT with Posttransplant Cyclophosphamide. Blood Adv. 2021, 5, 352–364. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Ganguly, S.; Zahurak, M.; Bolaños-Meade, J.; Thoburn, C.; Perkins, B.; Fuchs, E.J.; Jones, R.J.; Hess, A.D.; Luznik, L. Aldehyde Dehydrogenase Expression Drives Human Regulatory T Cell Resistance to Posttransplantation Cyclophosphamide. Sci. Transl. Med. 2013, 5, 211ra157. [Google Scholar] [CrossRef]
- Ganguly, S.; Ross, D.B.; Panoskaltsis-Mortari, A.; Kanakry, C.G.; Blazar, B.R.; Levy, R.B.; Luznik, L. Donor CD4+ Foxp3+ Regulatory T Cells Are Necessary for Posttransplantation Cyclophosphamide-Mediated Protection Against GVHD in Mice. Blood 2014, 124, 2131–2141. [Google Scholar] [CrossRef]
- Heo, B.Y.; Lee, M.W.; Choi, S.; Jung, Y.; Pham, T.T.D.; Jang, Y.; Park, J.H.; Kang, S.; Koh, J.S.; Jo, D.Y.; et al. Autoimmune Limbic Encephalitis in Patients with Hematologic Malignancies after Haploidentical Hematopoietic Stem Cell Transplantation with Post-Transplant Cyclophosphamide. Cells 2023, 12, 2049. [Google Scholar] [CrossRef]
- Sanz, J.; Galimard, J.E.; Labopin, M.; Afanasyev, B.; Angelucci, E.; Ciceri, F.; Blaise, D.; Cornelissen, J.J.; Meijer, E.; Diez-Martin, J.L.; et al. Post-Transplant Cyclophosphamide After Matched Sibling, Unrelated and Haploidentical Donor Transplants in Patients with Acute Myeloid Leukemia: A Comparative Study of the ALWP EBMT. J. Hematol. Oncol. 2020, 13, 46. [Google Scholar] [CrossRef]
- Spyridonidis, A.; Labopin, M.; Brissot, E.; Moiseev, I.; Cornelissen, J.; Choi, G.; Ciceri, F.; Vydra, J.; Reményi, P.; Rovira, M.; et al. Should Anti-Thymocyte Globulin Be Added in Post-Transplant Cyclophosphamide Based Matched Unrelated Donor Peripheral Blood Stem Cell Transplantation for Acute Myeloid Leukemia? A Study on Behalf of the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 1774–1780. [Google Scholar] [CrossRef]
- Duléry, R.; Brissot, E.; Mohty, M. Combining Post-Transplant Cyclophosphamide with Antithymocyte Globulin for Graft-Versus-Host Disease Prophylaxis in Hematological Malignancies. Blood Rev. 2023, 62, 101080. [Google Scholar] [CrossRef] [PubMed]
- Chorão, P.; Henriques, M.; Villalba, M.; Montoro, J.; Balaguer-Roselló, A.; González, E.M.; Gómez, M.D.; Gómez, I.; Solves, P.; Santiago, M.; et al. Cytomegalovirus Reactivations in Allogeneic Hematopoietic Stem Cell Transplantation from HLA-Matched and Haploidentical Donors with Post-Transplantation Cyclophosphamide. Transpl. Cell Ther. 2024, 30, e531–e538. [Google Scholar] [CrossRef]
- Bashey, A.; Zhang, M.J.; McCurdy, S.R.; St Martin, A.; Argall, T.; Anasetti, C.; Ciurea, S.O.; Fasan, O.; Gaballa, S.; Hamadani, M.; et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J. Clin. Oncol. 2017, 35, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, A.; Labopin, M.; Bacigalupo, A.; Afanasyev, B.; Cornelissen, J.J.; Elmaagacli, A.; Itälä-Remes, M.; Blaise, D.; Meijer, E.; Koc, Y.; et al. Post-Transplant Cyclophosphamide for Graft-Versus-Host Disease Prophylaxis in HLA Matched Sibling or Matched Unrelated Donor Transplant for Patients with Acute Leukemia, on Behalf of ALWP-EBMT. J. Hematol. Oncol. 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Yeon, S.H.; Seo, W.H.; Ryu, H.; Lee, H.J.; Yun, H.J.; Jo, D.Y.; Song, I.C. A Comparison of Post-Transplantation Cyclophosphamide Versus Antithymocyte-Globulin in Patients with Hematological Malignancies Undergoing HLA-Matched Unrelated Donor Transplantation. Medicine 2020, 99, e21571. [Google Scholar] [CrossRef]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Pretreatment with Anti-Thymocyte Globulin Versus No Anti-Thymocyte Globulin in Patients with Haematological Malignancies Undergoing Haemopoietic Cell Transplantation from Unrelated Donors: A Randomised, Controlled, Open-Label, Phase 3, Multicentre Trial. Lancet Oncol. 2016, 17, 164–173. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transpl. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring Therapeutic Response in Chronic Graft-Versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: IV. The 2014 Response Criteria Working Group report. Biol. Blood Marrow Transpl. 2015, 21, 984–999. [Google Scholar] [CrossRef]


| ATG (n = 28) | PTCy (n = 17) | p-Value | |
|---|---|---|---|
| Median Age, year (range) | 52.5 (21–66) | 57 (29–71) | 0.060 |
| Gender, M/F | 16/12 | 11/6 | 1.000 |
| Type of diseases | 0.824 | ||
| Acute myeloid leukemia | 18 (64.3%) | 10 (58.8%) | |
| Acute lymphoblastic leukemia | 6 (21.4%) | 3 (17.6%) | |
| Myelodysplastic syndrome | 4 (14.3%) | 4 (23.5%) | |
| Type of donors | <0.001 | ||
| HLA-matched sibling | 16 (57.1%) | 0 (0.0%) | |
| HLA-matched unrelated | 12 (42.9%) | 5 (29.4%) | |
| Haplo-identical | 0 (0.0%) | 12 (70.6%) | |
| Disease status at transplant | 0.830 | ||
| 1st CR | 18 (64.3%) | 11 (64.7%) | |
| 2nd CR | 4 (14.3%) | 1 (5.9%) | |
| MDS | 4 (14.3%) | 4 (23.5%) | |
| Persistent | 2 (7.1%) | 1 (5.9%) | |
| Poor risk * | 15 (53.6%) | 7 (43.8%) | 0.755 |
| HCT-CI | 0.434 | ||
| 0 | 20 (71.5%) | 13 (76.5%) | |
| 1–2 | 8 (28.6%) | 3 (17.6%) | |
| 3– | 0 (0.0%) | 1 (5.9%) | |
| CMV reactivation | 8 (28.6%) | 13 (76.5%) | 0.002 |
| Acute GVHD (evaluable) | 0.215 | ||
| Grade 0–I | 23 (82.1%) | 11 (37.6%) | |
| Grade II–IV | 5 (17.9%) | 6 (35.3%) | |
| Stem cell source | - | ||
| PB | 28 (100%) | 17 (100%) | |
| BM | 0 (0.0%) | 0 (0.0%) | |
| Conditioning regimen | 0.101 | ||
| MAC | 22 (78.6%) | 9 (52.9%) | |
| RIC | 6 (21.4%) | 8 (47.1%) | |
| Cell count, median (range) | |||
| TNC count (×108 cells/kg) | 11.97 (6.86–22.51) | 12.08 (6.87–20.60) | 0.926 |
| CD34 + cell (×106 cells/kg) | 7.94 (2.60–22.17) | 11.11 (2.17–36.00) | 0.216 |
| Median F/U duration, month (range) | 16.8 (3.8–23.3) | 11.5 (1.8–53.8) | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, B.-Y.; Koh, J.S.; Choi, S.-Y.; Pham, T.T.D.; Lee, S.-W.; Park, J.-H.; Jang, Y.; Lee, M.-W.; Lee, S.-B.; Seo, W.; et al. Comparison of Regulatory T-Cell Subpopulations in Antithymocytic Globulin Versus Post-Transplant Cyclophosphamide for Preventing Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation—A Retrospective Study. Int. J. Mol. Sci. 2025, 26, 2521. https://doi.org/10.3390/ijms26062521
Heo B-Y, Koh JS, Choi S-Y, Pham TTD, Lee S-W, Park J-H, Jang Y, Lee M-W, Lee S-B, Seo W, et al. Comparison of Regulatory T-Cell Subpopulations in Antithymocytic Globulin Versus Post-Transplant Cyclophosphamide for Preventing Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation—A Retrospective Study. International Journal of Molecular Sciences. 2025; 26(6):2521. https://doi.org/10.3390/ijms26062521
Chicago/Turabian StyleHeo, Bu-Yeon, Jeong Suk Koh, Su-Young Choi, Thi Thuy Duong Pham, Sang-Woo Lee, Jung-Hyun Park, Yunseon Jang, Myung-Won Lee, Seul-Bi Lee, Wonhyoung Seo, and et al. 2025. "Comparison of Regulatory T-Cell Subpopulations in Antithymocytic Globulin Versus Post-Transplant Cyclophosphamide for Preventing Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation—A Retrospective Study" International Journal of Molecular Sciences 26, no. 6: 2521. https://doi.org/10.3390/ijms26062521
APA StyleHeo, B.-Y., Koh, J. S., Choi, S.-Y., Pham, T. T. D., Lee, S.-W., Park, J.-H., Jang, Y., Lee, M.-W., Lee, S.-B., Seo, W., Jo, D.-Y., Kwon, J., & Song, I.-C. (2025). Comparison of Regulatory T-Cell Subpopulations in Antithymocytic Globulin Versus Post-Transplant Cyclophosphamide for Preventing Graft-Versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation—A Retrospective Study. International Journal of Molecular Sciences, 26(6), 2521. https://doi.org/10.3390/ijms26062521









