On-Resin Acetamidomethyl (Acm) Removal and Disulfide Formation in Cysteinyl Peptides Using N-Chlorosuccinimide (NCS) in the Presence of Other Cys-Protecting Groups
Abstract
1. Introduction
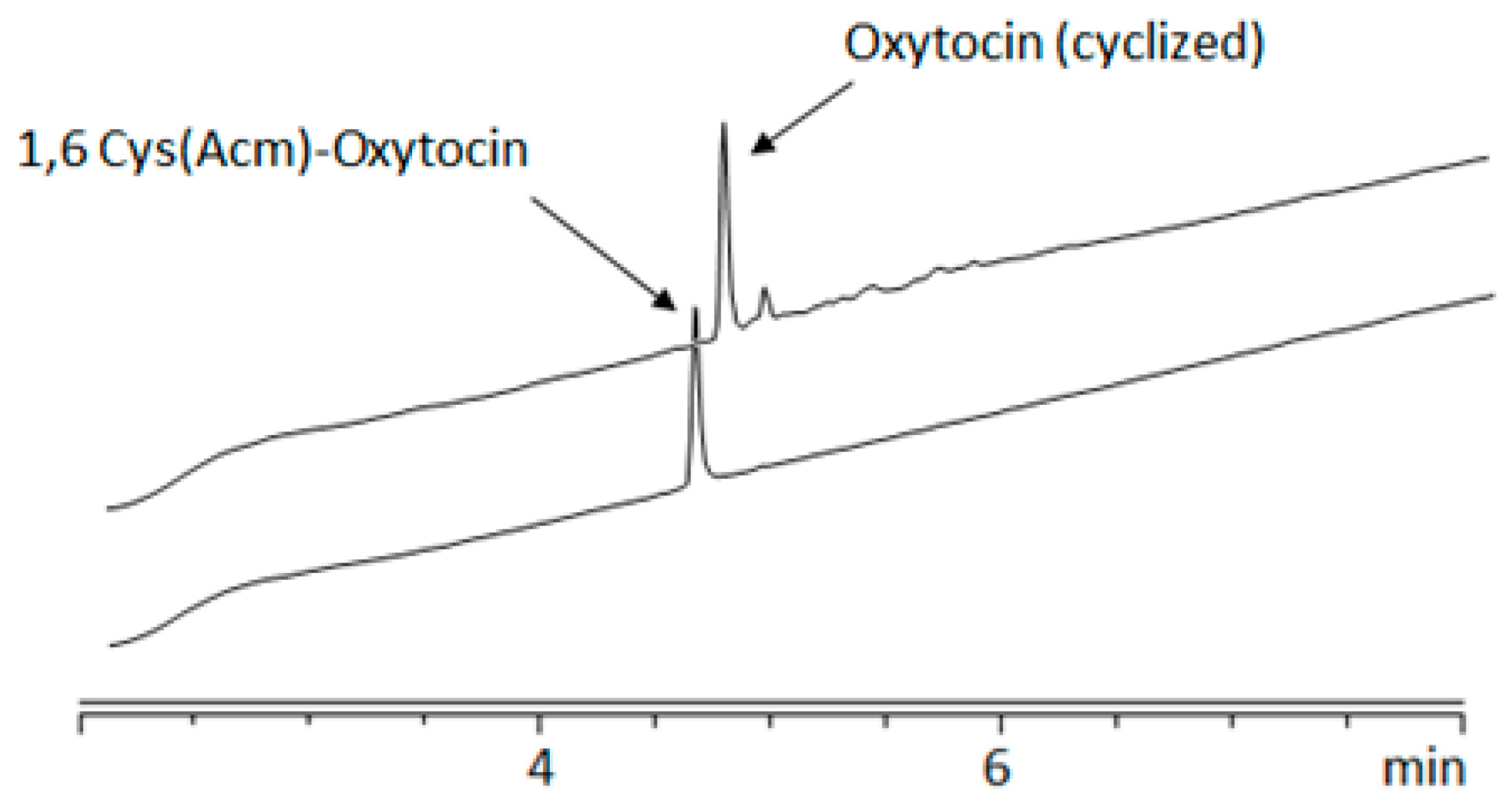
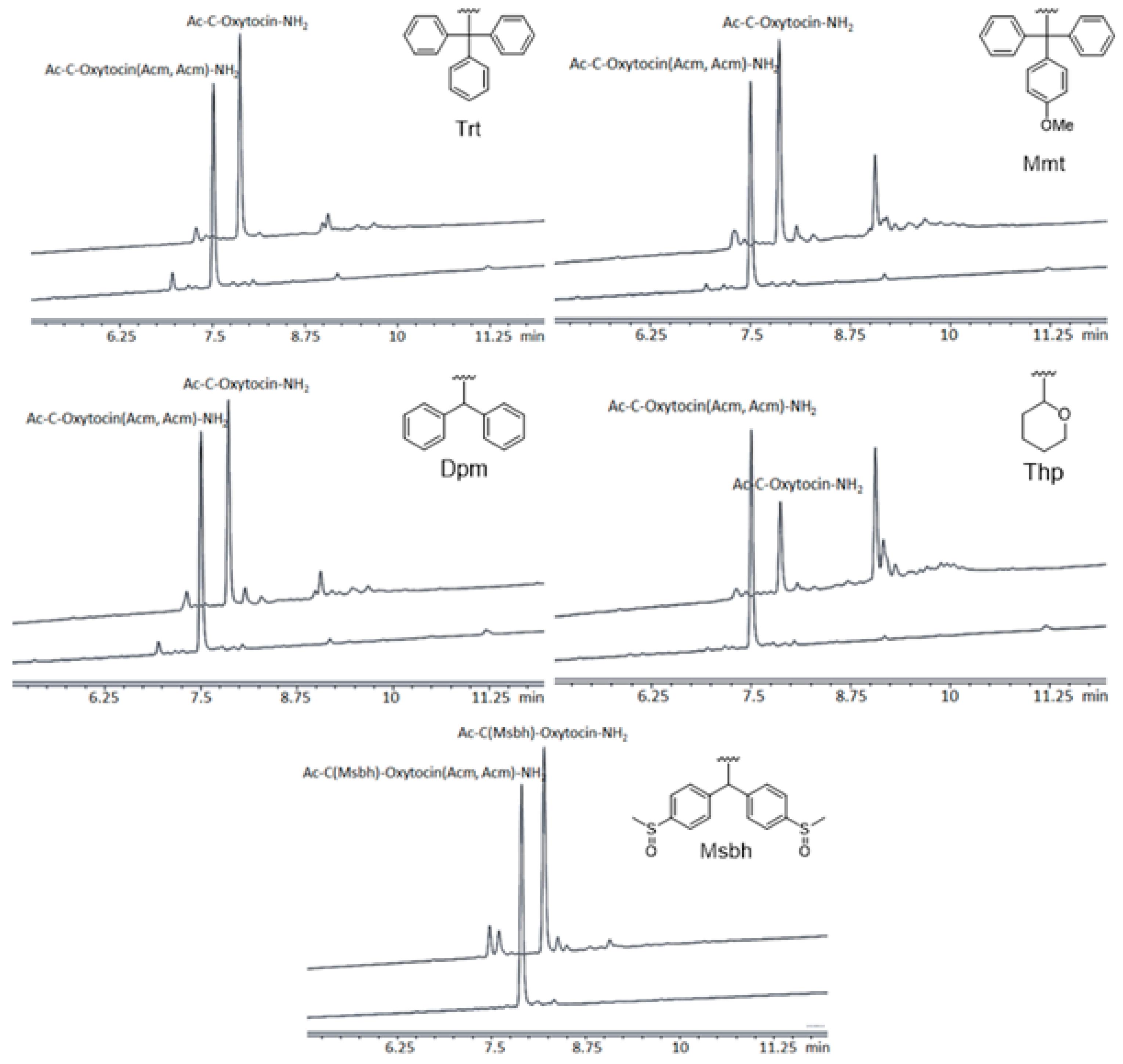
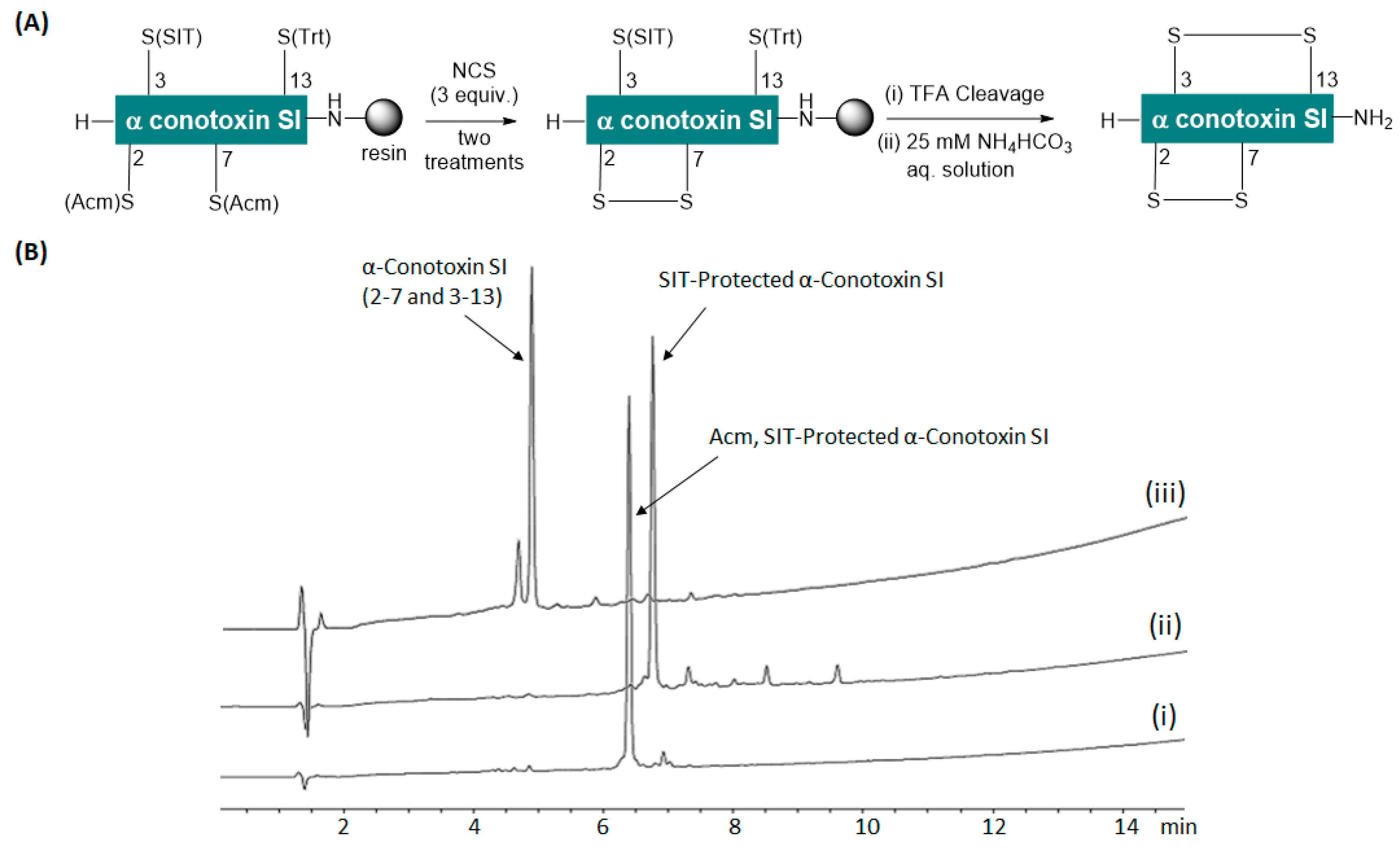
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Solid-Phase Peptide Synthesis
3.3. Cyclization of Acm-Protected Peptides on Resin
3.4. Cyclization of SIT-Protected Peptides in Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, H.J. A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef]
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2024, 29, 585. [Google Scholar] [CrossRef]
- Imhof, D.; Roy, D.; Albericio, F. Editorial: Chemical Design and Biomedical Applications of Disulfide-rich Peptides: Challenges and Opportunities. Front. Chem. 2020, 8, 586377. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, L.R. An insight into the pharmacology of cysteine/methionine containing peptide drugs. Eur. J. Med. Chem. 2024, 271, 116456. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Tulla-Puche, J.; Paradís-Bas, M.; Werbitzky, O.; Giraud, M.; Albericio, F. Optimized Fmoc solid-phase synthesis of the cysteine-rich peptide linaclotide. Pept. Sci. (Biopolym.) 2011, 96, 69–80. [Google Scholar] [CrossRef]
- Lembo, A.J.; Schneier, H.A.; Shiff, S.J.; Kurtz, C.B.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Lavins, B.J.; Currie, M.G.; Fitch, D.A. Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med. 2011, 365, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Yin, Y.; Suga, H. Macrocyclic Peptides as Drug Candidates: Recent Progress and Remaining Challenges. J. Am. Chem. Soc. 2019, 141, 4167–4181. [Google Scholar] [CrossRef]
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810. [Google Scholar] [CrossRef]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic Peptides for Drug Development. Angew. Chem. Int. Ed. 2024, 63, e202308251. [Google Scholar] [CrossRef] [PubMed]
- Postma, T.M.; Albericio, F. Disulfide Formation Strategies in Peptide Synthesis. Eur. J. Org. Chem. 2014, 2014, 3519–3530. [Google Scholar] [CrossRef]
- He, R.; Pan, J.; Mayer, J.P.; Liu, F. Stepwise Construction of Disulfides in Peptides. ChemBioChem 2020, 21, 1101–1111. [Google Scholar] [CrossRef]
- Spears, R.J.; McMahon, C.; Chudasama, V. Cysteine protecting groups: Applications in peptide and protein science. Chem. Soc. Rev. 2021, 50, 11098–11155. [Google Scholar] [CrossRef]
- Chakraborty, A.; Mthembu, S.N.; de la Torre, B.G.; Albericio, F. Ready to Use Cysteine Thiol Protecting Groups in SPPS, A Practical Overview. Org. Process Res. Dev. 2024, 28, 26–45. [Google Scholar] [CrossRef]
- Veber, D.; Milkowski, J.; Varga, S.; Denkewalter, R.; Hirschmann, R. Acetamidomethyl. A novel thiol protecting group for cysteine. J. Am. Chem. Soc. 1972, 94, 5456–5461. [Google Scholar] [CrossRef]
- Harris, K.M.; Flemer Jr, S.; Hondal, R.J. Studies on deprotection of cysteine and selenocysteine side-chain protecting groups. J. Pept. Sci. 2007, 13, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Kamber, B.; Hartmann, A.; Eisler, K.; Riniker, B.; Rink, H.; Sieber, P.; Rittel, W. The Synthesis of Cystine Peptides by Iodine Oxidation of S-Trityl-cysteine and S-Acetamidomethyl-cysteine Peptides. Helv. Chim. Acta 1980, 63, 899–915. [Google Scholar] [CrossRef]
- Chowdhury, A.; Gour, V.; Das, B.K.; Chatterjee, S.; Bandyopadhyay, A. Rapid and Highly Productive Assembly of a Disulfide Bond in Solid-Phase Peptide Macrocyclization. Org. Lett. 2023, 25, 1280–1284. [Google Scholar] [CrossRef]
- Laps, S.; Sun, H.; Kamnesky, G.; Brik, A. Palladium-Mediated Direct Disulfide Bond Formation in Proteins Containing S-Acetamidomethyl-cysteine under Aqueous Conditions. Angew. Chem. Int. Ed. 2019, 58, 5729–5733. [Google Scholar] [CrossRef] [PubMed]
- Schroll, A.L.; Hondal, R.J.; Flemer, S., Jr. 2,2′-Dithiobis(5-nitropyridine) (DTNP) as an effective and gentle deprotectant for common cysteine protecting groups. J. Pept. Sci. 2012, 18, 1–9. [Google Scholar] [CrossRef]
- Shih, H. New approaches to the synthesis of cystine peptides using N-iodosuccinimide in the construction of disulfide bridges. J. Org. Chem. 1993, 58, 3003–3008. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, Y.; Ma, D.; Shen, S.; Song, C.; Zhang, N.; Bo, T.; Shi, T.; Huo, S. N-Halosuccinimides mediated deprotection of cysteine-S protecting groups for one-pot regioselective synthesis of disulfide bonds in peptides under mild aqueous conditions. Tetrahedron Lett. 2023, 120, 154459. [Google Scholar] [CrossRef]
- Singh, P.R.; Rajopadhye, M.; Clark, S.L.; Williams, N.E. Effect of scavengers in acidolytic cleavage of Cys(Acm)-containing peptides from solid support: Isolation of an ethanedithiol disulfide adduct. Tetrahedron Lett. 1996, 37, 4117–4120. [Google Scholar] [CrossRef]
- Ste.Marie, E.J.; Hondal, R.J. Reduction of cysteine-S-protecting groups by triisopropylsilane. J. Pept. Sci. 2018, 24, e3130. [Google Scholar] [CrossRef]
- Tripathi, N.M.; Das, B.K.; Chowdhury, A.; Gour, V.; Bandyopadhyay, A. Introducing Regioselective Disulfide Linkages in Peptides under Pseudodilute Conditions by Harnessing Brønsted Acid-Activated N-Chlorosuccinimide. JACS Au 2025, 5, 802–810. [Google Scholar] [CrossRef]
- Postma, T.M.; Albericio, F. N-Chlorosuccinimide, an Efficient Reagent for On-Resin Disulfide Formation in Solid-Phase Peptide Synthesis. Org. Lett. 2013, 15, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Postma, T.M.; Albericio, F. N-chlorosuccinimide, an efficient peptide disulfide bond-forming reagent in aqueous solution. RSC Adv. 2013, 3, 14277–14280. [Google Scholar] [CrossRef]
- Hargittai, B.; Barany, G. Controlled syntheses of natural and disulfide-mispaired regioisomers of α-conotoxin SI. J. Pept. Res. 1999, 54, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Albericio, F.; de la Torre, B.G. Sec-isoamyl Mercaptan (SIT), a Multi-faceted Disulfide Based Protecting Group for Cysteine Thiol. Int. J. Pept. Res. Ther. 2024, 30, 72. [Google Scholar] [CrossRef]
- Shechter, Y.; Patchornik, A.; Burstein, Y. Selective chemical cleavage of tryptophanyl peptide bonds by oxidative chlorination with N-chlorosuccinimide. Biochemistry 1976, 15, 5071–5075. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, A.; Albericio, F.; de la Torre, B.G. On-Resin Acetamidomethyl (Acm) Removal and Disulfide Formation in Cysteinyl Peptides Using N-Chlorosuccinimide (NCS) in the Presence of Other Cys-Protecting Groups. Int. J. Mol. Sci. 2025, 26, 2523. https://doi.org/10.3390/ijms26062523
Chakraborty A, Albericio F, de la Torre BG. On-Resin Acetamidomethyl (Acm) Removal and Disulfide Formation in Cysteinyl Peptides Using N-Chlorosuccinimide (NCS) in the Presence of Other Cys-Protecting Groups. International Journal of Molecular Sciences. 2025; 26(6):2523. https://doi.org/10.3390/ijms26062523
Chicago/Turabian StyleChakraborty, Amit, Fernando Albericio, and Beatriz G. de la Torre. 2025. "On-Resin Acetamidomethyl (Acm) Removal and Disulfide Formation in Cysteinyl Peptides Using N-Chlorosuccinimide (NCS) in the Presence of Other Cys-Protecting Groups" International Journal of Molecular Sciences 26, no. 6: 2523. https://doi.org/10.3390/ijms26062523
APA StyleChakraborty, A., Albericio, F., & de la Torre, B. G. (2025). On-Resin Acetamidomethyl (Acm) Removal and Disulfide Formation in Cysteinyl Peptides Using N-Chlorosuccinimide (NCS) in the Presence of Other Cys-Protecting Groups. International Journal of Molecular Sciences, 26(6), 2523. https://doi.org/10.3390/ijms26062523







