Acute Effects of the Psychedelic Phenethylamine 25I-NBOMe in C57BL/6J Male Mice
Abstract
1. Introduction
2. Results
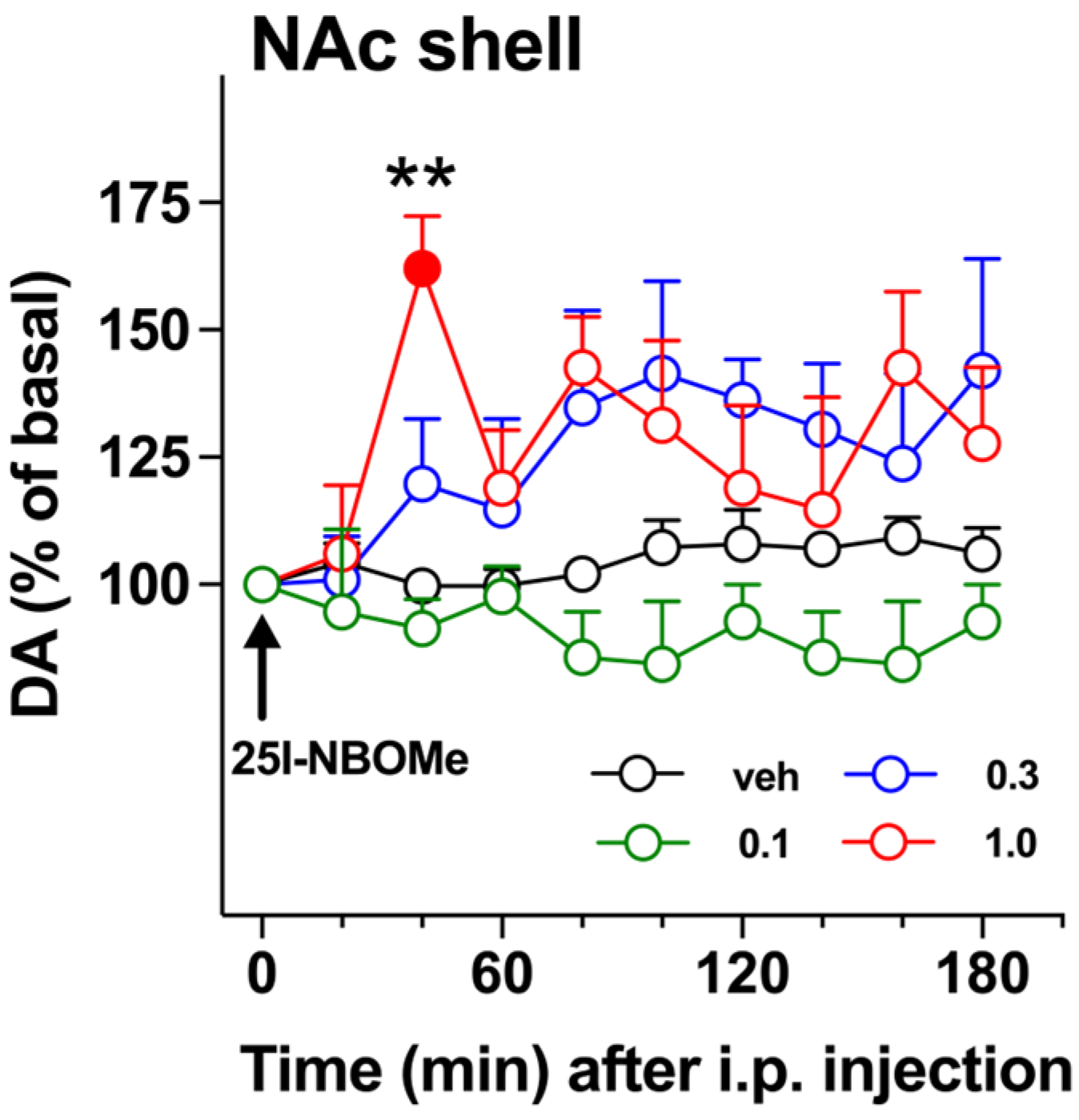
2.1. DA Transmission
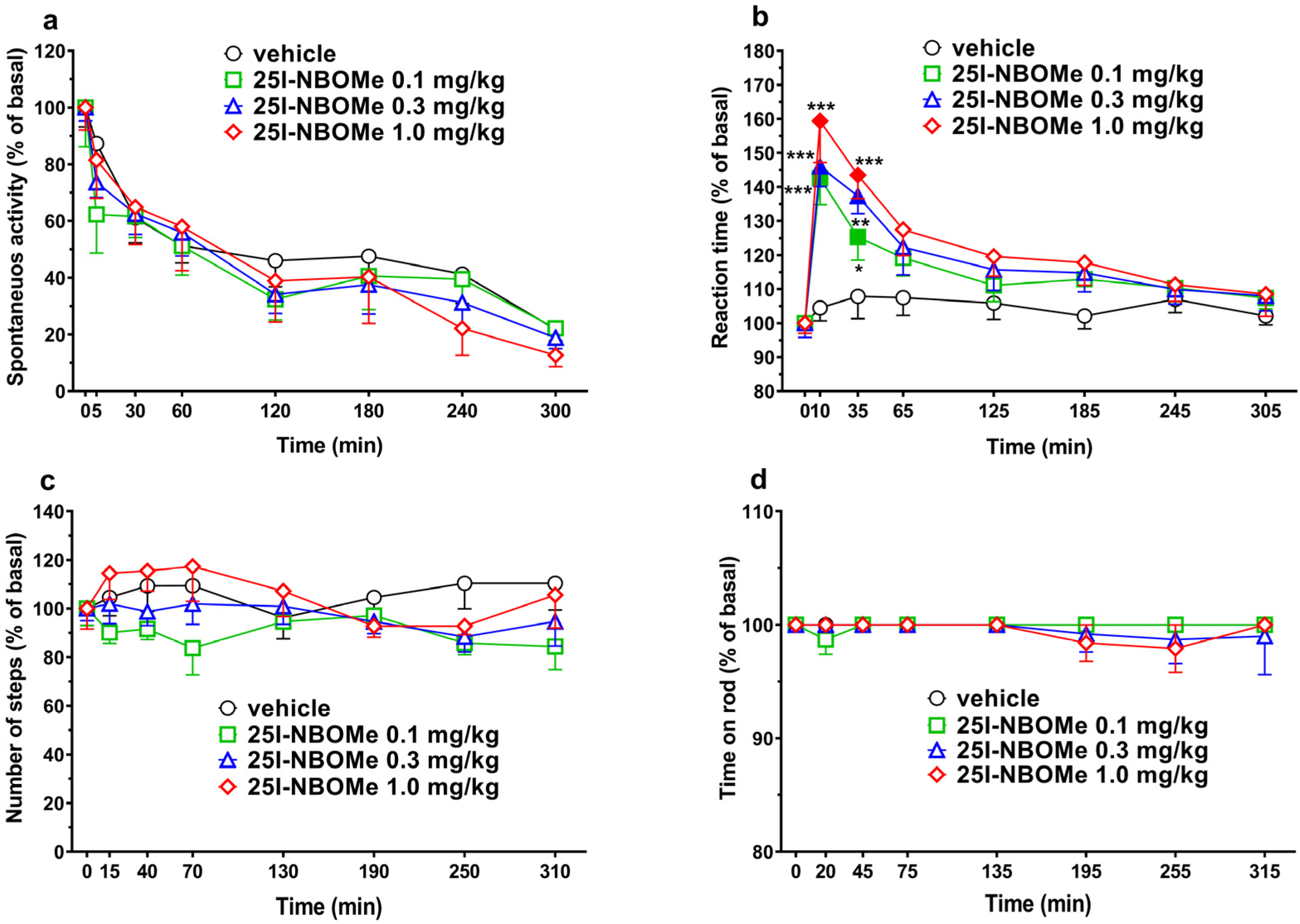
2.2. Behavioral Tests
2.2.1. Studies on Spontaneous Locomotor Activity in Mice
2.2.2. Reaction Time Test
2.2.3. Drag Test and Accelerod Test
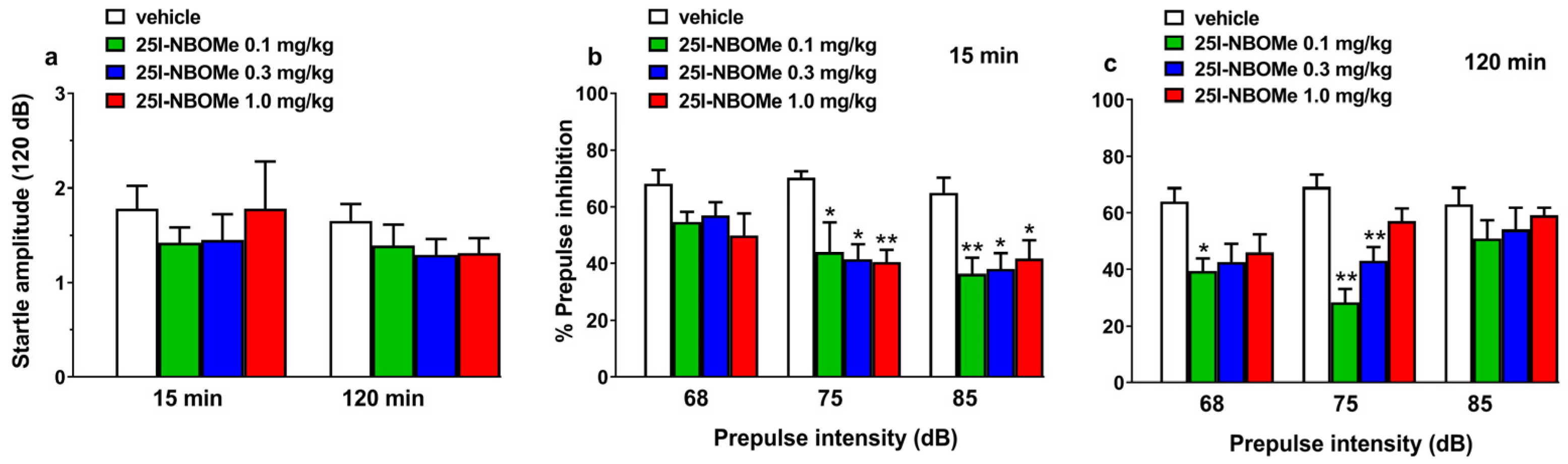
2.2.4. Prepulse Inhibition Studies
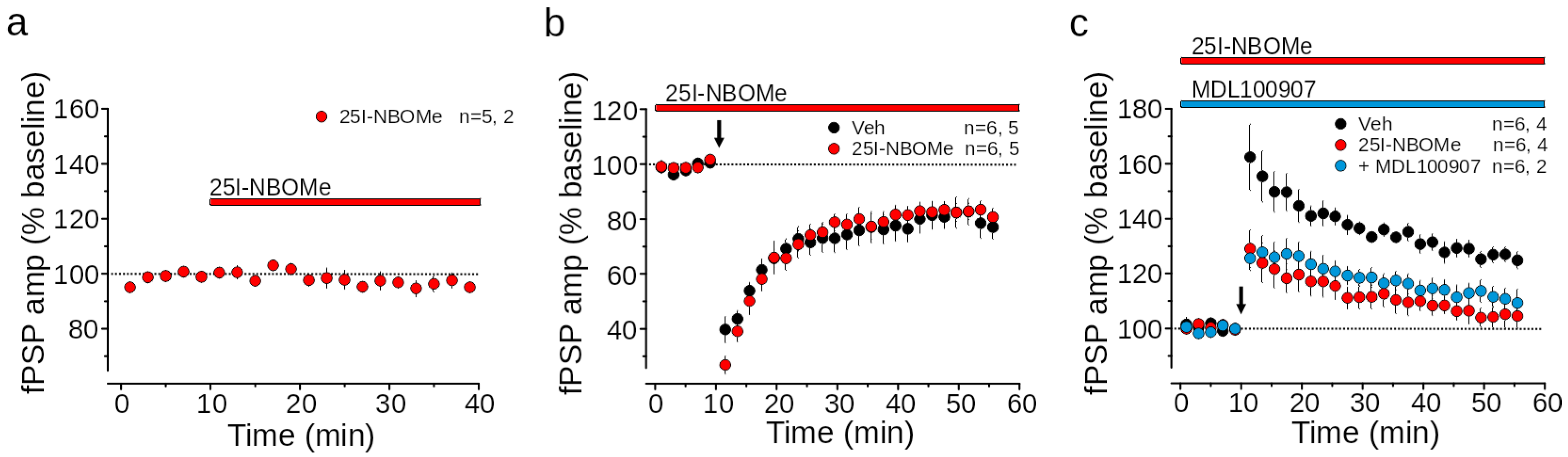
2.3. Extracellular Field Recordings of Synaptic Activity in the Medial Prefrontal Cortex (mPFC)
3. Discussion
3.1. 25I-NBOMe Influences the Dopaminergic Transmission in the NAc Shell
3.2. 25I-NBOMe Does Not Affect Locomotor Activity
3.3. 25I-NBOMe Impairs the Reaction Time Test and Disrupts the PPI
3.4. 25I-NBOMe Impairs LTP in the mPFC
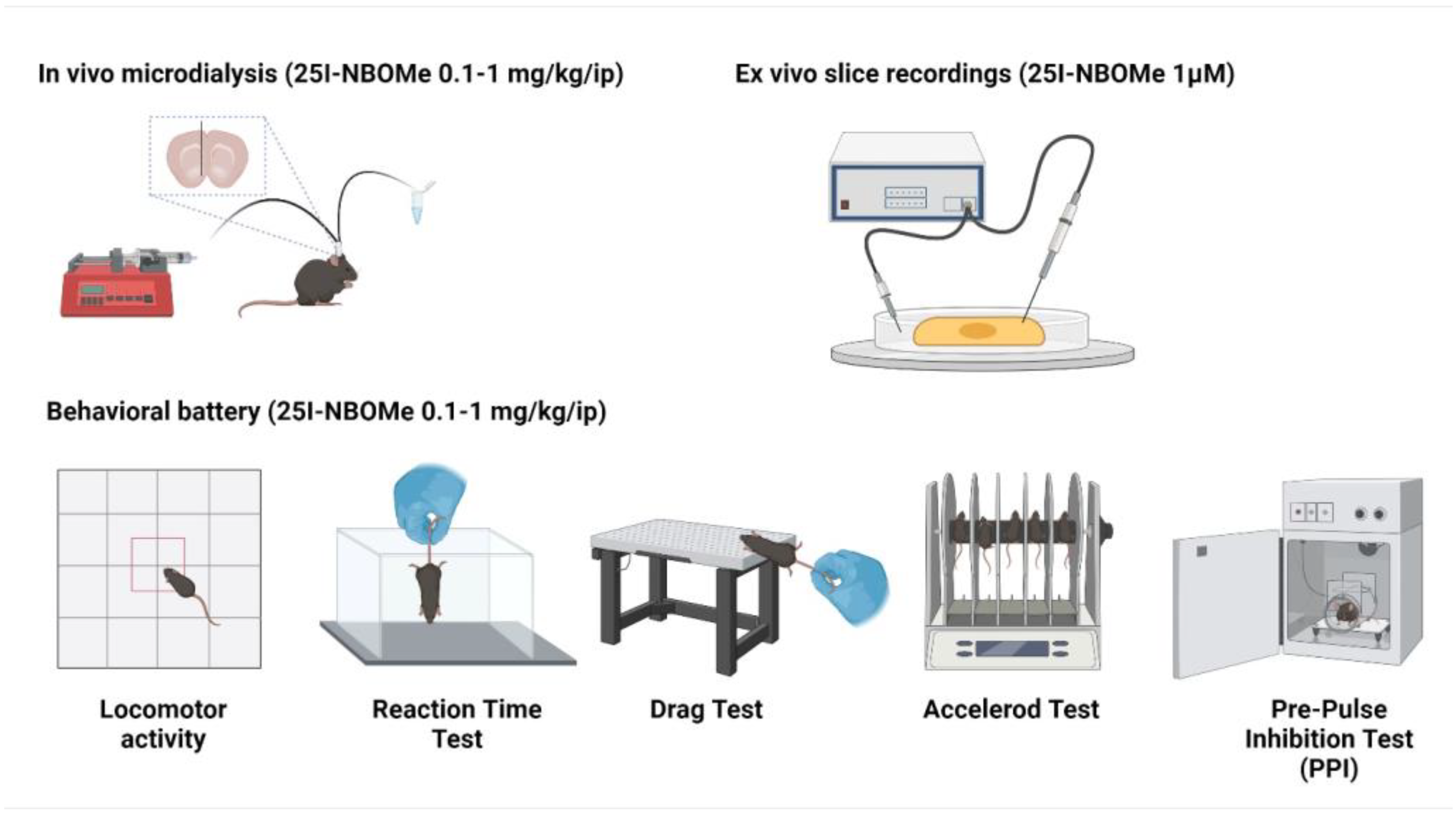
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. In Vivo Microdialysis
4.4. Behavioral Studies
4.4.1. Spontaneous Locomotor Activity
4.4.2. Reaction Time Test Assessment
4.4.3. Drag Test
4.4.4. Accelerod Test
4.4.5. Prepulse Inhibition Analysis
4.5. Electrophysiological Study
4.6. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 25I-NBOMe | 4-Iodo-2,5-dimethoxy-N-(2-methoxybenzyl) phenethylamine |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| mPFC | Medial prefrontal cortex |
| NAc | Nucleus Accumbens |
| NPSs | Novel Psychoactive Substances |
| PPI | Prepulse inhibition |
References
- Miliano, C.; Serpelloni, G.; Rimondo, C.; Mereu, M.; Marti, M.; De Luca, M.A. Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants. Front. Neurosci. 2016, 10, 153. [Google Scholar] [CrossRef]
- Schifano, F.; Orsolini, L.; Papanti, D.; Corkery, J. NPS: Medical Consequences Associated with Their Intake. Curr. Top. Behav. Neurosci. 2017, 32, 351–380. [Google Scholar] [PubMed]
- Miliano, C.; Margiani, G.; Fattore, L.; De Luca, M.A. Sales and Advertising Channels of New Psychoactive Substances (NPS): Internet, Social Networks, and Smartphone Apps. Brain Sci. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Tocco, G.; Papsun, D.M.; Mohr, A.L.; Fogarty, M.F.; Krotulski, A.J. U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market. Brain Sci. 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wojcieszak, J. An expanding world of new psychoactive substances-designer benzodiazepines. Neurotoxicology 2019, 73, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Chiappini, S.; Corkery, J.M.; Guirguis, A.; Papanti, D.; Schifano, F. The use of new psychoactive substances (NPS) in young people and their role in mental health care: A systematic review. Expert. Rev. Neurother. 2019, 19, 1253–1264. [Google Scholar] [CrossRef]
- Hansen, M.; Jacobsen, S.E.; Plunkett, S.; Liebscher, G.E.; McCorvy, J.D.; Brauner-Osborne, H.; Kristensen, J.L. Synthesis and pharmacological evaluation of N-benzyl substituted 4-bromo-2,5-dimethoxyphenethylamines as 5-HT2A/2C partial agonists. Bioorganic Med. Chem. 2015, 23, 3933–3937. [Google Scholar] [CrossRef]
- Negus, S.S.; Banks, M.L. Decoding the Structure of Abuse Potential for New Psychoactive Substances: Structure-Activity Relationships for Abuse-Related Effects of 4-Substituted Methcathinone Analogs. Curr. Top. Behav. Neurosci. 2017, 32, 119–131. [Google Scholar]
- Floresta, G.; Rescifina, A.; Abbate, V. Structure-Based Approach for the Prediction of Mu-opioid Binding Affinity of Unclassified Designer Fentanyl-Like Molecules. Int. J. Mol. Sci. 2019, 20, 2311. [Google Scholar] [CrossRef]
- De Luca, M.A.; Castelli, M.P.; Loi, B.; Porcu, A.; Martorelli, M.; Miliano, C.; Kellett, K.; Davidson, C.; Stair, J.L.; Schifano, F.; et al. Native CB1 receptor affinity, intrinsic activity and accumbens shell dopamine stimulant properties of third generation SPICE/K2 cannabinoids: BB-22, 5F-PB-22, 5F-AKB-48 and STS-135. Neuropharmacology 2016, 105, 630–638. [Google Scholar] [PubMed]
- Kronstrand, R.; Guerrieri, D.; Vikingsson, S.; Wohlfarth, A.; Green, H. Fatal Poisonings Associated with New Psychoactive Substances. Handb. Exp. Pharmacol. 2018, 252, 495–541. [Google Scholar]
- Kraemer, M.; Boehmer, A.; Madea, B.; Maas, A. Death cases involving certain new psychoactive substances: A review of the literature. Forensic Sci. Int. 2019, 298, 186–267. [Google Scholar] [PubMed]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [PubMed]
- Schifano, F.; Papanti, G.D.; Orsolini, L.; Corkery, J.M. Novel psychoactive substances: The pharmacology of stimulants and hallucinogens. Expert. Rev. Clin. Pharmacol. 2016, 9, 943–954. [Google Scholar]
- EMCDDA. European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- UNODC. World Drug Report 2024; United Nations Office On Drugs and Crime (UNODC): Vienna, Austria, 2024. [Google Scholar]
- Zawilska, J.B.; Kacela, M.; Adamowicz, P. NBOMes-Highly Potent and Toxic Alternatives of LSD. Front. Neurosci. 2020, 14, 78. [Google Scholar]
- Poulie CB, M.; Jensen, A.A.; Halberstadt, A.L.; Kristensen, J.L. DARK Classics in Chemical Neuroscience: NBOMes. ACS Chem. Neurosci. 2019, 11, 3860–3869. [Google Scholar]
- Drug Enforcement Administration. Schedules of Controlled Substances: Placement of Three Synthetic Phenethylamines Into Schedule I. Final rule. Fed. Regist. 2016, 81, 66181–66184. [Google Scholar]
- Kaminska, K.; Swit, P.; Malek, K. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOME): A Harmful Hallucinogen Review. J. Anal. Toxicol. 2021, 44, 947–956. [Google Scholar]
- Shanks, K.G.; Sozio, T.; Behonick, G.S. Fatal Intoxications with 25B-NBOMe and 25I-NBOMe in Indiana During 2014. J. Anal. Toxicol. 2015, 39, 602–606. [Google Scholar]
- Srisuma, S.; Bronstein, A.C.; Hoyte, C.O. NBOMe and 2C substitute phenylethylamine exposures reported to the National Poison Data System. Clin. Toxicol. 2015, 53, 624–628. [Google Scholar]
- Administration, D.E. 25I-NBOMe, 25C-NBOMe, and 25B-NBOMe (Street Names: N-bomb, Smiles, 25I, 25C, 25B). 2018. Available online: https://www.deadiversion.usdoj.gov/drug_chem_info/nbome.pdf (accessed on 11 March 2025).
- Schetz, D.; Schetz, A.; Kocic, I. A retrospective analysis of the “Neverending Trip” after administration of a potent full agonist of 5-HT2A receptor—25I-NBOMe. Biomed. Pharmacother. 2022, 146, 112295. [Google Scholar]
- Nichols, D.E.; Sassano, M.F.; Halberstadt, A.L.; Klein, L.M.; Brandt, S.D.; Elliott, S.P.; Fiedler, W.J. N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem. Neurosci. 2015, 6, 1165–1175. [Google Scholar] [PubMed]
- Elmore, J.S.; Decker, A.M.; Sulima, A.; Rice, K.C.; Partilla, J.S.; Blough, B.E.; Baumann, M.H. Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology 2018, 142, 240–250. [Google Scholar] [PubMed]
- Rickli, A.; Luethi, D.; Reinisch, J.; Buchy, D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015, 99, 546–553. [Google Scholar]
- Halberstadt, A.L.; Geyer, M.A. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 2014, 77, 200–207. [Google Scholar] [CrossRef]
- Zwartsen, A.; Verboven, A.H.A.; van Kleef, R.; Wijnolts, F.M.J.; Westerink, R.H.S.; Hondebrink, L. Measuring inhibition of monoamine reuptake transporters by new psychoactive substances (NPS) in real-time using a high-throughput, fluorescence-based assay. Toxicol. Vitr. 2017, 45 Pt 1, 60–71. [Google Scholar]
- Astrand, A.; Guerrieri, D.; Vikingsson, S.; Kronstrand, R.; Green, H. In vitro characterization of new psychoactive substances at the mu-opioid, CB1, 5HT(1A), and 5-HT(2A) receptors-On-target receptor potency and efficacy, and off-target effects. Forensic Sci. Int. 2020, 317, 110553. [Google Scholar]
- Deventer, M.H.; Persson, M.; Laus, A.; Pottie, E.; Cannaert, A.; Tocco, G.; Green, H.; Stove, C.P. Off-target activity of NBOMes and NBOMe analogs at the micro opioid receptor. Arch. Toxicol. 2023, 97, 1367–1384. [Google Scholar]
- Hill, S.L.; Doris, T.; Gurung, S.; Katebe, S.; Lomas, A.; Dunn, M.; Blain, P.; Thomas, S.H. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: Case series. Clin. Toxicol. 2013, 51, 487–492. [Google Scholar]
- Rose, S.R.; Poklis, J.L.; Poklis, A. A case of 25I-NBOMe (25-I) intoxication: A new potent 5-HT2A agonist designer drug. Clin. Toxicol. 2013, 51, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Stellpflug, S.J.; Kealey, S.E.; Hegarty, C.B.; Janis, G.C. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): Clinical case with unique confirmatory testing. J. Med. Toxicol. 2014, 10, 45–50. [Google Scholar] [CrossRef]
- Kyriakou, C.; Marinelli, E.; Frati, P.; Santurro, A.; Afxentiou, M.; Zaami, S.; Busardo, F.P. NBOMe: New potent hallucinogens--pharmacology, analytical methods, toxicities, fatalities: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3270–3281. [Google Scholar]
- Suzuki, J.; Dekker, M.A.; Valenti, E.S.; Arbelo Cruz, F.A.; Correa, A.M.; Poklis, J.L.; Poklis, A. Toxicities associated with NBOMe ingestion-a novel class of potent hallucinogens: A review of the literature. Psychosomatics 2015, 56, 129–139. [Google Scholar] [CrossRef]
- Hieger, M.A.; Rose, S.R.; Cumpston, K.L.; Stromberg, P.E.; Miller, S.; Wills, B.K. Severe poisoning after self-reported use of 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine, a novel substituted amphetamine: A case series. Am. J. Emerg. Med. 2015, 33, 1843.e1–1843.e3. [Google Scholar] [CrossRef]
- Bosak, A.; LoVecchio, F.; Levine, M. Recurrent seizures and serotonin syndrome following “2C-I” ingestion. J. Med. Toxicol. 2013, 9, 196–198. [Google Scholar] [CrossRef]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Morais, D.; Francisco da Cunha, K.; Betoni Rodrigues, T.; Lanaro, R.; de Melo Barbosa, L.; Jardim Zacca, J.; Nogueira Eberlin, M.; Costa, J.L. Triple quadrupole-mass spectrometry protocols for the analysis of NBOMes and NBOHs in blotter papers. Forensic Sci. Int. 2020, 309, 110184. [Google Scholar] [CrossRef] [PubMed]
- Elbardisy, H.M.; Foster, C.W.; Marron, J.; Mewis, R.E.; Sutcliffe, O.B.; Belal, T.S.; Talaat, W.; Daabees, H.G.; Banks, C.E. Quick Test for Determination of N-Bombs (Phenethylamine Derivatives, NBOMe) Using High-Performance Liquid Chromatography: A Comparison between Photodiode Array and Amperometric Detection. ACS Omega 2019, 4, 14439–14450. [Google Scholar] [CrossRef]
- Garrido, E.; Alfonso, M.; Diaz de Grenu, B.; Lozano-Torres, B.; Parra, M.; Gavina, P.; Marcos, M.D.; Martinez-Manez, R.; Sancenon, F. Nanosensor for Sensitive Detection of the New Psychedelic Drug 25I-NBOMe. Chemistry 2020, 26, 2813–2816. [Google Scholar] [CrossRef]
- Wohlfarth, A.; Roman, M.; Andersson, M.; Kugelberg, F.C.; Diao, X.; Carlier, J.; Eriksson, C.; Wu, X.; Konradsson, P.; Josefsson, M.; et al. 25C-NBOMe and 25I-NBOMe metabolite studies in human hepatocytes, in vivo mouse and human urine with high-resolution mass spectrometry. Drug Test. Anal. 2017, 9, 680–698. [Google Scholar] [PubMed]
- Waldman, W.; Kala, M.; Lechowicz, W.; Gil, D.; Anand, J.S. Severe clinical toxicity caused by 25I-NBOMe confirmed analytically using LC-MS-MS method. Acta Biochim. Pol. 2018, 65, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Leth-Petersen, S.; Petersen, I.N.; Jensen, A.A.; Bundgaard, C.; Baek, M.; Kehler, J.; Kristensen, J.L. 5-HT2A/5-HT2C Receptor Pharmacology and Intrinsic Clearance of N-Benzylphenethylamines Modified at the Primary Site of Metabolism. ACS Chem. Neurosci. 2016, 7, 1614–1619. [Google Scholar]
- Halberstadt, A.L. Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens. Curr. Top. Behav. Neurosci. 2017, 32, 283–311. [Google Scholar]
- Gatch, M.B.; Dolan, S.B.; Forster, M.J. Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behav. Pharmacol. 2017, 28, 375–385. [Google Scholar] [PubMed]
- Jeon, S.Y.; Kim, Y.H.; Kim, S.J.; Suh, S.K.; Cha, H.J. Abuse potential of 2-(4-iodo-2, 5-dimethoxyphenyl)N-(2-methoxybenzyl)ethanamine (25INBOMe); in vivo and ex vivo approaches. Neurochem. Int. 2019, 125, 74–81. [Google Scholar]
- Miliano, C.; Marti, M.; Pintori, N.; Castelli, M.P.; Tirri, M.; Arfe, R.; De Luca, M.A. Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe. Front. Pharmacol. 2019, 10, 1406. [Google Scholar]
- Herian, M.; Wojtas, A.; Kaminska, K.; Swit, P.; Wach, A.; Golembiowska, K. Hallucinogen-Like Action of the Novel Designer Drug 25I-NBOMe and Its Effect on Cortical Neurotransmitters in Rats. Neurotox. Res. 2019, 36, 91–100. [Google Scholar]
- Herian, M.; Wojtas, A.; Sobocinska, M.K.; Skawski, M.; Gonzalez-Marin, A.; Golembiowska, K. Contribution of serotonin receptor subtypes to hallucinogenic activity of 25I-NBOMe and to its effect on neurotransmission. Pharmacol. Rep. 2020, 72, 1593–1603. [Google Scholar]
- Herian, M.; Skawski, M.; Wojtas, A.; Sobocinska, M.K.; Noworyta, K.; Golembiowska, K. Tolerance to neurochemical and behavioral effects of the hallucinogen 25I-NBOMe. Psychopharmacology 2021, 238, 2349–2364. [Google Scholar] [CrossRef]
- Herian, M.; Wojtas, A.; Mackowiak, M.; Wawrzczak-Bargiela, A.; Solarz, A.; Bysiek, A.; Madej, K.; Golembiowska, K. Neurotoxicological profile of the hallucinogenic compound 25I-NBOMe. Sci. Rep. 2022, 12, 2939. [Google Scholar]
- Tirri, M.; Bilel, S.; Arfe, R.; Corli, G.; Marchetti, B.; Bernardi, T.; Boccuto, F.; Serpelloni, G.; Botre, F.; De-Giorgio, F.; et al. Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs. Front. Psychiatry 2022, 13, 875722. [Google Scholar]
- Bilel, S.; Tirri, M.; Arfe, R.; Stopponi, S.; Soverchia, L.; Ciccocioppo, R.; Frisoni, P.; Strano-Rossi, S.; Miliano, C.; De-Giorgio, F.; et al. Pharmacological and Behavioral Effects of the Synthetic Cannabinoid AKB48 in Rats. Front. Neurosci. 2019, 13, 1163. [Google Scholar]
- Bilel, S.; Zamberletti, E.; Caffino, L.; Tirri, M.; Mottarlini, F.; Arfe, R.; Barbieri, M.; Beggiato, S.; Boccuto, F.; Bernardi, T.; et al. Cognitive dysfunction and impaired neuroplasticity following repeated exposure to the synthetic cannabinoid JWH-018 in male mice. Br. J. Pharmacol. 2023, 180, 2777–2801. [Google Scholar]
- Corli, G.; Roda, E.; Tirri, M.; Bilel, S.; De Luca, F.; Strano-Rossi, S.; Gaudio, R.M.; De-Giorgio, F.; Fattore, L.; Locatelli, C.A. Sex-specific behavioural, metabolic, and immunohistochemical changes after repeated administration of the synthetic cannabinoid AKB48 in mice. Br. J. Pharmacol. 2024, 181, 1361–1382. [Google Scholar]
- Collins, D.; Zhang, Y.; Blendy, J.; Kreek, M.J. Murine model of OPRM1 A118G alters oxycodone self-administration and locomotor activation, but not conditioned place preference. Neuropharmacology 2020, 167, 107864. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.; Herian, M.; Skawski, M.; Sobocinska, M.; Gonzalez-Marin, A.; Noworyta-Sokolowska, K.; Golembiowska, K. Neurochemical and Behavioral Effects of a New Hallucinogenic Compound 25B-NBOMe in Rats. Neurotox. Res. 2021, 39, 305–326. [Google Scholar] [PubMed]
- Sipes, T.E.; Geyer, M.A. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997, 761, 97–104. [Google Scholar]
- Siegel, S.J.; Talpos, J.C.; Geyer, M.A. Animal models and measures of perceptual processing in schizophrenia. Neurosci. Biobehav. Rev. 2013, 37 Pt B, 2092–2098. [Google Scholar]
- Marti, M.; Neri, M.; Bilel, S.; Di Paolo, M.; La Russa, R.; Ossato, A.; Turillazzi, E. MDMA alone affects sensorimotor and prepulse inhibition responses in mice and rats: Tips in the debate on potential MDMA unsafety in human activity. Forensic Toxicol. 2019, 37, 132–144. [Google Scholar]
- Leyrer-Jackson, J.M.; Hood, L.E.; Olive, M.F. Drugs of Abuse Differentially Alter the Neuronal Excitability of Prefrontal Layer V Pyramidal Cell Subtypes. Front. Cell. Neurosci. 2021, 15, 703655. [Google Scholar]
- Rozas, C.; Loyola, S.; Ugarte, G.; Zeise, M.L.; Reyes-Parada, M.; Pancetti, F.; Rojas, P.; Morales, B. Acutely applied MDMA enhances long-term potentiation in rat hippocampus involving D1/D5 and 5-HT2 receptors through a polysynaptic mechanism. Eur. Neuropsychopharmacol. 2012, 22, 584–595. [Google Scholar] [PubMed]
- Shen, H.; Kalivas, P.W. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int. J. Neuropsychopharmacol. 2013, 16, 1165–1167. [Google Scholar]
- Ruan, H.; Yao, W.D. Loss of mGluR1-LTD following cocaine exposure accumulates Ca(2+)-permeable AMPA receptors and facilitates synaptic potentiation in the prefrontal cortex. J. Neurogenet. 2021, 35, 358–369. [Google Scholar] [PubMed]
- Tirri, M.; Arfe, R.; Bilel, S.; Corli, G.; Marchetti, B.; Fantinati, A.; Vincenzi, F.; De-Giorgio, F.; Camuto, C.; Mazzarino, M.; et al. In Vivo Bio-Activation of JWH-175 to JWH-018: Pharmacodynamic and Pharmacokinetic Studies in Mice. Int. J. Mol. Sci. 2022, 23, 8030. [Google Scholar] [CrossRef]
- Lafourcade, M.; Elezgarai, I.; Mato, S.; Bakiri, Y.; Grandes, P.; Manzoni, O.J. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE 2007, 2, e709. [Google Scholar]
- Braden, M.R.; Parrish, J.C.; Naylor, J.C.; Nichols, D.E. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol. Pharmacol. 2006, 70, 1956–1964. [Google Scholar]
- Palamar, J.J.; Acosta, P.; Sherman, S.; Ompad, D.C.; Cleland, C.M. Self-reported use of novel psychoactive substances among attendees of electronic dance music venues. Am. J. Drug Alcohol. Abuse 2016, 42, 624–632. [Google Scholar] [PubMed]
- Le Roux, G.; Bruneau, C.; Lelievre, B.; Bretaudeau Deguigne, M.; Turcant, A.; Harry, P.; Boels, D. Recreational phenethylamine poisonings reported to a French poison control center. Drug Alcohol. Depend. 2015, 154, 46–53. [Google Scholar]
- Di Chiara, G.; Bassareo, V.; Fenu, S.; De Luca, M.A.; Spina, L.; Cadoni, C.; Acquas, E.; Carboni, E.; Valentini, V.; Lecca, D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 2004, 47 (Suppl. S1), 227–241. [Google Scholar]
- De Luca, M.A.; Bimpisidis, Z.; Melis, M.; Marti, M.; Caboni, P.; Valentini, V.; Margiani, G.; Pintori, N.; Polis, I.; Marsicano, G.; et al. Stimulation of in vivo dopamine transmission and intravenous self-administration in rats and mice by JWH-018, a Spice cannabinoid. Neuropharmacology 2015, 99, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; van der Heijden, I.; Ruderman, M.A.; Risbrough, V.B.; Gingrich, J.A.; Geyer, M.A.; Powell, S.B. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 2009, 34, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Forster, M.J.; Wolfrum, K.M.; Johnson, R.A.; Janowsky, A.; Gatch, M.B. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: Mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology 2014, 231, 875–888. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Effect of Hallucinogens on Unconditioned Behavior. Curr. Top. Behav. Neurosci. 2018, 36, 159–199. [Google Scholar] [PubMed]
- Jo, C.; Joo, H.; Youn, D.H.; Kim, J.M.; Hong, Y.K.; Lim, N.Y.; Kim, K.S.; Park, S.J.; Choi, S.O. Rewarding and Reinforcing Effects of 25H-NBOMe in Rodents. Brain Sci. 2022, 12, 1490. [Google Scholar] [CrossRef]
- Viaro, R.; Marti, M.; Morari, M. Dual motor response to l-dopa and nociceptin/orphanin FQ receptor antagonists in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) treated mice: Paradoxical inhibition is relieved by D(2)/D(3) receptor blockade. Exp. Neurol. 2010, 223, 473–484. [Google Scholar] [CrossRef]
- Canazza, I.; Ossato, A.; Trapella, C.; Seri, C.; Rimondo, C.; Serpelloni, G.; Marti, M. 2C-B and 25I-NBOMe impair sensorimotor functions in mice. In Proceedings of the Italian Society of Pharmacology “Addictive disorders: From Neurobiology to Novel Therapeutic Strategies”, Palermo, Italy, 27–28 March 2015. [Google Scholar]
- Bassi, M.; Bilel, S.; Tirri, M.; Corli, G.; Di Rosa, F.; Gregori, A.; Alkilany, A.M.; Rachid, O.; Roda, E.; De Luca, F.; et al. The synthetic cathinones MDPHP and MDPV: Comparison of the acute effects in mice, in silico ADMET profiles and clinical reports. Neurotoxicology 2024, 103, 230–255. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health; HHS Publication No. SMA 17-5044, NSDUH Series H-52; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2017.
- United Nations Office on Drugs and Crime. Current NPS Threaths; United Nations Office on Drugs and Crime: Vienna, Austria, 2020; Volume III. [Google Scholar]
- United Nations Office on Drugs and Crime. Current NPS Treaths; United Nations Office on Drugs and Crime: Vienna, Austria, 2024; Volume VII. [Google Scholar]
- Rajotte, J.W.; Palmentier, J.F.P.; Wallage, H.R. Drug Recognition Evaluation and Chemical Confirmation of a 25C-NBOMe-Impaired Driver. J. Forensic Sci. 2017, 62, 1410–1413. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004, 47 (Suppl. S1), 33–46. [Google Scholar]
- Toth, A.; Petyko, Z.; Galosi, R.; Szabo, I.; Karadi, K.; Feldmann, A.; Peczely, L.; Kallai, V.; Karadi, Z.; Lenard, L. Neuronal coding of auditory sensorimotor gating in medial prefrontal cortex. Behav. Brain Res. 2017, 326, 200–208. [Google Scholar] [PubMed]
- Shao, L.X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544e4. [Google Scholar]
- Revenga, M.D.L.F.; Zhu, B.; Guevara, C.A.; Naler, L.B.; Saunders, J.M.; Zhou, Z.; Toneatti, R.; Sierra, S.; Wolstenholme, J.T.; Beardsley, P.M.; et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021, 37, 109836. [Google Scholar]
- Berthoux, C.; Barre, A.; Bockaert, J.; Marin, P.; Becamel, C. Sustained Activation of Postsynaptic 5-HT2A Receptors Gates Plasticity at Prefrontal Cortex Synapses. Cereb. Cortex 2019, 29, 1659–1669. [Google Scholar] [CrossRef]
- Xu, T.X.; Ma, Q.; Spealman, R.D.; Yao, W.D. Amphetamine modulation of long-term potentiation in the prefrontal cortex: Dose dependency, monoaminergic contributions, and paradoxical rescue in hyperdopaminergic mutant. J. Neurochem. 2010, 115, 1643–1654. [Google Scholar] [PubMed]
- Bilel, S.; Tirri, M.; Arfè, R.; Ossato, A.; Trapella, C.; Serpelloni, G.; Neri, M.; Fattore, L.; Marti, M. Novel halogenated synthetic cannabinoids impair sensorimotor functions in mice. Neurotoxicology 2020, 76, 17–32. [Google Scholar]
- Ossato, A.; Canazza, I.; Trapella, C.; Vincenzi, F.; De Luca, M.A.; Rimondo, C.; Varani, K.; Borea, P.A.; Serpelloni, G.; Marti, M. Effect of JWH-250, JWH-073 and their interaction on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 67, 31–50. [Google Scholar] [CrossRef]
- Ossato, A.; Uccelli, L.; Bilel, S.; Canazza, I.; Di Domenico, G.; Pasquali, M.; Pupillo, G.; De Luca, M.A.; Boschi, A.; Vincenzi, F.; et al. Psychostimulant Effect of the Synthetic Cannabinoid JWH-018 and AKB48: Behavioral, Neurochemical, and Dopamine Transporter Scan Imaging Studies in Mice. Front. Psychiatry 2017, 8, 130. [Google Scholar]
| Animal Model | Methods | Findings | Reference |
|---|---|---|---|
| C57BL/6J mice | 25I-NBOMe (0.1–1 mg/kg; s.c.) | 25I-NBOMe induced the head twitch response with 14-fold higher potency than 2C-I, and the effect was completely blocked by a selective 5HT2A antagonist | [29] |
| Swiss-Webster mice | 25I-NBOMe (1, 2.5, 5, 10, 25 mg/kg; i.p.) | 25I-NBOMe decreased locomotor activity time-dependently and dose-dependently | [48] |
| Sprague-Dawley rats | 25I-NBOMe (1, 2.5, 5, 10, 25 mg/kg; i.p.) | 25I-NBOMe failed to produce 80% drug-appropriate responding in DOM-trained rats; in MDMA-trained rats 25I-NBOMe produced considerable fluctuations in drug-appropriate responding | |
| Sprague-Dawley rats | 25I-NBOMe (0.01, 0.03, 0.1, 0.3 mg/kg; s.c.) | 25I-NBOMe induced wet dog shakes and back muscle contraction, and the effect was blocked by a selective 5HT2A antagonist | [27] |
| C57BL/6J mice | 25I-NBOMe (0.3, 1, 2 mg/kg; i.p.) | 25I-NBOMe increased conditioned place preference at the lowest dose tested | [49] |
| Sprague-Dawley rats | 25I-NBOMe (0.03 mg/kg; inf) | 25I-NBOMe did not induce intravenous self-administration | |
| Sprague-Dawley rats (male and female) | 25I-NBOMe (0.3, 1 mg/kg; i.p.): microdyalisis 25I-NBOMe (0.1, 0.3, 0.5, 1.0 mg/kg; i.p.): behavior | Both sexes: 25I-NBOMe evoked increases in DA levels in the NAc shell, it caused impaired visual responses, and it disrupted the PPI. Females: core temperature was heavily affected. Males: the highest dose exerted an analgesic effect | [50] |
| Wistar-Han rats | 25I-NBOMe (3 mg/kg; s.c.) | 25I-NBOMe increased DA and 5HT in the frontal cortex | [51] |
| Wistar-Han rats | 25I-NBOMe (1, 3 mg/kg; s.c.) for microdyalisis and behavioral test | 25I-NBOMe induced wet dog shakes, and the effect was reduced by selective 5HT2A and 5HT2C antagonists, but not by selective 5HT1A antagonist. 25I-NBOMe increased glutamate, DA and 5HT release and the effect was blocked by selective 5HT2A and 5HT2C and reduced by a selective 5HT1A antagonist. | [52] |
| Wistar-Han rats | 25I-NBOMe (0.3 mg/kg/day for 7 days; s.c.) for microdyalisis and behavioral tests | 25I-NBOMe increased levels of DA, 5HT, GLU, and Ach in the frontal cortex weakly with respect to acute injection. It enhanced DA and 5HT levels in the striatum and nucleus accumbens more deeply than acute injection. It increased Ach levels in the frontal cortex, striatum and nucleus accumbens. 25I-NBOMe induced a reduction in motor activity, impaired memory capability, and induced anxiety. | [53] |
| Wistar-Han rats | 25I-NBOMe (0.3 mg/kg/day for 7 days) | 25I-NBOMe decreased the number of astrocytes in the frontal cortex and mPFC and the number of microglia cells in the frontal cortex. | [54] |
| ICR (CD-1) mice | 25I-NBOMe (0.001, 0.01, 0.1, 1, 10 mg/kg; i.p.) | 25I-NBOMe induced a decrease in visual and acoustic responses and increased the reaction time. A 10 mg/kg dose induced a transient decrease in the total distance travelled. It altered the startle response (1 and 10 mg/kg) and disrupted the PPI (0.01–10 mg/kg). | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilel, S.; Miliano, C.; Corli, G.; Bassi, M.; Trusel, M.; Tonini, R.; De Luca, M.A.; Marti, M. Acute Effects of the Psychedelic Phenethylamine 25I-NBOMe in C57BL/6J Male Mice. Int. J. Mol. Sci. 2025, 26, 2815. https://doi.org/10.3390/ijms26062815
Bilel S, Miliano C, Corli G, Bassi M, Trusel M, Tonini R, De Luca MA, Marti M. Acute Effects of the Psychedelic Phenethylamine 25I-NBOMe in C57BL/6J Male Mice. International Journal of Molecular Sciences. 2025; 26(6):2815. https://doi.org/10.3390/ijms26062815
Chicago/Turabian StyleBilel, Sabrine, Cristina Miliano, Giorgia Corli, Marta Bassi, Massimo Trusel, Raffaella Tonini, Maria Antonietta De Luca, and Matteo Marti. 2025. "Acute Effects of the Psychedelic Phenethylamine 25I-NBOMe in C57BL/6J Male Mice" International Journal of Molecular Sciences 26, no. 6: 2815. https://doi.org/10.3390/ijms26062815
APA StyleBilel, S., Miliano, C., Corli, G., Bassi, M., Trusel, M., Tonini, R., De Luca, M. A., & Marti, M. (2025). Acute Effects of the Psychedelic Phenethylamine 25I-NBOMe in C57BL/6J Male Mice. International Journal of Molecular Sciences, 26(6), 2815. https://doi.org/10.3390/ijms26062815








