Modulation of the ETV6::RUNX1 Gene Fusion Prevalence in Newborns by Corticosteroid Use During Pregnancy
Abstract
1. Introduction
2. Results
2.1. High Adherence to Mediterranean Diet and Stress Reduction Intervention Is Associated with a Trend Toward Lower Prevalence of the ETV6::RUNX1 Fusion
2.2. Univariate Analysis Identifies Corticosteroid Application During Pregnancy as Significantly Associated with the Presence of ETV6::RUNX1 Fusions
2.3. Multivariate Regression Models Report a Higher Probability of ETV6::RUNX1 Occurrence for Corticosteroid Administration Before 26 Weeks of Gestation and Use of Betamethasone
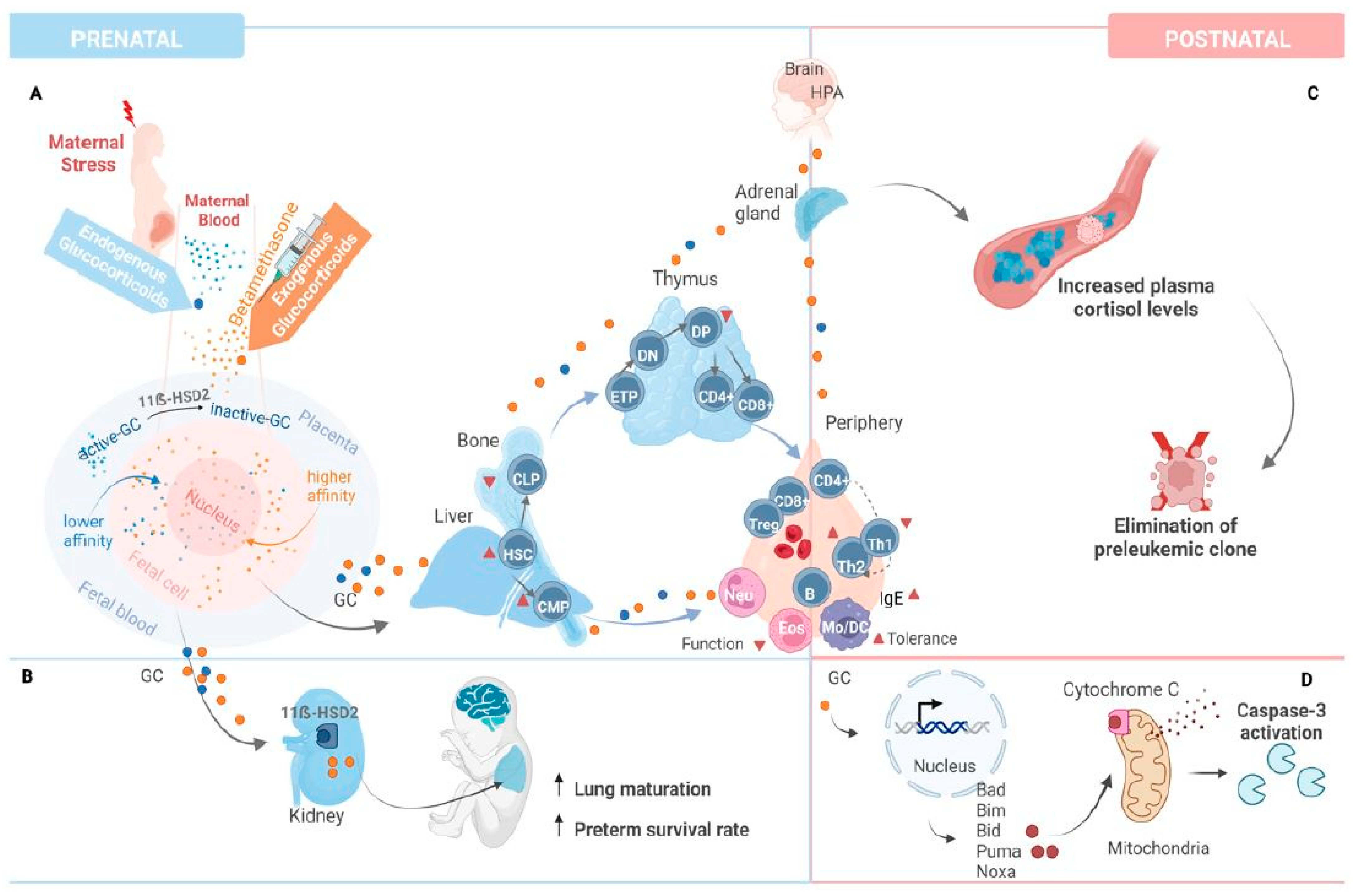
3. Discussion
4. Material and Methods
4.1. Study Design and Participant Selection
4.2. Interventions
4.3. Cord Blood Sample Collection
4.4. Genomic Inverse PCR for Exploration of Ligated Breakpoints (GIPFEL)
4.5. Predictive Variables
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute Lymphoblastic Leukemia |
| B-ALL | B-cell Acute Lymphoblastic Leukemia |
| CB | Cord Blood |
| CBGs | Cord Blood Genomes |
| CMP | Common Myeloid Progenitor |
| CRH | Corticotropin-Releasing Hormone |
| DNA | Deoxyribonucleic Acid |
| DP | Double-Positive (thymocytes) |
| ETV6::RUNX1 | Gene fusion associated with preleukemic clones |
| GCs | Glucocorticoids |
| GIPFEL | Genomic Inverse PCR for Exploration of Ligated Breakpoints |
| GR | Glucocorticoid Receptor |
| HPA | Hypothalamic–pituitary–adrenal (axis) |
| HSC | Hematopoietic Stem Cells |
| IMPACT-BCN | Improving Mothers for a Better Prenatal Care Trial Barcelona |
| MLL | Mixed-Lineage Leukemia (gene) |
| Mo | Monocyte |
| Neu | Neutrophils |
| NR3C1 | Nuclear Receptor Subfamily 3 Group C Member 1 (Glucocorticoid Receptor Gene) |
| OR | Odds Ratio |
| PBX1 | Pre-B-Cell Leukemia Homeobox 1 |
| PCR | Polymerase Chain Reaction |
| PREDIMED | Prevención con Dieta Mediterránea (Prevention with Mediterranean Diet) |
| RCT | Randomized Controlled Trial |
| RNA | Ribonucleic Acid |
| SGA | Small for Gestational Age |
| SP | Single-positive (thymocytes) |
| TCF3 | Transcription Factor 3 |
| Th | T-helper Cell |
| Topo II | DNA Topoisomerase II |
| t-AML | Therapy-Related Acute Myeloid Leukemia |
References
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [PubMed]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International Incidence of Childhood Cancer, 2001–10: A Population-Based Registry Study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [PubMed]
- Whitehead, T.P.; Metayer, C.; Wiemels, J.L.; Singer, A.W.; Miller, M.D. Childhood Leukemia and Primary Prevention. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 317–352. [Google Scholar] [PubMed]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can we prevent childhood Leukaemia? Leukemia 2021, 35, 1258–1264. [Google Scholar] [PubMed]
- Hauer, J.; Fischer, U.; Borkhardt, A. Towards prevention of childhood ALL by early-life immune training. Blood 2021, 138, 1412–1428. [Google Scholar]
- Knudson, A.G. The genetics of childhood cancer. Bull. Cancer 1988, 75, 135–138. [Google Scholar]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar]
- Bueno, C.; Tejedor, J.R.; Bashford-Rogers, R.; Gonzalez-Silva, L.; Valdes-Mas, R.; Agraz-Doblas, A.; Diaz de la Guardia, R.; Ribera, J.; Zamora, L.; Bilhou-Nabera, C.; et al. Natural history and cell of origin of TC F3-ZN F384 and PTPN11 mutations in monozygotic twins with concordant BCP-ALL. Blood 2019, 134, 900–905. [Google Scholar]
- Wiemels, J.L.; Cazzaniga, G.; Daniotti, M.; Eden, O.B.; Addison, G.M.; Masera, G.; Saha, V.; Biondi, A.; Greaves, M.F. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet 1999, 354, 1499–1503. [Google Scholar]
- Taub, J.W.; Konrad, M.A.; Ge, Y.; Naber, J.M.; Scott, J.S.; Matherly, L.H.; Ravindranath, Y. High frequency of leukemic clones in newborn screening blood samples of children with B-precursor acute lymphoblastic leukemia. Blood 2002, 99, 2992–2996. [Google Scholar] [PubMed]
- Eguchi-Ishimae, M.; Eguchi, M.; Ishii, E.; Miyazaki, S.; Ueda, K.; Kamada, N.; Mizutani, S. Breakage and fusion of the TEL (ETV6) gene in immature B lymphocytes induced by apoptogenic signals. Blood 2001, 97, 737–743. [Google Scholar]
- Mori, H.; Colman, S.M.; Xiao, Z.; Ford, A.M.; Healy, L.E.; Donaldson, C.; Hows, J.M.; Navarrete, C.; Greaves, M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc. Natl. Acad. Sci. USA 2002, 99, 8242–8247. [Google Scholar] [PubMed]
- Hein, D.; Borkhardt, A.; Fischer, U. Insights into the prenatal origin of childhood acute lymphoblastic leukemia. Cancer Metastasis Rev. 2020, 39, 161–171. [Google Scholar]
- Wiemels, J.L.; Ford, A.M.; Van Wering, E.R.; Postma, A.; Greaves, M. Protracted and variable latency of acute lymphoblastic leukemia after TEL-AML1 gene fusion in utero. Blood 1999, 94, 1057–1062. [Google Scholar] [PubMed]
- Papaemmanuil, E.; Rapado, I.; Li, Y.; Potter, N.E.; Wedge, D.C.; Tubio, J.; Alexandrov, L.B.; Van Loo, P.; Cooke, S.L.; Marshall, J.; et al. RAG-mediated recombination is the predominant driver of oncogenic rearrangement in ETV6-RUNX1 acute lymphoblastic leukemia. Nat. Genet. 2014, 46, 116–125. [Google Scholar] [PubMed]
- Benitez, L.; Castro-Barquero, S.; Crispi, F.; Youssef, L.; Crovetto, F.; Fischer, U.; Kameri, E.; Bueno, C.; Camos, M.; Menendez, P.; et al. Maternal Lifestyle and Prenatal Risk Factors for Childhood Leukemia: A Review of the Existing Evidence. Fetal Diagn. Ther. 2024, 51, 395–410. [Google Scholar]
- Marcotte, E.L.; Spector, L.G.; Mendes-de-Almeida, D.P.; Nelson, H.H. The Prenatal Origin of Childhood Leukemia: Potential Applications for Epidemiology and Newborn Screening. Front. Pediatr. 2021, 9, 639479. [Google Scholar]
- Fueller, E.; Schaefer, D.; Fischer, U.; Krell, P.F.; Stanulla, M.; Borkhardt, A.; Slany, R.K. Genomic inverse PCR for exploration of ligated breakpoints (GIPFEL), a new method to detect translocations in leukemia. PLoS ONE 2014, 9, e104419. [Google Scholar]
- Schafer, D.; Olsen, M.; Lahnemann, D.; Stanulla, M.; Slany, R.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood 2018, 131, 821–826. [Google Scholar]
- Hein, D.; Dreisig, K.; Metzler, M.; Izraeli, S.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. The preleukemic TCF3-PBX1 gene fusion can be generated in utero and is present in approximately 0.6% of healthy newborns. Blood 2019, 134, 1355–1358. [Google Scholar] [PubMed]
- Crovetto, F.; Crispi, F.; Casas, R.; Martin-Asuero, A.; Borras, R.; Vieta, E.; Estruch, R.; Gratacos, E.; Investigators, I.B.T. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to At-Risk Pregnant Individuals: The IMPACT BCN Randomized Clinical Trial. JAMA 2021, 326, 2150–2160. [Google Scholar]
- Jensen, C.D.; Block, G.; Buffler, P.; Ma, X.; Selvin, S.; Month, S. Maternal Dietary Risk Factors in Childhood Acute Lymphoblastic Leukemia (United States). Cancer Causes Control 2004, 15, 559–570. [Google Scholar] [CrossRef]
- Thompson, J.R.; Gerald, P.F.; Willoughby, M.L.; Armstrong, B.K. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: A case-control study. Lancet 2001, 358, 1935–1940. [Google Scholar]
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control 2015, 26, 1003–1017. [Google Scholar]
- MacArthur, A.C.; McBride, M.L.; Spinelli, J.J.; Tamaro, S.; Gallagher, R.P.; Theriault, G. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: The cross-Canada childhood leukemia study. Cancer Causes Control 2008, 19, 283–295. [Google Scholar] [PubMed]
- Metayer, C.; Milne, E.; Dockerty, J.D.; Clavel, J.; Pombo-de-Oliveira, M.S.; Wesseling, C.; Spector, L.G.; Schuz, J.; Petridou, E.; Ezzat, S.; et al. Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: A Childhood Leukemia International Consortium study. Epidemiology 2014, 25, 811–822. [Google Scholar] [PubMed]
- Karalexi, M.A.; Dessypris, N.; Thomopoulos, T.P.; Ntouvelis, E.; Kantzanou, M.; Diamantaras, A.A.; Moschovi, M.; Baka, M.; Hatzipantelis, E.; Kourti, M.; et al. Parental alcohol consumption and risk of leukemia in the offspring: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2017, 26, 433–441. [Google Scholar]
- O’Neill, K.A.; Murphy, M.F.; Bunch, K.J.; Puumala, S.E.; Carozza, S.E.; Chow, E.J.; Mueller, B.A.; McLaughlin, C.C.; Reynolds, P.; Vincent, T.J.; et al. Infant birthweight and risk of childhood cancer: International population-based case control studies of 40,000 cases. Int. J. Epidemiol. 2015, 44, 153–168. [Google Scholar] [PubMed]
- Klimentopoulou, A.; Antonopoulos, C.N.; Papadopoulou, C.; Kanavidis, P.; Tourvas, A.D.; Polychronopoulou, S.; Baka, M.; Athanasiadou-Piperopoulou, F.; Kalmanti, M.; Sidi, V.; et al. Maternal smoking during pregnancy and risk for childhood leukemia: A nationwide case-control study in Greece and meta-analysis. Pediatr. Blood Cancer 2012, 58, 344–351. [Google Scholar]
- Milne, E.; Royle, J.A.; Bennett, L.C.; de Klerk, N.H.; Bailey, H.D.; Bower, C.; Miller, M.; Attia, J.; Scott, R.J.; Kirby, M.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: Results from an Australian case-control study. Cancer Causes Control 2011, 22, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Menegaux, F.; Ripert, M.; Hemon, D.; Clavel, J. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: A French population-based case-control study. Paediatr. Perinat. Epidemiol. 2007, 21, 293–299. [Google Scholar] [CrossRef]
- Petridou, E.; Trichopoulos, D.; Kalapothaki, V.; Pourtsidis, A.; Kogevinas, M.; Kalmanti, M.; Koliouskas, D.; Kosmidis, H.; Panagiotou, J.P.; Piperopoulou, F.; et al. The risk profile of childhood leukaemia in Greece: A nationwide case-control study. Br. J. Cancer 1997, 76, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- McKinney, P.A.; Juszczak, E.; Findlay, E.; Smith, K.; Thomson, C.S. Pre- and perinatal risk factors for childhood leukaemia and other malignancies: A Scottish case control study. Br. J. Cancer 1999, 80, 1844–1851. [Google Scholar] [CrossRef]
- Jurek, A.M.; Greenland, S.; Spector, L.G.; Roesler, M.A.; Robison, L.L.; Ross, J.A. Self-report versus medical record—Perinatal factors in a study of infant leukaemia: A study from the Children’s Oncology Group. Paediatr. Perinat. Epidemiol. 2011, 25, 540–548. [Google Scholar] [CrossRef]
- Liang, D.-C.; Shih, L.-Y.; Yang, C.-P.; Hung, I.-J.; Liu, H.-C.; Jaing, T.-H.; Yeh, T.-C.; Liang, S.-T.; Chang, C.-L.; Lee, E.-H.; et al. Frequencies of ETV6-RUNX1 Fusion and Hyperdiploidy in Pediatric Acute Lymphoblastic Leukemia Are Lower in Far East than West. Pediatr. Blood Cancer 2010, 55, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-I.; Briggs, F.; Shao, X.; Metayer, C.; Wiemels, J.L.; Chokkalingam, A.P.; Barcellos, L.F. Pathway Analysis of Genome-Wide Association Study in Childhood Leukemia among Hispanics. Cancer Epidemiol. Biomark. Prev. 2016, 25, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Gaynon, P.S.; Lustig, R.H. The use of glucocorticoids in acute lymphoblastic leukemia of childhood. Molecular, cellular, and clinical considerations. J. Pediatr. Hematol. Oncol. 1995, 17, 1–12. [Google Scholar] [CrossRef]
- Kuster, L.; Grausenburger, R.; Fuka, G.; Kaindl, U.; Krapf, G.; Inthal, A.; Mann, G.; Kauer, M.; Rainer, J.; Kofler, R.; et al. ETV6/RUNX1-positive relapses evolve from an ancestral clone and frequently acquire deletions of genes implicated in glucocorticoid signaling. Blood 2011, 117, 2658–2667. [Google Scholar] [CrossRef]
- Akter, S.; Shimba, A.; Ikuta, K.; Mahmud, M.R.A.; Yamada, S.; Sasanuma, H.; Tsuda, M.; Sone, M.; Ago, Y.; Murai, K.; et al. Physiological concentrations of glucocorticoids induce pathological DNA double-strand breaks. Genes Cells 2023, 28, 53–67. [Google Scholar] [CrossRef]
- Brassesco, M.S.; Camparoto, M.L.; Tone, L.G.; Sakamoto-Hojo, E.T. Analysis of ETV6/RUNX1 fusions for evaluating the late effects of cancer therapy in ALL (acute lymphoblastic leukemia) cured patients. Cytogenet. Genome Res. 2004, 104, 346–351. [Google Scholar] [PubMed]
- Greaves, M.F. Aetiology of acute leukaemia. Lancet 1997, 349, 344–349. [Google Scholar] [PubMed]
- Kim, B.; Sasaki, A.; Murphy, K.; Matthews, S.G. DNA methylation signatures in human neonatal blood following maternal antenatal corticosteroid treatment. Transl. Psychiatry 2022, 12, 132. [Google Scholar] [PubMed]
- Timms, J.A.; Relton, C.L.; Sharp, G.C.; Rankin, J.; Strathdee, G.; McKay, J.A. Exploring a potential mechanistic role of DNA methylation in the relationship between in utero and post-natal environmental exposures and risk of childhood acute lymphoblastic leukaemia. Int. J. Cancer 2019, 145, 2933–2943. [Google Scholar]
- Nordlund, J.; Backlin, C.L.; Zachariadis, V.; Cavelier, L.; Dahlberg, J.; Ofverholm, I.; Barbany, G.; Nordgren, A.; Overnas, E.; Abrahamsson, J.; et al. DNA methylation-based subtype prediction for pediatric acute lymphoblastic leukemia. Clin. Epigenetics 2015, 7, 11. [Google Scholar]
- Berger, M.F.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220. [Google Scholar] [CrossRef]
- Lin, P.C.; Giannopoulou, E.G.; Park, K.; Mosquera, J.M.; Sboner, A.; Tewari, A.K.; Garraway, L.A.; Beltran, H.; Rubin, M.A.; Elemento, O. Epigenomic alterations in localized and advanced prostate cancer. Neoplasia 2013, 15, 373–383. [Google Scholar]
- Seckl Prenatal Glucocorticoids and Long-Term Programming. Eur. J. Endocrinol. 2004, 151, U49–U62. [CrossRef]
- Schmiegelow, K.; Vestergaard, T.; Nielsen, S.M.; Hjalgrim, H. Etiology of common childhood acute lymphoblastic leukemia: The adrenal hypothesis. Leukemia 2008, 22, 2137–2141. [Google Scholar]
- Ross, J.A.; Potter, J.D.; Reaman, G.H.; Pendergrass, T.W.; Robison, L.L. Maternal exposure to potential inhibitors of DNA topoisomerase II and infant leukemia (United States): A report from the Children’s Cancer Group. Cancer Causes Control 1996, 7, 581–590. [Google Scholar]
- Spector, L.G.; Xie, Y.; Robison, L.L.; Heerema, N.A.; Hilden, J.M.; Lange, B.; Felix, C.A.; Davies, S.M.; Slavin, J.; Potter, J.D.; et al. Maternal diet and infant leukemia: The DNA topoisomerase II inhibitor hypothesis: A report from the children’s oncology group. Cancer Epidemiol. Biomark. Prev. 2005, 14, 651–655. [Google Scholar]
- Bailey, H.D.; Miller, M.; Langridge, A.; de Klerk, N.H.; van Bockxmeer, F.M.; Attia, J.; Scott, R.J.; Armstrong, B.K.; Milne, E. Maternal dietary intake of folate and vitamins B6 and B12 during pregnancy and the risk of childhood acute lymphoblastic leukemia. Nutr. Cancer 2012, 64, 1122–1130. [Google Scholar]
- Chokkalingam, A.P.; Chun, D.S.; Noonan, E.J.; Pfeiffer, C.M.; Zhang, M.; Month, S.R.; Taggart, D.R.; Wiemels, J.L.; Metayer, C.; Buffler, P.A. Blood levels of folate at birth and risk of childhood leukemia. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1088–1094. [Google Scholar]
- Poole, C.; Greenland, S.; Luetters, C.; Kelsey, J.L.; Mezei, G. Socioeconomic status and childhood leukaemia: A review. Int. J. Epidemiol. 2006, 35, 370–384. [Google Scholar] [PubMed]
- Adam, M.; Rebholz, C.E.; Egger, M.; Zwahlen, M.; Kuehni, C.E. Childhood Leukaemia and Socioeconomic Status: What Is the Evidence? Radiat. Prot. Dosim. 2008, 132, 246–254. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Borras, R.; Paules, C.; Casas, R.; Martin-Asuero, A.; Arranz, A.; Vieta, E.; Estruch, R.; Gratacos, E. Mediterranean diet, Mindfulness-Based Stress Reduction and usual care during pregnancy for reducing fetal growth restriction and adverse perinatal outcomes: IMPACT BCN (Improving Mothers for a better PrenAtal Care Trial BarCeloNa): A study protocol for a randomized controlled trial. Trials 2021, 22, 362. [Google Scholar]
- Morris, R.K.; Johnstone, E.; Lees, C.; Morton, V.; Smith, G.; Royal College of Obstetricians and Gynaecologists. Investigation and Care of a Small-for-Gestational-Age Fetus and a Growth Restricted Fetus (Green-top Guideline No. 31). BJOG Int. J. Obstet. Gynaecol. 2024, 131, e31–e80. [Google Scholar]
- Toledo, E.; Salas-Salvado, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fito, M.; Hu, F.B.; Aros, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar]
- Ludwig, D.S.; Kabat-Zinn, J. Mindfulness in medicine. JAMA 2008, 300, 1350–1352. [Google Scholar]
- Juton, C.; Castro-Barquero, S.; Casas, R.; Freitas, T.; Ruiz-Leon, A.M.; Crovetto, F.; Domenech, M.; Crispi, F.; Vieta, E.; Gratacos, E.; et al. Reliability and Concurrent and Construct Validity of a Food Frequency Questionnaire for Pregnant Women at High Risk to Develop Fetal Growth Restriction. Nutrients 2021, 13, 1629. [Google Scholar] [CrossRef]

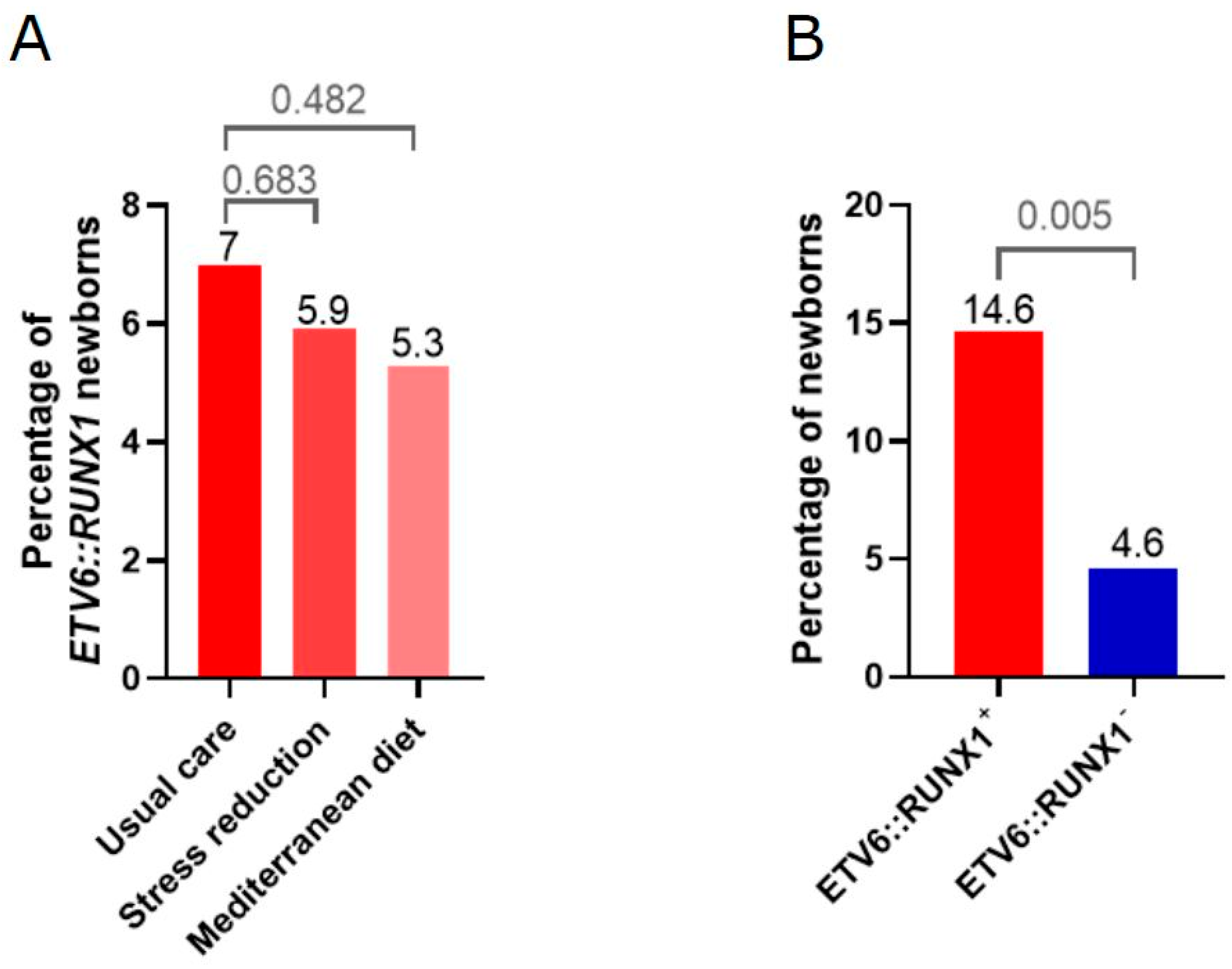

| Intervention Group | Neonatal ETV6::RUNX1+ No. (%) | Neonatal ETV6::RUNX1− No. (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Usual care | 17 (7.0) | 227 (93.0) | reference | NA |
| Stress reduction | 8 (5.9) | 128 (94.1) | 0.8 (0.3–2.0) | 0.683 |
| Mediterranean Diet | 9 (5.3) | 162 (94.7) | 0.7 (0.3–1.7) | 0.482 |
| No. (%) | Crude Odds Ratio for Neonatal ETV6::RUNX1+ | |||
|---|---|---|---|---|
| Neonatal ETV6::RUNX1 Positive (n = 48) | Neonatal ETV6::RUNX1 Negative (n = 693) | OR (95% CI) | p-Value | |
| Maternal Baseline Characteristics | ||||
| Maternal age (years) | 36.8 (5.0) | 36.8 (5.1) | 1.00 (0.95–1.06) | 0.969 |
| BMI before pregnancy (kg/m2) | 23.6 (4.0) | 24.1 (4.8) | 0.97 (0.9–1.04) | 0.427 |
| Ethnicity | ||||
| White | 34 (70.8) | 544 (78.5) | Reference | Reference |
| Black | 0 (0) | 14 (2.0) | NA | NA |
| Asian | 1 (2.1) | 15 (2.2) | 1.1 (0.1–8.3) | 0.951 |
| Indian | 0 (0) | 5 (0.7) | NA | NA |
| Latin American | 9 (18.8) | 110 (15.9) | 1.3 (0.6–2.8) | 0.489 |
| Maghreb | 4 (8.3) | 5 (0.7) | 12.8 (3.3–49.9) | <0.001 |
| Study class | ||||
| Primary/no studies | 5 (10.4) | 43 (6.2) | Reference | Reference |
| Secondary/technological | 9 (18.7) | 247 (35.6) | 0.3 (0.1–0.98) | 0.046 |
| University | 34 (70.8) | 403 (58.2) | 0.7 (0.3–1.9) | 0.525 |
| Socioeconomic status ψ | ||||
| Low | 5 (10.4) | 43 (6.2) | Reference | Reference |
| Medium | 12 (25.0) | 270 (39.0) | 0.4 (0.1–1.1) | 0.084 |
| High | 31 (64.6) | 380 (54.8) | 0.7 (0.3–1.9) | 0.485 |
| Nulliparous | 27 (56.3) | 399 (57.6) | 0.95 (0.5–1.7) | 0.857 |
| Pregestational diabetes | 5 (10.4) | 38 (5.5) | 2.0 (0.8–5.4) | 0.165 |
| Thyroid disorder | 4 (8.3) | 90 (13.0) | 0.6 (0.2–1.7) | 0.353 |
| Autoimmune disease | 6 (12.5) | 112 (16.2) | 0.7 (0.3–1.8) | 0.504 |
| Chronic hypertension | 1 (2.1) | 29 (4.2) | 0.5 (0.1–3.7) | 0.484 |
| Chronic kidney disease | 1 (2.1) | 17 (2.5) | 0.8 (0.1–6.5) | 0.872 |
| Obesity ф | 3 (6.3) | 85 (12.3) | 0.5 (0.1–1.6) | 0.223 |
| Pregnancy and prenatal characteristics | ||||
| Intervention group | ||||
| Usual care | 17 (35.4) | 228 (32.9) | Reference | Reference |
| Stress reduction | 16 (33.3) | 230 (33.3) | 0.9 (0.5–1.9) | 0.847 |
| Mediterranean Diet | 15 (31.3) | 235 (33.9) | 0.85 (0.4–1.8) | 0.671 |
| Cigarette smoking | 2 (4.2) | 54 (7.8) | 0.5 (0.1–2.2) | 0.367 |
| Alcohol intake | 0 (0) | 17 (2.5) | NA | NA |
| Recreational drug consumption | 0 (0) | 3 (0.4) | NA | NA |
| Folate supplementation | 31 (64.6) | 532 (76.8) | 0.6 (0.3–1.2) | 0.059 |
| Exogenous corticosteroids | 7 (14.6) | 32 (4.6) | 3.5 (1.5–8.5) | 0.005 |
| Gestational diabetes | 3 (6.3) | 81 (11.7) | 0.5 (0.2–1.7) | 0.259 |
| Preterm birth | 4 (8.3) | 37 (5.3) | 1.6 (0.5–4.7) | 0.386 |
| Preeclampsia | 0 (0) | 57 (8.2) | NA | NA |
| Small for gestational age Υ | 7 (14.6) | 27 (3.9) | 0.8 (0.3–1.8) | 0.591 |
| Gestational age at delivery (weeks) | 39.2 (1.9) | 39.4 (1.7) | 0.9 (0.8–1.1) | 0.404 |
| Neonatal sex | 0.367 | |||
| Female | 20 (41.7) | 335 (48.4) | Reference | |
| Male | 28 (58.3) | 357 (51.6) | 1.3 (0.7–2.4) | |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Crude OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | |
| Maghreb ethnicity | 12.8 (3.3–9.9) | <0.001 | 14.8 (3.4–63.9) | <0.001 | 10.5 (2.4–45.4) | 0.002 |
| Exogenous corticosteroids | 3.5 (1.5–8.5) | 0.005 | 3.4 (1.4–8.4) | 0.007 | 3.9 (1.6–9.8) | 0.003 |
| Study class Primary/no studies Secondary/technological University | Reference 0.3 (0.1–0.98) 0.7 (0.3–1.9) | 0.0367 | Reference 0.5 (0.2–1.9) 1.4 (0.4–4.6) | 0.03 | Reference 0.5 (0.1–1.9) 1.4 (0.4–4.4) | 0.306 |
| Folate supplementation | 0.6 (0.3–1.2) | 0.059 | 0.6 (0.3–1.1) | 0.10 | 0.6 (0.3–1.1) | 0.083 |
| Exogenous Corticosteroid Class | Neonatal ETV6::RUNX1+ (n = 7) | Neonatal ETV6::RUNX1− (n = 32) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Betamethasone (GR:MR = 25:1) | 5 | 20 | 4.0 (1.4–11.3) | 0.008 |
| Metilprednisone (GR:MR = 11:1) | 1 | 0 | NA | NA |
| Prednisone (GR:MR = 5:1) | 1 | 10 | 1.6 (0.2–12.9) | 0.653 |
| Topical corticosteroids (GR:MR = 10–100:1, according to steroid class) | 0 | 2 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, L.; Fischer, U.; Crispi, F.; Castro-Barquero, S.; Crovetto, F.; Larroya, M.; Youssef, L.; Kameri, E.; Castillo, H.; Bueno, C.; et al. Modulation of the ETV6::RUNX1 Gene Fusion Prevalence in Newborns by Corticosteroid Use During Pregnancy. Int. J. Mol. Sci. 2025, 26, 2971. https://doi.org/10.3390/ijms26072971
Benítez L, Fischer U, Crispi F, Castro-Barquero S, Crovetto F, Larroya M, Youssef L, Kameri E, Castillo H, Bueno C, et al. Modulation of the ETV6::RUNX1 Gene Fusion Prevalence in Newborns by Corticosteroid Use During Pregnancy. International Journal of Molecular Sciences. 2025; 26(7):2971. https://doi.org/10.3390/ijms26072971
Chicago/Turabian StyleBenítez, Leticia, Ute Fischer, Fàtima Crispi, Sara Castro-Barquero, Francesca Crovetto, Marta Larroya, Lina Youssef, Ersen Kameri, Helena Castillo, Clara Bueno, and et al. 2025. "Modulation of the ETV6::RUNX1 Gene Fusion Prevalence in Newborns by Corticosteroid Use During Pregnancy" International Journal of Molecular Sciences 26, no. 7: 2971. https://doi.org/10.3390/ijms26072971
APA StyleBenítez, L., Fischer, U., Crispi, F., Castro-Barquero, S., Crovetto, F., Larroya, M., Youssef, L., Kameri, E., Castillo, H., Bueno, C., Casas, R., Borras, R., Vieta, E., Estruch, R., Menéndez, P., Borkhardt, A., & Gratacós, E. (2025). Modulation of the ETV6::RUNX1 Gene Fusion Prevalence in Newborns by Corticosteroid Use During Pregnancy. International Journal of Molecular Sciences, 26(7), 2971. https://doi.org/10.3390/ijms26072971









