Therapeutic Potential of Extracellular Vesicles in Oral Inflammation
Abstract
:1. Introduction
1.1. Overview of Oral Inflammation
1.2. EVs: Definition and Classification
- Exosomes:
- Microvesicles:
- Apoptotic Bodies:
1.3. Search Strategy
2. Mechanisms of Inflammation in the Oral Environment
2.1. Role of the Immune System in Oral Inflammation
2.2. Cellular and Molecular Pathways in Periodontitis
2.3. Current Anti-Inflammatory Strategies in Oral Disease
3. EVs: Characteristics and Therapeutic Potential
3.1. Biogenesis, Composition, and Functional Roles of EVs
3.2. Sources of EVs for Therapeutic Applications
3.3. Mechanisms of EV Action in Modulating Inflammation
4. Evidence of EV Therapeutics in Oral Inflammation
4.1. EVs in Periodontitis: Experimental Studies
4.2. EVs in Gingivitis and Other Oral Inflammatory Conditions
4.3. Comparative Studies of EVs and Conventional Therapies
5. Human Platelet-Derived EVs in Periodontitis
5.1. Extraction and Chararcterisation of hPLT-EVs
5.2. Anti-Inflammatory Properties of hPLT-EVs
5.3. Preclinical Evidence in Ligature-Induce Periodontitis Models: In Vitro and In Vivo Studies
6. Advantages and Challenges of EV-Based Therapies
6.1. Benefits of EVs over Traditional Treatments
6.1.1. Biocompatibility and Low Immunogenicity
6.1.2. Targeted Delivery and Enhanced Stability
6.1.3. Ability to Cross Biological Barriers
6.1.4. Cell-Free Therapy
6.1.5. Intrinsic Cargo Diversity
6.2. Challenges in EV Manufacturing and Standardisation
6.2.1. Isolation and Purification
6.2.2. Scalability and Yield
6.2.3. Characterisation and Quality Control
6.2.4. Storage and Stability
6.3. Ethical and Regulatory Considerations
6.3.1. Safety and Efficacy
6.3.2. Source and Donor Considerations
6.3.3. Regulatory Frameworks
6.3.4. Cost and Accessibility
7. Future Perspective
7.1. Potential Applications in Oral Health and Systemic Therapies
7.2. Emerging Technologies in EV Isolation, Engineering, and Characterisation
7.3. Knowledge Gaps and Research Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| gDNA | Genomic DNA |
| sEV | Small extracellular vesicle |
| MVs | Microvesicles |
| bEVs | Bacterial extracellular vesicles |
| hPLT-EVs | Human platelet-derived extracellular vesicles |
| TLR | Toll-like receptor |
| NF-kB | Nuclear factor kappa B |
| RANKL | Nuclear factor kappa B ligand |
| NSAIDs | Nonsteroidal anti-inflammatory medications |
| TRAP | Tartrate-resistant acid phosphatase |
| OSCC | Oral squamous cell carcinoma |
| CAFs | Cancer-associated fibroblasts |
| GMSCs | Gingival mesenchymal stem cell-derived exosomes |
| PDLSCs | Periodontal ligament stem cells |
| MSCs | Mesenchymal stem cells |
| hPLT | Human platelet lysate |
| NTA | Nanoparticle tracking analysis |
| TEM | Transmission electron microscopy |
| HA | Hyaluronic acid |
| MHC | Major histocompatibility complex |
| RVG | Rabies virus glycoprotein |
| BBB | Blood–brain barrier |
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| SHED | Human exfoliated deciduous teeth |
| VEGF | Vascular endothelial growth factor |
| SEC | Size-exclusion chromatography |
| PBS-HAT | Phosphate-buffered saline supplemented with human serum albumin and trehalose |
| FDA | Food and Drug Administration |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| 3D | Three-dimensional |
| UC-MSCs | Umbilical cord-derived mesenchymal stem cells |
References
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [PubMed]
- Boyapati, R.; Vudathaneni, V.K.P.; Nadella, S.B.; Bollepalli, A.C.; Marella, Y.; Adurty, C. Reflex Gastroesophageal Disorders and Functional Dyspepsia: Potential Confounding Variables for the Progression of Chronic Periodontitis: A Clinical Study. Int. J. Prev. Med. 2020, 11, 138. [Google Scholar] [PubMed]
- Han, P.; Lai, A.; Salomon, C.; Ivanovski, S. Detection of Salivary Small Extracellular Vesicles Associated Inflammatory Cytokines Gene Methylation in Gingivitis. Int. J. Mol. Sci. 2020, 21, 5273. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Jiang, Z.; Marka, N.; Wolff, L.F. Periodontal Disease, Tooth Loss, and Systemic Conditions: An Exploratory Study. Int. Dent. J. 2024, 74, 207–215. [Google Scholar]
- Preußer, C.; Hung, L.H.; Schneider, T.; Schreiner, S.; Hardt, M.; Moebus, A.; Santoso, S.; Bindereif, A. Selective release of circRNAs in platelet-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1424473. [Google Scholar]
- Vismara, M.; Negri, S.; Scolari, F.; Brunetti, V.; Trivigno, S.M.G.; Faris, P.; Galgano, L.; Soda, T.; Berra-Romani, R.; Canobbio, I.; et al. Platelet-Derived Extracellular Vesicles Stimulate Migration through Partial Remodelling of the Ca2+ Handling Machinery in MDA-MB-231 Breast Cancer Cells. Cells 2022, 11, 3120. [Google Scholar] [CrossRef]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E.; Moses, M.A.; et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar]
- Parvaneh, M.; Witting, P.K.; Ku, J.; Moradi, T.; Eroglu, E.; Freedman, B.; Sutherland, G.T.; McCorkindale, A.; Guennewig, B.; Choowong, P.; et al. Periodontitis induces endothelial dysfunction in mice. Sci. Rep. 2021, 11, 14993. [Google Scholar]
- Santonocito, S.; Polizzi, A.; Palazzo, G.; Isola, G. The Emerging Role of microRNA in Periodontitis: Pathophysiology, Clinical Potential and Future Molecular Perspectives. Int. J. Mol. Sci. 2021, 22, 5456. [Google Scholar] [CrossRef]
- Ha, J.Y.; Seok, J.; Kim, S.J.; Jung, H.J.; Ryu, K.Y.; Nakamura, M.; Jang, S.; Hong, S.H.; Lee, Y.; Lee, H.J. Periodontitis promotes bacterial extracellular vesicle-induced neuroinflammation in the brain and trigeminal ganglion. PLoS Pathog. 2023, 19, e1011743. [Google Scholar]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Vizio, D.D.; Driendonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [PubMed]
- Lee, Y.; El Andaloussi, S.; Wood, M.J.A. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar]
- Atkin-Smith, G.K.; Poon, I.K.H. Disassembly of the Dying: Mechanisms and Functions. Trends Cell Biol. 2017, 27, 151–162. [Google Scholar]
- Radic, M. Clearance of Apoptotic Bodies, NETs, and Biofilm DNA: Implications for Autoimmunity. Front. Immunol. 2014, 5, 365. Available online: http://journal.frontiersin.org/article/10.3389/fimmu.2014.00365/abstract (accessed on 14 March 2025).
- Muttiah, B.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles. Biomedicines 2024, 12, 1850. [Google Scholar] [CrossRef]
- Aatonen, M.T.; Öhman, T.; Nyman, T.A.; Laitinen, S.; Grönholm, M.; Siljander, P.R.-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicle 2014, 3, 24692. [Google Scholar]
- Zhang, Z.; Zheng, Y.; Bian, X.; Wang, M.; Chou, J.; Liu, H.; Wang, Z. Identification of Key Genes and Pathways Associated with Oxidative Stress in Periodontitis. Oxidative Med. Cell. Longev. 2022, 2022, 9728172. [Google Scholar]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar]
- Zhao, Q.; Wang, K.; Hou, L.; Guo, L.; Liu, X. Based on network pharmacology and molecular docking to explore the potential mechanism of shikonin in periodontitis. BMC Oral Health. 2024, 24, 839. [Google Scholar] [CrossRef] [PubMed]
- Puletic, M.; Velikic, G.; Maric, D.M.; Supic, G.; Maric, D.L.; Radovic, N.; Amromov, S.; Vojvodic, D. Clinical Efficacy of Extracellular Vesicle Therapy in Periodontitis: Reduced Inflammation and Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 5753. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar]
- Jang, J.H.; Shin, H.W.; Lee, J.M.; Lee, H.W.; Kim, E.C.; Park, S.H. An Overview of Pathogen Recognition Receptors for Innate Immunity in Dental Pulp. Agrawal, A., editor. Mediat. Inflamm. 2015, 2015, 794143. [Google Scholar]
- Huang, H.; Yang, R.; Shi, B. The potential role of cfDNA-related innate immune responses in postoperative bone loss after alveolar bone grafting. Front. Immunol. 2023, 13, 1068186. [Google Scholar] [CrossRef]
- Yu, Y.; Yue, Z.; Xu, M.; Zhang, M.; Shen, X.; Ma, Z.; Li, J.; Xie, X. Macrophages play a key role in tissue repair and regeneration. PeerJ 2022, 10, e14053. [Google Scholar]
- Gao, Z.; Guan, L.; Liu, Z.; Yan, F.; Fang, S.; Zhang, X.; Gao, C. Using extracellular vesicles derived from human umbilical cord mesenchymal stem cells for a topical coating promotes oral mucositis healing in rats. Ann. Transl. Med. 2022, 10, 290. [Google Scholar]
- Calixto, G.; Fonseca-Santos, B.; Chorilli, M.; Bernegossi, J. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 3719. [Google Scholar]
- Diomede, F.; Fonticoli, L.; Guarnieri, S.; Della Rocca, Y.; Rajan, T.S.; Fontana, A.; Trubiani, O.; Marconi, G.D.; Pizzicannella, J. The Effect of Liposomal Curcumin as an Anti-Inflammatory Strategy on Lipopolysaccharide e from Porphyromonas gingivalis Treated Endothelial Committed Neural Crest Derived Stem Cells: Morphological and Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 7534. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Sherman, C.D.; Lodha, S.; Sahoo, S. EV Cargo Sorting in Therapeutic Development for Cardiovascular Disease. Cells 2021, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Saviana, M.; Romano, G.; Le, P.; Acunzo, M.; Nana-Sinkam, P. Extracellular Vesicles in Lung Cancer Metastasis and Their Clinical Applications. Cancers 2021, 13, 5633. [Google Scholar] [CrossRef]
- Foers, A.D.; Garnham, A.L.; Chatfield, S.; Smyth, G.K.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Extracellular Vesicles in Synovial Fluid from Rheumatoid Arthritis Patients Contain miRNAs with Capacity to Modulate Inflammation. IJMS 2021, 22, 4910. [Google Scholar]
- Liu, X.; Li, W.; Fu, X.; Xu, Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front. Immunol. 2017, 8, 645. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2017.00645/full (accessed on 13 February 2025).
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Luo, H.; Birjandi, A.A.; Ren, F.; Sun, T.; Sharpe, P.T.; Sun, H.; An, Z. Advances in oral mesenchymal stem cell-derived extracellular vesicles in health and disease. Genes Dis. 2023, 11, 346–357. [Google Scholar]
- Bahmani, L.; Ullah, M. Different Sourced Extracellular Vesicles and Their Potential Applications in Clinical Treatments. Cells 2022, 11, 1989. [Google Scholar] [CrossRef]
- Tang, T.T.; Wang, B.; Lv, L.L.; Liu, B.C. Extracellular vesicle-based Nanotherapeutics: Emerging frontiers in anti-inflammatory therapy. Theranostics 2020, 10, 8111–8129. [Google Scholar]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Kou, X.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Kong, Q.; He, H.; Sun, J.; Qiu, W.; Zhang, L.; Yang, M. Extracellular Vesicle Preparation and Analysis: A State-of-the-Art Review. Adv. Sci. 2024, 11, 2401069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Hou, L.; Zhu, Z.; Jiang, F.; Zhao, J.; Jia, Q.; Jiang, Q.; Wang, H.; Xue, W.; Wang, Y.; Tian, L. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles alleviated silica induced lung inflammation and fibrosis in mice via circPWWP2A/miR-223–3p/NLRP3 axis. Ecotoxicol. Environ. Saf. 2023, 251, 114537. [Google Scholar] [CrossRef]
- Dourado, M.R.; Korvala, J.; Åström, P.; De Oliveira, C.E.; Cervigne, N.K.; Mofatto, L.S.; Bastos, D.C.; Messetti, A.C.P.; Graner, E.; Leme, A.F.P.; et al. Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. J. Extracell. Vesicles 2019, 8, 1578525. [Google Scholar] [CrossRef]
- Arebro, J.; Towle, R.; Lee, C.M.; Bennewith, K.L.; Garnis, C. Extracellular vesicles promote activation of pro-inflammatory cancer-associated fibroblasts in oral cancer. Front. Cell Dev. Biol. 2023, 11, 1240159. [Google Scholar] [CrossRef]
- Qin, X.; Guo, H.; Wang, X.; Zhu, X.; Yan, M.; Wang, X.; Shi, J.; Lu, E.; Chen, W.; Zhang, J. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019, 20, 12. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef]
- Han, P.; Bartold, P.M.; Ivanovski, S. The emerging role of small extracellular vesicles in saliva and gingival crevicular fluid as diagnostics for periodontitis. J. Periodontal Res. 2022, 57, 219–231. [Google Scholar] [CrossRef]
- Cai, R.; Wang, L.; Zhang, W.; Liu, B.; Wu, Y.; Pang, J.; Ma, C. The role of extracellular vesicles in periodontitis: Pathogenesis, diagnosis, and therapy. Front. Immunol. 2023, 14, 1151322. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1151322/full (accessed on 24 December 2024).
- Naito, Y.; Yamamoto, Y.; Sakamoto, N.; Shimomura, I.; Kogure, A.; Kumazaki, M.; Yokoi, A.; Yashiro, M.; Kinoyo, T.; Yanagihara, K.; et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene 2019, 38, 5566–5579. [Google Scholar] [PubMed]
- Peng, Y.; Jaar, J.; Tran, S.D. Gingival mesenchymal stem cells: Biological properties and therapeutic applications. J. Oral Biol. Craniofacial Res. 2024, 14, 547–569. [Google Scholar]
- Shi, Q.; Huo, N.; Wang, X.; Yang, S.; Wang, J.; Zhang, T. Exosomes from oral tissue stem cells: Biological effects and applications. Cell Biosci. 2020, 10, 108. [Google Scholar]
- Zhang, H.C.; Liu, X.B.; Huang, S.; Bi, X.Y.; Wang, H.X.; Xie, L.X.; Wang, Y.Q.; Cao, X.F.; LV, J.; Xiao, F.J.; et al. Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Stimulated by Hypoxia Promote Angiogenesis Both In Vitro and In Vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar]
- Zeng, M.; Liu, M.; Tao, X.; Yin, X.; Shen, C.; Wang, X. Emerging Trends in the Application of Extracellular Vesicles as Novel Oral Delivery Vehicles for Therapeutics in Inflammatory Diseases. Int. J. Nanomed. 2024, 19, 8573–8601. [Google Scholar]
- Antich-Rosselló, M.; Munar-Bestard, M.; Forteza-Genestra, M.A.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications. Int. J. Mol. Sci. 2022, 23, 7668. [Google Scholar] [CrossRef]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnology 2023, 21, 231. [Google Scholar]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W.; Baccarelli, A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. 2021, 169, 10–3791. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. Available online: https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/0471143030.cb0322s30 (accessed on 14 February 2025).
- Sharma, K.D.; Doktorova, M.; Waxham, M.N.; Heberle, F.A. Cryo-EM images of phase-separated lipid bilayer vesicles analyzed with a machine-learning approach. Biophys. J. 2024, 123, 2877–2891. [Google Scholar] [PubMed]
- Sun, J.; Hu, Y.; Fu, Y.; Zou, D.; Lu, J.; Lyu, C. Emerging roles of platelet concentrates and platelet-derived extracellular vesicles in regenerative periodontology and implant dentistry. APL Bioeng. 2022, 6, 031503. [Google Scholar]
- Huang, X.; Wang, H.; Wang, C.; Cao, Z. The Applications and Potentials of Extracellular Vesicles from Different Cell Sources in Periodontal Regeneration. Int. J. Mol. Sci. 2023, 24, 5790. [Google Scholar] [CrossRef]
- Shi, M.; Lu, Q.; Zhao, Y.; Ding, Z.; Yu, S.; Li, J.; Ji, M.; Fan, H.; Hou, S. miR-223: A key regulator of pulmonary inflammation. Front. Med. 2023, 10, 1187557. [Google Scholar]
- Boldin, M.P.; Taganov, K.D.; Rao, D.S.; Yang, L.; Zhao, J.L.; Kalwani, M.; Garcia-Flores, Y.; Luong, M.; Devrekanli, A.; Xu, J.; et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011, 208, 1189–1201. [Google Scholar]
- O’Boyle, C.; Haley, M.J.; Lemarchand, E.; Smith, C.J.; Allan, S.M.; Konkel, J.E.; Lawrence, C.B. Ligature-induced periodontitis induces systemic inflammation but does not alter acute outcome after stroke in mice. Int. J. Stroke 2020, 15, 175–187. [Google Scholar]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018, 8, 1171. [Google Scholar]
- Beetler, D.J.; Di Florio, D.N.; Law, E.W.; Groen, C.M.; Windebank, A.J.; Peterson, Q.P.; Fairweather, D. The Evolving Regulatory Landscape in Regenerative Medicine. Mol. Asp. Med. 2023, 91, 101138. [Google Scholar]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, 2403199. [Google Scholar]
- Lu, X.; Fan, S.; Cao, M.; Liu, D.; Xuan, K.; Liu, A. Extracellular vesicles as drug delivery systems in therapeutics: Current strategies and future challenges. J. Pharm. Investig. 2024, 54, 785–802. [Google Scholar]
- Cieślik, M.; Bryniarski, K.; Nazimek, K. Biodelivery of therapeutic extracellular vesicles: Should mononuclear phagocytes always be feared? Front. Cell Dev. Biol. 2023, 11, 1211833. [Google Scholar]
- Lara, P.; Chan, A.B.; Cruz, L.J.; Quest, A.F.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef]
- Shao, M.; Rodrigues, J.; Sousa-Oliveira, I.; Moradialvand, M.; Asadollahi, P.; Veiga, F.; Hameed, H.; Jha, N.K.; Sillanpaa, M.; Sethi, G.; et al. Revolutionizing cancer treatment via bioengineered extracellular vesicles: Exploring nanovesicles to fully synthetic solutions. Appl. Mater. Today 2024, 40, 102395. [Google Scholar]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar]
- Cheng, W.; Xu, C.; Su, Y.; Shen, Y.; Yang, Q.; Zhao, Y.; Zhao, Y.; Liu, Y. Engineered Extracellular Vesicles: A potential treatment for regeneration. iScience 2023, 26, 108282. [Google Scholar] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Segaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509. [Google Scholar]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [PubMed]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar]
- Wu, J.; Chen, L.; Wang, R.; Song, Z.; Shen, Z.; Zhao, Y.; Huang, S.; Lin, Z. Exosomes Secreted by Stem Cells from Human Exfoliated Deciduous Teeth Promote Alveolar Bone Defect Repair through the Regulation of Angiogenesis and Osteogenesis. ACS Biomater. Sci. Eng. 2019, 5, 3561–3571. [Google Scholar]
- Adlerz, K.; Patel, D.; Rowley, J.; Ng, K.; Ahsan, T. Strategies for scalable manufacturing and translation of MSC-derived extracellular vesicles. Stem Cell Res. 2020, 48, 101978. [Google Scholar]
- Kanellopoulos, J.M.; Rieux-Laucat, F.; Ojcius, D.M. Biological functions of extracellular vesicles from mammalian cells. Biomed. J. 2024, 47, 100788. [Google Scholar]
- Ateeq, M.; Broadwin, M.; Sellke, F.W.; Abid, M.R. Extracellular Vesicles’ Role in Angiogenesis and Altering Angiogenic Signaling. Med. Sci. 2024, 12, 4. [Google Scholar] [CrossRef]
- Phillips, W.; Willms, E.; Hill, A.F. Understanding extracellular vesicle and nanoparticle heterogeneity: Novel methods and considerations. Proteomics 2021, 21, 2000118. [Google Scholar]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [PubMed]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar]
- Syromiatikova, V.; Prokopeva, A.; Gomzikova, M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 10522. [Google Scholar] [CrossRef]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef]
- Bachurski, D.; Schuldner, M.; Nguyen, P.H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hensen, H.P.; Hallek, M.; et al. Extracellular vesicle measurements with nanoparticle tracking analysis—An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar]
- Malenica, M.; Vukomanović, M.; Kurtjak, M.; Masciotti, V.; dal Zilio, S.; Greco, S.; Lazzarino, M.; Krusic, V.; Percic, M.; Badovinac, I.J.; et al. Perspectives of Microscopy Methods for Morphology Characterisation of Extracellular Vesicles from Human Biofluids. Biomedicines 2021, 9, 603. [Google Scholar] [CrossRef]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical Translation of Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, 2301010. [Google Scholar]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; Andaloussi, S.E. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [PubMed]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar]
- Wang, Q.; Sun, J.; Jiang, H.; Yu, M. Emerging roles of extracellular vesicles in oral and maxillofacial areas. Int. J. Oral. Sci. 2025, 17, 11. [Google Scholar]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Sjoqvist, S.; Kasai, Y.; Shimura, D.; Ishikawa, T.; Ali, N.; Iwata, T.; Kanai, N. Oral keratinocyte-derived exosomes regulate proliferation of fibroblasts and epithelial cells. Biochem. Biophys. Res. Commun. 2019, 514, 706–712. [Google Scholar]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef]
- Brezgin, S.; Danilik, O.; Yudaeva, A.; Kachanov, A.; Kostyusheva, A.; Karandashov, I.; Ponomareva, N.; Zamyatnin, A.A.; Parodi, A.; Chulanov, V.; et al. Basic Guide for Approaching Drug Delivery with Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 10401. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.K.; van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, C.; Gao, X.; Ding, L.; Wang, P.; Ren, Y.; Hou, X.; Yao, Y.; Zhang, C.; Yang, X.; et al. One-Minute Iodine Isotope Labeling Technology Enables Noninvasive Tracking and Quantification of Extracellular Vesicles in Tumor Lesions and Intact Animals. Mol. Pharm. 2023, 20, 3672–3682. [Google Scholar] [PubMed]
- Rai, A.; Claridge, B.; Lozano, J.; DW, G. The Discovery of Extracellular Vesicles and Their Emergence as a Next-Generation Therapy. Circ. Res. 2024, 135, 198–221. [Google Scholar]
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020, 53, e12857. [Google Scholar] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
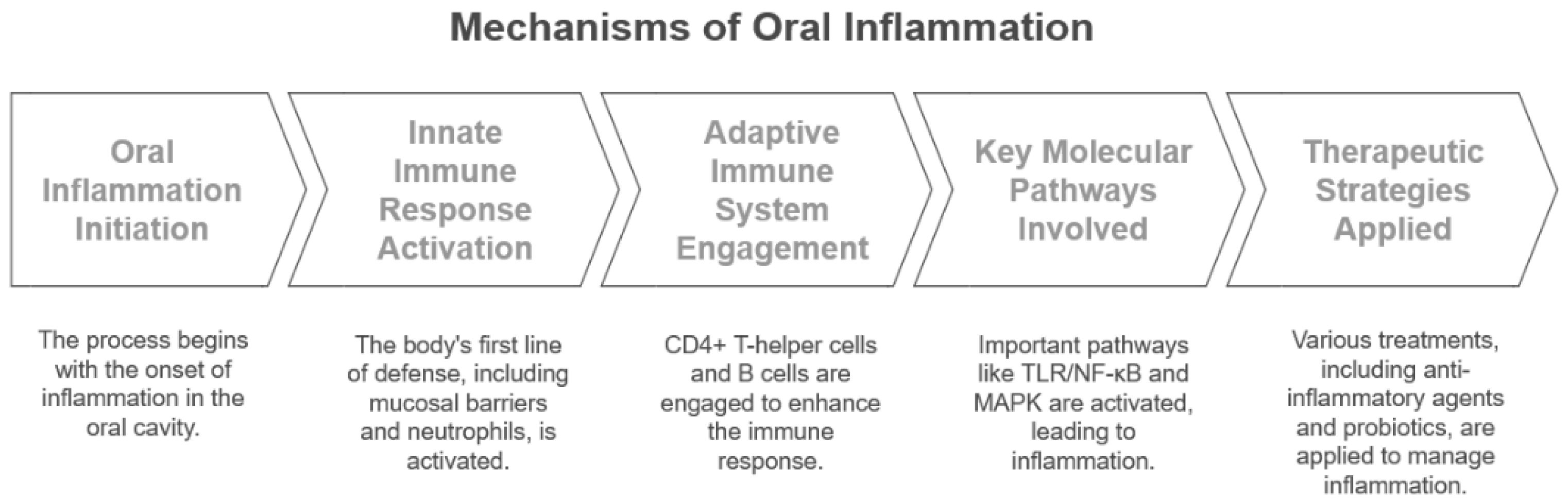


| Key Component of the Innate Immune System | Function | Citation |
|---|---|---|
| Saliva and Mucosal Barriers | Contain antimicrobial proteins like lysozyme and defensins; form a physical barrier against pathogens. | [23] |
| Neutrophils | First responders that migrate to infection sites, releasing enzymes and reactive oxygen species (ROS). | [24] |
| Macrophages and Dendritic Cells | Detect pathogens and trigger adaptive immunity by acting as antigen-presenting cells (APCs). | [20] |
| Toll-like Receptors (TLRs) | Identify microbiological components and initiate the synthesis of inflammatory cytokines. | [25] |
| Key Components of the Adaptive Immune System | Function | Citation |
|---|---|---|
| CD4+ T-Helper Cells (Th1, Th2, Th17) | Regulate inflammation; Th1 promotes cell-mediated immunity, Th2 enhances humoral immunity, and Th17 recruits neutrophils. | [23] |
| B Cells and Antibodies | Produce antibodies (IgA, IgG, IgM) to neutralise pathogens and mediate the immune response. | [20] |
| Regulatory T Cells (Tregs) | Suppress an overabundance of immunological responses to avoid tissue harm. | [26] |
| Therapeutic Strategy | Mechanism of Action | Citation |
|---|---|---|
| Anti-inflammatory Agents | NSAIDs, corticosteroids, and cytokine inhibitors reduce inflammation. | [26] |
| Host Modulation Therapy | Drugs like doxycycline and bisphosphonates regulate immune responses. | [20] |
| Probiotics and Prebiotics | Promote beneficial oral microbiota to maintain immune homeostasis. | [23] |
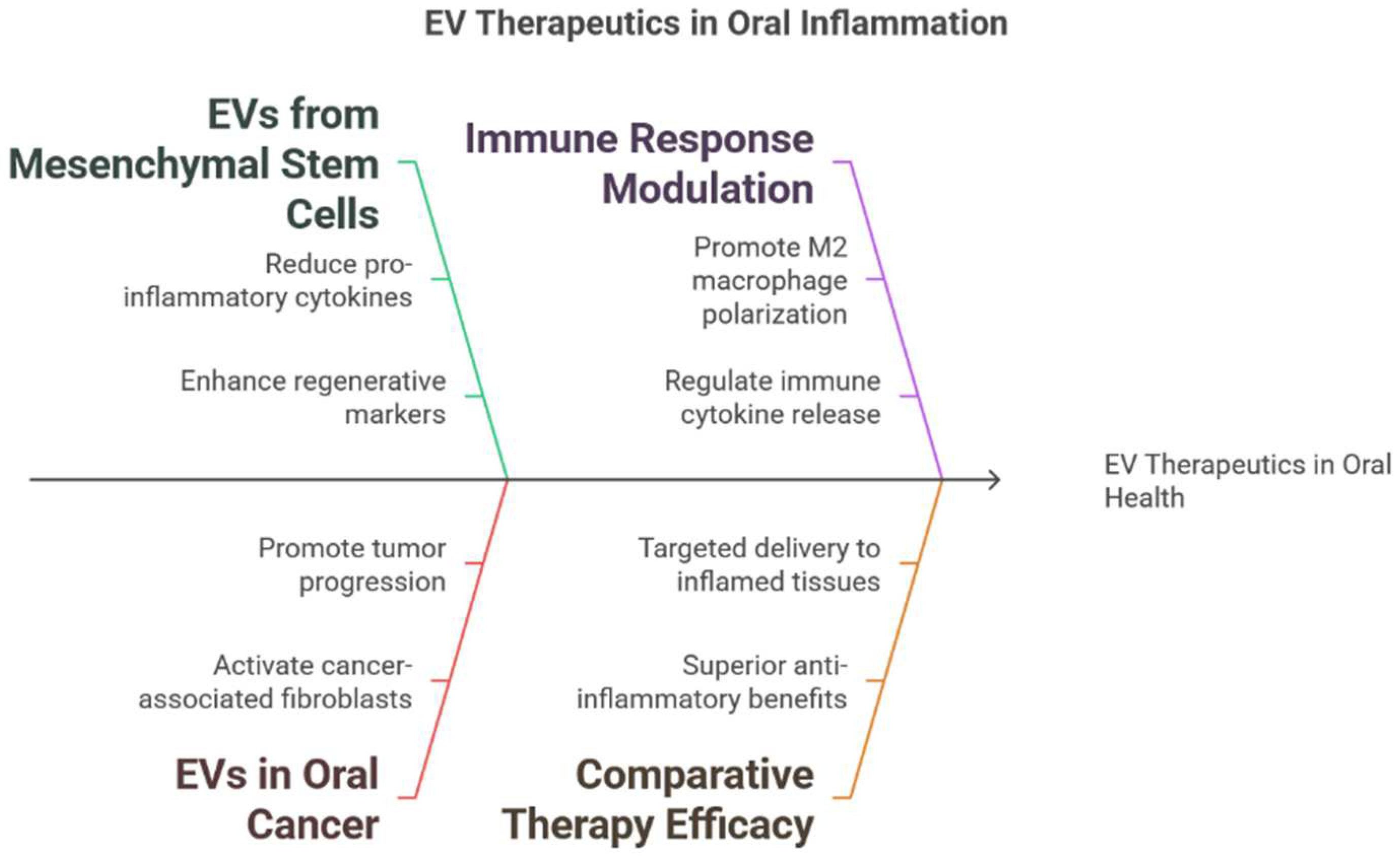
| Source of EVs | Key Functions | Citation |
|---|---|---|
| Mesenchymal Stem Cell (MSC)-Derived EVs | Promote tissue repair, modulate immune responses, and reduce inflammation. | [19] |
| Platelet-Derived EVs | Enhance wound healing, stimulate fibroblast proliferation, and support vascular regeneration. | [35] |
| Immune Cell-Derived EVs | Modulate immune responses, alter cytokine production, and facilitate antigen presentation. | [36] |
| Epithelial Cell-Derived EVs | Regulate host–microbiome interactions and influence immune responses in oral health. | [28] |
| Stem Cell-Derived EVs from Other Sources | Exhibit regenerative properties similar to MSC-derived EVs but face ethical and production challenges. | [21] |
| Synthetic and Engineered EVs | Designed for targeted drug delivery, to carry therapeutic molecules, and enhance treatment efficacy. | [35] |
| Oral Condition | EV Source | Mechanism of Action | Preclinical Studies |
|---|---|---|---|
| Periodontitis | Mesenchymal Stem Cell-derived EVs (MSC-EVs) | Modulation of the inflammatory microenvironment (e.g., downregulation of TNF-α and IL-6) and promotion of periodontal tissue regeneration. | Animal studies showing reduced inflammatory infiltration and improved periodontal ligament integrity. |
| Oral Mucositis | Oral Keratinocyte-derived Exosomes | Stimulation of re-epithelialisation and acceleration of mucosal wound closure. | Models demonstrating faster mucosal healing and reduced inflammatory cytokine levels. |
| Prevention of Malignant Transformation in Premalignant Lesions | EVs from various sources (MSC and keratinocytes) | Modulation of cell signalling pathways to suppress dysplastic progression. | Emerging experimental data suggesting EVs can help maintain normal cell behaviour in high-risk oral tissues. |
| Precision Drug Delivery in Periodontal Therapy | Engineered EVs from MSCs and other cells | Targeted delivery of anti-inflammatory or regenerative agents specifically to periodontal pockets. | Studies demonstrating enhanced local efficacy and reduced systemic side effects. |
| Craniofacial Tissue Regeneration | Engineered EVs combined with biomaterials | Promotion of osteogenic differentiation and integration of bone and soft tissue. | Preclinical models of craniofacial defects showing improved regeneration outcomes. |
| Systemic Therapy for Neurodegenerative Disorders | MSC-EVs engineered for CNS delivery | Ability to cross the blood–brain barrier and deliver neuroprotective agents, reducing neuroinflammation. | Preclinical studies linking oral inflammation with neurodegeneration and demonstrating targeted delivery in Alzheimer’s models. |
| Salivary EV Diagnostics | Salivary EVs | Non-invasive collection of EVs that contain diagnostic biomarkers (e.g., proteins and microRNAs) reflecting both oral and systemic health. | Studies correlating exosomal microRNA profiles with periodontitis severity and systemic conditions (diabetes and cardiovascular diseases). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farm, Y.R.; Chuah, B.H.; Law, J.X.; Leong, X.F.; Razali, M.; Ng, S.L. Therapeutic Potential of Extracellular Vesicles in Oral Inflammation. Int. J. Mol. Sci. 2025, 26, 3031. https://doi.org/10.3390/ijms26073031
Farm YR, Chuah BH, Law JX, Leong XF, Razali M, Ng SL. Therapeutic Potential of Extracellular Vesicles in Oral Inflammation. International Journal of Molecular Sciences. 2025; 26(7):3031. https://doi.org/10.3390/ijms26073031
Chicago/Turabian StyleFarm, Yan Rou, Bing Huan Chuah, Jia Xian Law, Xin Fang Leong, Masfueh Razali, and Sook Luan Ng. 2025. "Therapeutic Potential of Extracellular Vesicles in Oral Inflammation" International Journal of Molecular Sciences 26, no. 7: 3031. https://doi.org/10.3390/ijms26073031
APA StyleFarm, Y. R., Chuah, B. H., Law, J. X., Leong, X. F., Razali, M., & Ng, S. L. (2025). Therapeutic Potential of Extracellular Vesicles in Oral Inflammation. International Journal of Molecular Sciences, 26(7), 3031. https://doi.org/10.3390/ijms26073031







