The Microbial Assay for Risk Assessment (MARA) in the Assessment of the Antimicrobial Activity of Ofloxacin and Its Photoproducts
Abstract
1. Introduction
2. Results
2.1. OFLX and Floxal Toxicity
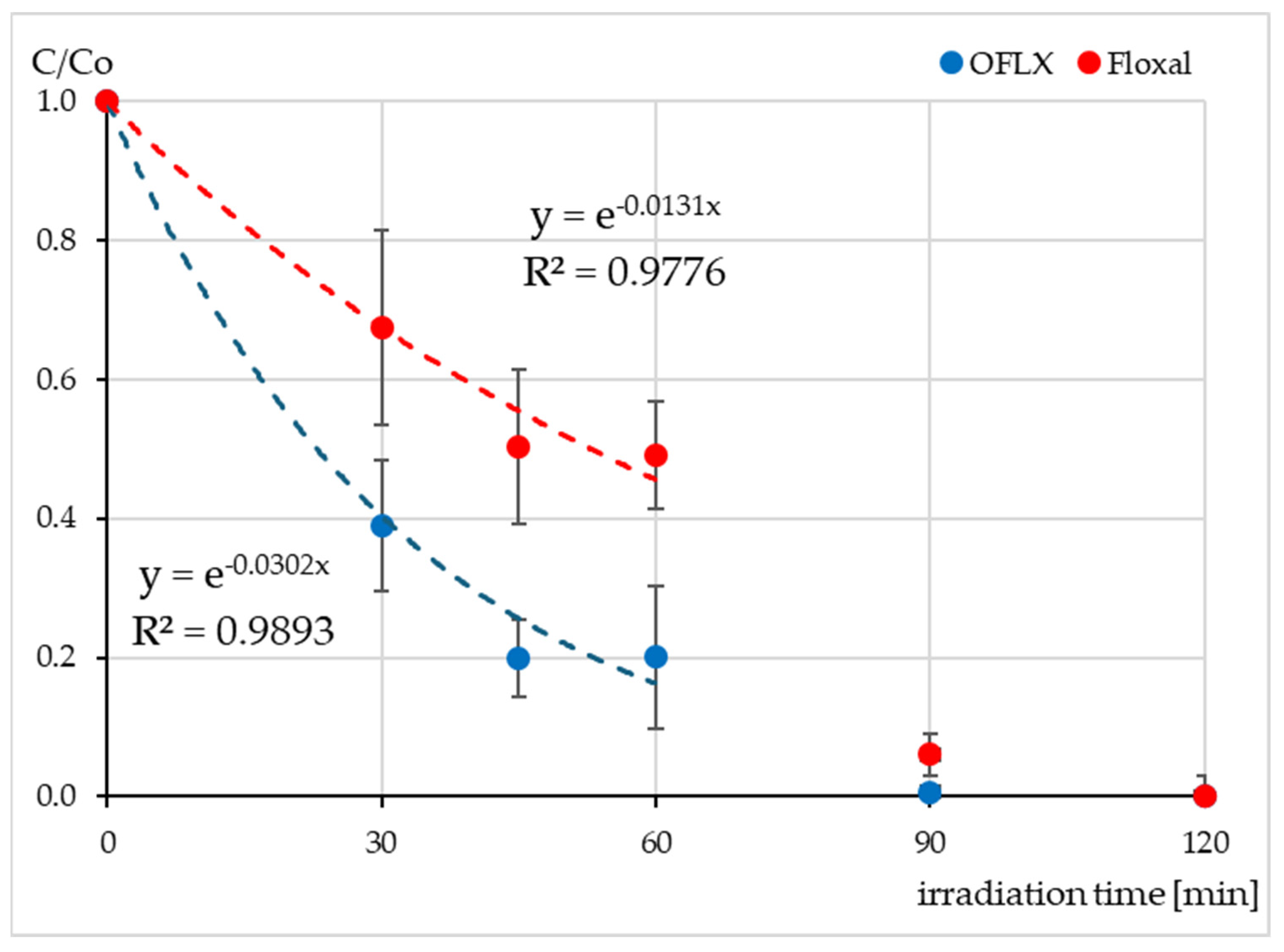
2.2. The Concentration of OFLX During Irradiation
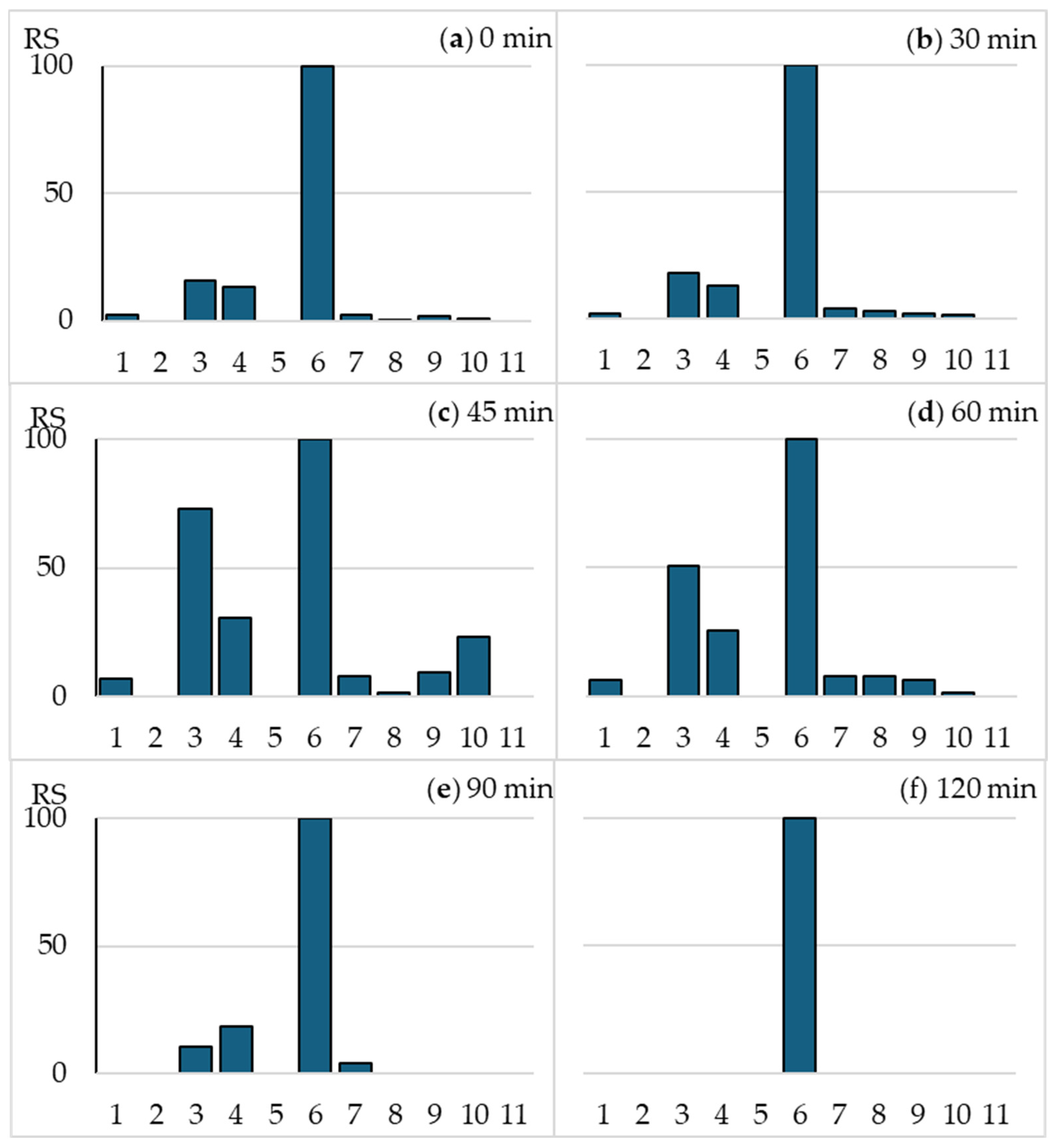
2.3. The Effect of Irradiation on the OFLX and Floxal Toxicity in the MARA Assay
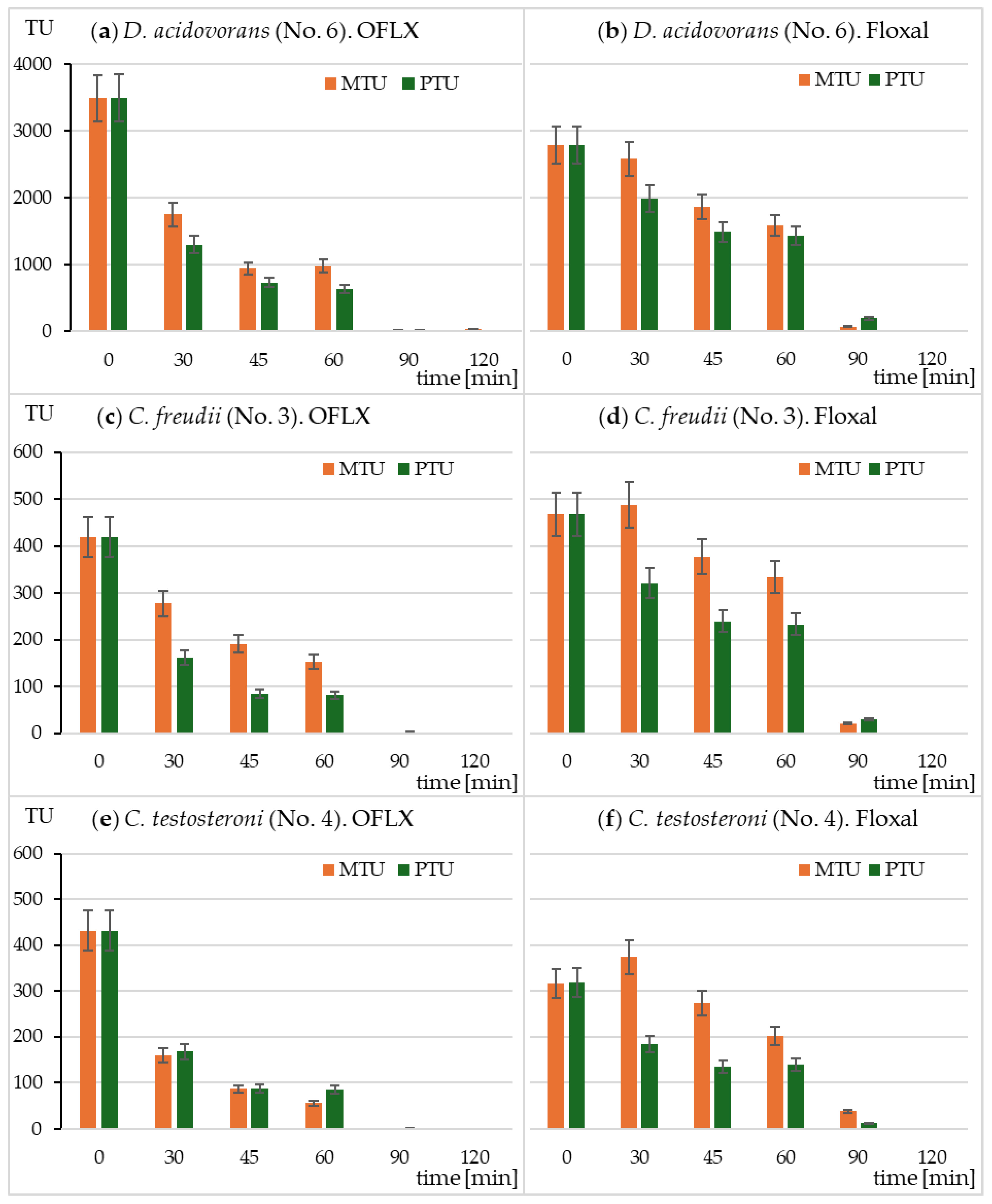
2.4. The Effect of Irradiation on OFLX and Floxal Toxicity in Microtox® and Spirotox Assays
2.5. Analysis of Photodegradation Products with UPLC-MS/MS
3. Discussion
3.1. OFLX’s Antibacterial Activity
3.2. Photodegradation of OFLX
3.3. Toxicity of OFLX Photodegradation Products
4. Materials and Methods
4.1. Chemicals
4.2. Photodegradation Experiment
4.3. Liquid Chromatography with Photodiode Array Detector
4.4. Liquid Chromatography with Mass Spectrometer Detector
4.5. Microbial Assay for Risk Assessment
4.6. Microtox®
4.7. Spirotox Assay
4.8. Data Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kane, S.P. ClinCalc DrugStats: Most Commonly Prescribed Medications in 2021. Available online: https://clincalc.com/blog/2024/01/clincalc-drugstats-most-commonly-prescribed-medications-in-2021/ (accessed on 31 December 2024).
- World Health Organization. Model List of Essential Medicines. Available online: https://list.essentialmeds.org (accessed on 4 November 2024).
- Al-Omar, M.A. Chapter 6—Ofloxacin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 34, pp. 265–298. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. Chem. Life Sci. 2018, 351, e1800141. [Google Scholar] [CrossRef]
- Maris, A.S.; Mody, P.; Brewer, D.J.; Humphries, R.M. The fluoroquinolones: An update for the clinical microbiologist. Clin. Microbiol. Newsl. 2021, 43, 97–107. [Google Scholar] [CrossRef]
- Mendoza, A.; Aceña, J.; Pérez, S.; López de Alda, M.; Barceló, D.; Gil, A.; Valcárcel, Y. Pharmaceuticals and iodinated contrast media in a hospital wastewater: A case study to analyse their presence and characterize their environmental risk and hazard. Environ. Res. 2015, 140, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, M.; Guo, C.; An, D.; Xu, J.; Zhang, Y.; Xi, B. Distribution and ecological risk of antibiotics in a typical effluent-receiving river (Wangyang River) in north China. Chemosphere 2014, 112, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxeus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Environmental risk assessment of six human pharmaceuticals: Are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ. Toxicol. Chem. 2004, 23, 1344–1354. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Wang, B.; Sun, T.; Wu, B.; Wang, Y. Photocatalytic degradation of some typical antibiotics: Recent advances and future outlooks. Int. J. Mol. Sci. 2022, 23, 8130. [Google Scholar] [CrossRef]
- Parezanović, G.Š.; Lalic-Popovic, M.; Golocorbin-Kon, S.; Vasovic, V.; Milijašević, B.; Al-Salami, H.; Mikov, M. Environmental Transformation of Pharmaceutical Formulations: A Scientific Review. Arch. Environ. Contam. Toxicol. 2019, 77, 155–161. [Google Scholar] [CrossRef]
- Hubicka, U.; Żmudzki, P.; Talik, P.; Żuromska-Witek, B.; Krzek, J. Photodegradation assessment of ciprofloxacin, moxifloxacin, norfloxacin and ofloxacin in the presence of excipients from tablets by PULC-MS/MS and DSC. Chem. Cent. J. 2013, 7, 133. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Profumo, A.; Fasani, E.; Albini, A.; Migliavacca, R.; Nucleo, E. Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water Res. 2012, 46, 5575–5582. [Google Scholar] [CrossRef]
- Dhiman, P.; Sharma, G.; Alodhayb, A.N.; Kumar, A.; Rana, G.; Sithole, T.; ALOthman, Z.A. Constructing a visible-active CoFe2O4@Bi2O3/NiO nanoheterojunction as magnetically recoverable photocatalyst with boosted ofloxacin degradation efficiency. Molecules 2022, 27, 8234. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, J.; Du, Z.; Zhao, Z.; Gu, Y.; Guo, Z.; Huang, Y.; Tang, C.; Chen, G.; Fang, Y. Construction of novel ternary MoSe2/ZnO/p-BN photocatalyst for efficient ofloxacin degradation under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131050. [Google Scholar] [CrossRef]
- Frąckowiak, A.; Kamiński, B.; Urbaniak, B.; Dereziński, P.; Klupczyńska, A.; Darul-Duszkiewicz, M.; Kokot, Z.J. A study of ofloxacin and levofloxacin photostability in aqueous solutions. J. Med. Sci. 2016, 85, 238–244. [Google Scholar] [CrossRef]
- Li, R.; Lu, X.; Gao, J.; Chen, Y.; Pan, S. Activation of peracetic acid by CoFe2O4 for efficient degradation of ofloxacin: Reactive species and mechanism. Molecules 2023, 28, 7906. [Google Scholar] [CrossRef]
- Ni, T.; Yang, Z.; Zhang, H.; Zhou, L.; Guo, W.; Pan, L.; Yang, Z.; Chang, K.; Ge, C.; Liu, D. Peroxymonosulfate activation by Co3O4/SnO2 for efficient degradation of ofloxacin under visible light. J. Colloid Interface Sci. 2022, 615, 650–662. [Google Scholar] [CrossRef]
- Xing, C.; Chen, K.; Hu, L.; Liu, L. Efficient degradation of ofloxacin by magnetic CuFe2O4 coupled PMS system: Optimization, degradation pathways and toxicity evaluation. Toxics 2024, 12, 731. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, L. The antibacterial activity of fluoroquinolone derivatives: An update (2018–2021). Eur. J. Med. Chem. 2021, 224, 113741. [Google Scholar] [CrossRef]
- Gabrielson, J.; Kühn, I.; Colque-Navarro, P.; Hart, M.; Iversen, A.; McKenzie, D.; Möllby, R. Microplate-based microbial assay for risk assessment and (eco)toxic fingerprinting of chemicals. Anal. Chim. Acta 2003, 485, 121–130. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Wadhia, K.; Adomas, B.; Piotrowicz-Cieślak, A.I.; Sawicki, J. Application of microbial assay for risk assessment biotest in evaluation of toxicity of human and veterinary antibiotics. Environ. Toxicol. 2010, 25, 487–494. [Google Scholar] [CrossRef]
- Wadhia, K. ISTA13—International interlaboratory comparative evaluation of Microbial Assay for Risk Assessment (MARA). Environ. Toxicol. 2008, 23, 626–633. [Google Scholar] [CrossRef]
- Adamek, E.; Masternak, E.; Sapińska, D.; Baran, W. Degradation of the selected antibiotic in an aqueous solution by the Fenton process: Kinetics, products and ecotoxicity. Int. J. Mol. Sci. 2022, 23, 15676. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W. Degradation of veterinary antibiotics by the ozonation process: Product identification and ecotoxicity assessment. J. Hazard. Mater. 2024, 469, 134026. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G. Spirotox—Spirostomum ambiguum acute toxicity test—10 years of experience. Environ. Toxicol. 2004, 19, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ji, Y.; Chovelon, J.-M.; Lu, J. Fluoroquinolone antibiotics sensitized photodegradation of isoproturon. Water Res. 2021, 198, 117136. [Google Scholar] [CrossRef]
- Ding, P.; Li, J.; Guo, M.; Ji, H.; Li, P.; Liu, W.; Tressel, J.; Gao, S.; Wang, Q.; Chen, S. Visible light-driven degradation of ofloxacin by graphene oxide-supported titania/zirconia ternary nanocomposites. Inorg. Chem. Commun. 2023, 155, 111001. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, M.; Gminski, R.; Spangenberg, B.; Debiak, M.; Bürkle, A.; Mersch-Sundermann, V.; Kümmerer, K.; Giere, R. Antibiotics and sweeteners in the aquatic environment: Biodegradability, formation of phototransformation products, and in vitro toxicity. Environ. Sci. Pollut. Res. 2015, 22, 18017–18030. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.N.; Chow, A.T. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119. [Google Scholar] [CrossRef]
- Wierzyńska, E.; Nałęcz-Jawecki, G.; Giebułtowicz, J.; Drobniewska, A.; Łęcki, T.; Zarębska, K.; Skompska, M. From aromatic rings to aliphatic compounds—Degradation of duloxetine with the use of visible light driven Z-scheme photocatalyst. J. Environ. Chem. Eng. 2024, 12, 114270. [Google Scholar] [CrossRef]
- Makota, O.; Yankovych, H.B.; Bondarchuk, O.; Saldan, I.; Melnyk, I. Sphere-shaped ZnO photocatalyst synthesis for enhanced degradation of the Quinolone antibiotic, Ofloxacin, under UV irradiation. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.A.; Fasani, E.; Albini, A. Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl. Catal. B Environ. 2012, 119–120, 32–39. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, H.; Yin, S.; Sun, L.; Lu, H. Unraveling the toxicity associated with ciprofloxacin biodegradation in biological wastewater treatment. Environ. Sci. Technol. 2022, 56, 15941–15952. [Google Scholar] [CrossRef] [PubMed]
- Zgadzaj, A.; Skrzypczak, A.; Welenc, I.; Ługowska, A.; Parzonko, A.; Siedlecka, E.; Sommer, S.; Sikorska, K.; Nałęcz-Jawecki, G. Evaluation of photodegradation, phototoxicity and photogenotoxicity of ofloxacin in ointments with sunscreens and in solutions. J. Photochem. Photobiol. B Biol. 2015, 144, 76–84. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Sawicki, J. Toxicity of inorganic compounds in the Spirotox test: A miniaturized version of the Spirostomum ambiguum test. Arch. Environ. Contam. Toxicol. 1998, 34, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Nałęcz-Jawecki, G.; Giebułtowicz, J.; Drzewicz, P. Degradation of oxytetracycline by ferrate(VI): Treatment optimization, UHPLC-MS/MS and toxicological studies of the degradation products, and impact of urea and creatinine on the removal. Chem. Eng. J. 2024, 485, 149802. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G. Spirotox Test—Spirostomum ambiguum Acute Toxicity Test. In Small-Scale Freshwater Toxicity Investigations: Toxicity Test Methods; Blaise, C., Férard, J.-F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 299–322. [Google Scholar]

| Microplate Column | Species | Phylogenetic Group | Sample | No 1 | MTC [μg L−1] | MTC [nM] |
|---|---|---|---|---|---|---|
| 6 | Delftia acidovorans | Gram− β-proteobacteria | OFLX | 1 | 7.6 | 21.0 |
| 2 | 10.6 | 29.3 | ||||
| Floxal | 1 | 21.2 | 58.6 | |||
| 2 | 9.4 | 26.0 | ||||
| 3 | Citrobacter freudii | Gram− γ-proteobacteria | OFLX | 1 | 75.8 | 210 |
| 2 | 68.6 | 190 | ||||
| Floxal | 1 | 89.4 | 247 | |||
| 2 | 68.6 | 190 | ||||
| 4 | Comamonas testosteroni | Gram− α-proteobacteria | OFLX | 1 | 74.1 | 205 |
| 2 | 65.9 | 182 | ||||
| Floxal | 1 | 109 | 303 | |||
| 2 | 121 | 334 | ||||
| 7 | Kurthia gibsonii | Gram+ | OFLX | 1 | 539 | 1492 |
| 2 | 417 | 1154 | ||||
| Floxal | 1 | 439 | 1215 | |||
| 2 | 493 | 1364 | ||||
| 1 | Microbacterium sp | Gram+ | OFLX | 1 | 539 | 1492 |
| 2 | 605 | 1675 | ||||
| Floxal | 1 | 602 | 1667 | |||
| 2 | 271 | 749 | ||||
| 9 | Pseudomonas aurantiaca | Gram− γ-proteobacteria | OFLX | 1 | 674 | 1865 |
| 2 | 484 | 1340 | ||||
| Floxal | 1 | 1551 | 4293 | |||
| 2 | 433 | 1199 | ||||
| 8 | Staphylococcus warneri | Gram+ | OFLX | 1 | NT 2 | |
| 2 | 605 | 1675 | ||||
| Floxal | 1 | NT 2 | ||||
| 2 | 1065 | 2947 | ||||
| 2 | Brevundimonas diminuta | Gram− α-proteobacteria | OFLX | 1, 2 | NT 2 | |
| Floxal | 1, 2 | NT 2 | ||||
| 5 | Enterococcus casseliflavus | Gram+ | OFLX | 1, 2 | NT 2 | |
| Floxal | 1, 2 | NT 2 | ||||
| 10 | Serratia rubidaea | Gram− γ-proteobacteria | OFLX | NT 2 | ||
| Floxal | NT 2 | |||||
| 11 | Pichia anomala | Yeast | OFLX | NT 2 | ||
| Floxal | NT 2 |
| Irradiation Time [min] | Microtox® EC50 [%] | Spirotox EC50 [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| OFLX | Floxal | OFLX | Floxal | |||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| 0 | NT 1 | NT | NT | NT | NT | NT | NT | NT |
| 30 | NT | NT | NT | NT | 44.5 | 69.4 | 70.7 | 70.7 |
| 45 | NT | NT | 97.4 | NT | 35.4 | 35.4 | 42.6 | 36.0 |
| 60 | NT | NT | 80.8 | NT | 32.4 | 35.4 | 35.4 | 34.0 |
| 90 | 97.4 | 85.1 | 76.8 | 70.8 | 17.7 | 26.6 | 33.3 | 32.4 |
| 120 | 76.1 | 76.4 | 67.7 | 64.8 | 17.7 | 29.4 | 32.4 | 19.3 |
| Name | Formula | Calc. MW 1 | Δ Mass [ppm] | RT [min] | RDBE 2 | H/C 3 | Selected MS/MS Fragments [m/z] |
|---|---|---|---|---|---|---|---|
| P391 | C18H18FN3O6 | 391.1180 | 0.06 | 11.4 | 11 | 1.0 | 86.0599; 237.0308; 279.0772; 374.1139 |
| P363 | C17H18FN3O5 | 363.1229 | −0.42 | 4.8 | 10 | 1.1 | 86.0599; 235.0877; 289.0977; 333.0877 |
| P349 | C16H16FN3O5 | 349.1071 | −0.81 | 4.8 | 10 | 1.0 | 72.0445; 279.0774; 289.0981; 330.1092 |
| P348 | C17H20N2O6 | 348.1316 | −1.47 | 4.7 | 9 | 1.2 | 70.0651; 175.0726; 279.0757 |
| P347 | C17H18FN3O4 | 347.1283 | 0.64 | 4.9 | 10 | 1.1 | 70.0652; 221.0718; 261.1032; 304.1452 |
| P345 | C17H16FN3O4 | 345.1128 | 0.93 | 4.9 | 11 | 0.9 | 70.0651; 221.0718; 261.1033; 302.1292 |
| P319b | C15H17N3O5 | 319.1168 | −0.10 | 4.4 | 9 | 1.1 | 218.0917; 244.0715; 262.0817; 276.0967 |
| P319a | C14H13N3O6 | 319.0806 | 0.60 | 4.3 | 10 | 0.9 | 70.0287; 72.0443; 143.0238; 161.0688 |
| P306 | C14H11FN2O5 | 306.0650 | −0.66 | 10.9 | 10 | 0.8 | 238.0377; 261.0668; 279.0781 |
| P304 | C15H13FN2O4 | 304.0860 | 0.27 | 4.7 | 10 | 0.9 | 207.0564; 219.0561; 235.0875; 261.1040 |
| P289 | C14H15N3O4 | 289.1066 | 1.01 | 4.2 | 9 | 1.1 | 58.0654; 145.5602; 202.0971; 233.0556 |
| P283 | C12H13NO7 | 283.0690 | −0.61 | 4.4 | 7 | 1.1 | 58.0654; 180.0291; 208.0241; 226.0344 |
| P278 | C13H11FN2O4 | 278.0702 | −0.44 | 12.2 | 9 | 0.8 | 191.0250; 219.0199; 238.0376; 261.0668 |
| P275 | C13H13N3O4 | 275.0910 | 1.39 | 4.2 | 9 | 1.0 | 118.0286; 138.5522; 189.0657; 233.0550 |
| P267 | C12H13NO6 | 267.0741 | −0.68 | 4.5 | 7 | 1.1 | 59.0494; 182.0449; 192.0296; 210.0398 |
| P263 | C13H10FNO4 | 263.0594 | 0.00 | 13.1 | 9 | 0.8 | 202.0671; 224.0339; 246.0558 |
| P255 | C11H13NO6 | 255.0740 | −1.10 | 4.8 | 6 | 1.2 | 180.0290; 220.0603; 210.0754; 238.0707 |
| P249 | C12H11NO5 | 249.0635 | −0.77 | 4.4 | 8 | 0.9 | 149.0601; 164.0341; 192.0288; 232.0602 |
| P225 | C10H11NO5 | 225.0635 | −1.16 | 4.7 | 6 | 1.1 | 59.0493; 108.0444; 152.0342; 210.0758 |
| P128 | C5H8N2O2 | 128.0585 | −0.55 | 4.5 | 3 | 1.6 | 111.0554; 85.0761; 58.0655 |
| P119 | C4H9NO3 | 119.0583 | 0.34 | 4.5 | 1 | 2.2 | 56.0499; 74.0600; 102.0545 |
| P100 | C5H12N2 | 100.1000 | −0.10 | 4.1 | 1 | 2.4 | 58.0654; 70.0653; 84.0806; 99.0915 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nałęcz-Jawecki, G.; Mielniczek, J.; Wawryniuk, M.; Giebułtowicz, J.; Drobniewska, A. The Microbial Assay for Risk Assessment (MARA) in the Assessment of the Antimicrobial Activity of Ofloxacin and Its Photoproducts. Int. J. Mol. Sci. 2025, 26, 2595. https://doi.org/10.3390/ijms26062595
Nałęcz-Jawecki G, Mielniczek J, Wawryniuk M, Giebułtowicz J, Drobniewska A. The Microbial Assay for Risk Assessment (MARA) in the Assessment of the Antimicrobial Activity of Ofloxacin and Its Photoproducts. International Journal of Molecular Sciences. 2025; 26(6):2595. https://doi.org/10.3390/ijms26062595
Chicago/Turabian StyleNałęcz-Jawecki, Grzegorz, Jakub Mielniczek, Milena Wawryniuk, Joanna Giebułtowicz, and Agata Drobniewska. 2025. "The Microbial Assay for Risk Assessment (MARA) in the Assessment of the Antimicrobial Activity of Ofloxacin and Its Photoproducts" International Journal of Molecular Sciences 26, no. 6: 2595. https://doi.org/10.3390/ijms26062595
APA StyleNałęcz-Jawecki, G., Mielniczek, J., Wawryniuk, M., Giebułtowicz, J., & Drobniewska, A. (2025). The Microbial Assay for Risk Assessment (MARA) in the Assessment of the Antimicrobial Activity of Ofloxacin and Its Photoproducts. International Journal of Molecular Sciences, 26(6), 2595. https://doi.org/10.3390/ijms26062595






