Effect of Small Extracellular Vesicles Produced by Mesenchymal Stem Cells on 5xFAD Mice Hippocampal Cultures
Abstract
:1. Introduction
2. Results
2.1. Characterization of sEVs
2.2. Estimation of sEV Cytotoxicity in Hippocampal Cultures
2.3. Interaction of sEVs with Tg and Non-Transgenic Hippocampal Cells
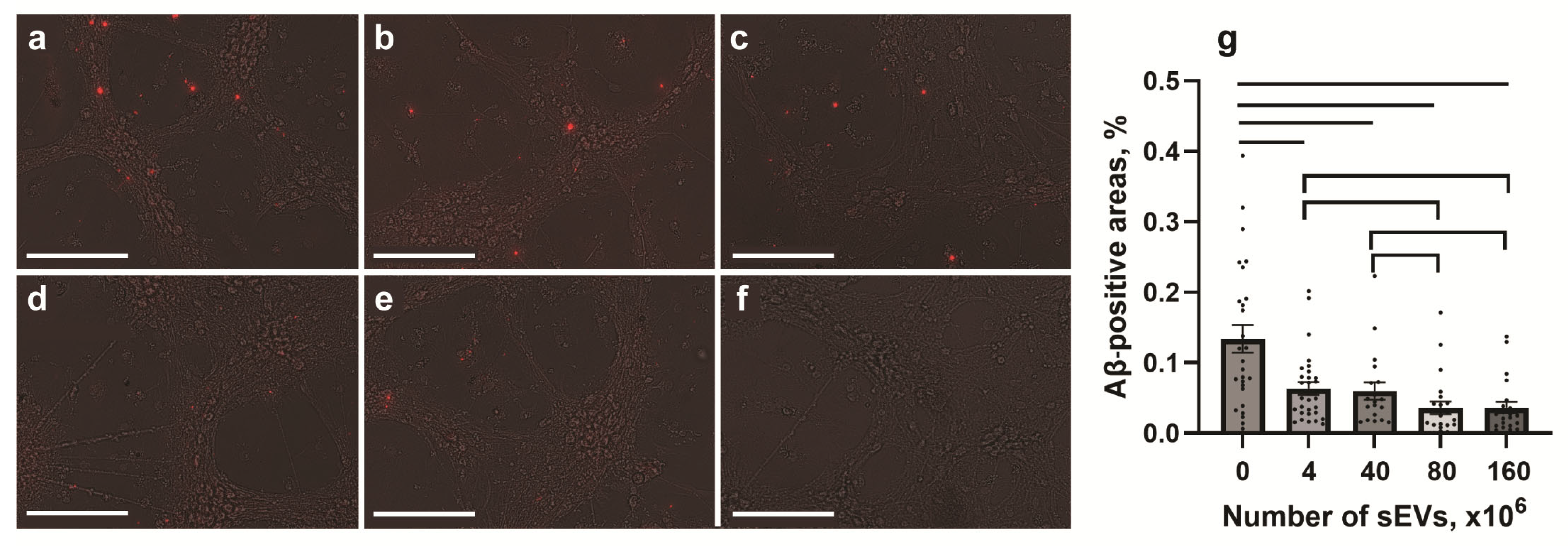
2.4. Effect of sEVs on the Expression of Aβ
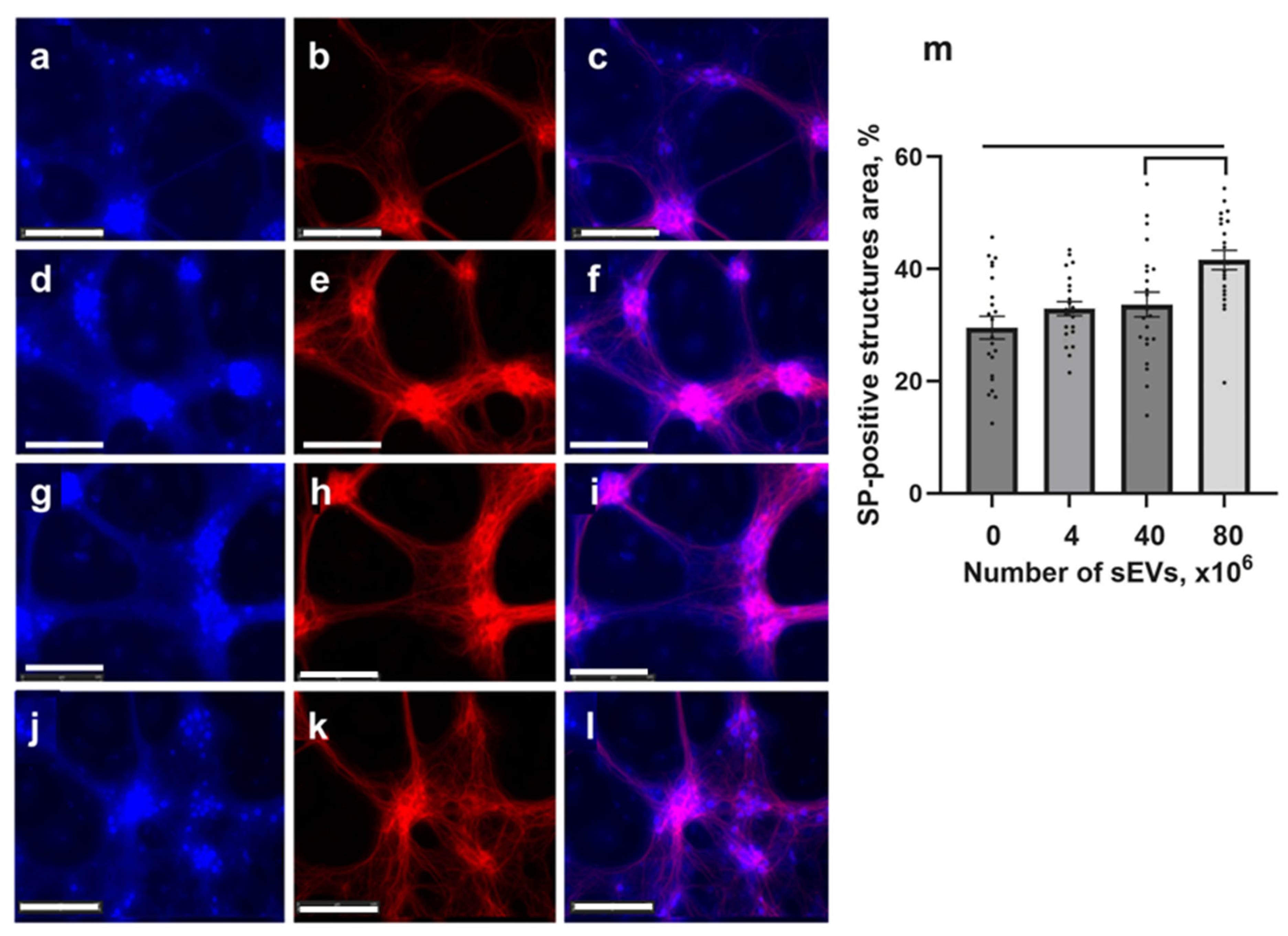
2.5. Expression of Synaptophysin in Tg Cells
3. Discussion
4. Materials and Methods
4.1. Mesenchymal Stromal Cells (MSCs)
4.2. Production and Characterization of the MSC-Derived sEVs
4.3. PAAG Gel-Electrophoresis and Western Blotting
4.4. Transmission Electron Microscopy (TEM)
4.5. Nanoparticle Tracking Analysis (NTA)
4.6. ζ-Potential
4.7. Confocal Microscopy
4.8. Multiplex Analysis of Cytokines
4.9. Animals
4.10. Primary Culture of 5xFAD Mouse Hippocampi
4.11. Staining of sEVs from MSCs
4.12. Cell Viability Analysis
4.13. Effect of sEVs on Synaptogenesis and Immunoreactivity to Aβ
4.14. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| BBB | Blood–brain barrier |
| MFI | Median fluorescence intensity |
| MSCs | Mesenchymal stem cells |
| NTA | Nanoparticle tracking analysis |
| PAAG | Polyacrylamide gel electrophoresis |
| RRIDs | Research resource identifiers |
| SEM | Scanning electron microscopy |
| sEVs | Small extracellular vesicles |
| Tg | Transgenic |
| 5xFAD | Transgenic mice carrying mutations involved in familial Alzheimer’s disease |
References
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The Biological Pathways of Alzheimer Disease: A Review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Sig. Transduct. Target Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Nursing Home Placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) Trial: Secondary and Post-Hoc Analyses. Lancet Neurol. 2015, 14, 1171–1181. [Google Scholar] [CrossRef]
- Bond, M.; Rogers, G.; Peters, J.; Anderson, R.; Hoyle, M.; Miners, A.; Moxham, T.; Davis, S.; Thokala, P.; Wailoo, A.; et al. The Effectiveness and Cost-Effectiveness of Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer’s Disease (Review of Technology Appraisal No. 111): A Systematic Review and Economic Model. Health Technol. Assess. 2012, 16, 1–470. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Berthier, M.L.; Rami, L. Donepezil Provides Greater Benefits in Mild Compared to Moderate Alzheimer’s Disease: Implications for Early Diagnosis and Treatment. Arch. Gerontol. Geriatr. 2011, 52, 18–22. [Google Scholar] [CrossRef]
- Wu, T.; Lin, D.; Cheng, Y.; Jiang, S.; Riaz, M.W.; Fu, N.; Mou, C.; Ye, M.; Zheng, Y. Amyloid Cascade Hypothesis for the Treatment of Alzheimer’s Disease: Progress and Challenges. Aging Dis. 2022, 13, 1745. [Google Scholar] [CrossRef]
- Piller, C. Research Backing Experimental Alzheimer’s Drug Was First Target of Suspicion. Science 2022, 377, 363. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Huang, J.; Huang, S.; Bai, Y.; Li, Y.; Huang, W. Efficacy of Antidepressant Drugs in the Treatment of Depression in Alzheimer Disease Patients: A Systematic Review and Network Meta-Analysis. J. Psychopharmacol. 2021, 35, 901–909. [Google Scholar] [CrossRef]
- Joshi, A.; Todd, S.; Finn, D.P.; McClean, P.L.; Wong-Lin, K. Multi-Dimensional Relationships among Dementia, Depression and Prescribed Drugs in England and Wales Hospitals. BMC Med. Inf. Decis. Mak. 2022, 22, 262. [Google Scholar] [CrossRef]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Hijroudi, F.; Rahbarghazi, R.; Sadigh-Eteghad, S.; Bahlakeh, G.; Hassanpour, M.; Shimia, M.; Karimipour, M. Neural Stem Cells Secretome Increased Neurogenesis and Behavioral Performance and the Activation of Wnt/β-Catenin Signaling Pathway in Mouse Model of Alzheimer’s Disease. Neuromol. Med. 2022, 24, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Zriek, F.; Di Battista, J.A.; Alaaeddine, N. Mesenchymal Stromal Cell Secretome: Immunomodulation, Tissue Repair and Effects on Neurodegenerative Conditions. CSCR 2021, 16, 656–669. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Yang, L.-P.; Zhao, L. Stem Cell Therapy for Alzheimer’s Disease. WJSC 2020, 12, 787–802. [Google Scholar] [CrossRef]
- Lu, M.-H.; Ji, W.-L.; Chen, H.; Sun, Y.-Y.; Zhao, X.-Y.; Wang, F.; Shi, Y.; Hu, Y.-N.; Liu, B.-X.; Wu, J.; et al. Intranasal Transplantation of Human Neural Stem Cells Ameliorates Alzheimer’s Disease-Like Pathology in a Mouse Model. Front. Aging Neurosci. 2021, 13, 650103. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Zhang, G.-X.; Ma, C. Intranasal Delivery of Stem Cells as Therapy for Central Nervous System Disease. Exp. Mol. Pathol. 2015, 98, 145–151. [Google Scholar] [CrossRef]
- Nakano, M.; Fujimiya, M. Potential Effects of Mesenchymal Stem Cell Derived Extracellular Vesicles and Exosomal miRNAs in Neurological Disorders. Neural Regen. Res. 2021, 16, 2359. [Google Scholar] [CrossRef]
- Kim, J.; Song, Y.; Park, C.H.; Choi, C. Platform Technologies and Human Cell Lines for the Production of Therapeutic Exosomes. EVCNA 2021, 2, 3–17. [Google Scholar] [CrossRef]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 317. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.; Hernández-Sapiéns, M.; Gutiérrez-Mercado, Y.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A. Mesenchymal Stem Cell-Derived Exosomes Promote Neurogenesis and Cognitive Function Recovery in a Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, D.Y.; Poltavtseva, R.A.; Svirshchevskaya, E.V.; Bobkova, N.V. Effect of Intranasal Administration of Multipotent Mesenchymal Stromal Cell Exosomes on Memory of Mice in Alzheimer’s Disease Model. Bull. Exp. Biol. Med. 2021, 170, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Gu, H.; Wang, J.; Zhang, J.; Zen, K.; Li, D. Comparative Analyses of Human Exosome Proteomes. Protein J. 2023, 42, 365–373. [Google Scholar] [CrossRef]
- Gho, Y.S.; Lee, C. Emergent Properties of Extracellular Vesicles: A Holistic Approach to Decode the Complexity of Intercellular Communication Networks. Mol. BioSyst. 2017, 13, 1291–1296. [Google Scholar] [CrossRef]
- Chitti, S.V.; Gummadi, S.; Kang, T.; Shahi, S.; Marzan, A.L.; Nedeva, C.; Sanwlani, R.; Bramich, K.; Stewart, S.; Petrovska, M.; et al. Vesiclepedia 2024: An extracellular vesicles and extracellular particles repository. Nucleic Acids Res. 2024, 52, D1694–D1698. [Google Scholar] [CrossRef]
- Borrelli, D.A.; Yankson, K.; Shukla, N.; Vilanilam, G.; Ticer, T.; Wolfram, J. Extracellular Vesicle Therapeutics for Liver Disease. J. Control. Release 2018, 273, 86–98. [Google Scholar] [CrossRef]
- Pascual, M.; Ibáñez, F.; Guerri, C. Exosomes as Mediators of Neuron-Glia Communication in Neuroinflammation. Neural Regen. Res. 2020, 15, 796. [Google Scholar] [CrossRef]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch Iv, T.L.; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p Mediates Cardioprotection by Mesenchymal Stem Cells. J. Mol. Cell. Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef]
- Hayashi, T.; Hoffman, M.P. Exosomal microRNA Communication between Tissues during Organogenesis. RNA Biol. 2017, 14, 1683–1689. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, Y.; Zhang, H.; Niu, Y.; Liu, C.; Wang, J.; Zen, K.; Zhang, C.-Y.; Li, D. Comprehensive Evolutionary Analysis of the Major RNA-Induced Silencing Complex Members. Sci. Rep. 2018, 8, 14189. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, H.; Hashimoto, S.; Watamura, N.; Sato, K.; Takamura, R.; Nagata, K.; Tsubuki, S.; Ohshima, T.; Yoshiki, A.; Sato, K.; et al. Recent Advances in the Modeling of Alzheimer’s Disease. Front. Neurosci. 2022, 16, 807473. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Alldred, M.J.; Che, S. Gene Expression Levels Assessed by CA1 Pyramidal Neuron and Regional Hippocampal Dissections in Alzheimer’s Disease. Neurobiol. Dis. 2012, 45, 99–107. [Google Scholar] [CrossRef]
- De Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In Vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Victor, M.B.; Tsai, L.-H. Dissecting the Complexities of Alzheimer Disease with in Vitro Models of the Human Brain. Nat. Rev. Neurol. 2022, 18, 25–39. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-Enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef]
- De Godoy, M.A.; Saraiva, L.M.; De Carvalho, L.R.P.; Vasconcelos-dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; Silva, L.R.D.P.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal Stem Cells and Cell-Derived Extracellular Vesicles Protect Hippocampal Neurons from Oxidative Stress and Synapse Damage Induced by Amyloid-β Oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Dasgupta, S.; Wang, G.; Zhu, G.; Bieberich, E. Exosome Reduction In Vivo Is Associated with Lower Amyloid Plaque Load in the 5XFAD Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 1792–1800. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lu, C.-H.; Ke, C.-C.; Chiu, S.-J.; Jeng, F.-S.; Chang, C.-W.; Yang, B.-H.; Liu, R.-S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Morris, M.; Coste, G.I.; Redding-Ochoa, J.; Guo, H.; Graves, A.R.; Troncoso, J.C.; Huganir, R.L. Hippocampal Synaptic Alterations Associated with Tau Pathology in Primary Age-Related Tauopathy. J. Neuropathol. Exp. Neurol. 2023, 82, 836–844. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. The Beneficial Effects of Mesenchymal Stem Cells and Their Exosomes on Myocardial Infarction and Critical Considerations for Enhancing Their Efficacy. Ageing Res. Rev. 2023, 89, 101980. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC Exosome Works through a Protein-Based Mechanism of Action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Chamling, X.; Zack, D.J.; Ahmed, Z.; Tomarev, S. TNFα-Mediated Priming of Mesenchymal Stem Cells Enhances Their Neuroprotective Effect on Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human Adipose Tissue-Derived Mesenchymal Stem Cells Secrete Functional Neprilysin-Bound Exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alnaaim, S.A.; Alexiou, A.; Papadakis, M.; Khalifa, A.A.; Saad, H.M.; Batiha, G.E. Neprilysin Inhibitors and Risk of Alzheimer’s Disease: A Future Perspective. J. Cell. Mol. Medi. 2024, 28, e17993. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Wang, Y.; Huang, H.; Zhao, Y.; Zhou, H. Meta-Analysis of Expression and Function of Neprilysin in Alzheimer’s Disease. Neurosci. Lett. 2017, 657, 69–76. [Google Scholar] [CrossRef]
- Alam, R.; Mrad, Y.; Hammoud, H.; Saker, Z.; Fares, Y.; Estephan, E.; Bahmad, H.F.; Harati, H.; Nabha, S. New Insights into the Role of Fibroblast Growth Factors in Alzheimer’s Disease. Mol. Biol. Rep. 2022, 49, 1413–1427. [Google Scholar] [CrossRef]
- Kalehua, A.N.; Nagel, J.E.; Whelchel, L.M.; Gides, J.J.; Pyle, R.S.; Smith, R.J.; Kusiak, J.W.; Taub, D.D. Monocyte Chemoattractant Protein-1 and Macrophage Inflammatory Protein-2 Are Involved in Both Excitotoxin-Induced Neurodegeneration and Regeneration. Exp. Cell Res. 2004, 297, 197–211. [Google Scholar] [CrossRef]
- Finneran, D.J.; Nash, K.R. Neuroinflammation and Fractalkine Signaling in Alzheimer’s Disease. J. Neuroinflammation. 2019, 16, 30. [Google Scholar] [CrossRef]
- Zheng, L.; Ishii, Y.; Tokunaga, A.; Hamashima, T.; Shen, J.; Zhao, Q.; Ishizawa, S.; Fujimori, T.; Nabeshima, Y.; Mori, H.; et al. Neuroprotective Effects of PDGF against Oxidative Stress and the Signaling Pathway Involved. J. Neurosci. Res. 2010, 88, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Góra-Kupilas, K.; Jośko, J. The Neuroprotective Function of Vascular Endothelial Growth Factor (VEGF). Folia Neuropathol. 2005, 43, 31–39. [Google Scholar] [PubMed]
- Bodart-Santos, V.; De Carvalho, L.R.P.; De Godoy, M.A.; Batista, A.F.; Saraiva, L.M.; Lima, L.G.; Abreu, C.A.; De Felice, F.G.; Galina, A.; Mendez-Otero, R.; et al. Extracellular Vesicles Derived from Human Wharton’s Jelly Mesenchymal Stem Cells Protect Hippocampal Neurons from Oxidative Stress and Synapse Damage Induced by Amyloid-β Oligomers. Stem. Cell Res. Ther. 2019, 10, 332. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Herron, S.; Botros, M.B.; Ikezu, T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019, 42, 361–372. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution Proteomic and Lipidomic Analysis of Exosomes and Microvesicles from Different Cell Sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Bobkova, N.V.; Garbuz, D.G.; Nesterova, I.; Medvinskaya, N.; Samokhin, A.; Alexandrova, I.; Yashin, V.; Karpov, V.; Kukharsky, M.S.; Ninkina, N.N.; et al. Therapeutic Effect of Exogenous Hsp70 in Mouse Models of Alzheimer’s Disease. JAD 2013, 38, 425–435. [Google Scholar] [CrossRef]
- Evgen’ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. JAD 2017, 59, 1415–1426. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Avetisyan, A.V.; Samokhin, A.N.; Alexandrova, I.Y.; Zinovkin, R.A.; Simonyan, R.A.; Bobkova, N.V. Mitochondrial Dysfunction in Neocortex and Hippocampus of Olfactory Bulbectomized Mice, a Model of Alzheimer’s Disease. Biochem. Mosc. 2016, 81, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kim, D.; Kim, Y.; Gho, Y.S. Proteomics, Transcriptomics and Lipidomics of Exosomes and Ectosomes. Proteomics 2013, 13, 1554–1571. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Otgontenger, U.; Jamsranjav, A.; Kim, S.S. Deleterious Alteration of Glia in the Brain of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6676. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High Complement Levels in Astrocyte-derived Exosomes of Alzheimer Disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef]
- Zanjani, H.; Finch, C.E.; Kemper, C.; Atkinson, J.; McKeel, D.; Morris, J.C.; Price, J.L. Complement Activation in Very Early Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2005, 19, 55–66. [Google Scholar] [CrossRef]
- Ailawadi, S.; Wang, X.; Gu, H.; Fan, G.-C. Pathologic Function and Therapeutic Potential of Exosomes in Cardiovascular Disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 1–11. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Chopp, M. Exosomes/miRNAs as Mediating Cell-Based Therapy of Stroke. Front. Cell. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef]
- Koutsis, G.; Siasos, G.; Spengos, K. The Emerging Role of microRNA in Stroke. CTMC 2013, 13, 1573–1588. [Google Scholar] [CrossRef]
- Li, D.-B.; Liu, J.-L.; Wang, W.; Li, R.-Y.; Yu, D.-J.; Lan, X.-Y.; Li, J.-P. Plasma Exosomal miR-422a and miR-125b-2-3p Serve as Biomarkers for Ischemic Stroke. CNR 2018, 14, 330–337. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Dammer, E.B.; Ren, R.-J.; Wang, G. The Endosomal-Lysosomal System: From Acidification and Cargo Sorting to Neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef]
- Polanco, J.C.; Li, C.; Durisic, N.; Sullivan, R.; Götz, J. Exosomes Taken up by Neurons Hijack the Endosomal Pathway to Spread to Interconnected Neurons. Acta Neuropathol. Commun. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.S.; Chuprov-Netochin, R.N.; Tsydenzhapova, E.; Svirshchevskaya, E.V.; Poltavtseva, R.A.; Merdalimova, A.; Yashchenok, A.; Keshelava, A.; Sorokin, K.; Keshelava, V.; et al. Asymmetric Depth-filtration: A Versatile and Scalable Method for High-yield Isolation of Extracellular Vesicles with Low Contamination. J. Extracell. Vesicles 2022, 11, e12256. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhdanova, D.Y.; Bobkova, N.V.; Chaplygina, A.V.; Svirshchevskaya, E.V.; Poltavtseva, R.A.; Vodennikova, A.A.; Chernyshev, V.S.; Sukhikh, G.T. Effect of Small Extracellular Vesicles Produced by Mesenchymal Stem Cells on 5xFAD Mice Hippocampal Cultures. Int. J. Mol. Sci. 2025, 26, 4026. https://doi.org/10.3390/ijms26094026
Zhdanova DY, Bobkova NV, Chaplygina AV, Svirshchevskaya EV, Poltavtseva RA, Vodennikova AA, Chernyshev VS, Sukhikh GT. Effect of Small Extracellular Vesicles Produced by Mesenchymal Stem Cells on 5xFAD Mice Hippocampal Cultures. International Journal of Molecular Sciences. 2025; 26(9):4026. https://doi.org/10.3390/ijms26094026
Chicago/Turabian StyleZhdanova, Daria Y., Natalia V. Bobkova, Alina V. Chaplygina, Elena V. Svirshchevskaya, Rimma A. Poltavtseva, Anastasia A. Vodennikova, Vasiliy S. Chernyshev, and Gennadiy T. Sukhikh. 2025. "Effect of Small Extracellular Vesicles Produced by Mesenchymal Stem Cells on 5xFAD Mice Hippocampal Cultures" International Journal of Molecular Sciences 26, no. 9: 4026. https://doi.org/10.3390/ijms26094026
APA StyleZhdanova, D. Y., Bobkova, N. V., Chaplygina, A. V., Svirshchevskaya, E. V., Poltavtseva, R. A., Vodennikova, A. A., Chernyshev, V. S., & Sukhikh, G. T. (2025). Effect of Small Extracellular Vesicles Produced by Mesenchymal Stem Cells on 5xFAD Mice Hippocampal Cultures. International Journal of Molecular Sciences, 26(9), 4026. https://doi.org/10.3390/ijms26094026






