Erectile Dysfunction and Oxidative Stress: A Narrative Review
Abstract
1. Introduction
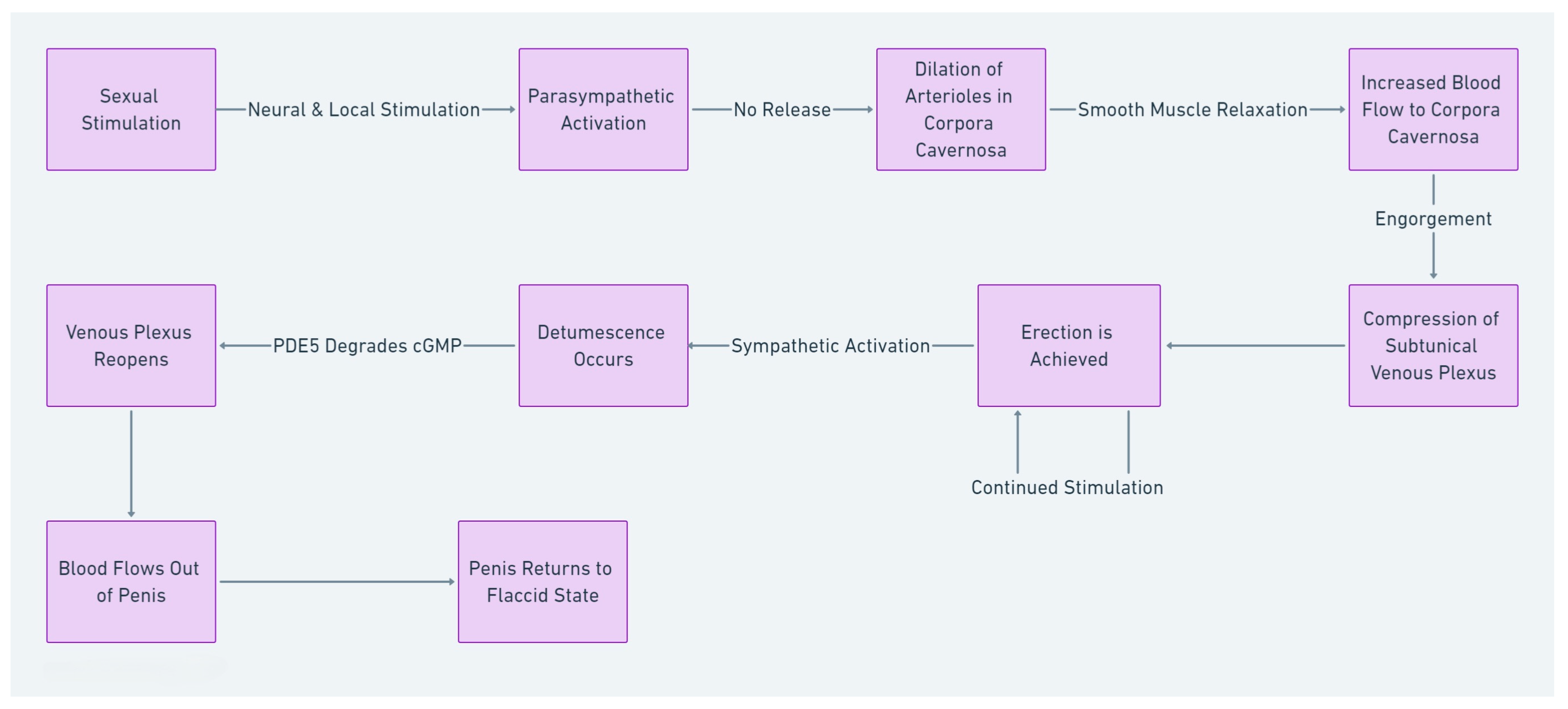
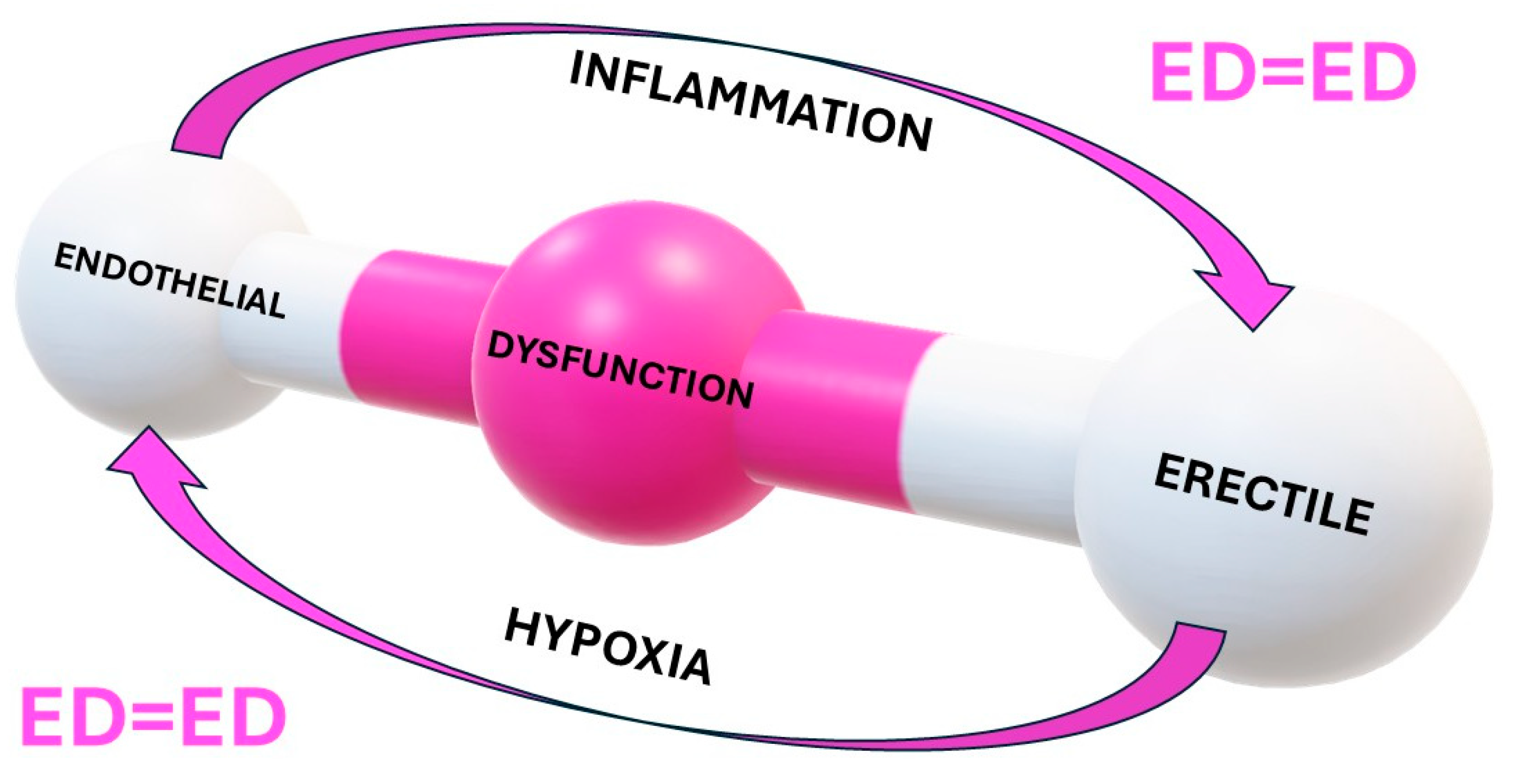
2. Endothelial Function and ED
3. Reactive Oxygen Species
3.1. Sources of ROS
3.1.1. Endogenous Sources of ROS
3.1.2. Exogenous Sources of ROS
3.1.3. Degenerated Products of Antioxidant Defenses
3.2. ROS Scavengers and Antioxidants
3.3. Chemical Reactivity of ROS
4. Environmental and Lifestyle Contributions
4.1. Psychological Distress and OS
4.2. Smoking
4.3. Obesity
4.4. Environmental Contributors
5. OS and Non-Communicable Diseases
5.1. Hypertension
5.2. Diabetes Mellitus
5.3. Hyperlipidemia
5.4. Male Hypogonadism
5.5. Lower Urinary Tract Symptoms (LUTS)
5.6. Gout
6. Other Sexual and Reproductive Dysfunctions
6.1. Premature Ejaculation
6.2. Male Infertility
7. Conclusions: Reducing Oxidative Stress to Improve Erectile Function
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ED | Erectile dysfunction |
| CVD | Cardiovascular disease |
| NCD | Non-Communicable Disease |
| LCEE | Loss of control of erection and ejaculation |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS | Neuronal nitric oxide synthase |
| eNOS | Endothelial nitric oxide synthase |
| PDE5 | Phosphodiesterase Type 5 |
| OS | Oxidative stress |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| ONOOH | Peroxynitrous acid |
| ONOO– | Peroxynitrite |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| NOX | NADPH oxidase |
| XO | Xanthine oxidase |
| iNOS | Inducible nitric oxide synthase |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| MAPK | Mitogen-activated protein kinases |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| MDD | Major depressive disorder |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| TNF | Tumor Necrosis Factor |
| HPG | Hypothalamic–pituitary–gonadal |
| T2DM | Type 2 diabetes mellitus |
| LUTS | Lower urinary tract symptoms |
| BPH | Benign Prostate Hyperplasia |
| XOR | Xanthine oxidoreductase |
| PE | Premature ejaculation |
| CP/CPPS | Chronic prostatitis/chronic pelvic pain syndrome |
References
- Impotence. NIH Consens. Statement 1992, 10, 1–33.
- Mark, K.P.; Arenella, K.; Girard, A.; Herbenick, D.; Fu, J.; Coleman, E. Erectile dysfunction prevalence in the United States: Report from the 2021 National Survey of Sexual Wellbeing. J. Sex. Med. 2024, 21, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Hussain, N.H.N.; Noor, N.M.; Mohamed, M.; Sidi, H.; Ismail, S.B. Epidemiology of Male Sexual Dysfunction in Asian and European Regions: A Systematic Review. Am. J. Men’s Health 2020, 14, 1557988320937200. [Google Scholar] [CrossRef]
- Jannini, E.A.; McCabe, M.P.; Salonia, A.; Montorsi, F.; Sachs, B.D. Organic vs. psychogenic? The Manichean diagnosis in sexual medicine. J. Sex. Med. 2010, 7, 1726–1733. [Google Scholar] [CrossRef]
- Corona, G.; Cucinotta, D.; Di Lorenzo, G.; Ferlin, A.; Giagulli, V.A.; Gnessi, L.; Isidori, A.M.; Maiorino, M.I.; Miserendino, P.; Murrone, A.; et al. The Italian Society of Andrology and Sexual Medicine (SIAMS), along with ten other Italian Scientific Societies, guidelines on the diagnosis and management of erectile dysfunction. J. Endocrinol. Investig. 2023, 46, 1241–1274. [Google Scholar] [CrossRef]
- Dewitte, M.; Bettocchi, C.; Carvalho, J.; Corona, G.; Flink, I.; Limoncin, E.; Pascoal, P.; Reisman, Y.; Van Lankveld, J. A Psychosocial Approach to Erectile Dysfunction: Position Statements from the European Society of Sexual Medicine (ESSM). Sex. Med. 2021, 9, 100434. [Google Scholar] [CrossRef]
- Sansone, A.; Romanelli, F.; Gianfrilli, D.; Lenzi, A. Endocrine evaluation of erectile dysfunction. Endocrine 2014, 46, 423–430. [Google Scholar] [CrossRef]
- Isidori, A.M.; Corona, G.; Aversa, A.; Gianfrilli, D.; Jannini, E.A.; Foresta, C.; Maggi, M.; Lenzi, A.; Group, S.-E.S. The SIAMS-ED Trial: A National, Independent, Multicentre Study on Cardiometabolic and Hormonal Impairment of Men with Erectile Dysfunction Treated with Vardenafil. Int. J. Endocrinol. 2014, 2014, 858715. [Google Scholar] [CrossRef]
- Sansone, A.; Reisman, Y.; Jannini, E.A. Relationship between hyperuricemia with deposition and sexual dysfunction in males and females. J. Endocrinol. Investig. 2022, 45, 691–703. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Limoncin, E.; Colonnello, E.; Jannini, E.A. Sexual Dysfunction in Men and Women with Diabetes: A Reflection of their Complications? Curr. Diabetes Rev. 2022, 18, e030821192147. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Zhang, W.; Liu, G.; Jiang, H.; Huang, H.; Zhang, X. Erectile Dysfunction in Multiple Sclerosis: A Prevalence Meta-Analysis and Systematic Review. J. Sex. Med. 2022, 19, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Konstantinidis, C. Neurogenic Erectile Dysfunction. Where Do We Stand? Medicines 2021, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Valles-Antuna, C.; Fernandez-Gomez, J.; Fernandez-Gonzalez, F. Peripheral neuropathy: An underdiagnosed cause of erectile dysfunction. BJU Int. 2011, 108, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.G. Erectile dysfunction. Intern. Med. J. 2014, 44, 18–26. [Google Scholar] [CrossRef]
- Jannini, E.A.; Lenzi, A.; Isidori, A.; Fabbri, A. Subclinical erectile dysfunction: Proposal for a novel taxonomic category in sexual medicine. J. Sex. Med. 2006, 3, 787–794. [Google Scholar] [CrossRef]
- Jannini, T.B.; Sansone, A.; Rossi, R.; Di Lorenzo, G.; Toscano, M.; Siracusano, A.; Jannini, E.A. Pharmacological strategies for sexual recovery in men undergoing antipsychotic treatment. Expert. Opin. Pharmacother. 2022, 23, 1065–1080. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Hagen, M.; Kriston, L.; Soares-Weiser, K.; Maayan, N.; Berner, M.M. Management of sexual dysfunction due to antipsychotic drug therapy. Cochrane Database Syst. Rev. 2012, 11, CD003546. [Google Scholar] [CrossRef]
- Trinchieri, M.; Trinchieri, M.; Perletti, G.; Magri, V.; Stamatiou, K.; Cai, T.; Montanari, E.; Trinchieri, A. Erectile and Ejaculatory Dysfunction Associated with Use of Psychotropic Drugs: A Systematic Review. J. Sex. Med. 2021, 18, 1354–1363. [Google Scholar] [CrossRef]
- Farmakis, I.T.; Pyrgidis, N.; Doundoulakis, I.; Mykoniatis, I.; Akrivos, E.; Giannakoulas, G. Effects of Major Antihypertensive Drug Classes on Erectile Function: A Network Meta-analysis. Cardiovasc. Drugs Ther. 2022, 36, 903–914. [Google Scholar] [CrossRef]
- Corona, G.; Vena, W.; Pizzocaro, A.; Salvio, G.; Sparano, C.; Sforza, A.; Maggi, M. Anti-hypertensive medications and erectile dysfunction: Focus on beta-blockers. Endocrine 2025, 87, 11–26. [Google Scholar] [CrossRef]
- Shindel, A.W.; Lue, T.F. Medical and Surgical Therapy of Erectile Dysfunction. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Mollaioli, D.; Ciocca, G.; Limoncin, E.; Di Sante, S.; Gravina, G.L.; Carosa, E.; Lenzi, A.; Jannini, E.A.F. Lifestyles and sexuality in men and women: The gender perspective in sexual medicine. Reprod. Biol. Endocrinol. 2020, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Tostes, R.C. Cigarette smoking and erectile dysfunction: An updated review with a focus on pathophysiology, e-cigarettes, and smoking cessation. Sex. Med. Rev. 2023, 11, 61–73. [Google Scholar] [CrossRef]
- Jannini, E.A. SM = SM: The Interface of Systems Medicine and Sexual Medicine for Facing Non-Communicable Diseases in a Gender-Dependent Manner. Sex. Med. Rev. 2017, 5, 349–364. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Isidori, A.M.; Pivonello, R.; Bettocchi, C.; Reisman, Y.; Sforza, A.; Maggi, M. Erectile dysfunction and cardiovascular risk: A review of current findings. Expert. Rev. Cardiovasc. Ther. 2020, 18, 155–164. [Google Scholar] [CrossRef]
- Corona, G. Erectile dysfunction and premature ejaculation: A continuum movens supporting couple sexual dysfunction. J. Endocrinol. Investig. 2022, 45, 2029–2041. [Google Scholar] [CrossRef]
- Colonnello, E.; Ciocca, G.; Limoncin, E.; Sansone, A.; Jannini, E.A. Redefining a sexual medicine paradigm: Subclinical premature ejaculation as a new taxonomic entity. Nat. Rev. Urol. 2021, 18, 115–127. [Google Scholar] [CrossRef]
- Colonnello, E.; Limoncin, E.; Ciocca, G.; Sansone, A.; Mollaioli, D.; Balercia, G.; Porst, H.; Zhang, H.; Yu, X.; Zhang, Y.; et al. The Lost Penis Syndrome: A New Clinical Entity in Sexual Medicine. Sex. Med. Rev. 2022, 10, 113–129. [Google Scholar] [CrossRef]
- Perelman, M.A. Psychosexual therapy for delayed ejaculation based on the Sexual Tipping Point model. Transl. Androl. Urol. 2016, 5, 563–575. [Google Scholar] [CrossRef]
- Wyllie, M.G. The underlying pathophysiology and causes of erectile dysfunction. Clin. Cornerstone 2005, 7, 19–27. [Google Scholar] [CrossRef]
- Dean, R.C.; Lue, T.F. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol. Clin. N. Am. 2005, 32, 379–395. [Google Scholar] [CrossRef]
- Ramírez-González, J.A.; Sansone, A. Male reproductive system. In Fertility, Pregnancy, and Wellness; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 23–36. [Google Scholar] [CrossRef]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Buvat, J.; Corona, G.; Goldstein, I.; Jannini, E.A.; Lenzi, A.; Porst, H.; Salonia, A.; Traish, A.M.; Maggi, M. A critical analysis of the role of testosterone in erectile function: From pathophysiology to treatment-a systematic review. Eur. Urol. 2014, 65, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Alwaal, A.; Breyer, B.N.; Lue, T.F. Normal male sexual function: Emphasis on orgasm and ejaculation. Fertil. Steril. 2015, 104, 1051–1060. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [PubMed]
- Dolci, S.; Belmonte, A.; Santone, R.; Giorgi, M.; Pellegrini, M.; Carosa, E.; Piccione, E.; Lenzi, A.; Jannini, E.A. Subcellular localization and regulation of type-1C and type-5 phosphodiesterases. Biochem. Biophys. Res. Commun. 2006, 341, 837–846. [Google Scholar] [CrossRef]
- Cesarini, V.; Guida, E.; Campolo, F.; Crescioli, C.; Di Baldassarre, A.; Pisano, C.; Balistreri, C.R.; Ruvolo, G.; Jannini, E.A.; Dolci, S. Type 5 phosphodiesterase (PDE5) and the vascular tree: From embryogenesis to aging and disease. Mech. Ageing Dev. 2020, 190, 111311. [Google Scholar] [CrossRef]
- Gravina, G.L.; Guida, E.; Dri, M.; Massoud, R.; Di Stasi, S.M.; Fucci, G.; Sansone, A.; Dolci, S.; Jannini, E.A. Measurement of PDE5 concentration in human serum: Proof-of-concept and validation of methodology in control and prostate cancer patients. J. Endocrinol. Investig. 2025, 48, 153–160. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Dimitriadis, F.; Sheshi, D.; Politis, M.; Moustakli, E.; Symeonidis, E.N.; Chrisofos, M.; Sofikitis, N.; Zachariou, A. Oxidative Stress and Erectile Dysfunction: Pathophysiology, Impacts, and Potential Treatments. Curr. Issues Mol. Biol. 2024, 46, 8807–8834. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Tostes, R.C.; Carneiro, F.S.; Carvalho, M.H.; Reckelhoff, J.F. Reactive oxygen species: Players in the cardiovascular effects of testosterone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1–R14. [Google Scholar] [CrossRef]
- Krylatov, A.V.; Maslov, L.N.; Voronkov, N.S.; Boshchenko, A.A.; Popov, S.V.; Gomez, L.; Wang, H.; Jaggi, A.S.; Downey, J.M. Reactive Oxygen Species as Intracellular Signaling Molecules in the Cardiovascular System. Curr. Cardiol. Rev. 2018, 14, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, A.; Miranda-Diaz, A.G.; Cardona-Munoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Usta, M.F.; Champion, H.C.; Kadowitz, P.J.; Hellstrom, W.J. Endothelial dysfunction in erectile dysfunction: Role of the endothelium in erectile physiology and disease. J. Androl. 2003, 24, S17–S37. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Guida, E.; Dolci, S.; Frangione, V.; Asso, A.; Bellia, G.; Jannini, E.A. Future perspectives for PDE5 inhibitors bridging the gap between cardiovascular health and psychological status. Basic Clin. Androl. 2025, 35, 3. [Google Scholar] [CrossRef]
- Guay, A.T. ED2: Erectile dysfunction = endothelial dysfunction. Endocrinol. Metab. Clin. N. Am. 2007, 36, 453–463. [Google Scholar] [CrossRef]
- Diaconu, C.C.; Manea, M.; Marcu, D.R.; Socea, B.; Spinu, A.D.; Bratu, O.G. The erectile dysfunction as a marker of cardiovascular disease: A review. Acta Cardiol. 2020, 75, 286–292. [Google Scholar] [CrossRef]
- Montorsi, P.; Ravagnani, P.M.; Galli, S.; Rotatori, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Montorsi, F. The artery size hypothesis: A macrovascular link between erectile dysfunction and coronary artery disease. Am. J. Cardiol. 2005, 96, 19M–23M. [Google Scholar] [CrossRef]
- Corona, G.; Forti, G.; Maggi, M. Why can patients with erectile dysfunction be considered lucky? The association with testosterone deficiency and metabolic syndrome. Aging Male 2008, 11, 193–199. [Google Scholar] [CrossRef]
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84, 104802. [Google Scholar] [CrossRef]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Witting, P.K.; Drummond, G.R. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008, 10, 1713–1765. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging. Mutat. Res. 1992, 275, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Manoj, K.M.; Bazhin, N.M. The murburn precepts for aerobic respiration and redox homeostasis. Progress Biophys. Mol. Biol. 2021, 167, 104–120. [Google Scholar] [CrossRef]
- Manoj, K.M.; Jaeken, L. Synthesis of theories on cellular powering, coherence, homeostasis and electro-mechanics: Murburn concept and evolutionary perspectives. J. Cell. Physiol. 2023, 238, 931–953. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Chapter 1. Overview of Reactive Oxygen Species. In Singlet Oxygen; The Royal Society of Chemistry: London, UK, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef]
- Siekevitz, P. Powerhouse of the Cell. Sci. Am. 1957, 197, 131–144. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Stowe, D.F.; Camara, A.K. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antioxid. Redox Signal. 2009, 11, 1373–1414. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Vanhoutte, P.M. Superoxide anion is an endothelium-derived contracting factor. Am. J. Physiol. 1989, 257, H33–H37. [Google Scholar] [CrossRef]
- Schroder, K. NADPH oxidases: Current aspects and tools. Redox Biol. 2020, 34, 101512. [Google Scholar] [CrossRef]
- Duan, J.; Gao, S.; Tu, S.; Lenahan, C.; Shao, A.; Sheng, J. Pathophysiology and Therapeutic Potential of NADPH Oxidases in Ischemic Stroke-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 6631805. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Wood, K.C.; Hsu, L.L.; Gladwin, M.T. Sickle cell disease vasculopathy: A state of nitric oxide resistance. Free Radic. Biol. Med. 2008, 44, 1506–1528. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive oxygen species in vascular biology: Role in arterial hypertension. Expert. Rev. Cardiovasc. Ther. 2003, 1, 91–106. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31 (Suppl. S2), S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Schmidt, H.M.; DeVallance, E.R.; Lewis, S.E.; Wood, K.C.; Annarapu, G.K.; Carreno, M.; Hahn, S.A.; Seman, M.; Maxwell, B.A.; Hileman, E.A.; et al. Release of hepatic xanthine oxidase (XO) to the circulation is protective in intravascular hemolytic crisis. Redox Biol. 2023, 62, 102636. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, V.; Reiss, K.; Kollau, A.; Mayer, B.; Gorren, A.C.F. Site and mechanism of uncoupling of nitric-oxide synthase: Uncoupling by monomerization and other misconceptions. Nitric Oxide 2019, 89, 14–21. [Google Scholar] [CrossRef]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef]
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.A.; Zweier, J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 2014, 53, 3679–3688. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef]
- Bayo Jimenez, M.T.; Frenis, K.; Hahad, O.; Steven, S.; Cohen, G.; Cuadrado, A.; Münzel, T.; Daiber, A. Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressors. Free Radic. Biol. Med. 2022, 187, 72–91. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef] [PubMed]
- Norma Francenia, S.-S.; Raúl, S.-C.; Claudia, V.-C.; Beatriz, H.-C. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Emad, S., Ed.; IntechOpen: Rijeka, Croatia, 2019; 418p. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.B.; Crack, P.J.; Flentjar, N.; Iannello, R.C.; Hertzog, P.J.; Kola, I. An imbalance in antioxidant defense affects cellular function: The pathophysiological consequences of a reduction in antioxidant defense in the glutathione peroxidase-1 (Gpx1) knockout mouse. Redox Rep. 2003, 8, 69–79. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
- Imlay, J.A.; Fridovich, I. Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 1991, 266, 6957–6965. [Google Scholar]
- Traber, M.G.; Manor, D. Vitamin E. Adv. Nutr. 2012, 3, 330–331. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, Y.; Liehn, E.A.; Rusu, M. Vitamin C as Scavenger of Reactive Oxygen Species during Healing after Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 3114. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.I.; Bokov, A.; Van Remmen, H.; Mele, J.; Ran, Q.; Ikeno, Y.; Richardson, A. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta 2009, 1790, 1005–1014. [Google Scholar] [CrossRef]
- Zhang, Y.; Ikeno, Y.; Qi, W.; Chaudhuri, A.; Li, Y.; Bokov, A.; Thorpe, S.R.; Baynes, J.W.; Epstein, C.; Richardson, A.; et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jedrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Carboneau, B.A.; Krzysik-Walker, S.M.; de Cabo, R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012, 11, 390–398. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Ravussin, E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003, 78, 361–369. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance exercise as a countermeasure for aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef]
- Brotto, L.; Atallah, S.; Johnson-Agbakwu, C.; Rosenbaum, T.; Abdo, C.; Byers, E.S.; Graham, C.; Nobre, P.; Wylie, K. Psychological and Interpersonal Dimensions of Sexual Function and Dysfunction. J. Sex. Med. 2016, 13, 538–571. [Google Scholar] [CrossRef]
- Maestre-Loren, F.; Castillo-Garayoa, J.A.; Lopez, I.M.X.; Sarquella-Geli, J.; Andres, A.; Cifre, I. Psychological Distress in Erectile Dysfunction: The Moderating Role of Attachment. Sex. Med. 2021, 9, 100436. [Google Scholar] [CrossRef]
- Lee, L.Y.; Loscalzo, J. Chapter 3—Systems Biology and network medicine: An integrated approach to redox biology and Pathobiology. In Oxidative Stress; Academic Press: New York, NY, USA, 2020; pp. 29–49. [Google Scholar] [CrossRef]
- Sansone, A.; Jannini, E.A.; Romanelli, F. Antioxidants in Male Sexual Dysfunctions. In Antioxidants in Andrology; Springer: Cham, Switzerland, 2017; pp. 71–79. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Aschbacher, K.; O’Donovan, A.; Wolkowitz, O.M.; Dhabhar, F.S.; Su, Y.; Epel, E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013, 38, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, L.; Cen, M.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J. Affect. Disord. 2023, 334, 205–212. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Rehman, S.; Kamal, M.A.; da Rocha, J.B.T. Association of Oxidative Stress with Neurological Disorders. Curr. Neuropharmacol. 2022, 20, 1046–1072. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhaes, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Tursich, M.; Neufeld, R.W.; Frewen, P.A.; Harricharan, S.; Kibler, J.L.; Rhind, S.G.; Lanius, R.A. Association of trauma exposure with proinflammatory activity: A transdiagnostic meta-analysis. Transl. Psychiatry 2014, 4, e413. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Dikalov, S.; Itani, H.; Richmond, B.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; Dikalova, A. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Cheng, C.; Wang, Y.; Xue, Y.; Li, W.; Li, X. Dose-related effect of secondhand smoke on cardiovascular disease in nonsmokers: Systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2020, 228, 113546. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.; Chen, F.; Zhang, C.; Guo, W.; Dai, S. Environmental tobacco smoke and carotid intima-media thickness in healthy children and adolescents: A systematic review. Open Heart 2022, 9, e001790. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Ho, D.R.; Shi, C.S.; Chen, C.S.; Li, J.M.; Huang, Y.C. The influence of smoking exposure and cessation on penile hemodynamics and corporal tissue in a rat model. Transl. Androl. Urol. 2020, 9, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; El Sayed, I.E.T.; Ali, M.M.A.; Wang, Y.; El Baz, A.; Elarabany, N.; Khalil, A.A. Oxidative stress and inflammation: Elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bull. Natl. Res. Cent. 2024, 48, 16. [Google Scholar] [CrossRef]
- Cao, S.; Gan, Y.; Dong, X.; Liu, J.; Lu, Z. Association of quantity and duration of smoking with erectile dysfunction: A dose-response meta-analysis. J. Sex. Med. 2014, 11, 2376–2384. [Google Scholar] [CrossRef]
- Allen, M.S.; Walter, E.E. Health-Related Lifestyle Factors and Sexual Dysfunction: A Meta-Analysis of Population-Based Research. J. Sex. Med. 2018, 15, 458–475. [Google Scholar] [CrossRef]
- Cao, S.; Yin, X.; Wang, Y.; Zhou, H.; Song, F.; Lu, Z. Smoking and risk of erectile dysfunction: Systematic review of observational studies with meta-analysis. PLoS ONE 2013, 8, e60443. [Google Scholar] [CrossRef]
- Corona, G.; Sansone, A.; Pallotti, F.; Ferlin, A.; Pivonello, R.; Isidori, A.M.; Maggi, M.; Jannini, E.A. People smoke for nicotine, but lose sexual and reproductive health for tar: A narrative review on the effect of cigarette smoking on male sexuality and reproduction. J. Endocrinol. Investig. 2020, 43, 1391–1408. [Google Scholar] [CrossRef]
- Sansone, A.; Limoncin, E.; Colonnello, E.; Mollaioli, D.; Ciocca, G.; Corona, G.; Jannini, E.A. Harm Reduction in Sexual Medicine. Sex. Med. Rev. 2022, 10, 3–22. [Google Scholar] [CrossRef]
- Moon, K.H.; Park, S.Y.; Kim, Y.W. Obesity and Erectile Dysfunction: From Bench to Clinical Implication. World J. Men’s Health 2019, 37, 138–147. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Chakraborty, S.; Choudhury, A.P.; Das, A.; Jha, N.K.; Slama, P.; Nath, M.; Massanyi, P.; Ruokolainen, J.; Kesari, K.K. Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction. Antioxidants 2021, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Drzezdzon, J.; Jacewicz, D.; Chmurzynski, L. The impact of environmental contamination on the generation of reactive oxygen and nitrogen species—Consequences for plants and humans. Environ. Int. 2018, 119, 133–151. [Google Scholar] [CrossRef]
- Lakey, P.S.; Berkemeier, T.; Tong, H.; Arangio, A.M.; Lucas, K.; Poschl, U.; Shiraiwa, M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016, 6, 32916. [Google Scholar] [CrossRef]
- Hsie, A.W.; Recio, L.; Katz, D.S.; Lee, C.Q.; Wagner, M.; Schenley, R.L. Evidence for reactive oxygen species inducing mutations in mammalian cells. Proc. Natl. Acad. Sci. USA 1986, 83, 9616–9620. [Google Scholar] [CrossRef]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3. [Google Scholar] [CrossRef]
- Francati, S.; Fiore, M.; Ferraguti, G. The janus face of oxidative stress in health and disease: The cause or the cure? Biomed. Rev. 2023, 34, 13–26. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Carrier, A. Metabolic Syndrome and Oxidative Stress: A Complex Relationship. Antioxid. Redox Signal. 2017, 26, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Falcon, D.; Gomez-Sanchez, L.; Gomez-Sanchez, M.; Rodriguez-Sanchez, E.; Tamayo-Morales, O.; Lugones-Sanchez, C.; Gonzalez-Sanchez, S.; Garcia-Ortiz, L.; Diaz, M.; Gomez-Marcos, M.A.; et al. Progression in Central Blood Pressure and Hemodynamic Parameters and Relationship With Cardiovascular Risk Factors in a Spanish Population: EVA Follow-Up Study. Am. J. Hypertens. 2024, 38, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Patil, S.M.; Dasgupta, A.; Podder, A.; Kumar, J.; Sindwani, P.; Karumuri, P. Unravelling the Intricate Relationship Between Oxidative Stress and Endothelial Dysfunction in Hypertension. Cureus 2024, 16, e61245. [Google Scholar] [CrossRef]
- Higashi, Y.; Kihara, Y.; Noma, K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 2012, 35, 1039–1047. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Ward, N.C.; Croft, K.D. Hypertension and oxidative stress. Clin. Exp. Pharmacol. Physiol. 2006, 33, 872–876. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Touyz, R.M. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: Role of angiotensin II. Exp. Physiol. 2005, 90, 449–455. [Google Scholar] [CrossRef]
- Corona, D.G.; Vena, W.; Pizzocaro, A.; Rastrelli, G.; Sparano, C.; Sforza, A.; Vignozzi, L.; Maggi, M. Metabolic syndrome and erectile dysfunction: A systematic review and meta-analysis study. J. Endocrinol. Investig. 2023, 46, 2195–2211. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- De Young, L.; Yu, D.; Bateman, R.M.; Brock, G.B. Oxidative stress and antioxidant therapy: Their impact in diabetes-associated erectile dysfunction. J. Androl. 2004, 25, 830–836. [Google Scholar] [CrossRef]
- Li, R.; Cui, K.; Wang, T.; Wang, S.; Li, X.; Qiu, J.; Yu, G.; Liu, J.; Wen, B.; Rao, K. Hyperlipidemia impairs erectile function in rats by causing cavernosal fibrosis. Andrologia 2017, 49, e12693. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, J.H.; Lee, Y.J. Effects of experimental hyperlipidemia on the pharmacokinetics of tadalafil in rats. J. Pharm. Pharm. Sci. 2012, 15, 528–537. [Google Scholar] [CrossRef]
- Huang, Y.C.; Ning, H.; Shindel, A.W.; Fandel, T.M.; Lin, G.; Harraz, A.M.; Lue, T.F.; Lin, C.S. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J. Sex. Med. 2010, 7, 1391–1400. [Google Scholar] [CrossRef]
- Musicki, B.; Liu, T.; Lagoda, G.A.; Strong, T.D.; Sezen, S.F.; Johnson, J.M.; Burnett, A.L. Hypercholesterolemia-induced erectile dysfunction: Endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J. Sex. Med. 2010, 7, 3023–3032. [Google Scholar] [CrossRef]
- Isidori, A.M.; Aversa, A.; Calogero, A.; Ferlin, A.; Francavilla, S.; Lanfranco, F.; Pivonello, R.; Rochira, V.; Corona, G.; Maggi, M. Adult- and late-onset male hypogonadism: The clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2022, 45, 2385–2403. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Corona, G.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men. Aging Male 2021, 24, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gul, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Castellana, M.; Lisco, G.; Triggiani, V. Critical evaluation of different available guidelines for late-onset hypogonadism. Andrology 2020, 8, 1628–1641. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, F.; Sansone, A.; Lenzi, A. Erectile dysfunction in aging male. Acta Biomed. 2010, 81 (Suppl. S1), 89–94. [Google Scholar]
- Corona, G.; Goulis, D.G.; Huhtaniemi, I.; Zitzmann, M.; Toppari, J.; Forti, G.; Vanderschueren, D.; Wu, F.C. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology 2020, 8, 970–987. [Google Scholar] [CrossRef]
- Leisegang, K.; Roychoudhury, S.; Slama, P.; Finelli, R. The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease. Antioxidants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Traish, A.M.; Miner, M.M.; Morgentaler, A.; Zitzmann, M. Testosterone deficiency. Am. J. Med. 2011, 124, 578–587. [Google Scholar] [CrossRef]
- Corpas, F.J.; Rio, L.A.D.; Palma, J.M. Impact of Nitric Oxide (NO) on the ROS Metabolism of Peroxisomes. Plants 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Elrashidy, R.A.; Li, B.; Liu, G. Oxidative Stress: A Putative Link Between Lower Urinary Tract Symptoms and Aging and Major Chronic Diseases. Front. Med. 2022, 9, 812967. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burgio, G.; Condorelli, R.A.; Cannarella, R.; La Vignera, S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male 2019, 22, 12–19. [Google Scholar] [CrossRef]
- Fusco, F.; D’Anzeo, G.; Sessa, A.; Pace, G.; Rossi, A.; Capece, M.; d’Emmanuele di Villa Bianca, R. BPH/LUTS and ED: Common pharmacological pathways for a common treatment. J. Sex. Med. 2013, 10, 2382–2393. [Google Scholar] [CrossRef]
- De Nunzio, C.; Lombardo, R.; Tema, G.; Tubaro, A. Erectile Dysfunction and Lower Urinary Tract Symptoms. Curr. Urol. Rep. 2018, 19, 61. [Google Scholar] [CrossRef]
- Andersson, K.E. Oxidative Stress and Its Relation to Lower Urinary Tract Symptoms. Int. Neurourol. J. 2022, 26, 261–267. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y.H.; Yamada, Y.; Daneshgari, F. External urethral sphincter activity in diabetic rats. Neurourol. Urodyn. 2008, 27, 429–434. [Google Scholar] [CrossRef]
- Kracochansky, M.; Reis, L.O.; Lorenzetti, F.; Ortiz, V.; Dambros, M. Impact of castration with or without alpha-tocopherol supplementation on the urethral sphincter of rats. Int. Braz. J. Urol. 2012, 38, 277–283. [Google Scholar] [CrossRef]
- Yang, Z.; Dolber, P.C.; Fraser, M.O. Diabetic urethropathy compounds the effects of diabetic cystopathy. J. Urol. 2007, 178, 2213–2219. [Google Scholar] [CrossRef]
- Kullisaar, T.; Turk, S.; Punab, M.; Mandar, R. Oxidative stress--cause or consequence of male genital tract disorders? Prostate 2012, 72, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Arsova-Sarafinovska, Z.; Sayal, A.; Eken, A.; Erdem, O.; Erten, K.; Ozgok, Y.; Dimovski, A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin. Biochem. 2006, 39, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Gecit, I.; Meral, I.; Aslan, M.; Kocyigit, A.; Celik, H.; Taskin, A.; Kaba, M.; Pirincci, N.; Gunes, M.; Taken, K.; et al. Peripheral mononuclear leukocyte DNA damage, plasma prolidase activity, and oxidative status in patients with benign prostatic hyperplasia. Redox Rep. 2015, 20, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef]
- Krishnan, E. Inflammation, oxidative stress and lipids: The risk triad for atherosclerosis in gout. Rheumatology 2010, 49, 1229–1238. [Google Scholar] [CrossRef]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Roddy, E.; Zhang, W.; Doherty, M. Is gout associated with reduced quality of life? A case-control study. Rheumatology 2007, 46, 1441–1444. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, J.; Guo, J.; Chen, H.; Zhang, X.; Gu, Z.; Zhou, F.; Dong, C. Health-related quality of life assessed by Gout Impact Scale (GIS) in Chinese patients with gout. Curr. Med. Res. Opin. 2020, 36, 2071–2078. [Google Scholar] [CrossRef]
- Chandratre, P.; Mallen, C.; Richardson, J.; Muller, S.; Hider, S.; Rome, K.; Blagojevic-Bucknall, M.; Roddy, E. Health-related quality of life in gout in primary care: Baseline findings from a cohort study. Semin. Arthritis Rheum. 2018, 48, 61–69. [Google Scholar] [CrossRef]
- Singh, J.A. Gout and sexual function: Patient perspective of how gout affects personal relationships and intimacy. BMC Rheumatol. 2019, 3, 8. [Google Scholar] [CrossRef]
- Sansone, A.; Reisman, Y.; Meto, S.; Dolci, S.; Jannini, E.A. The Role of the “Anti-Inflammatory” Couple for the Management of Hyperuricemia With Deposition. Sex. Med. 2022, 10, 100562. [Google Scholar] [CrossRef] [PubMed]
- Reach, G. Treatment adherence in patients with gout. Jt. Bone Spine 2011, 78, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Ciocca, G.; Limoncin, E.; Mollaioli, D.; Di Sante, S.; Gianfrilli, D.; Lombardo, F.; Lenzi, A. Premature ejaculation: Old story, new insights. Fertil. Steril. 2015, 104, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Amar, E.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Montorsi, F.; Vardi, Y.; Wespes, E.; European Association of, U. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. Eur. Urol. 2010, 57, 804–814. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Limoncin, E.; Sforza, A.; Jannini, E.A.; Maggi, M. Interplay Between Premature Ejaculation and Erectile Dysfunction: A Systematic Review and Meta-Analysis. J. Sex. Med. 2015, 12, 2291–2300. [Google Scholar] [CrossRef]
- Sansone, A.; Romanelli, F.; Jannini, E.A.; Lenzi, A. Hormonal correlations of premature ejaculation. Endocrine 2015, 49, 333–338. [Google Scholar] [CrossRef]
- Corona, G.; Jannini, E.A.; Vignozzi, L.; Rastrelli, G.; Maggi, M. The hormonal control of ejaculation. Nat. Rev. Urol. 2012, 9, 508–519. [Google Scholar] [CrossRef]
- Jannini, E.A.; Isidori, A.M.; Aversa, A.; Lenzi, A.; Althof, S.E. Which is first? The controversial issue of precedence in the treatment of male sexual dysfunctions. J. Sex. Med. 2013, 10, 2359–2369. [Google Scholar] [CrossRef]
- Min, S.; Xu, J.; Ren, C.; Cai, Z.; Li, H.; Wang, Z. The correlation between premature ejaculation and a high incidence of erectile dysfunction and its research progress: A narrative review. Transl. Androl. Urol. 2024, 13, 2338–2350. [Google Scholar] [CrossRef]
- Sansone, A.; Aversa, A.; Corona, G.; Fisher, A.D.; Isidori, A.M.; La Vignera, S.; Limoncin, E.; Maggi, M.; Merico, M.; Jannini, E.A. Management of premature ejaculation: A clinical guideline from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2021, 44, 1103–1118. [Google Scholar] [CrossRef]
- Abdel-Hameed, S.S.; El-Daly, M.; Ahmed, A.F.; Bekhit, A.A.; Heeba, G.H. Dapoxetine prevents neuronal damage and improves functional outcomes in a model of ischemic stroke through the modulation of inflammation and oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Yuan, J.; Hou, G.; Zhang, L.; Gao, M.; Zhang, Z.; Jiang, H.; Wang, F.; Guo, J.; Geng, Q.; et al. From Waterloo to the Great Wall: A retrospective, multicenter study on the clinical practice and cultural attitudes in the management of premature ejaculation, in China. Andrology 2024, 12, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. Male Infertility. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wischmann, T.H. Sexual disorders in infertile couples. J. Sex. Med. 2010, 7, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Pu, Z.; Wang, Y.; Zhang, Y.; Dong, C.; Zeng, Y.; Zhou, S. Sexual Dysfunction in Infertile Men: A Systematic Review and Meta-Analysis. Sex. Med. 2022, 10, 100528. [Google Scholar] [CrossRef]
- Luca, G.; Parrettini, S.; Sansone, A.; Calafiore, R.; Jannini, E.A. The Inferto-Sex Syndrome (ISS): Sexual dysfunction in fertility care setting and assisted reproduction. J. Endocrinol. Investig. 2021, 44, 2071–2102. [Google Scholar] [CrossRef]
- Griveau, J.F.; Le Lannou, D. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef]
- Lopes, S.; Jurisicova, A.; Sun, J.G.; Casper, R.F. Reactive oxygen species: Potential cause for DNA fragmentation in human spermatozoa. Hum. Reprod. 1998, 13, 896–900. [Google Scholar] [CrossRef]
- Kao, S.H.; Chao, H.T.; Chen, H.W.; Hwang, T.I.S.; Liao, T.L.; Wei, Y.H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Kierspel, E.; Stalf, T.; Mehnert, C.; Menkveld, R.; Tinneberg, H.R.; Schill, W.B.; Kruger, T.F. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil. Steril. 2005, 83, 635–642. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Nada, E.A.; El-Tonsy, M.H.; Sharma, R.K.; Meyer, A.; Nelson, D.R.; Thomas, A.J. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil. Steril. 2003, 79 (Suppl. S3), 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Sansone, A.; Romanelli, F.; Paoli, D.; Gandini, L.; Lenzi, A. The role of antioxidant therapy in the treatment of male infertility: An overview. Asian J. Androl. 2011, 13, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Aversa, A.; La Vignera, S.; Corona, G.; Ferlin, A. The use of nutraceuticals in male sexual and reproductive disturbances: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2017, 40, 1389–1397. [Google Scholar] [CrossRef]
- Gianfrilli, D.; Ferlin, A.; Isidori, A.M.; Garolla, A.; Maggi, M.; Pivonello, R.; Santi, D.; Sansone, A.; Balercia, G.; Granata, A.R.M.; et al. Risk behaviours and alcohol in adolescence are negatively associated with testicular volume: Results from the Amico-Andrologo survey. Andrology 2019, 7, 769–777. [Google Scholar] [CrossRef]
- Sansone, A.; Sansone, M.; Vaamonde, D.; Sgro, P.; Salzano, C.; Romanelli, F.; Lenzi, A.; Di Luigi, L. Sport, doping and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 114. [Google Scholar] [CrossRef]
- Jannini, E.A.; Reisman, Y. Medicine Without Sexual Medicine Is Not Medicine: An MJCSM and ESSM Petition on Sexual Health to the Political and University Authorities. J. Sex. Med. 2019, 16, 943–945. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Ockaili, R.; Salloum, F.; Yin, C.; Hawkins, J.; Das, A.; Xi, L. Cardioprotection with phosphodiesterase-5 inhibition--a novel preconditioning strategy. J. Mol. Cell Cardiol. 2004, 36, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Deyoung, L.; Chung, E.; Kovac, J.R.; Romano, W.; Brock, G.B. Daily use of sildenafil improves endothelial function in men with type 2 diabetes. J. Androl. 2012, 33, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Tzoumas, N.; Farrah, T.E.; Dhaun, N.; Webb, D.J. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br. J. Pharmacol. 2020, 177, 5467–5488. [Google Scholar] [CrossRef] [PubMed]
- Cupone, I.E.; Sansone, A.; Marra, F.; Giori, A.M.; Jannini, E.A. Orodispersible Film (ODF) Platform Based on Maltodextrin for Therapeutical Applications. Pharmaceutics 2022, 14, 2011. [Google Scholar] [CrossRef]
- Jannini, E.A.; Vignesh, S.O.; Hassan, T. Next-generation pharmaceuticals: The rise of sildenafil citrate ODF for the treatment of men with erectile dysfunction. Ther. Deliv. 2025, 1–14. [Google Scholar] [CrossRef]
- Sansone, A.; Frangione, V.; Lanzarotti, A.; Cocci, A.; Ceruti, C.; De Sio, M.; Imbimbo, C.; Mirone, V.; Schips, L.; Terrone, C.; et al. Effect of the new 75-mg orodispersible film of sildenafil on erection and sexual quality of life: Insights from an observational study. Sex. Med. 2023, 11, qfac007. [Google Scholar] [CrossRef]
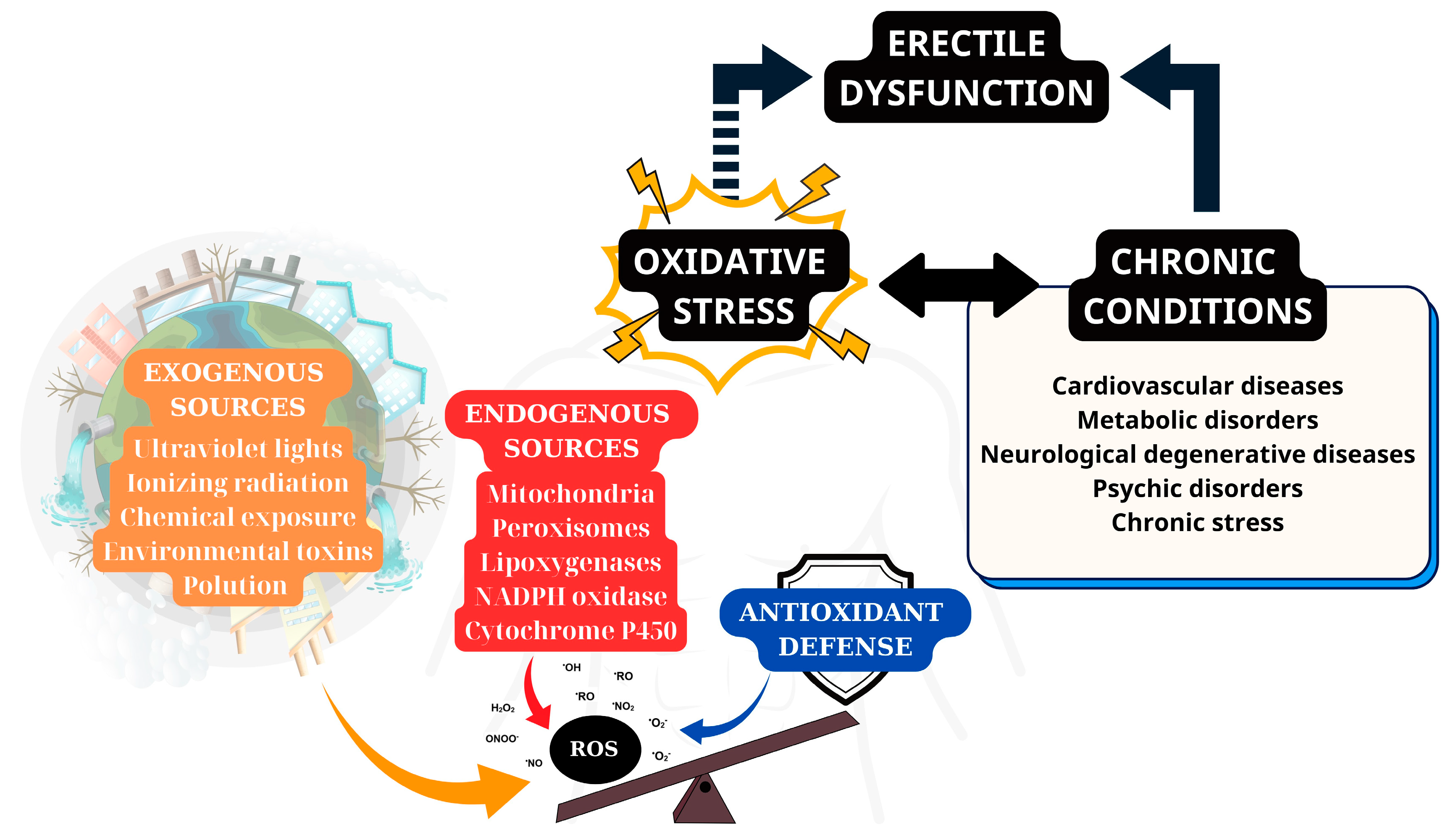
| Category | Source | Description | Example/Notes |
|---|---|---|---|
| Endogenous Sources | Mitochondria | Electron leakage during oxidative phosphorylation generates superoxide. | Major contributor to oxidative damage in tissues; linked to ED via reduced NO availability and endothelial dysfunction. |
| NADPH Oxidase (NOX) | Transmembrane enzymes transfer electrons from NADPH to O2, producing superoxide. | Critical for immune defense (e.g., neutrophils), but excess ROS causes tissue damage (e.g., hypertension, diabetes). | |
| Xanthine Oxidase (XO) | Catalyzes hypoxanthine → xanthine → uric acid, releasing ROS. | Elevated in liver damage, hypoxia, or inflammation; unclear role in ED. | |
| Uncoupled NOS | NOS isoforms (eNOS, nNOS, iNOS) produce superoxide instead of NO when uncoupled. | eNOS uncoupling in penile tissue contributes to ED and local oxidative stress. | |
| Exogenous Sources | Environmental Pollutants | Heavy metals (Fe, Cu, Cd, Hg), industrial solvents, air pollution. | Trigger ROS via redox cycling or direct oxidation. |
| Lifestyle Factors | Smoking, alcohol, UV/X-ray radiation, poor diet. | Directly induce ROS production or impair antioxidant defenses. | |
| Drugs | Halothane, doxorubicin, and metronidazole | Generate ROS as a side effect, exacerbating oxidative stress. | |
| Degenerated Antioxidant Defenses | Dysfunctional Antioxidant Enzymes | SOD, catalase, glutathione peroxidase lose activity due to mutations or damage. | Failure to neutralize ROS leads to accumulation (e.g., SOD dysfunction in aging). |
| Autoxidation of Antioxidants | Reduced glutathione (GSH) or vitamin C reacts with transition metals (Fe, Cu). | Paradoxically generate ROS (e.g., Fenton reaction). | |
| Degradation of Antioxidants | Polyphenols, carotenoids, or flavonoids degrade into pro-oxidants. | Prolonged heat/light exposure converts antioxidants into ROS-promoting compounds. | |
| Antioxidant Imbalance | Excess antioxidants react with ROS to form unstable intermediates. | “Antioxidant paradox”: High antioxidant levels may exacerbate oxidative stress. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Pham, Q.M.; Wang, C.; Colonnello, E.; Yannas, D.; Nguyen, B.H.; Zhang, Y.; Jannini, E.A.; Sansone, A. Erectile Dysfunction and Oxidative Stress: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3073. https://doi.org/10.3390/ijms26073073
Zhu D, Pham QM, Wang C, Colonnello E, Yannas D, Nguyen BH, Zhang Y, Jannini EA, Sansone A. Erectile Dysfunction and Oxidative Stress: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(7):3073. https://doi.org/10.3390/ijms26073073
Chicago/Turabian StyleZhu, Dake, Quan Minh Pham, Chunlin Wang, Elena Colonnello, Dimitri Yannas, Bac Hoai Nguyen, Yan Zhang, Emmanuele A. Jannini, and Andrea Sansone. 2025. "Erectile Dysfunction and Oxidative Stress: A Narrative Review" International Journal of Molecular Sciences 26, no. 7: 3073. https://doi.org/10.3390/ijms26073073
APA StyleZhu, D., Pham, Q. M., Wang, C., Colonnello, E., Yannas, D., Nguyen, B. H., Zhang, Y., Jannini, E. A., & Sansone, A. (2025). Erectile Dysfunction and Oxidative Stress: A Narrative Review. International Journal of Molecular Sciences, 26(7), 3073. https://doi.org/10.3390/ijms26073073








