Study of the Reaction Mechanism of the Excessive Adsorption of Mn2+ from Water by In Situ Synthesis of MnO2@SiO2 Colloid as an Adsorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Characterization
2.1.1. SEM and EDS
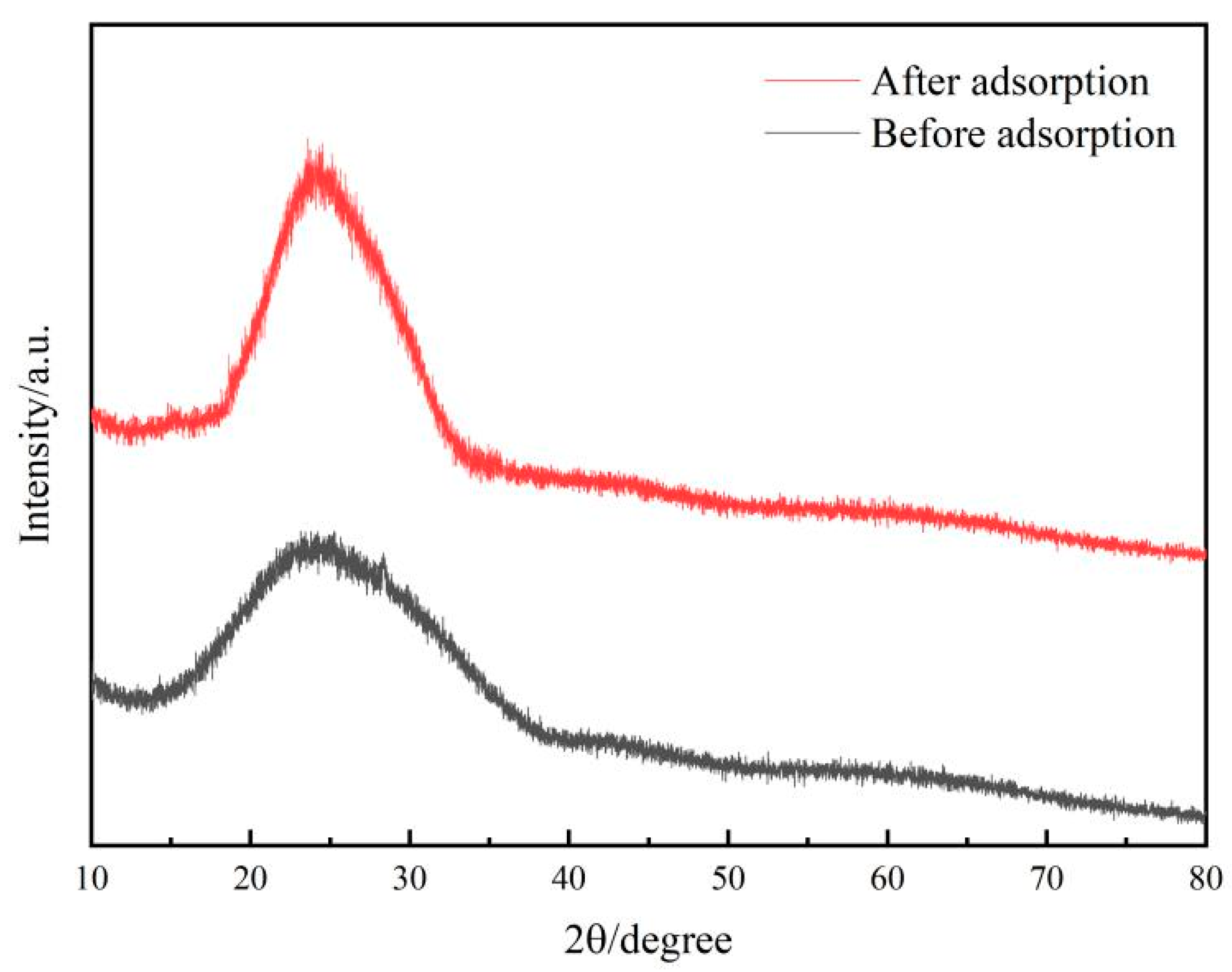
2.1.2. XRD
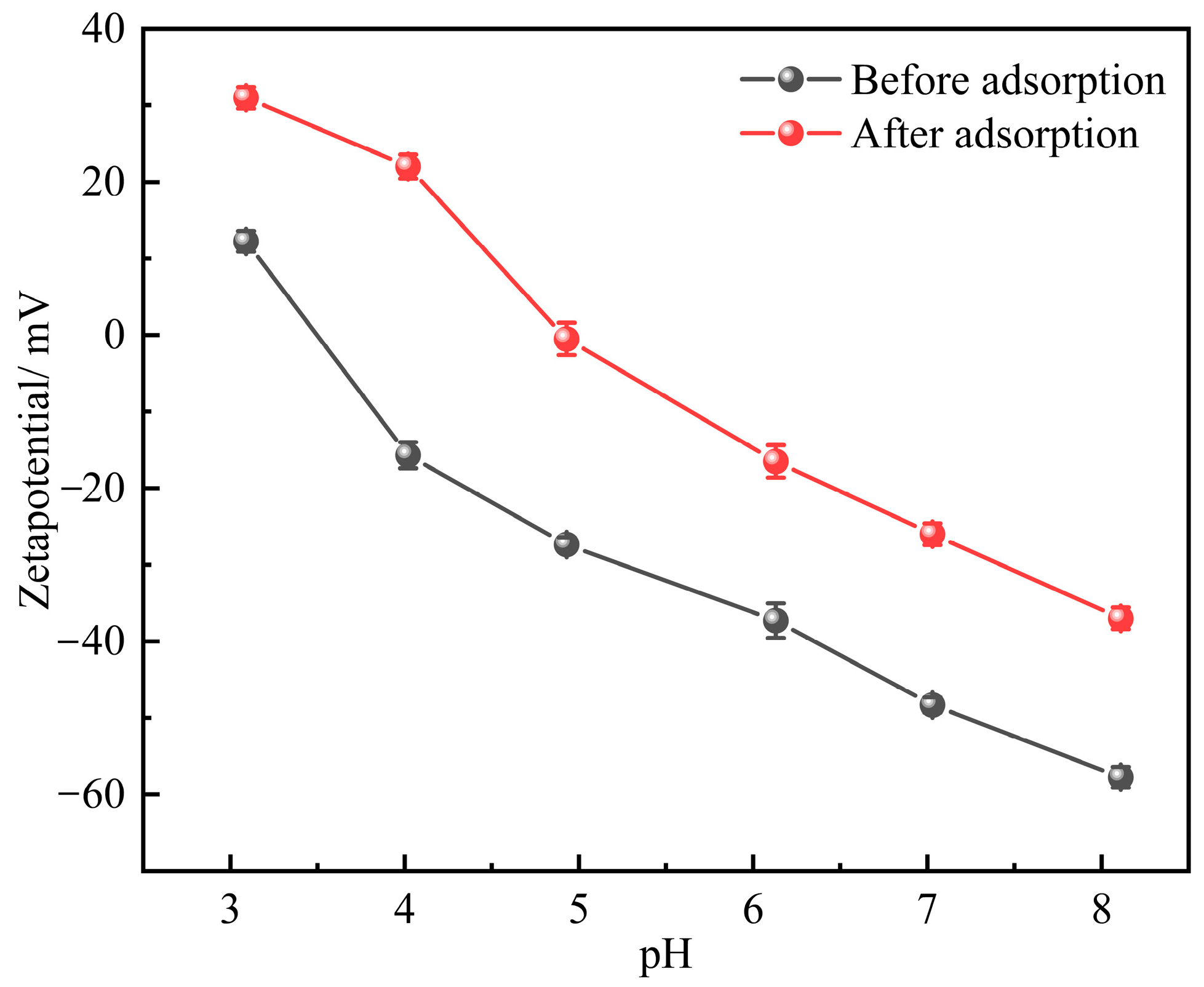
2.1.3. Zeta Potential
2.2. Conclusion of the Experiments
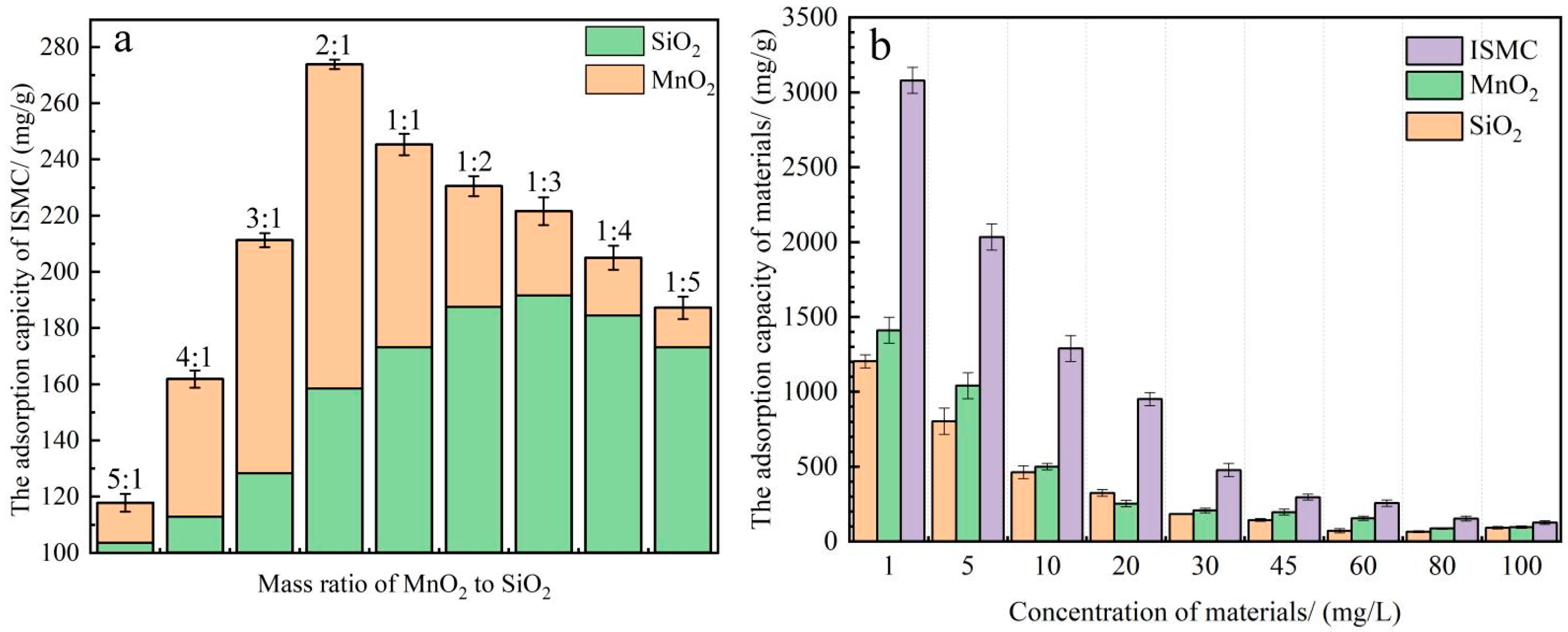
2.2.1. Effect of the MnO2-to-SiO2 Mass Ratio in Pristine Materials and Comparison of the Adsorption Capacities of the Three Materials
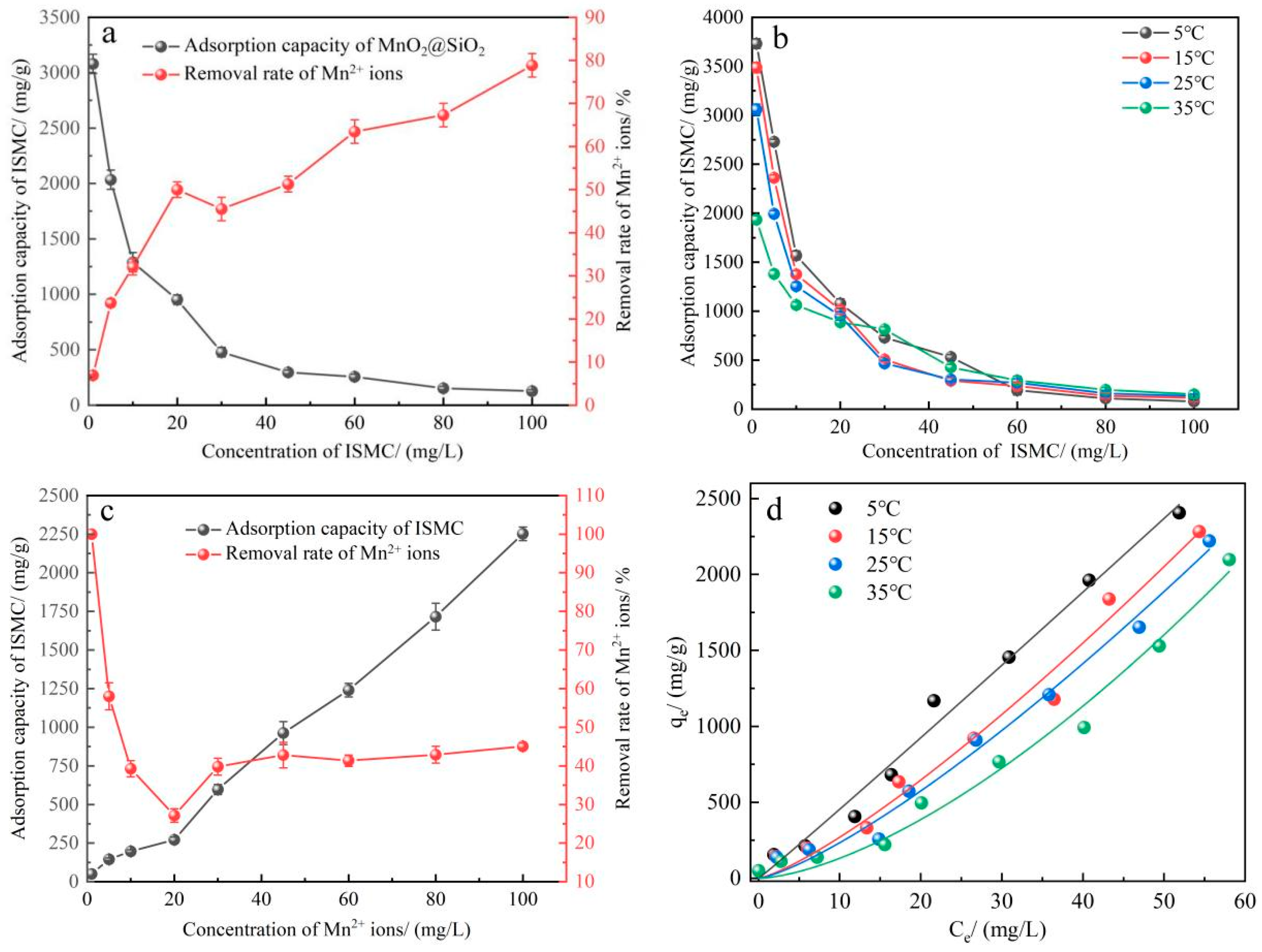
2.2.2. Effect of Adsorbent Dosage, Concentration of Mn2+ Ions, and Temperature
2.2.3. Adsorption Isotherms and Adsorption Thermodynamics
2.2.4. Adsorption Kinetic Study
2.2.5. Effect of pH
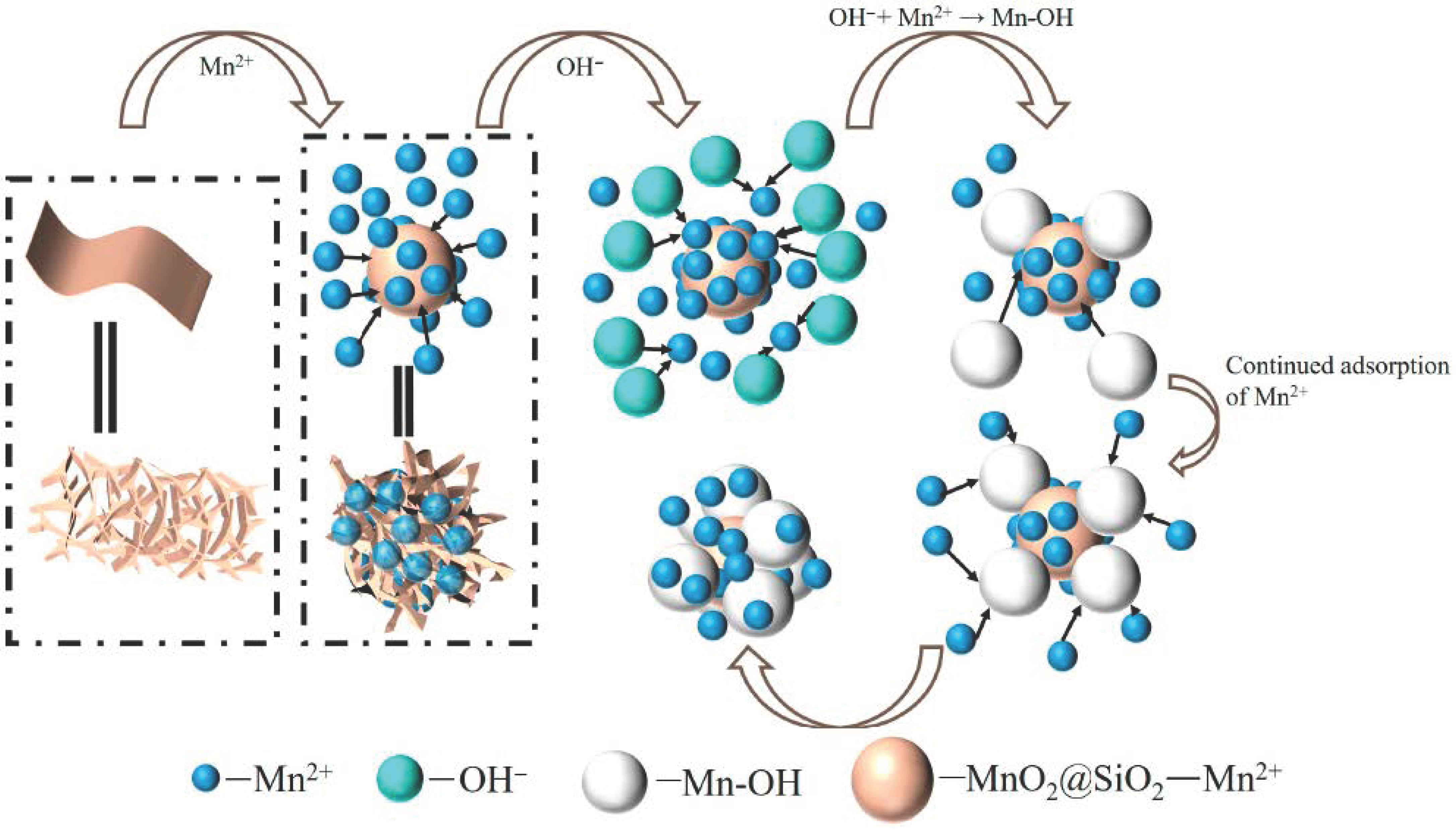
2.3. Adsorption Mechanism
2.3.1. XPS
2.3.2. FTIR
2.3.3. Adsorption Process
3. Experimental Section
3.1. Experimental Reagents
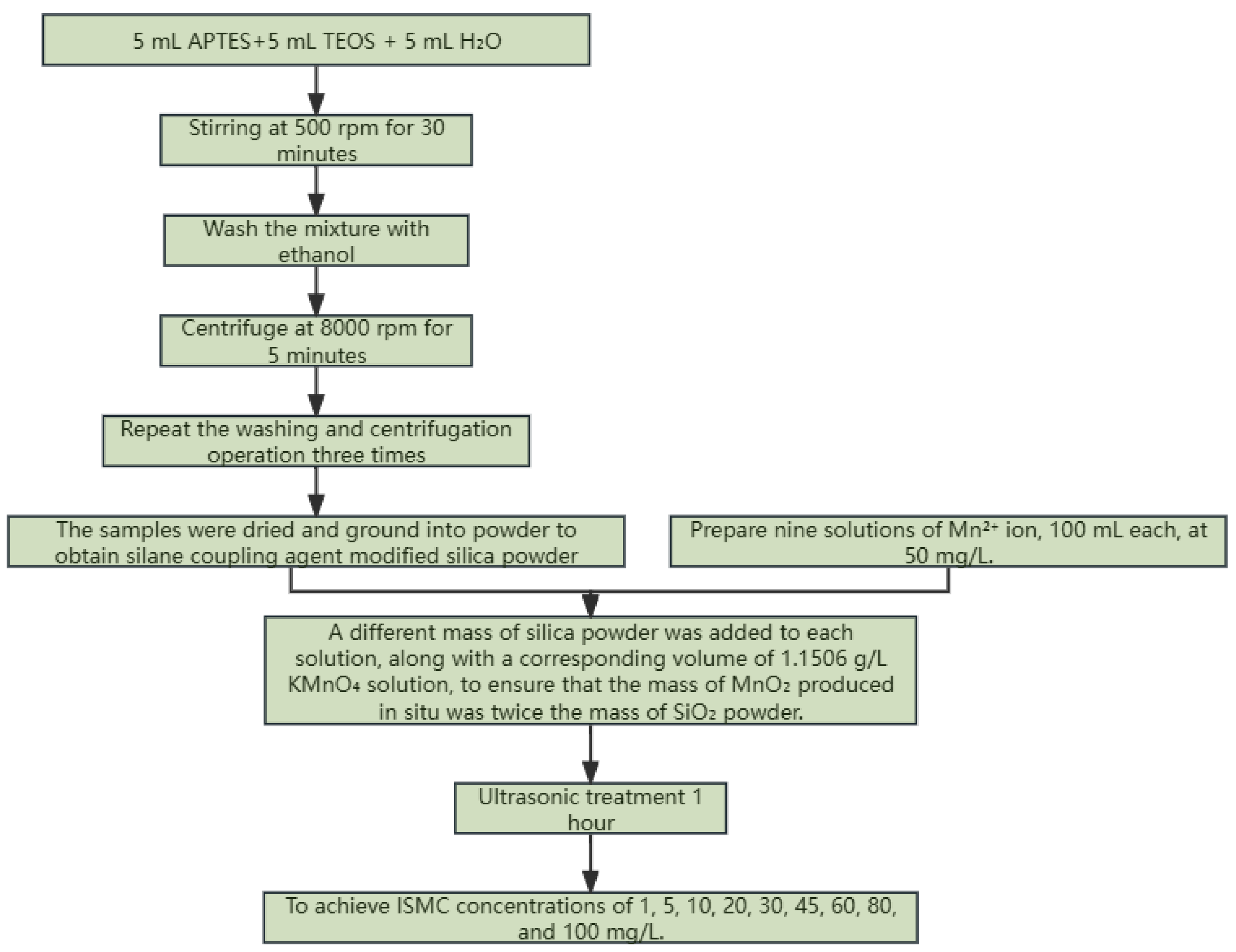
3.2. Preparation of ISMCs
3.3. Characterization
3.4. Determination Method of Mn2+ Ions
3.5. Batch Adsorption Experiments
3.5.1. Calculation of the Adsorption Capacity
3.5.2. Effect of the MnO2-to-SiO2 Mass Ratio in Raw Materials
3.5.3. Comparison of the Adsorption Capacities of the Three Materials
3.5.4. Effect of Adsorbent Dosage
3.5.5. Adsorption Isotherms and Thermodynamics
3.5.6. Effect of the Adsorption Temperature
3.5.7. Adsorption Kinetics Study
3.5.8. Effect of pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Zhang, T.Y.; Yu, X.W.; Mkandawire, V.; Ma, J.D.; Li, X.L. Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles. Materials 2022, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Zheng, J.; Zhang, Z.Q.; Long, Q.; Zhang, Q.Y. Application of annealed red mud to Mn2+ ion adsorption from aqueous solution. Water Sci. Technol. 2016, 73, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Wu, Y.; Ji, Y.; Chen, M.; Wu, H.; Gao, Y.; Wei, L.; Zhao, L.; Huo, T.; Liu, R. A new electrochemical method for simultaneous removal of Mn2+ and NH4+-N in wastewater with Cu plate as cathode. Ecotoxicol. Environ. Safe 2020, 206, 10. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kamarudin, S.K.; Kofli, N.T.; Anuar, N. Simultaneous NH4+-N and Mn2+ removal from drinking water using a biological aerated filter system: Effects of different aeration rates. Sep. Purif. Technol. 2013, 118, 547–556. [Google Scholar] [CrossRef]
- Cheng, Q.F.; Liu, Z.Y.; Huang, Y.; Li, F.J.; Nengzi, L.C.; Zhang, J. Influence of temperature on CODMn and Mn2+ removal and microbial community structure in pilot-scale biofilter. Bioresour. Technol. 2020, 316, 8. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, G.T.; Çelik, S.; Durak, S.G.; Acarer, S.; Çetin, E.; Demir, S.A.; Tüfekci, N. Effects of Fe(OH)3 and MnO2 Flocs on Iron/Manganese Removal and Fouling in Aerated Submerged Membrane Systems. Polymers 2021, 13, 22. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kamarudin, S.K.; Kofli, N.T. Response surface methodology for optimization of simultaneous COD, NH4+-N and Mn2+ removal from drinking water by biological aerated filter. Desalination 2011, 275, 50–61. [Google Scholar] [CrossRef]
- Attia, N.F.; Diab, M.A.; Attia, A.S.; El-Shahat, M.F. Greener approach for fabrication of antibacterial graphene-polypyrrole nanoparticle adsorbent for removal of Mn2+ from aqueous solution. Synth. Met. 2021, 282, 8. [Google Scholar] [CrossRef]
- Shafaei, A.; Rezayee, M.; Arami, M.; Nikazar, M. Removal of Mn2+ ions from synthetic wastewater by electrocoagulation process. Desalination 2010, 260, 23–28. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Yang, H.; Yan, Z.; Du, X.; Bai, L.; Yu, H.; Ding, A.; Li, G.; Liang, H.; Aminabhavi, T.M. Removal of manganese from groundwater in the ripened sand filtration: Biological oxidation versus chemical auto-catalytic oxidation. Chem. Eng. J. 2020, 382, 9. [Google Scholar] [CrossRef]
- Pakarinen, J.; Paatero, E. Recovery of manganese from iron containing sulfate solutions by precipitation. Miner. Eng. 2011, 24, 1421–1429. [Google Scholar] [CrossRef]
- Jez-Walkowiak, J.; Dymaczewski, Z.; Weber, L. Iron and manganese removal from groundwater by filtration through a chalcedonite bed. J. Water Supply Res. Technol. Aqua 2015, 64, 19–34. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin. Hydrometallurgy 2021, 206, 10. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Hybrid liquid membrane—Electrodialysis process for extraction of manganese(II). Desalination 2011, 274, 220–225. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 18. [Google Scholar] [CrossRef]
- Gao, J.X.; Li, X.C.; Shi, Y.Y.; Jia, S.Y.; Ye, X.W.; Long, Y.Z. The adsorption model of the adsorption process of CH4 on coal and its thermodynamic characteristics. Colloid Surf. A Physicochem. Eng. Asp. 2022, 632, 14. [Google Scholar] [CrossRef]
- Vievard, J.; Alem, A.; Pantet, A.; Ahfir, N.-D.; Arellano-Sánchez, M.G.; Devouge-Boyer, C.; Mignot, M. Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review. Toxics 2023, 11, 33. [Google Scholar] [CrossRef]
- Tran, B.; Watts, S.; Valentin, J.D.P.; Raßmann, N.; Papastavrou, G.; Ramstedt, M.; Salentinig, S. pH-Responsive Virus-Based Colloidal Crystals for Advanced Material Platforms. Adv. Funct. Mater. 2024, 34, 12. [Google Scholar] [CrossRef]
- He, H.; Zhong, M.; Konkolewicz, D.; Yacatto, K.; Rappold, T.; Sugar, G.; David, N.E.; Gelb, J.; Kotwal, N.; Merkle, A.; et al. Three-Dimensionally Ordered Macroporous Polymeric Materials by Colloidal Crystal Templating for Reversible CO2 Capture. Adv. Funct. Mater. 2013, 23, 4720–4728. [Google Scholar] [CrossRef]
- Xu, X.; He, J.; Li, Y.; Fu, G.; Cao, Q.; Zhang, D.; Tan, Y.H.; Gao, M.; Li, W.; Li, C.; et al. Integration of surface modified aqueous ink for multi-functional material extrusion. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131137. [Google Scholar] [CrossRef]
- Losch, P.; Huang, W.; Goodman, E.D.; Wrasman, C.J.; Holm, A.; Riscoe, A.R.; Schwalbe, J.A.; Cargnello, M. Colloidal nanocrystals for heterogeneous catalysis. Nano Today 2019, 24, 15–47. [Google Scholar] [CrossRef]
- Maity, P.; Ghosh, H.N. Strategies for extending charge separation in colloidal nanostructured quantum dot materials. Phys. Chem. Chem. Phys. 2019, 21, 23283–23300. [Google Scholar] [CrossRef] [PubMed]
- Rosas, C.A.C.; Franzreb, M.; Valenzuela, F.; Höll, W.H. Magnetic manganese dioxide as an amphoteric adsorbent for removal of harmful inorganic contaminants from water. React. Funct. Polym. 2010, 70, 516–520. [Google Scholar] [CrossRef]
- Lisha, K.P.; Maliyekkal, S.M.; Pradeep, T. Manganese dioxide nanowhiskers: A potential adsorbent for the removal of Hg(II) from water. Chem. Eng. J. 2010, 160, 432–439. [Google Scholar] [CrossRef]
- Cheng, M.M.; Yao, C.X.; Su, Y.; Liu, J.L.; Xu, L.J.; Hou, S.F. Synthesis of membrane-type graphene oxide immobilized manganese dioxide adsorbent and its adsorption behavior for lithium ion. Chemosphere 2021, 279, 9. [Google Scholar] [CrossRef]
- Kim, C.; Zhang, Z.F.; Wang, L.S.; Sun, T.; Hu, X.M. Core-shell magnetic manganese dioxide nanocomposites modified with citric acid for enhanced adsorption of basic dyes. J. Taiwan Inst. Chem. Eng. 2016, 67, 418–425. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, G.; Huang, H.; Qin, Y.; Fu, M.; Tu, X.; Ye, D.; Wu, J. Insights into the Role of Nanorod-Shaped MnO2 and CeO2 in a Plasma Catalysis System for Methanol Oxidation. Nanomaterials 2023, 13, 17. [Google Scholar] [CrossRef]
- Shi, C.; Fan, Y.; Zhang, Z.; Deng, X.; Yu, J.; Zhou, H.; Meng, F.; Feng, J. Development of core-shell SiO2@A-TiO2 abrasives and novel photocatalytic chemical machinal polishing for atomic surface of fused silica. Appl. Surf. Sci. 2024, 652, 9. [Google Scholar] [CrossRef]
- Yang, H.M.; Deng, Y.L.; Shu, J.C.; Chen, M.J.; Yang, Y.; Deng, Z.Y. Effect of KMnO4 on the migration and transformation of Mn2+ and NH4+-N in electrolytic manganese residue: Autocatalytic system of manganese. Chem. Eng. Sci. 2023, 282, 12. [Google Scholar] [CrossRef]
- STaffarel, R.; Rubio, J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner. Eng. 2010, 23, 1131–1138. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Tan, X.; Xu, X.; Li, P.; Tian, D.; Jiang, Y.; Xie, J.; Xiao, H.; Huang, X.; et al. Synthesis of cellulose II-based spherical nanoparticle microcluster adsorbent for removal of toxic hexavalent chromium. Int. J. Biol. Macromol. 2022, 221, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Gopi, T.; Swetha, G.; Shekar, S.C.; Ramakrishna, C.; Saini, B.; Krishna, R.; Rao, P.V.L. Catalytic decomposition of ozone on nanostructured potassium and proton containing δ-MnO2 catalysts. Catal. Commun. 2017, 92, 51–55. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Pan, Y.; Wang, F.; Sun, Y.; Wang, S.; Wang, Z.; Wu, A.; Zhang, Y. Efficient removal of heavy metal ions from wastewater and fixation of heavy metals in soil by manganese dioxide nanosorbents with tailored hollow mesoporous structure. Chem. Eng. J. 2023, 459, 11. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhang, X.F.; He, X.; Zhang, W.; Zhang, X.X.; Lu, C.H. In situ synthesis of MnO2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohydr. Polym. 2014, 110, 302–308. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, B.; Xian, W.; Liu, M.; Wu, J.; Liu, S.; Qin, S.; Wang, Y.; Liu, Y. Unveiling the synergistic interaction: Investigating the enhanced mechanism of 4H-SiC chemical mechanical polishing with the addition of sodium silicate and manganese dioxide. Mater. Sci. Semicond. Process. 2024, 184, 10. [Google Scholar] [CrossRef]
- Lu, X.J.; Liu, Z.; Wang, W.T.; Wang, X.; Ma, H.C.; Cao, M.W. Synthesis and Evaluation of Peptide-Manganese Dioxide Nanocomposites as Adsorbents for the Removal of Strontium Ions. Nanomaterials 2024, 14, 14. [Google Scholar] [CrossRef]
- Michna, A. Macroion adsorption-electrokinetic and optical methods. Adv. Colloid Interface Sci. 2017, 250, 95–131. [Google Scholar] [CrossRef]
- Abuhaikal, M.; Loannidou, K.; Petersen, T.; Pellenq, R.J.M.; Ulm, F.J. Le Chatelier’s conjecture: Measurement of colloidal eigenstresses in chemically reactive materials. J. Mech. Phys. Solids 2018, 112, 334–344. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Jiao, S.; Chen, R.; Zhou, Q.; Shung, K.K.; Wang, L.V.; Zhang, H.F. Saturation effect in functional photoacoustic imaging. J. Biomed. Opt. 2010, 15, 5. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 23. [Google Scholar] [CrossRef]
- Cosic, M.; Hadzijojic, M.; Rymzhanov, R.; Petrovic, S.; Bellucci, S. Investigation of the graphene thermal motion by rainbow scattering. Carbon 2019, 145, 161–174. [Google Scholar] [CrossRef]
- Dilokekunakul, W.; Klomkliang, N.; Phadungbut, P.; Chaemchuen, S.; Supasitmongkol, S. Effects of functional group concentration, type, and configuration on their saturation of methanol adsorption on functionalized graphite. Appl. Surf. Sci. 2020, 501, 10. [Google Scholar] [CrossRef]
- Cheng, H.J.; Yang, T.; Jiang, J.; Lu, X.H.; Wang, P.X.; Ma, J. Mn2+ effect on manganese oxides (MnOx) nanoparticles aggregation in solution: Chemical adsorption and cation bridging. Environ. Pollut. 2020, 267, 9. [Google Scholar] [CrossRef]
- Goncalves, W.D.; Iost, R.M.; Crespilho, F.N. Diffusion Mechanisms in Nanoelectrodes: Evaluating the Edge Effect. Electrochim. Acta 2014, 123, 66–71. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P.; Akram, M.; Tiberg, C. Predicting sulphate adsorption/desorption in forest soils: Evaluation of an extended Freundlich equation. Chemosphere 2015, 119, 83–89. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.N.; Tsygankova, L.E. Analytical Continuation within the Freundlich Adsorption Model. Comment on Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. Processes 2021, 9, 1251. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 12. [Google Scholar] [CrossRef]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
- Zou, C.L.; Xu, Z.W.; Nie, F.H.; Guan, K.; Li, J.C. Application of hydroxyapatite-modified carbonized rice husk for the adsorption of Cr(VI) from aqueous solution. J. Mol. Liq. 2023, 371, 11. [Google Scholar] [CrossRef]
- Dzoujo, H.T.; Shikuku, V.O.; Tome, S.; Akiri, S.; Kengne, N.M.; Abdpour, S.; Janiak, C.; Etoh, M.A.; Dina, D. Synthesis of pozzolan and sugarcane bagasse derived geopolymer-biochar composites for methylene blue sequestration from aqueous medium. J Environ. Manag. 2022, 318, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Wang, J.W.; Gou, B.B.; Fu, D.J.; Wang, H.F.; Zhao, P.Y. Relationship between Surface Hydroxyl Complexation and Equi-Acidity Point pH of MnO2 and Its Adsorption for Co2+ and Ni2+. ACS Omega 2022, 7, 9602–9613. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.B.; Wang, Z.B.; Wu, A.G.; Zhang, Y.J. Synthesis of hollow mesoporous manganese dioxide nanoadsorbents with strong negative charge and their ultra-efficient adsorption for cationic dyes. Sep. Purif. Technol. 2022, 295, 10. [Google Scholar] [CrossRef]
- Saha, S.; Pal, A. Microporous assembly of MnO2 nanosheets for malachite green degradation. Sep. Purif. Technol. 2014, 134, 26–36. [Google Scholar] [CrossRef]
- Sim, H.; Jo, C.; Yu, T.; Lim, E.; Yoon, S.; Lee, J.H.; Yoo, J.; Lee, J.; Lim, B. Reverse Micelle Synthesis of Colloidal Nickel-Manganese Layered Double Hydroxide Nanosheets and Their Pseudocapacitive Properties. Chem. Eur. J. 2014, 20, 14880–14884. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ruan, C.; Shi, Y.; Chen, G.; Ma, Y.; Dai, H.; Chen, X.; Yang, X. Insight to hydrophobic SiO2 encapsulated SiO2 gel: Preparation and application in fire extinguishing. J. Hazard. Mater. 2021, 405, 12. [Google Scholar] [CrossRef] [PubMed]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl. Sci. 2021, 11, 7. [Google Scholar] [CrossRef]
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloid Surf. B Biointerfaces 2020, 191, 10. [Google Scholar] [CrossRef]
- Yao, C.C.; Chen, T.J. An improved regression method for kinetics of adsorption from aqueous solutions. J. Water Process. Eng. 2019, 31, 8. [Google Scholar] [CrossRef]
- Yao, C.C.; Zhu, C.X. A new multi-mechanism adsorption kinetic model and its relation to mass transfer coefficients. Surf. Interfaces 2021, 26, 6. [Google Scholar] [CrossRef]


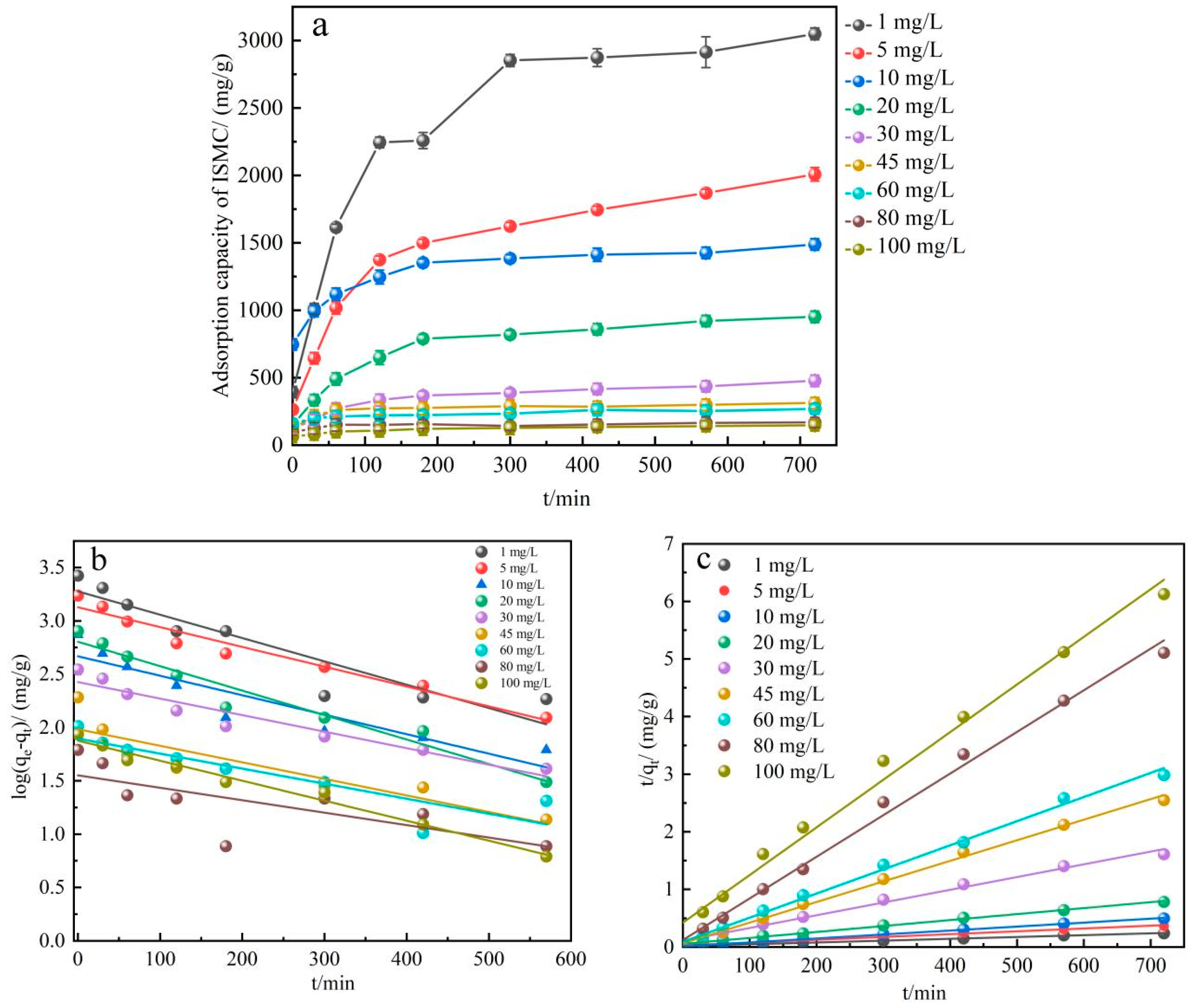
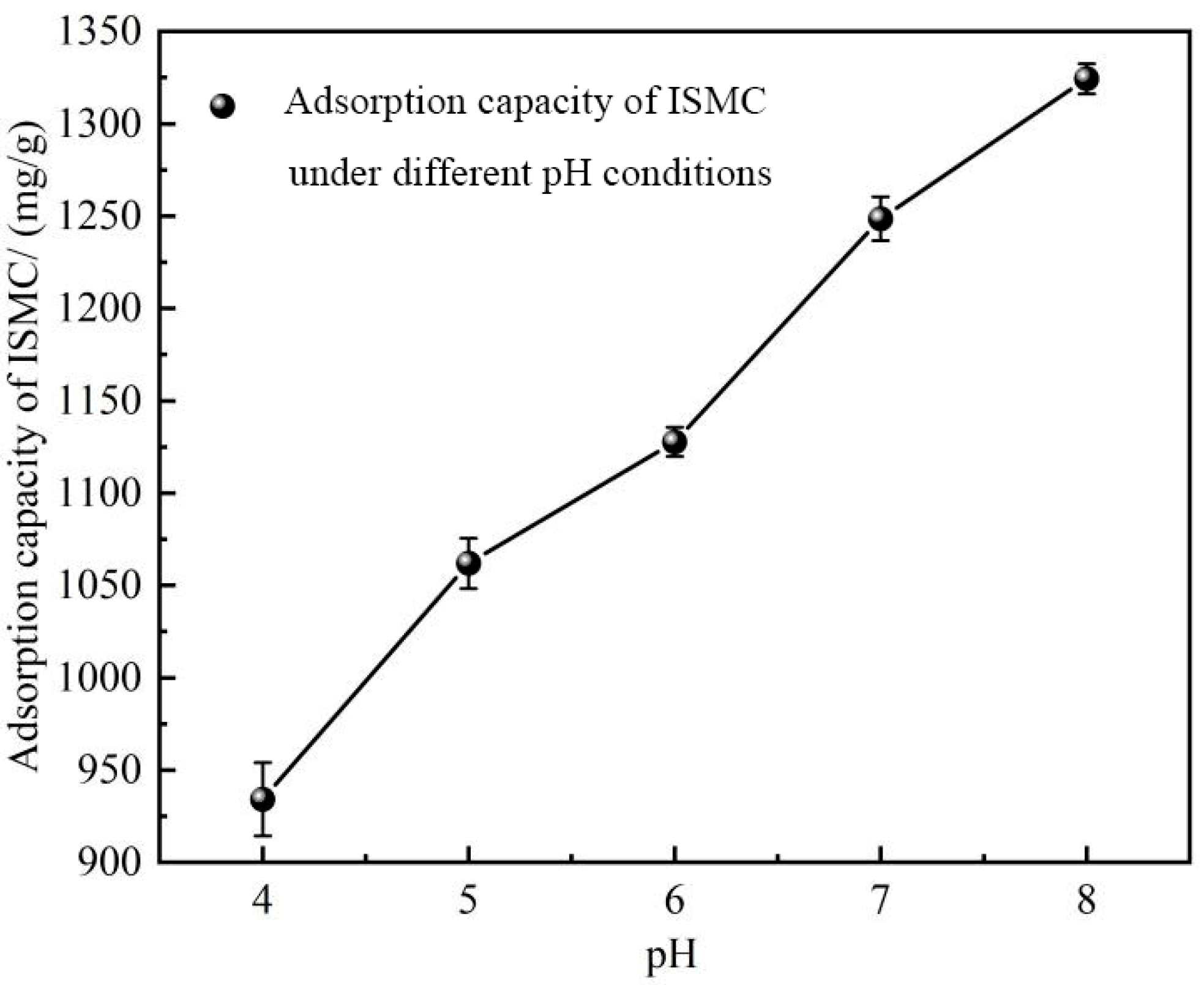
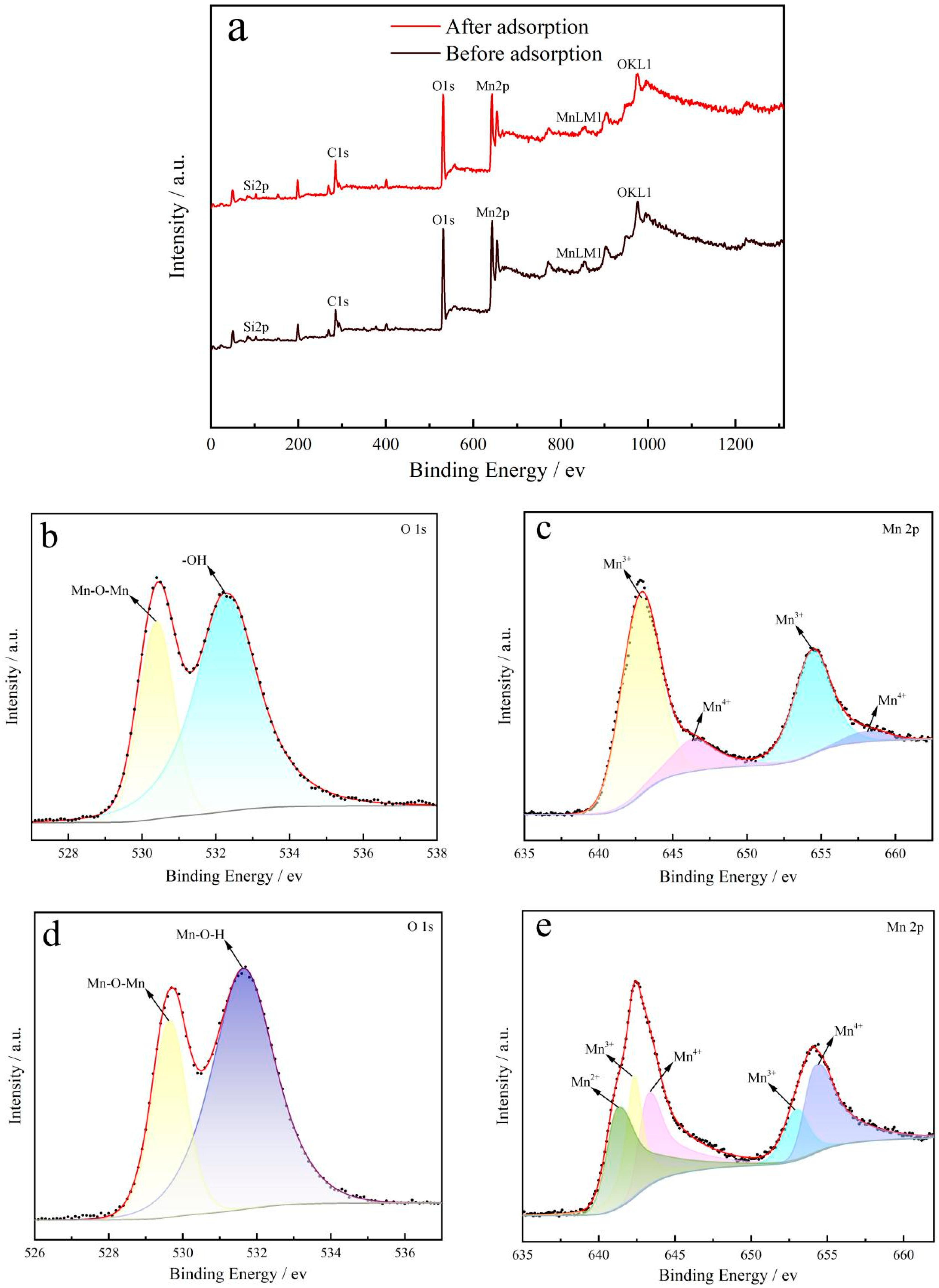
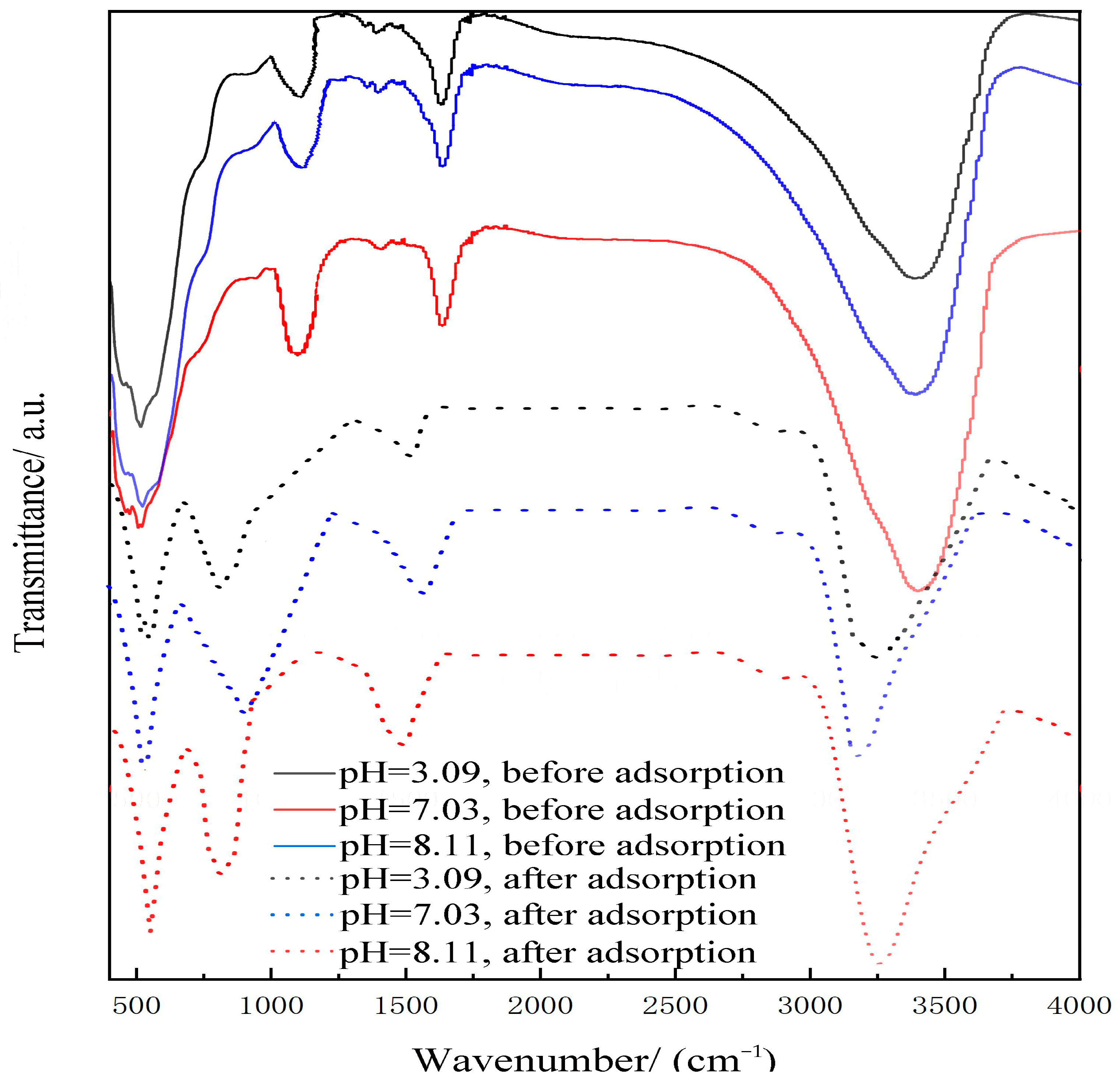
| Adsorption Temperature/°C | k | n | R2 |
|---|---|---|---|
| 5 | 42.9111 | 0.9752 | 0.9887 |
| 15 | 14.9024 | 0.7942 | 0.9829 |
| 25 | 11.8464 | 0.7713 | 0.9886 |
| 35 | 3.7118 | 0.6446 | 0.9852 |
| Concentration of ISMC/(mg/L) | Pseudo-First-Order Dynamic Model | Pseudo-Second-Order Dynamic Model | ||||
|---|---|---|---|---|---|---|
| K1 | qe/(mg/L) | R2 | K2 | qe/(mg/L) | R2 | |
| 1 | 0.0050 | 1891.39 | 0.8676 | 0.000007 | 3147.44 | 0.9920 |
| 5 | 0.0043 | 1342.61 | 0.9599 | 0.000009 | 2044.05 | 0.9892 |
| 10 | 0.0042 | 465.93 | 0.8564 | 0.000041 | 1458.57 | 0.9985 |
| 20 | 0.0053 | 637.81 | 0.9547 | 0.000020 | 961.54 | 0.9921 |
| 30 | 0.0036 | 266.91 | 0.9293 | 0.000045 | 452.49 | 0.9893 |
| 45 | 0.0036 | 96.42 | 0.8029 | 0.000192 | 280.11 | 0.9966 |
| 60 | 0.0033 | 78.74 | 0.7943 | 0.000195 | 238.66 | 0.9948 |
| 80 | 0.0027 | 35.62 | 0.5378 | 0.004342 | 138.31 | 0.9943 |
| 100 | 0.0043 | 75.23 | 0.9827 | 0.001619 | 120.92 | 0.9880 |
| Parameters | T (K) | ∆G (kJ/mol) | ∆S (kJ/(mol·K)) | ∆H (kJ/mol) |
|---|---|---|---|---|
| ISMC | 278.15 | −8.61 | −0.16 | −53.88 |
| 288.15 | −6.98 | |||
| 298.15 | −5.35 | |||
| 308.15 | −3.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Tang, Y.; Zhang, X.; Huang, X.; Zhang, B. Study of the Reaction Mechanism of the Excessive Adsorption of Mn2+ from Water by In Situ Synthesis of MnO2@SiO2 Colloid as an Adsorbent. Int. J. Mol. Sci. 2025, 26, 2928. https://doi.org/10.3390/ijms26072928
Wang K, Tang Y, Zhang X, Huang X, Zhang B. Study of the Reaction Mechanism of the Excessive Adsorption of Mn2+ from Water by In Situ Synthesis of MnO2@SiO2 Colloid as an Adsorbent. International Journal of Molecular Sciences. 2025; 26(7):2928. https://doi.org/10.3390/ijms26072928
Chicago/Turabian StyleWang, Kun, Yuchao Tang, Xinyu Zhang, Xianhuai Huang, and Beiping Zhang. 2025. "Study of the Reaction Mechanism of the Excessive Adsorption of Mn2+ from Water by In Situ Synthesis of MnO2@SiO2 Colloid as an Adsorbent" International Journal of Molecular Sciences 26, no. 7: 2928. https://doi.org/10.3390/ijms26072928
APA StyleWang, K., Tang, Y., Zhang, X., Huang, X., & Zhang, B. (2025). Study of the Reaction Mechanism of the Excessive Adsorption of Mn2+ from Water by In Situ Synthesis of MnO2@SiO2 Colloid as an Adsorbent. International Journal of Molecular Sciences, 26(7), 2928. https://doi.org/10.3390/ijms26072928






