Can Humanized Immune System Mouse and Rat Models Accelerate the Development of Cytomegalovirus-Based Vaccines Against Infectious Diseases and Cancers?
Abstract
1. Introduction
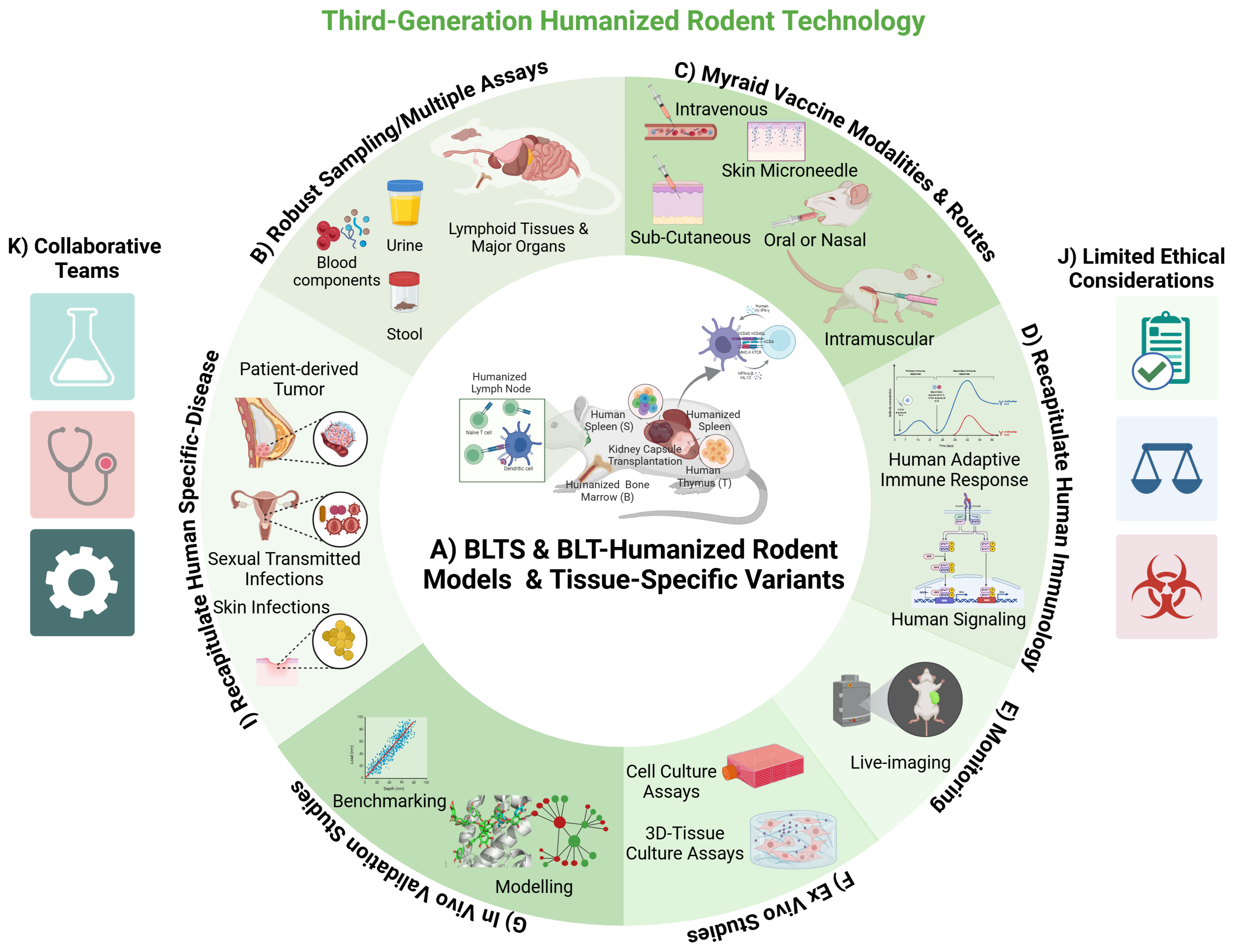
2. Humanized Immune System (HIS) Rodent Models and Related Variants
3. Modeling Pathogen Infections and Human Cancer in HIS Rodent Models and Related Variants
4. HIS-Rodent Models of HCMV Infection and Immune Response
5. The Biology of HCMV-Based Viral Vectored Vaccines
6. Vaccinology Considerations for HCMV-Based Viral Vectored Vaccines
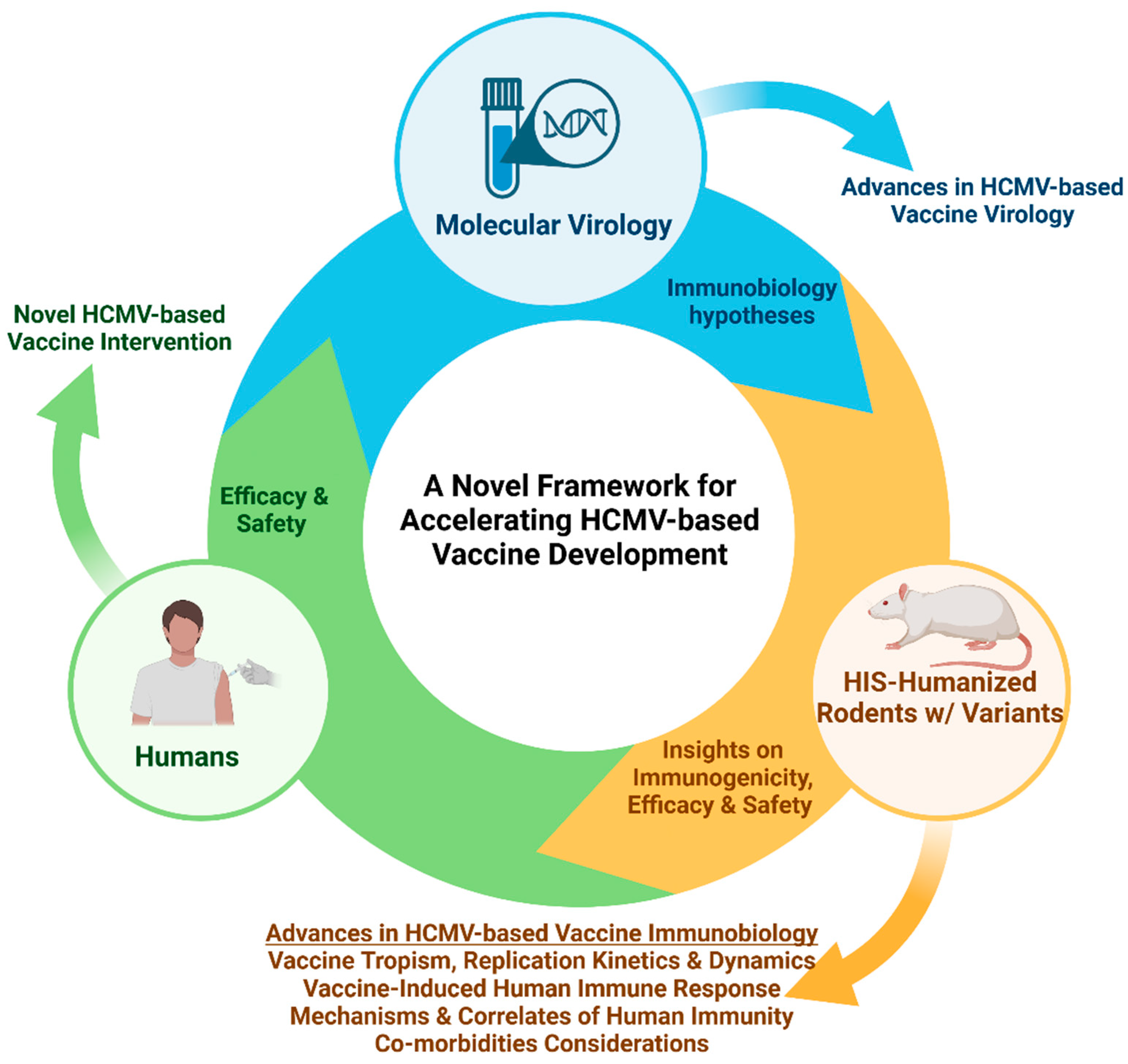
7. Application of HIS-Rodent Models in Accelerating the Development of HCMV-Based Viral Vector Vaccines Against Infectious Diseases and Cancers
8. Future Directions for Immunobiology Modeling in HIS-Rodents and Related Variants
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agarwal, Y.; Beatty, C.; Biradar, S.; Castronova, I.; Ho, S.; Melody, K.; Bility, M.T. Moving beyond the mousetrap: Current and emerging humanized mouse and rat models for investigating prevention and cure strategies against HIV infection and associated pathologies. Retrovirology 2020, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Akkina, R.; Barber, D.L.; Bility, M.T.; Bissig, K.-D.; Burwitz, B.J.; Eichelberg, K.; Endsley, J.J.; Garcia, J.V.; Hafner, R.; Karakousis, P.C.; et al. Small Animal Models for Human Immunodeficiency Virus (HIV), Hepatitis B, and Tuberculosis: Proceedings of an NIAID Workshop. Curr. HIV Res. 2020, 18, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Leucci, E. Humanized mouse models for anti-cancer therapy. In Methods in Cell Biology; Abhishek, G., Lorenzo, G., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 183, pp. 317–333. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Li, D.; Zhou, W.; Yan, F.; Wang, W. Humanized mouse models: A valuable platform for preclinical evaluation of human cancer. Biotechnol. Bioeng. 2023, 121, 835–852. [Google Scholar] [CrossRef]
- Yahata, T.; Ando, K.; Nakamura, Y.; Ueyama, Y.; Shimamura, K.; Tamaoki, N.; Kato, S.; Hotta, T. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor γ null mice. J. Immunol. 2002, 169, 204–209. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/γcnull mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Stripecke, R.; Münz, C.; Schuringa, J.J.; Bissig, K.; Soper, B.; Meeham, T.; Yao, L.; Di Santo, J.P.; Brehm, M.; Rodriguez, E.; et al. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol. Med. 2020, 12, e8662. [Google Scholar] [CrossRef]
- Theobald, S.J.; Kreer, C.; Khailaie, S.; Bonifacius, A.; Eiz-Vesper, B.; Figueiredo, C.; Mach, M.; Backovic, M.; Ballmaier, M.; Koenig, J.; et al. Repertoire characterization and validation of gB-specific human IgGs directly cloned from humanized mice vaccinated with dendritic cells and protected against HCMV. PLoS Pathog. 2020, 16, e1008560. [Google Scholar] [CrossRef]
- Sun, Z.; Denton, P.W.; Estes, J.D.; Othieno, F.A.; Wei, B.L.; Wege, A.K.; Melkus, M.W.; Padgett-Thomas, A.; Zupancic, M.; Haase, A.T.; et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 2007, 204, 705–714. [Google Scholar] [CrossRef]
- Denton, P.W.; Estes, J.D.; Sun, Z.; Othieno, F.A.; Wei, B.L.; Wege, A.K.; Powell, D.A.; Payne, D.; Haase, A.T.; Garcia, J.V. Antiretroviral Pre-exposure Prophylaxis Prevents Vaginal Transmission of HIV-1 in Humanized BLT Mice. PLoS Med. 2008, 5, e16. [Google Scholar] [CrossRef]
- Jangalwe, S.; Shultz, L.D.; Mathew, A.; Brehm, M.A. Improved B cell development in humanized NOD-scid IL2Rγnull mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 2016, 4, 427–440. [Google Scholar] [CrossRef]
- Brehm, M.A.; Jouvet, N.; Greiner, D.L.; Shultz, L.D. Humanized mice for the study of infectious diseases. Curr. Opin. Immunol. 2013, 25, 428–435. [Google Scholar] [CrossRef]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Macchiarini, F.; Manz, M.G.; Palucka, A.K.; Shultz, L.D. Humanized mice: Are we there yet? J. Exp. Med. 2005, 202, 1307–1311. [Google Scholar] [CrossRef]
- Denayer, T.; Stöhr, T.; Van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef]
- Crawford, L.B.; Tempel, R.; Streblow, D.N.; Kreklywich, C.; Smith, P.; Picker, L.J.; Nelson, J.A.; Caposio, P. Human Cytomegalovirus Induces Cellular and Humoral Virus-specific Immune Responses in Humanized BLT Mice. Sci. Rep. 2017, 7, 937. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Theobald, S.J.; Stripecke, R. Modeling Human Cytomegalovirus in Humanized Mice for Vaccine Testing. Vaccines 2020, 8, 89. [Google Scholar] [CrossRef]
- Barrenäs, F.; Hansen, S.G.; Law, L.; Driscoll, C.; Green, R.R.; Smith, E.; Chang, J.; Golez, I.; Urion, T.; Peng, X.; et al. Interleukin-15 response signature predicts RhCMV/SIV vaccine efficacy. PLoS Pathog. 2021, 17, e1009278. [Google Scholar] [CrossRef]
- Picker, L.J.; Lifson, J.D.; Gale, M.; Hansen, S.G.; Früh, K. Programming cytomegalovirus as an HIV vaccine. Trends Immunol. 2023, 44, 287–304. [Google Scholar] [CrossRef]
- Bility, M.T.; Cheng, L.; Zhang, Z.; Luan, Y.; Li, F.; Chi, L.; Zhang, L.; Tu, Z.; Gao, Y.; Fu, Y.; et al. Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages. PLoS Pathog. 2014, 10, e1004032. [Google Scholar] [CrossRef]
- Bility, M.T.; Curtis, A.; Su, L. A chimeric mouse model to study immunopathogenesis of HCV infection. Methods Mol Biol. 2014, 1213, 379–388. [Google Scholar] [CrossRef]
- Bility, M.T.; Li, F.; Cheng, L.; Su, L. Liver immune-pathogenesis and therapy of human liver tropic virus infection in humanized mouse models. J. Gastroenterol. Hepatol. 2013, 28, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bility, M.T.; Nio, K.; Li, F.; McGivern, D.R.; Lemon, S.M.; Feeney, E.R.; Chung, R.T.; Su, L. Chronic hepatitis C infection–induced liver fibrogenesis is associated with M2 macrophage activation. Sci. Rep. 2016, 6, 39520. [Google Scholar] [CrossRef]
- Biradar, S.; Agarwal, Y.; Lotze, M.T.; Bility, M.T.; Mailliard, R.B. The BLT Humanized Mouse Model as a Tool for Studying Human Gamma Delta T Cell-HIV Interactions In Vivo. Front. Immunol. 2022, 13, 881607. [Google Scholar] [CrossRef]
- Biradar, S.; Agarwal, Y.; Das, A.; Shu, S.T.; Samal, J.; Ho, S.; Kelly, N.; Mahesh, D.; Teredesai, S.; Castronova, I.; et al. Nef defect attenuates HIV viremia and immune dysregulation in the bone marrow-liver-thymus-spleen (BLTS) humanized mouse model. Virology 2024, 598, 110192. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, G.; Li, D.; Zhang, Z.; Li, F.; Zurawski, S.; Zurawski, G.; Levy, Y.; Su, L. A novel therapeutic vaccination delays cART-resistant HIV-1 reservoir rebound in HIV-1 infected humanized mice. J. Immunol. 2016, 196, 76.7. [Google Scholar] [CrossRef]
- Dash, P.K.; Kaminski, R.; Bella, R.; Su, H.; Mathews, S.; Ahooyi, T.M.; Chen, C.; Mancuso, P.; Sariyer, R.; Ferrante, P.; et al. Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat. Commun. 2019, 10, 2753. [Google Scholar] [CrossRef]
- Godot, V.; Tcherakian, C.; Gil, L.; Cervera-Marzal, I.; Li, G.; Cheng, L.; Ortonne, N.; Lelièvre, J.-D.; Pantaleo, G.; Fenwick, C.; et al. TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice. PLoS Pathog. 2020, 16, e1009025. [Google Scholar] [CrossRef]
- Ménoret, S.; Renart-Depontieu, F.; Martin, G.; Thiam, K.; Anegon, I. Efficient generation of human immune system rats using human CD34+ cells. Stem Cell Rep. 2024, 19, 1255–1263. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Agarwal, Y.; Beatty, C.; Ho, S.; Thurlow, L.; Das, A.; Kelly, S.; Castronova, I.; Salunke, R.; Biradar, S.; Yeshi, T.; et al. Development of humanized mouse and rat models with full-thickness human skin and autologous immune cells. Sci. Rep. 2020, 10, 14598. [Google Scholar] [CrossRef]
- Kaushik, S.; Kumari, L.; Deepak, R.K. Humanized mouse model for vaccine evaluation: An overview. Clin. Exp. Vaccine Res. 2024, 13, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ménoret, S.; Ouisse, L.-H.; Tesson, L.; Remy, S.; Usal, C.; Guiffes, A.; Chenouard, V.; Royer, P.-J.; Evanno, G.; Vanhove, B.; et al. In Vivo Analysis of Human Immune Responses in Immunodeficient Rats. Transplantation 2020, 104, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Chenouard, V.; Remy, S.; Tesson, L.; Ménoret, S.; Ouisse, L.-H.; Cherifi, Y.; Anegon, I. Advances in Genome Editing and Application to the Generation of Genetically Modified Rat Models. Front. Genet. 2021, 12, 615491. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Douam, F.; Ziegler, C.G.K.; Hrebikova, G.; Fant, B.; Leach, R.; Parsons, L.; Wang, W.; Gaska, J.M.; Winer, B.Y.; Heller, B.; et al. Selective expansion of myeloid and NK cells in humanized mice yields human-like vaccine responses. Nat. Commun. 2018, 9, 5031. [Google Scholar] [CrossRef]
- Lysenko, V.; van Wijk, N.W.-V.; Zimmermann, K.; Weller, M.-C.; Bühler, M.; Wildschut, M.H.E.; Schürch, P.; Fritz, C.; Wagner, U.; Calabresi, L.; et al. Enhanced engraftment of human myelofibrosis stem and progenitor cells in MISTRG mice. Blood Adv. 2020, 4, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Radtke, S.; Chan, Y.-Y.; Sippel, T.R.; Kiem, H.-P.; Rongvaux, A. MISTRG mice support engraftment and assessment of nonhuman primate hematopoietic stem and progenitor cells. Exp. Hematol. 2019, 70, 31–41.e1. [Google Scholar] [CrossRef]
- Samal, J.; Kelly, S.; Na-Shatal, A.; Elhakiem, A.; Das, A.; Ding, M.; Sanyal, A.; Gupta, P.; Melody, K.; Roland, B.; et al. Human immunodeficiency virus infection induces lymphoid fibrosis in the BM-liver-thymus-spleen humanized mouse model. J. Clin. Investig. 2018, 3, e120430. [Google Scholar] [CrossRef]
- Wahl, A.; De, C.; Fernandez, M.A.; Lenarcic, E.M.; Xu, Y.; Cockrell, A.S.; Cleary, R.A.; Johnson, C.E.; Schramm, N.J.; Rank, L.M.; et al. Precision mouse models with expanded tropism for human pathogens. Nat. Biotechnol. 2019, 37, 1163–1173. [Google Scholar] [CrossRef]
- Yang, D.; Beddows, I.; Tang, H.; Cascio, S.; McGonigal, S.C.; Bai, S.; Johnson, B.K.; Powers, J.J.; Acharya, R.; Bao, R.; et al. A Novel Humanized Immune Stroma PDX Cancer Model for Therapeutic Studies. bioRxiv 2023. [Google Scholar] [CrossRef]
- Claiborne, D.T.; Dudek, T.E.; Maldini, C.R.; Power, K.A.; Ghebremichael, M.; Seung, E.; Mellors, E.F.; Vrbanac, V.D.; Krupp, K.; Bisesi, A.; et al. Immunization of BLT Humanized Mice Redirects T Cell Responses to Gag and Reduces Acute HIV-1 Viremia. J. Virol. 2019, 93, e00814-19. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Coppin, E.; Sundarasetty, B.S.; Rahmig, S.; Blume, J.; Verheyden, N.A.; Bahlmann, F.; Ravens, S.; Schubert, U.; Schmid, J.; Ludwig, S.; et al. Enhanced differentiation of functional human T cells in NSGW41 mice with tissue-specific expression of human interleukin-7. Leukemia 2021, 35, 3561–3567. [Google Scholar] [CrossRef]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r γnull humanized mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Klicznik, M.M.; Benedetti, A.; Gail, L.M.; Varkhande, S.R.; Holly, R.; Laimer, M.; Stoecklinger, A.; Sir, A.; Reitsamer, R.; Neuper, T.; et al. A novel humanized mouse model to study the function of human cutaneous memory T cells in vivo in human skin. Sci. Rep. 2020, 10, 11164. [Google Scholar] [CrossRef]
- Kooreman, N.G.; de Almeida, P.E.; Stack, J.P.; Nelakanti, R.V.; Diecke, S.; Shao, N.-Y.; Swijnenburg, R.-J.; Sanchez-Freire, V.; Matsa, E.; Liu, C.; et al. Alloimmune Responses of Humanized Mice to Human Pluripotent Stem Cell Therapeutics. Cell Rep. 2017, 20, 1978–1990. [Google Scholar] [CrossRef]
- Chupp, D.P.; Rivera, C.E.; Zhou, Y.; Xu, Y.; Ramsey, P.S.; Xu, Z.; Zan, H.; Casali, P. A humanized mouse that mounts mature class-switched, hypermutated and neutralizing antibody responses. Nat. Immunol. 2024, 25, 1489–1506. [Google Scholar] [CrossRef]
- Wunderlich, M.; Chou, F.S.; Sexton, C.; Presicce, P.; Chougnet, C.A.; Aliberti, J.; Mulloy, J.C. Improved multilineage human hemato-poietic reconstitution and function in NSGS mice. PLoS ONE 2018, 13, e0209034. [Google Scholar] [CrossRef]
- Chang, H.; Biswas, S.; Tallarico, A.S.; Sarkis, P.T.N.; Geng, S.; Panditrao, M.M.; Zhu, Q.; Marasco, W.A. Human B-cell ontogeny in humanized NOD/SCID γcnull mice generates a diverse yet auto/poly- and HIV-1-reactive antibody repertoire. Genes Immun. 2012, 13, 399–410. [Google Scholar] [CrossRef]
- Chen, Q.; Khoury, M.; Chen, J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc. Natl. Acad. Sci. USA 2009, 106, 21783–21788. [Google Scholar] [CrossRef]
- Yong, K.S.M.; Her, Z.; Tan, S.Y.; Tan, W.W.S.; Liu, M.; Lai, F.; Heng, S.M.; Fan, Y.; Chang, K.T.E.; Wang, C.-I.; et al. Humanized Mouse as a Tool to Predict Immunotoxicity of Human Biologics. Front. Immunol. 2020, 11, 553362. [Google Scholar] [CrossRef] [PubMed]
- Weß, V.; Schuster-Winkelmann, P.; Karatekin, Y.H.; Malik, S.; Beigel, F.; Kühn, F.; Gropp, R. Humanized NSG Mouse Models as a Preclinical Tool for Translational Research in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2023, 24, 12348. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Feuer, G.; Bourré, L. Humanized Mouse Models for Immuno-oncology Drug Discovery. Curr. Protoc. 2023, 3, e852. [Google Scholar] [CrossRef] [PubMed]
- Karnik, I.; Her, Z.; Neo, S.H.; Liu, W.N.; Chen, Q. Emerging Preclinical Applications of Humanized Mouse Models in the Discovery and Validation of Novel Immunotherapeutics and Their Mechanisms of Action for Improved Cancer Treatment. Pharmaceutics 2023, 15, 1600. [Google Scholar] [CrossRef] [PubMed]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Melody, K.; Roy, C.N.; Kline, C.; Cottrell, M.L.; Evans, D.; Shutt, K.; Pennings, P.S.; Keele, B.F.; Bility, M.; Kashuba, A.D.M.; et al. Long-Acting Rilpivirine (RPV) Preexposure Prophylaxis Does Not Inhibit Vaginal Transmission of RPV-Resistant HIV-1 or Select for High-Frequency Drug Resistance in Humanized Mice. J. Virol. 2020, 94, e01912-19. [Google Scholar] [CrossRef]
- Washburn, M.L.; Bility, M.T.; Zhang, L.; Kovalev, G.I.; Buntzman, A.; Frelinger, J.A.; Barry, W.; Ploss, A.; Rice, C.M.; Su, L. A Humanized Mouse Model to Study Hepatitis C Virus Infection, Immune Response, and Liver Disease. Gastroenterology 2011, 140, 1334–1344. [Google Scholar] [CrossRef]
- Kaur, K.; Jewett, A. Supercharged NK Cell-Based Immuotherapy in Humanized Bone Marrow Liver and Thymus (Hu-BLT) Mice Model of Oral, Pancreatic, Glioblastoma, Hepatic, Melanoma and Ovarian Cancers. Crit. Rev. Immunol. 2023, 43, 13–25. [Google Scholar] [CrossRef]
- Zafar, S.; Basnet, S.; Launonen, I.-M.; Quixabeira, D.C.A.; Santos, J.; Hemminki, O.; Malmstedt, M.; Cervera-Carrascon, V.; Aronen, P.; Kalliokoski, R.; et al. Oncolytic Adenovirus Type 3 Coding for CD40L Facilitates Dendritic Cell Therapy of Prostate Cancer in Humanized Mice and Patient Samples. Hum. Gene Ther. 2021, 32, 192–202. [Google Scholar] [CrossRef]
- Bility, M.T.; Zhang, L.; Washburn, M.L.; Curtis, T.A.; Kovalev, G.I.; Su, L. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nat. Protoc. 2012, 7, 1608–1617. [Google Scholar] [CrossRef]
- Poling, H.M.; Sundaram, N.; Fisher, G.W.; Singh, A.; Shiley, J.R.; Nattamai, K.; Govindarajah, V.; Cortez, A.R.; Krutko, M.O.; Ménoret, S.; et al. Human pluripotent stem cell-derived organoids repair damaged bowel in vivo. Cell Stem Cell 2024, 31, 1513–1523.e7. [Google Scholar] [CrossRef] [PubMed]
- Adigbli, G.M.; Ménoret, S.B.; Cross, A.R.; Hester, J.; Issa, F.F.; Anegon, I. Humanization of Immunodeficient Animals for the Modeling of Transplantation, Graft Versus Host Disease, and Regenerative Medicine. Transplantation 2020, 104, 2290–2306. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.R.; Poling, H.M.; Brown, N.E.; Singh, A.; Mahe, M.M.; Helmrath, M.A. Transplantation of human intestinal organoids into the mouse mesentery: A more physiologic and anatomic engraftment site. Surgery 2018, 164, 643–650. [Google Scholar] [CrossRef]
- Ng, S.S.; Saeb-Parsy, K.; Blackford, S.J.; Segal, J.M.; Serra, M.P.; Horcas-Lopez, M.; No, D.Y.; Mastoridis, S.; Jassem, W.; Frank, C.W.; et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials 2018, 182, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Kumar, D.M.; Curlin, J.; Remling-Mulder, L.; Stenglein, M.; O’connor, S.; Marx, P.; Akkina, R. Modeling the evolution of SIV sooty mangabey progenitor virus towards HIV-2 using humanized mice. Virology 2017, 510, 175–184. [Google Scholar] [CrossRef]
- Yi, G.; Xu, X.; Abraham, S.; Petersen, S.; Guo, H.; Ortega, N.; Shankar, P.; Manjunath, N. A DNA Vaccine Protects Human Immune Cells against Zika Virus Infection in Humanized Mice. EBioMedicine 2017, 25, 87–94. [Google Scholar] [CrossRef]
- Bohórquez, J.A.; Adduri, S.; Ansari, D.; John, S.; Florence, J.; Adejare, O.; Singh, G.; Konduru, N.V.; Jagannath, C.; Yi, G.; et al. A novel humanized mouse model for HIV and tuberculosis co-infection studies. Front. Immunol. 2024, 15, 1395018. [Google Scholar] [CrossRef]
- Denton, P.W.; Olesen, R.; Choudhary, S.K.; Archin, N.M.; Wahl, A.; Swanson, M.D.; Chateau, M.; Nochi, T.; Krisko, J.F.; Spagnuolo, R.A.; et al. Generation of HIV Latency in Humanized BLT Mice. J. Virol. 2012, 86, 630–634. [Google Scholar] [CrossRef]
- Denton, P.W.; Garcia, J.V. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol. 2012, 20, 268–274. [Google Scholar] [CrossRef]
- Denton, P.W.; Krisko, J.F.; Powell, D.A.; Mathias, M.; Kwak, Y.T.; Martinez-Torres, F.; Zou, W.; Payne, D.A.; Estes, J.D.; Garcia, J.V. Systemic Administration of Antiretrovirals Prior to Exposure Prevents Rectal and Intravenous HIV-1 Transmission in Humanized BLT Mice. PLoS ONE 2010, 5, e8829. [Google Scholar] [CrossRef]
- Dash, P.K.; Chen, C.; Kaminski, R.; Su, H.; Mancuso, P.; Sillman, B.; Zhang, C.; Liao, S.; Sravanam, S.; Liu, H.; et al. CRISPR editing of CCR5 and HIV-1 facilitates viral elimination in antiretroviral drug-suppressed virus-infected humanized mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2217887120. [Google Scholar] [CrossRef] [PubMed]
- Seung, E.; Dudek, T.E.; Allen, T.M.; Freeman, G.J.; Luster, A.D.; Tager, A.M. PD-1 Blockade in Chronically HIV-1-Infected Humanized Mice Suppresses Viral Loads. PLoS ONE 2013, 8, e77780. [Google Scholar] [CrossRef] [PubMed]
- Sungur, C.M.; Wang, Q.; Ozantürk, A.N.; Gao, H.; Schmitz, A.J.; Cella, M.; Yokoyama, W.M.; Shan, L. Human NK cells confer protection against HIV-1 infection in humanized mice. J. Clin. Investig. 2022, 132, e162694. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.A.; Halper-Stromberg, A.; Mouquet, H.; Gitlin, A.D.; Tretiakova, A.; Eisenreich, T.R.; Malbec, M.; Gravemann, S.; Billerbeck, E.; Dorner, M.; et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc. Natl. Acad. Sci. USA 2013, 110, 16538–16543. [Google Scholar] [CrossRef]
- Halper-Stromberg, A.; Lu, C.-L.; Klein, F.; Horwitz, J.A.; Bournazos, S.; Nogueira, L.; Eisenreich, T.R.; Liu, C.; Gazumyan, A.; Schaefer, U.; et al. Broadly Neutralizing Antibodies and Viral Inducers Decrease Rebound from HIV-1 Latent Reservoirs in Humanized Mice. Cell 2014, 158, 989–999. [Google Scholar] [CrossRef]
- Hur, E.M.; Patel, S.N.; Shimizu, S.; Rao, D.S.; Gnanapragasam, P.N.P.; An, D.S.; Yang, L.; Baltimore, D. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood 2012, 120, 4571–4582. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.X.; Shi, W.; Ma, A.H.; De Vere White, R.W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- Okada, F.; Izutsu, R.; Goto, K.; Osaki, M. Inflammation-Related Carcinogenesis: Lessons from Animal Models to Clinical Aspects. Cancers 2021, 13, 921. [Google Scholar] [CrossRef]
- Noto, F.K.; Sangodkar, J.; Adedeji, B.T.; Moody, S.; McClain, C.B.; Tong, M.; Ostertag, E.; Crawford, J.; Gao, X.; Hurst, L.; et al. The SRG rat, a Sprague-Dawley Rag2/Il2rg double-knockout validated for human tumor oncology studies. PLoS ONE 2020, 15, e0240169. [Google Scholar] [CrossRef]
- He, D.; Zhang, J.; Wu, W.; Yi, N.; He, W.; Lu, P.; Li, B.; Yang, N.; Wang, D.; Xue, Z.; et al. A novel immunodeficient rat model supports human lung cancer xenografts. FASEB J. 2018, 33, 140–150. [Google Scholar] [CrossRef]
- Kim, J.-I.; Lim, H.-J.; Kwon, E.; Mashimo, T.; Kang, B.-C. Immune deficiency phenotypes of Il2rg, Rag2 or Il2rg/Rag2 double knockout rats; establishment of human leukemia xenograft models. Lab. Anim. Res. 2024, 40, 43. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Huang, Y.; Krajina, B.A.; McBirney, M.; Doak, A.E.; Qu, S.; Wang, C.L.; Haffner, M.C.; Cheung, K.J. Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7. Proc. Natl. Acad. Sci. USA 2023, 120, e2214888120. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Takizawa, A.; Kobayashi, J.; Kunihiro, Y.; Yoshimi, K.; Ishida, S.; Tanabe, K.; Yanagi, A.; Tachibana, A.; Hirose, J.; et al. Generation and Characterization of Severe Combined Immunodeficiency Rats. Cell Rep. 2012, 2, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, J.; He, J.; Liu, J.; Wang, H.; Liu, Y.; Jiang, T.; Zhang, Q.; Fu, X.; Xu, Y. An Immune System-Modified Rat Model for Human Stem Cell Transplantation Research. Stem Cell Rep. 2018, 11, 514–521. [Google Scholar] [CrossRef]
- Caposio, P.; Worm, S.v.D.; Crawford, L.; Perez, W.; Kreklywich, C.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ratts, R.; Marshall, E.E.; et al. Characterization of a live-attenuated HCMV-based vaccine platform. Sci. Rep. 2019, 9, 19236. [Google Scholar] [CrossRef]
- Hakki, M.; Goldman, D.C.; Streblow, D.N.; Hamlin, K.L.; Krekylwich, C.N.; Fleming, W.H.; Nelson, J.A. HCMV Infection of Humanized Mice after Transplantation of G-CSF–Mobilized Peripheral Blood Stem Cells from HCMV-Seropositive Donors. Biol. Blood Marrow Transplant. 2013, 20, 132–135. [Google Scholar] [CrossRef]
- Theobald, S.J.; Khailaie, S.; Meyer-Hermann, M.; Volk, V.; Olbrich, H.; Danisch, S.; Gerasch, L.; Schneider, A.; Sinzger, C.; Schaudien, D.; et al. Signatures of T and B Cell Development, Functional Responses and PD-1 Upregulation After HCMV Latent Infections and Reactivations in Nod.Rag.Gamma Mice Humanized With Cord Blood CD34+ Cells. Front. Immunol. 2018, 9, 2734. [Google Scholar] [CrossRef]
- Crawford, L.B.; Streblow, D.N.; Hakki, M.; Nelson, J.A.; Caposio, P. Humanized mouse models of human cytomegalovirus infection. Curr. Opin. Virol. 2015, 13, 86–92. [Google Scholar] [CrossRef]
- Crawford, L.B.; Tempel, R.; Streblow, D.N.; Yurochko, A.D.; Goodrum, F.D.; Nelson, J.A.; Caposio, P. Human Cytomegalovirus Infection Suppresses CD34+ Progenitor Cell Engraftment in Humanized Mice. Microorganisms 2020, 8, 525. [Google Scholar] [CrossRef]
- Smith, M.S.; Goldman, D.C.; Bailey, A.S.; Pfaffle, D.L.; Kreklywich, C.N.; Spencer, D.B.; Othieno, F.A.; Streblow, D.N.; Garcia, J.V.; Fleming, W.H.; et al. Granulocyte-Colony Stimulating Factor Reactivates Human Cytomegalovirus in a Latently Infected Humanized Mouse Model. Cell Host Microbe 2010, 8, 284–291. [Google Scholar] [CrossRef]
- Brown, J.M.; Kaneshima, H.; Mocarski, E.S. Dramatic Interstrain Differences in the Replication of Human Cytomegalovirus in SCID-hu Mice. J. Infect. Dis. 1995, 171, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Dulal, K.; Cheng, T.; Yang, L.; Wang, W.; Huang, Y.; Silver, B.; Selariu, A.; Xie, C.; Wang, D.; Espeseth, A.; et al. Functional analysis of human cytomegalovirus UL/b′ region using SCID-hu mouse model. J. Med. Virol. 2016, 88, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Kern, E.R.; Rybak, R.J.; Hartline, C.B.; Bidanset, D.J. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir. Chem. Chemother. 2001, 12, 149–156. [Google Scholar] [PubMed]
- Mocarski, E.S.; Bonyhadi, M.; Salimi, S.; McCune, J.M.; Kaneshima, H. Human cytomegalovirus in a SCID-hu mouse: Thymic epithelial cells are prominent targets of viral replication. Proc. Natl. Acad. Sci. USA 1993, 90, 104–108. [Google Scholar] [CrossRef]
- Maidji, E.; Kosikova, G.; Joshi, P.; Stoddart, C.A. Impaired Surfactant Production by Alveolar Epithelial Cells in a SCID-hu Lung Mouse Model of Congenital Human Cytomegalovirus Infection. J. Virol. 2012, 86, 12795–12805. [Google Scholar] [CrossRef]
- McCune, J.M.; Namikawa, R.; Kaneshima, H.; Shultz, L.D.; Lieberman, M.; Weissman, I.L. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science 1988, 241, 1632–1639. [Google Scholar] [CrossRef]
- Crawford, L.B.; Diggins, N.L.; Caposio, P.; Hancock, M.H. Advances in Model Systems for Human Cytomegalovirus Latency and Reactivation. mBio 2022, 13, e01724-21. [Google Scholar] [CrossRef]
- Crawford, L.B.; Caposio, P. Development of a huBLT Mouse Model to Study HCMV Latency, Reactivation, and Immune Response. Methods Mol Biol. 2021, 2244, 343–363. [Google Scholar] [CrossRef]
- Fleming, J.; Ginn, S.L.; Weinberger, R.P.; Trahair, T.N.; Smythe, J.A.; Alexander, I.E. Adeno-Associated Virus and Lentivirus Vectors Mediate Efficient and Sustained Transduction of Cultured Mouse and Human Dorsal Root Ganglia Sensory Neurons. Hum. Gene Ther. 2001, 12, 77–86. [Google Scholar] [CrossRef]
- Galimi, F.; Saez, E.; Gall, J.; Hoong, N.; Cho, G.; Evans, R.M.; Verma, I.M. Development of Ecdysone-Regulated Lentiviral Vectors. Mol. Ther. 2005, 11, 142–148. [Google Scholar] [CrossRef]
- Ginn, S.L.; Fleming, J.; Rowe, P.B.; Alexander, I.E. Promoter Interference Mediated by the U3 Region in Early-Generation HIV-1–Derived Lentivirus Vectors Can Influence Detection of Transgene Expression in a Cell-Type and Species-Specific Manner. Hum. Gene Ther. 2003, 14, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Klimstra, W.B.; Ryman, K.D.; Heidner, H.W. Sindbis Virus Vectors Designed To Express a Foreign Protein as a Cleavable Component of the Viral Structural Polyprotein. J. Virol. 2003, 77, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.M.; Weitzman, M.D. GENE THERAPY: Twenty-First Century Medicine. Annu. Rev. Biochem. 2005, 74, 711–738. [Google Scholar] [CrossRef]
- Ge, X.; Jaijyan, D.K.; Wang, W.; Cheng, T.; Tang, Q.; Wu, F.; Jin, T.; Zhu, H. Rationally designed synthetic vectors for therapeutic nucleic acid delivery against human cytomegalovirus infection. J. Med. Virol. 2023, 95, e28586. [Google Scholar] [CrossRef]
- Liu, J.; Jaijyan, D.K.; Tang, Q.; Zhu, H. Promising Cytomegalovirus-Based Vaccine Vector Induces Robust CD8+ T-Cell Response. Int. J. Mol. Sci. 2019, 20, 4457. [Google Scholar] [CrossRef]
- Zeng, J.; Jaijyan, D.K.; Yang, S.; Pei, S.; Tang, Q.; Zhu, H. Exploring the Potential of Cytomegalovirus-Based Vectors: A Review. Viruses 2023, 15, 2043. [Google Scholar] [CrossRef]
- Armstrong, N.; Tang, Q. Congenital cytomegalovirus infection and advances in murine models of neuropathogenesis. Virol. Sin. 2022, 37, 318–320. [Google Scholar] [CrossRef]
- Plotkin, S. The history of vaccination against cytomegalovirus. Med. Microbiol. Immunol. 2015, 204, 247–254. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; Wang, M.; Cao, D.; Jaijyan, D.K.; Enescu, N.; Liu, J.; Wu, S.; Wang, S.; Sun, W.; et al. Circular RNAs Represent a Novel Class of Human Cytomegalovirus Transcripts. Microbiol. Spectr. 2022, 10, e01106-22. [Google Scholar] [CrossRef]
- Hansen, S.G.; Womack, J.; Scholz, I.; Renner, A.; Edgel, K.A.; Xu, G.; Ford, J.C.; Grey, M.; Laurent, B.S.; Turner, J.M.; et al. Cytomegalovirus vectors expressing Plasmodium knowlesi antigens induce immune responses that delay parasitemia upon sporozoite challenge. PLoS ONE 2019, 14, e0210252. [Google Scholar] [CrossRef]
- Tsuda, Y.; Parkins, C.J.; Caposio, P.; Feldmann, F.; Botto, S.; Ball, S.; Messaoudi, I.; Cicin-Sain, L.; Feldmann, H.; Jarvis, M.A. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine 2015, 33, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Murphy, A.A.; Feldmann, F.; Parkins, C.J.; Haddock, E.; Hanley, P.W.; Emery, M.J.; Engelmann, F.; Messaoudi, I.; Feldmann, H.; et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci. Rep. 2016, 6, 21674. [Google Scholar] [CrossRef]
- Hansen, S.G.; Marshall, E.E.; Malouli, D.; Ventura, A.B.; Hughes, C.M.; Ainslie, E.; Ford, J.C.; Morrow, D.; Gilbride, R.M.; Bae, J.Y.; et al. A live-attenuated RhCMV/SIV vaccine shows long-term efficacy against heterologous SIV challenge. Sci. Transl. Med. 2019, 11, eaaw2607. [Google Scholar] [CrossRef]
- Liu, J.; Jaijyan, D.K.; Chen, Y.; Feng, C.; Yang, S.; Xu, Z.; Zhan, N.; Hong, C.; Li, S.; Cheng, T.; et al. Cytomegalovirus-vectored COVID-19 vaccines elicit neutralizing antibodies against the SARS-CoV-2 Omicron variant (BA.2) in mice. Microbiol. Spectr. 2023, 11, e02463-23. [Google Scholar] [CrossRef]
- Zheng, X.; Oduro, J.D.; Boehme, J.D.; Borkner, L.; Ebensen, T.; Heise, U.; Gereke, M.; Pils, M.C.; Krmpotic, A.; Guzmán, C.A.; et al. Mucosal CD8+ T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge. PLoS Pathog. 2019, 15, e1008036. [Google Scholar] [CrossRef]
- Abdelaziz, M.O.; Ossmann, S.; Kaufmann, A.M.; Leitner, J.; Steinberger, P.; Willimsky, G.; Raftery, M.J.; Schönrich, G. Development of a Human Cytomegalovirus (HCMV)-Based Therapeutic Cancer Vaccine Uncovers a Previously Unsuspected Viral Block of MHC Class I Antigen Presentation. Front. Immunol. 2019, 10, 1776. [Google Scholar] [CrossRef]
- Schwartz, M.; Stern-Ginossar, N. Rethinking human cytomegalovirus latency reservoir. Ann. N. Y. Acad. Sci. 2023, 1524, 30–36. [Google Scholar] [CrossRef]
- Zeng, J.; Cao, D.; Yang, S.; Jaijyan, D.K.; Liu, X.; Wu, S.; Cruz-Cosme, R.; Tang, Q.; Zhu, H. Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review. Viruses 2023, 15, 1703. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Malouli, D.; Hansen, S.G.; Hancock, M.H.; Hughes, C.M.; Ford, J.C.; Gilbride, R.M.; Ventura, A.B.; Morrow, D.; Randall, K.T.; Taher, H.; et al. Cytomegaloviral determinants of CD8 + T cell programming and RhCMV/SIV vaccine efficacy. Sci. Immunol. 2021, 6, eabg5413. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Hancock, M.H.; Malouli, D.; Marshall, E.E.; Hughes, C.M.; Randall, K.T.; Morrow, D.; Ford, J.C.; Gilbride, R.M.; Selseth, A.N.; et al. Myeloid cell tropism enables MHC-E–restricted CD8 + T cell priming and vaccine efficacy by the RhCMV/SIV vaccine. Sci. Immunol. 2022, 7, eabn9301. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.F.; Verweij, M.C.; Nair, S.S.; Morrow, D.; Mansouri, M.; Chakravarty, D.; Beechwood, T.; Meyer, C.; Uebelhoer, L.; Lauron, E.J.; et al. CD8+ T cell targeting of tumor antigens presented by HLA-E. Sci. Adv. 2024, 10, eadm7515. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Vieville, C.; Whizin, N.; Coyne-Johnson, L.; Siess, D.C.; Drummond, D.D.; Legasse, A.W.; Axthelm, M.K.; Oswald, K.; Trubey, C.M.; et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009, 15, 293–299. [Google Scholar] [CrossRef]
- Wang, E.C.Y.; Pjechova, M.; Nightingale, K.; Vlahava, V.-M.; Patel, M.; Ruckova, E.; Forbes, S.K.; Nobre, L.; Antrobus, R.; Roberts, D.; et al. Suppression of costimulation by human cytomegalovirus promotes evasion of cellular immune defenses. Proc. Natl. Acad. Sci. USA 2018, 115, 4998–5003. [Google Scholar] [CrossRef] [PubMed]
- Arav-Boger, R.; Wojcik, G.L.; Duggal, P.; Ingersoll, R.G.; Beaty, T.; Pass, R.F.; Yolken, R.H. Polymorphisms in Toll-like receptor genes influence antibody responses to cytomegalovirus glycoprotein B vaccine. BMC Res. Notes 2012, 5, 140. [Google Scholar] [CrossRef]
- Alfi, O.; From, I.; Yakirevitch, A.; Drendel, M.; Wolf, M.; Meir, K.; Zakay-Rones, Z.; Nevo, Y.; Elgavish, S.; Ilan, O.; et al. Human Nasal Turbinate Tissues in Organ Culture as a Model for Human Cytomegalovirus Infection at the Mucosal Entry Site. J. Virol. 2020, 94, e01258-20. [Google Scholar] [CrossRef]
- Abad-Fernandez, M.; Goonetilleke, N. Human cytomegalovirus-vectored vaccines against HIV. Curr. Opin. HIV AIDS 2019, 14, 137–142. [Google Scholar] [CrossRef]
- Al-Talib, M.; Dimonte, S.; Humphreys, I.R. Mucosal T-cell responses to chronic viral infections: Implications for vaccine design. Cell. Mol. Immunol. 2024, 21, 982–998. [Google Scholar] [CrossRef]
- Harris, E. Trial Launches of New HIV Vaccine Candidate With CMV Vector. JAMA 2023, 330, 1515. [Google Scholar] [CrossRef]
- Kim, Y.; Zheng, X.; Eschke, K.; Chaudhry, M.Z.; Bertoglio, F.; Tomić, A.; Krmpotić, A.; Hoffmann, M.; Bar-On, Y.; Boehme, J.; et al. MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination. Cell. Mol. Immunol. 2022, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Gbedande, K.; Ibitokou, S.A.; Ong, M.L.; Degli-Esposti, M.A.; Brown, M.G.; Stephens, R. Boosting Live Malaria Vaccine with Cytomegalovirus Vector Can Prolong Immunity through Innate and Adaptive Mechanisms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Barry, P.A.; Deere, J.D.; Yue, Y.; Chang, W.W.; Schmidt, K.A.; Wussow, F.; Chiuppesi, F.; Diamond, D.J.; Sparger, E.E.; Walter, M.R.; et al. Cytomegalovirus-vectored vaccines for HIV and other pathogens. AIDS 2020, 34, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, R.; Ivic, S.; Pepper, M.S.; Gers-Huber, G.; Li, D.; Audigé, A.; Rochat, M.-A.; Jaquet, V.; Regenass, S.; Manz, M.G.; et al. Lentivector Knockdown of CCR5 in Hematopoietic Stem and Progenitor Cells Confers Functional and Persistent HIV-1 Resistance in Humanized Mice. J. Virol. 2015, 89, 6761–6772. [Google Scholar] [CrossRef]
- Terahara, K.; Ishige, M.; Ikeno, S.; Mitsuki, Y.-Y.; Okada, S.; Kobayashi, K.; Tsunetsugu-Yokota, Y. Expansion of Activated Memory CD4+ T Cells Affects Infectivity of CCR5-Tropic HIV-1 in Humanized NOD/SCID/JAK3null Mice. PLoS ONE 2013, 8, e53495. [Google Scholar] [CrossRef]
- Terahara, K.; Ishige, M.; Ikeno, S.; Okada, S.; Kobayashi-Ishihara, M.; Ato, M.; Tsunetsugu-Yokota, Y. Humanized mice dually challenged with R5 and X4 HIV-1 show preferential R5 viremia and restricted X4 infection of CCR5+CD4+ T cells. Microbes Infect. 2015, 17, 378–386. [Google Scholar] [CrossRef]
- Gray, G.; Buchbinder, S.; Duerr, A. Overview of STEP and Phambili trial results: Two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr. Opin. HIV AIDS 2010, 5, 357–361. [Google Scholar] [CrossRef]
- Sekaly, R.P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef]
- Xu, S.X.; Leontyev, D.; Kaul, R.; Gray-Owen, S.D. Neisseria gonorrhoeae co-infection exacerbates vaginal HIV shedding without affecting systemic viral loads in human CD34+ engrafted mice. PLoS ONE 2018, 13, e0191672. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Crim, M.J.; Franklin, C.L. A brief history of animal modeling. Mo. Med. 2013, 110, 201–205. [Google Scholar]
- Snowden, F.M. Emerging and reemerging diseases: A historical perspective. Immunol. Rev. 2008, 225, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J. The 50-Year War on Cancer Revisited: Should We Continue to Fight the Enemy Within? J. Cancer Prev. 2021, 26, 219–223. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craft, K.; Amanor, A.; Barnett, I.; Donaldson, C.; Anegon, I.; Madduri, S.; Tang, Q.; Bility, M.T. Can Humanized Immune System Mouse and Rat Models Accelerate the Development of Cytomegalovirus-Based Vaccines Against Infectious Diseases and Cancers? Int. J. Mol. Sci. 2025, 26, 3082. https://doi.org/10.3390/ijms26073082
Craft K, Amanor A, Barnett I, Donaldson C, Anegon I, Madduri S, Tang Q, Bility MT. Can Humanized Immune System Mouse and Rat Models Accelerate the Development of Cytomegalovirus-Based Vaccines Against Infectious Diseases and Cancers? International Journal of Molecular Sciences. 2025; 26(7):3082. https://doi.org/10.3390/ijms26073082
Chicago/Turabian StyleCraft, Kaci, Athina Amanor, Ian Barnett, Clarke Donaldson, Ignacio Anegon, Srinivas Madduri, Qiyi Tang, and Moses T. Bility. 2025. "Can Humanized Immune System Mouse and Rat Models Accelerate the Development of Cytomegalovirus-Based Vaccines Against Infectious Diseases and Cancers?" International Journal of Molecular Sciences 26, no. 7: 3082. https://doi.org/10.3390/ijms26073082
APA StyleCraft, K., Amanor, A., Barnett, I., Donaldson, C., Anegon, I., Madduri, S., Tang, Q., & Bility, M. T. (2025). Can Humanized Immune System Mouse and Rat Models Accelerate the Development of Cytomegalovirus-Based Vaccines Against Infectious Diseases and Cancers? International Journal of Molecular Sciences, 26(7), 3082. https://doi.org/10.3390/ijms26073082






