Halved Dose of Antipsychotics Versus High-Dose Antipsychotic Therapy for Relapse in Patients with Schizophrenia Receiving High-Dose Antipsychotic Therapy: A Randomized Single-Blind Trial
Abstract
:1. Introduction
2. Results
2.1. Comparison of the Halved-Dose Group and the High-Dose Group
2.2. Safety
2.3. Changes in PANSS and DIEPSS Scores During Relapse
3. Discussion
3.1. Dopamine (DA) Neuron Firing, DA Transients, and DA Function
3.2. Model of Delusion Formation in Schizophrenia
3.3. Increased DA Release and Relapse in Patients with Schizophrenia
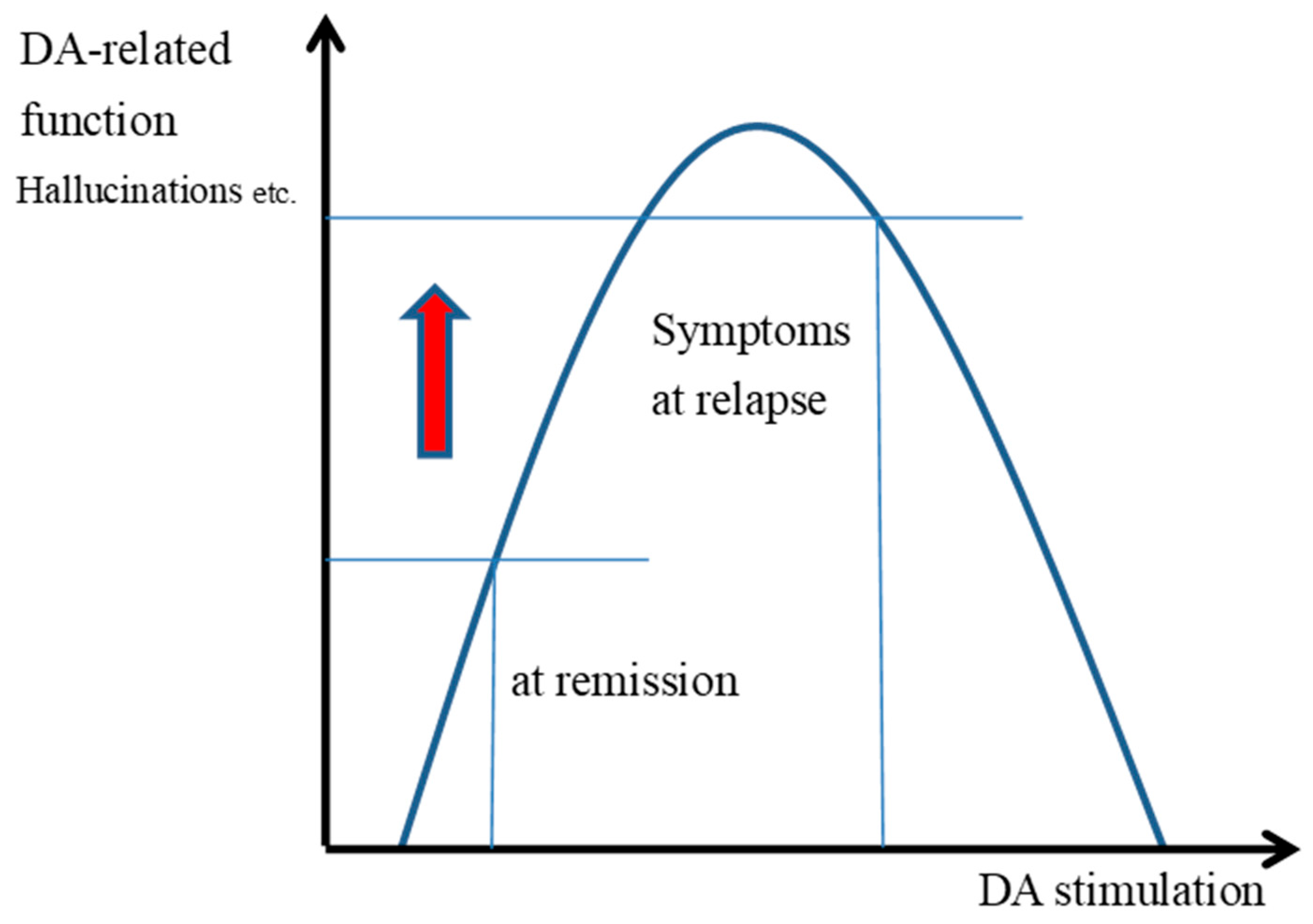
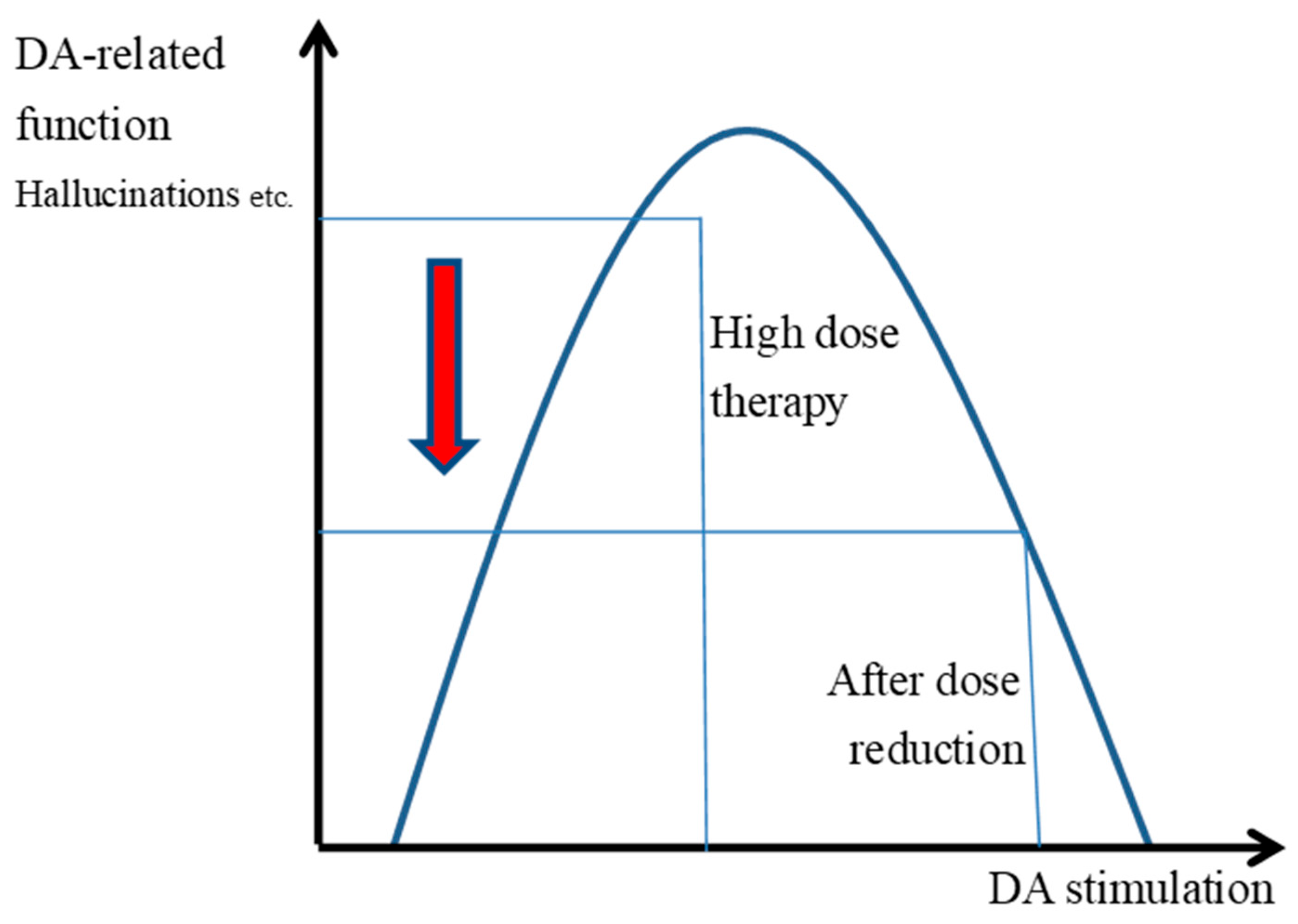
3.4. The Inverted U-Shaped Relationship Between the Transmission and Functioning of DAD2 Receptors
3.5. Symptom Changes and Improvement Associated with Halving the Dose of Antipsychotics
3.6. Increased Stress Exacerbates Relapse
3.7. Increased Stress Impairs Prefrontal Cortex Functioning
3.8. Improved Functioning of DAD2 Autoreceptors Acts to Improve After Relapse
4. Methods
4.1. Study Design
4.2. Ethics Approval and Recruitment
4.3. Participants
4.4. Sample Size
4.5. Randomization
4.6. Primary Outcome
4.7. Secondary Outcomes
4.8. Intervention
4.8.1. Antipsychotic Dose Reduction
4.8.2. Anticholinergic Dose Reduction
4.8.3. Relapse
4.8.4. Remission
4.9. Data Analysis
4.10. Safety Monitoring
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| DA | Dopamine |
| PFC | Prefrontal cortex |
| CP | Chlorpromazine |
| PANSS | Positive and Negative Syndrome Scale |
| EPS | Extrapyramidal symptoms |
| DIEPSS | Drug-induced Extrapyramidal Symptoms Scale |
| NE | Norepinephrine |
References
- Kuhs, H.; Eikelmann, B. Suspension of neuroleptic therapy in acute schizophrenia. Pharmacopsychiatry 1988, 21, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Thaker, G.K.; Wagman, A.M.; Kirkpatrick, B.; Tamminga, C.A. Alterations in sleep polygraphy after neuroleptic withdrawal: A putative supersensitive dopaminergic mechanism. Biol. Psychiatry 1989, 25, 75–86. [Google Scholar] [CrossRef]
- Kirch, D.G.; Jaskiw, G.; Linnoila, M.; Weiberger, D.R.; Wyatt, R.J. Plasma amine metabolites before and after withdrawal from neuroleptic treatment in chronic schizophrenic inpatients. Psychiatry Res. 1988, 25, 233–242. [Google Scholar] [CrossRef]
- Dufresne, R.L.; Wagner, R.L. Antipsychotic-withdrawal akathisia versus antipsychotic-induced akathisia: Further evidence for the existence of tardive akathisia. J. Clin. Psychiatry 1988, 49, 435–438. [Google Scholar]
- Breitenstein, C.; Korsukewitz, C.; Flöel, A. Tonic dopaminergic stimulation impairs associative learning in healthy subjects. Neuropsychopharmacology 2006, 31, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Frank, M.J.; Gibbs, S.E.; Miyakawa, A.; Jagust, W.; D’Esposito, M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J. Neurosci. 2009, 29, 1538–1543. [Google Scholar] [CrossRef]
- Graef, S.; Biele, G.; Krugel, L.K.; Marzinzik, F.; Wahl, M.; Wotka, J.; Klostermann, F.; Heekeren, H.R. Differential influence of levodopa on reward-based learning in Parkinson’s disease. Front. Hum. Neurosci. 2010, 4, 169. [Google Scholar] [CrossRef]
- Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kambeitz, J.; Kim, E.; Stahl, D.; Slifstein, M.; Abi-Dargham, A.; Kapur, S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 2012, 69, 776–786. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Meyer-Lindenberg, A. Striatal presynaptic dopamine in schizophrenia, part II: Meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr. Bull. 2013, 39, 33–42. [Google Scholar] [CrossRef]
- Guillin, O.; Abi-Dargham, A.; Laruelle, M. Neurobiology of dopamine in schizophrenia. Int. Rev. Neurobiol. 2007, 78, 1–39. [Google Scholar] [PubMed]
- Egerton, A.; Howes, O.D.; Houle, S.; McKenzie, K.; Valmaggia, L.R.; Bagby, M.R.; Tseng, H.H.; Bloomfield, M.A.P.; Kenk, M.; Bhattacharyya, S.; et al. Elevated striatal dopamine function in immigrants and their children: A risk mechanism for psychosis. Schizophr. Bull. 2017, 43, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jimenez, M.; Priede, A.; Hetrick, S.E.; Bendall, S.; Killackey, E.; Parker, A.G.; Mcgorry, P.D.; Gleeson, J.F. Risk factors for relapse following treatment for first episode psychosis: A systematic review and meta-analysis of longitudinal studies. Schizophr. Res. 2012, 139, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Tardy, M.; Komossa, K.; Heres, S.; Kissling, W.; Salanti, G.; Davis, J.M. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta-analysis. Lancet 2012, 379, 2063–2071. [Google Scholar] [CrossRef]
- Laruelle, M.; Abi-Dargham, A.; Gil, R.; Kegeles, L.; Innis, R. Increased dopamine transmission in schizophrenia: Relationship to illness phases. Biol. Psychiatry 1999, 46, 56–72. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Champagne, F.; Meaney, M.J.; Dagher, A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: A positron emission tomography study using [11C] raclopride. J. Neurosci. 2004, 24, 2825–2831. [Google Scholar] [CrossRef]
- Brunelin, J.; d’Amato, T.; Van Os, J.; Costes, N.; Chagny, M.F.S.; Saoud, M. Increased left striatal dopamine transmission in unaffected siblings of schizophrenia patients in response to acute metabolic stress. Psychiatry Res. 2010, 181, 130–135. [Google Scholar] [CrossRef]
- Mizrahi, R.; Addington, J.; Rusjan, P.M.; Suridjan, I.; Ng, A.; Boileau, I.; Pruessner, J.C.; Remington, G.; Houle, S.; Wilsonet, A.A. Increased stress-induced dopamine release in psychosis. Biol. Psychiatry 2012, 71, 561–567. [Google Scholar] [CrossRef]
- Howes, O.D.; Montgomery, A.J.; Asselin, M.C.; Murray, R.M.; Valli, I.; Tabraham, P.; Bramon-Bosch, E.; Valmaggia, L.; Johns, L.; Broome, M.; et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry 2009, 66, 13–20. [Google Scholar] [CrossRef]
- Gold, J.M.; Waltz, J.A.; Frank, M.J. Effort cost computation in schizophrenia: A commentary on the recent literature. Biol. Psychiatry 2015, 78, 747–753. [Google Scholar] [CrossRef]
- Vaessen, T.; Hernaus, D.; Myin-Germeys, I.; van Amelvoort, T. The dopaminergic response to acute stress in health and psychopathology: A systematic review. Neurosci. Biobehav. Rev. 2015, 56, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Remington, G.; Foussias, G.; Agid, O.; Fervaha, G.; Tekeuchi, H. The neurobiology of relapse in schizophrenia. Schizophr. Res. 2014, 152, 381–390. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.M.; Birnbaum, M.L.; Bloomfield, M.A.P.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T. Treatment resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.E.; Slotema, C.W.; Daskalakis, Z.J.; Derks, E.M.; Blom, J.D.; Gaag, M.V.D. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr. Bull. 2012, 38, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef]
- LeSauter, J.; Balsam, P.D.; Simpson, E.H.; Silver, R. Overexpression of striatal D2 receptors reduces motivation thereby decreasing food anticipatory activity. Eur. J. Neurosci. 2020, 51, 71–81. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Aosaki, T.; Flaherty, A.W.; Kimura, M. The basal ganglia and adaptive motor control. Science 1994, 265, 1826–1831. [Google Scholar] [CrossRef]
- Yin, H.H.; Knowlton, B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006, 7, 464–476. [Google Scholar] [CrossRef]
- Ljungberg, T.; Apicella, P.; Schultz, W. Responses of monkey dopamine neurons during learning of behavioral reactions. J. Neurophysiol. 1992, 67, 145–163. [Google Scholar] [CrossRef]
- Schultz, W.; Apicella, P.; Ljungberg, T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993, 13, 900–913. [Google Scholar] [CrossRef]
- Montague, P.R.; Dayan, P.; Sejnowski, T.J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 1996, 16, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Brischoux, F.; Chakraborty, S.; Brierley, D.I.; Ungless, M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 4894–4899. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007, 447, 1111–1115. [Google Scholar] [CrossRef]
- Andreou, C.; Bozikas, V.P.; Luedtke, T.; Moritz, S. Associations between visual perception accuracy and confidence in a dopaminergic manipulation study. Front. Psychol. 2015, 6, 414. [Google Scholar] [CrossRef]
- Lou, H.C.; Skewes, J.C.; Thomsen, K.R.; Overgaard, M.; Lau, H.C.; Mouridsen, K.; Roepstorff, A. Dopaminergic stimulation enhances confidence and accuracy in seeing rapidly presented words. J. Vis. 2011, 11, 15. [Google Scholar] [CrossRef]
- Diederen, K.M.; Spencer, T.; Vestergaard, M.D.; Fletcher, P.C.; Schultz, W. Adaptive prediction error coding in the human midbrain and striatum facilitates behavioral adaptation and learning efficiency. Neuron 2016, 90, 1127–1138. [Google Scholar] [CrossRef]
- Fiorillo, C.D.; Tobler, P.N.; Schultz, W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science 2003, 299, 1898–1902. [Google Scholar] [CrossRef]
- Sombers, L.A.; Beyene, M.; Carelli, R.M.; Wightman, R.M. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J. Neurosci. 2009, 29, 1735–1742. [Google Scholar] [CrossRef]
- Heinz, A.; Schlagenhauf, F. Dopaminergic dysfunction in schizophrenia: Salience attribution revisited. Schizophr. Bull. 2010, 36, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Keiflin, R.; Janak, P.H. Dopamine prediction errors in reward learning and addiction: From theory to neural circuitry. Neuron 2015, 88, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Maia, T.V.; Frank, M.J. An integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry 2017, 81, 52–66. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar]
- Heinz, A. Dopaminergic dysfunction in alcoholism and schizophrenia—Psychopathological and behavioral correlates. Eur. Psychiatry 2002, 17, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tobler, P.N.; Fiorillo, C.D.; Schultz, W. Adaptive coding of reward value by dopamine neurons. Science 2005, 307, 1642–1645. [Google Scholar] [CrossRef]
- Fletcher, P.C.; Frith, C.D. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 2009, 10, 48–58. [Google Scholar] [CrossRef]
- Mathys, C.; Daunizeau, J.; Friston, K.J.; Stephan, K.E. A Bayesian foundation for individual learning under uncertainty. Front. Hum. Neurosci. 2011, 5, 39. [Google Scholar] [CrossRef]
- Moritz, S.; Woodward, T. Plausibility judgment in schizophrenic patients: Evidence for a liberal acceptance bias. Ger. J. Psychiatry 2004, 7, 66–74. [Google Scholar]
- Howes, O.; Bose, S.; Turkheimer, F.; Valli, I.; Egerton, A.; Stahl, D.; Valmaggia, L.; Allen, P.; Murray, R.; Mcguire, P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: A PET study. Mol. Psychiatry 2011, 16, 885–886. [Google Scholar] [CrossRef]
- Saunders, B.T. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 2018, 21, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R.; Laruelle, M. Neurochemical and neuropharmacological imaging in schizophrenia. In Neuropsychopharmacology: The Fifth Generation of Progress; Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 833–855. [Google Scholar]
- Parga, A. Cortical Auditory Functional Activation by Corticostriato-Thalamo-Cortical Circuits. Unpublished. Doctoral Dissertation, Arizona State University, Tempe, AZ, USA, 2014. [Google Scholar]
- Parga, A.; Munoz, G.; Hammer, R.P. Excessive striatal dopamine activates auditory cortex via striato-pallido-thalamo-cortical projections in the rat. Biol. Psychiatry 2015, 77, 62S. [Google Scholar]
- Laruelle, M.; Abi-Dargham, A.; van Dyck, C.H.; Gil, R.; D’Souza, C.D.; Erdos, J.; McCane, E.; Rosenblatt, W.; Fingado, C.; Zoghbi, S.S.; et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA 1996, 93, 9235–9240. [Google Scholar] [CrossRef]
- Lim, S.J.; Fiez, J.A.; Holt, L.L. How may the basal ganglia contribute to auditory categorization and speech perception? Front. Neurosci. 2014, 8, 230. [Google Scholar] [CrossRef]
- Shipp, S. The functional logic of corticostriatal connections. Brain Struct. Funct. 2017, 222, 669–706. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lanciego, J.; Kerkerian-Le-Goff, L.; Coulon, P.; Salin, P.; Kachidian, P.; Lei, W.; Del Mar, N.; Reiner, A. Differential organization of cortical inputs to striatal projection neurons of the matrix compartment in rats. Front. Syst. Neurosci. 2015, 9, 51. [Google Scholar] [CrossRef]
- Dobbs, L.K.; Kaplan, A.R.; Lemos, J.C.; Matsui, A.; Rubinstein, M.; Alvarez, V.A. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 2016, 90, 1100–1113. [Google Scholar] [CrossRef]
- Burke, D.A.; Rotstein, H.G.; Alvarez, V.A. Striatal local circuitry: A new framework for lateral inhibition. Neuron 2017, 96, 267–284. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia. Dopamine and the striatum: From biology to symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- Cazorla, M.; Carvalho, F.D.; Chohan, M.O.; Shegda, M.; Chuhma, N.; Rayport, S.; Ahmari, S.E.; Moore, H.; Kellendonk, C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 2014, 81, 153–164. [Google Scholar] [CrossRef]
- Birnbaum, S.; Gobeske, K.T.; Auerbach, J.; Taylor, J.R.; Arnsten, A.F. A role for norepinephrine in stressinduced cognitive deficits: α-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiatry 1999, 46, 1266–1274. [Google Scholar] [CrossRef]
- Arnsten, A.F. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 2011, 69, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Li, B.M.; Mao, Z.M.; Wang, M.; Mei, Z.T. Alpha-2 adrenergicmodulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacolog 1999, 21, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Bentley, P.; Driver, J.; Dolana, R.J. Cholinergic modulation of cognition: Insights from human pharmacological functional neuroimaging. Prog. Neurobiol. 2011, 94, 360–388. [Google Scholar] [CrossRef]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex 2001, 11, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Pawlak, A.P.; Cho, J.; Root, D.H.; Barker, D.J.; Westa, M.O. Amphetamin’s dose-dependent effects on dorsolateral striatum sensorimotor neuron firing. Behav. Brain Res. 2013, 244, 152–161. [Google Scholar] [CrossRef]
- Shen, W.; Flajolet, M.; Greengard, P.; Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008, 321, 848–851. [Google Scholar] [CrossRef]
- Daberkow, D.P.; Brown, H.D.; Bunner, K.D.; Kraniotis, S.A.; Doellman, M.A.; Ragozzino, M.E.; Garris, P.A.; Roitman, M.F. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J. Neurosci. 2013, 33, 452–463. [Google Scholar] [CrossRef]
- Kegeles, L.S.; Abi-Dargham, A.; Frankle, W.G.; Gil, R.; Cooper, T.B.; Slifstein, M.; Hwang, D.R.; Huang, Y.; Haber, S.N.; Laruelle, M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 231–239. [Google Scholar] [CrossRef]
- Morrens, M.; Hulstijn, W.; Sabbe, B. Psychomotor slowing in schizophrenia. Schizophr. Bull. 2007, 33, 1038–1053. [Google Scholar] [CrossRef]
- Mizrahi, R.; Rusjan, P.; Agid, O.; Graff, A.; Mamo, D.C.; Zipursky, R.B.; Kapur, S. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: A PET study in schizophrenia. Am. J. Psychiatry 2007, 164, 630–637. [Google Scholar] [CrossRef]
- Haan, L.; Lavalaye, J.; van Bruggen, M.; van Nimwegen, L.; Booij, J.; van Amelsvoort, T.; Linszen, D. Subjective experience and dopamine D2 receptor occupancy in patients treated with antipsychotics: Clinical implications. Can. J. Psychiatry 2004, 49, 290–296. [Google Scholar] [CrossRef]
- Kapur, S.; Zipursky, R.; Jones, C.; Remington, C.; Houle, S. Relationship between dopamine D2 occupancy, clinical response, and side effects: A double-blind PET study of first-episode schizophrenia. Am. J. Psychiatry 2000, 157, 514–520. [Google Scholar] [CrossRef]
- Holtzman, C.W.; Trotman, H.D.; Goulding, S.M.; Ryan, A.T.; Macdonald, A.N.; Shapiro, D.I.; Brasfield, J.L.; Walker, E.F. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience 2013, 249, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Sinha, R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci. Biobehav. Rev. 2008, 32, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, H.T.; Barbas, H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 2002, 115, 1261–1279. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T.; Raskind, M.A.; Taylor, F.B.; Connor, D.F. The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol. Stress 2015, 1, 89–99. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Wang, M.; Paspalas, C.D. Dopamine’s actions in primate prefrontal cortex: Challenges for treating cognitive disorders. Pharmacol. Rev. 2015, 67, 681–696. [Google Scholar] [CrossRef]
- Piazza, P.V.; Barrot, M.; Rougé-Pont, F.; Marinelli, M.; Maccari, S.; Abrous, D.N.; Simon, H.; Moal, M.L. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc. Natl. Acad. Sci. USA 1996, 93, 15445–15450. [Google Scholar] [CrossRef]
- Bello, E.P.; Mateo, Y.; Gelman, D.M.; Noaín, D.; Shin, J.H.; Low, M.J.; Alvarez, V.A.; Lovinger, D.M.; Rubinstein, M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 2011, 14, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Uchida, H.; Tanaka, K.F.; Tomita, M.; Tsunoda, K.; Nomura, K.; Takano, H.; Tanabe, A.; Watanabe, K.; Yagi, G.; et al. Reducing the dose of antipsychotic medications for those who had been treated with high-dose antipsychotic polypharmacy: An open study of dose reduction for chronic schizophrenia. Int. Clin. Psychopharmacol. 2003, 18, 323–329. [Google Scholar] [PubMed]
- Yamanouchi, Y.; Sukegawa, T.; Inagaki, A.; Inada, T.; Yoshio, T.; Yoshimura, R.; Iwata, N. Evaluation of the individual safe correction of antipsychotic agent polypharmacy in Japanese patients with chronic schizophrenia: Validation of safe corrections for antipsychotic polypharmacy and the high-dose method. Int. J. Neuropsychopharmacol. 2014, 18, 1–8. [Google Scholar] [CrossRef]
- Essock, S.M.; Schooler, N.R.; Stroup, T.S.; McEvoy, J.P.; Rojas, I.; Jackson, C.; Covell, N.H. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am. J. Psychiatry 2011, 168, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C.; Carpenter, W.T., Jr.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef]


| Halved-Dose Group (n = 27) (Number of Relapse = 14) | High-Dose Group (n = 27) (Number of Relapse = 15) | T (Relapsed Patients) | p-Value (Relapsed Patients) | |
|---|---|---|---|---|
| Age | 50.2 ± 10.5 (49.6 ± 7.9) | 47.6 ± 10.0 (45.2 ± 9.3) | 0.90 (1.27) | 0.37 (0.22) |
| Sex | Male: 14 (6) Female: 13 (8) | Male: 14 (7), Female: 13 (8) | 0.00 (0.37) | 1.00 (0.71) |
| Dose of antipsychotics (CP eq./mg) | 1695 ± 492 (1793 ± 393) | 1618 ± 429 (1836 ± 413) | 0.54 (0.26) | 0.59 (0.79) |
| PANSS | 66.5 ± 13.1 (68.7 ± 12.6) | 68.3 ± 11.2 (70.4 ± 9.8) | 0.37 (0.86) | 0.71 (0.40) |
| DIEPSS | 10.2 ± 5.8 (10.8 ± 3.6) | 9.5 ± 4.8 (9.3 ± 4.8) | 0.87 (0.76) | 0.39 (0.46) |
| Halved-Dose Group | High-Dose Group | T (Relapsed Patients) | p-Value | |
|---|---|---|---|---|
| PANSS | 69.2 ± 6.8 | 68.1 ± 7.2 | 0.11 | 0.91 |
| DIEPSS | 8.0 ± 2.7 | 9.9 ± 4.2 | 0.88 | 0.12 |
| Dose of antipsychotics (CP eq./mg) | 1014 ± 241 | 1771 ± 454 | 4.83 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataniya, R.; Koike, T.; Inamoto, A. Halved Dose of Antipsychotics Versus High-Dose Antipsychotic Therapy for Relapse in Patients with Schizophrenia Receiving High-Dose Antipsychotic Therapy: A Randomized Single-Blind Trial. Int. J. Mol. Sci. 2025, 26, 4003. https://doi.org/10.3390/ijms26094003
Ataniya R, Koike T, Inamoto A. Halved Dose of Antipsychotics Versus High-Dose Antipsychotic Therapy for Relapse in Patients with Schizophrenia Receiving High-Dose Antipsychotic Therapy: A Randomized Single-Blind Trial. International Journal of Molecular Sciences. 2025; 26(9):4003. https://doi.org/10.3390/ijms26094003
Chicago/Turabian StyleAtaniya, Ryota, Takeshi Koike, and Atsuko Inamoto. 2025. "Halved Dose of Antipsychotics Versus High-Dose Antipsychotic Therapy for Relapse in Patients with Schizophrenia Receiving High-Dose Antipsychotic Therapy: A Randomized Single-Blind Trial" International Journal of Molecular Sciences 26, no. 9: 4003. https://doi.org/10.3390/ijms26094003
APA StyleAtaniya, R., Koike, T., & Inamoto, A. (2025). Halved Dose of Antipsychotics Versus High-Dose Antipsychotic Therapy for Relapse in Patients with Schizophrenia Receiving High-Dose Antipsychotic Therapy: A Randomized Single-Blind Trial. International Journal of Molecular Sciences, 26(9), 4003. https://doi.org/10.3390/ijms26094003







