Bamboo Leaf Flavonoids from Phyllostachys glauca McClure Suppress the Progression of Alzheimer’s Disease Induced by Circadian Rhythm Disruption Through Regulating Hif3α/Rab7/TNFα/IL1β Pathway
Abstract
1. Introduction
2. Results
2.1. Brain Index and Biochemical Indicators in Mouse Serum
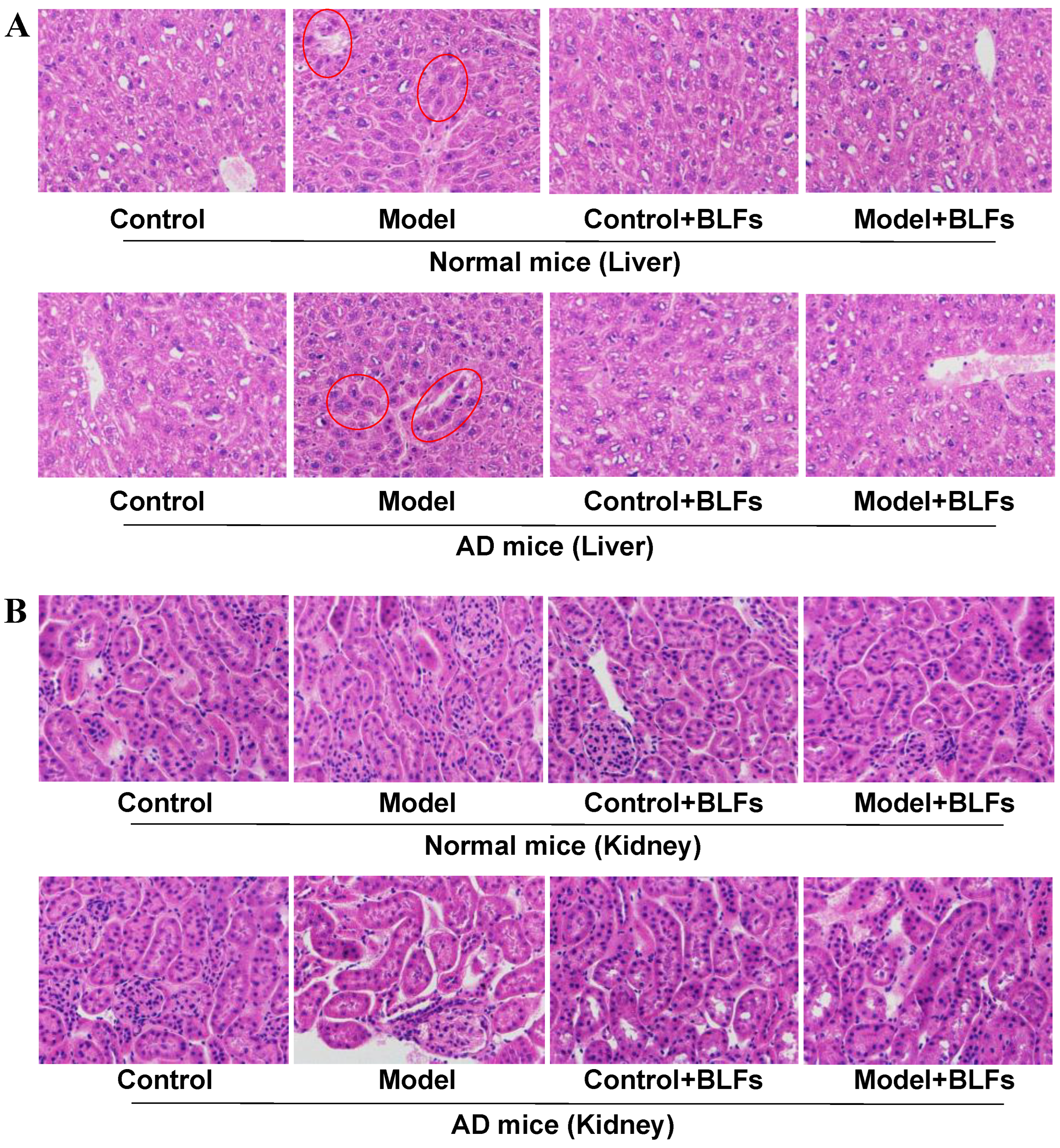
2.2. Histopathological Observation of Liver and Kidney Tissue
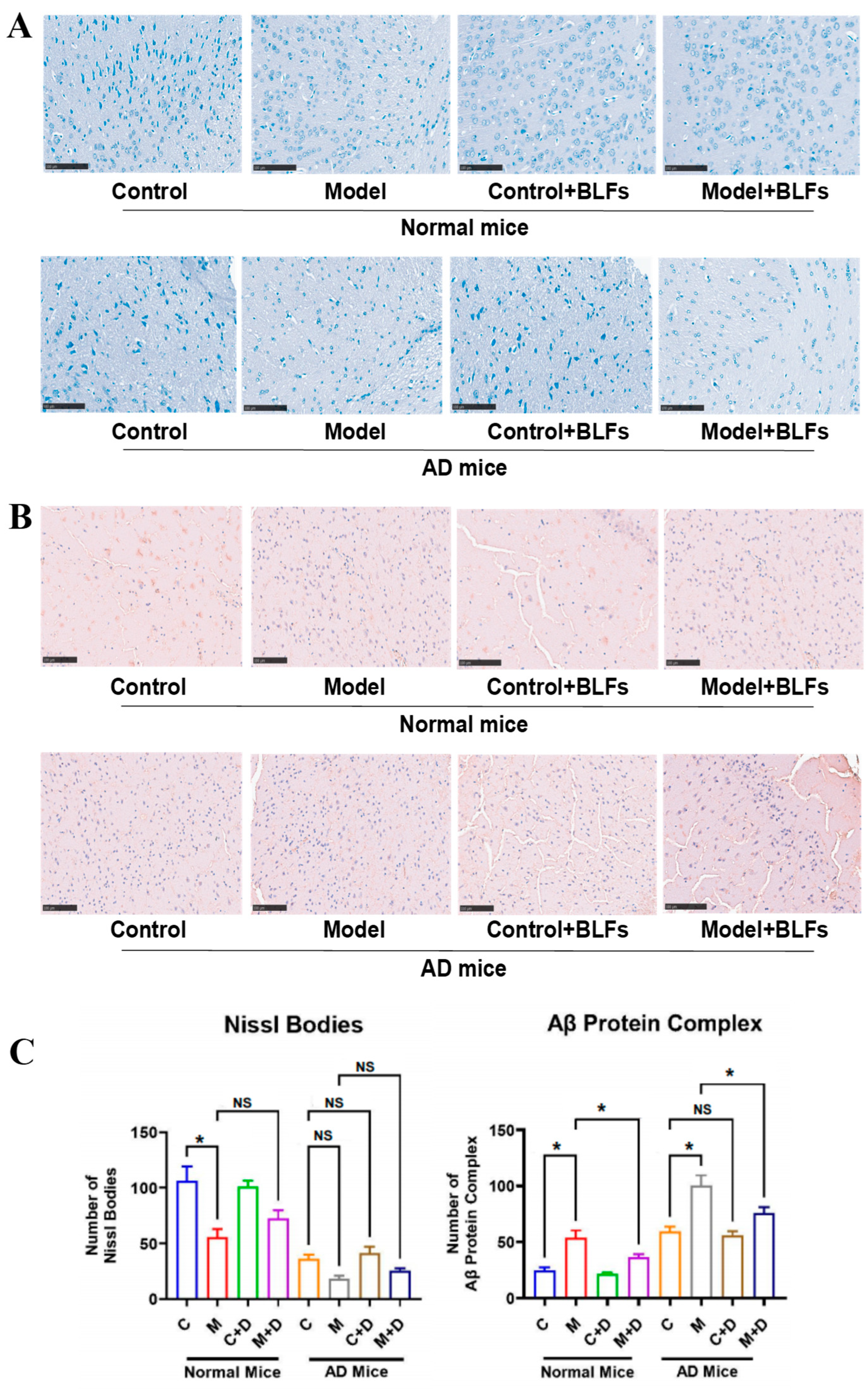
2.3. Staining of Special Markers in Brain Tissue: Nissl Bodies and Amyloid-β (Aβ) Protein
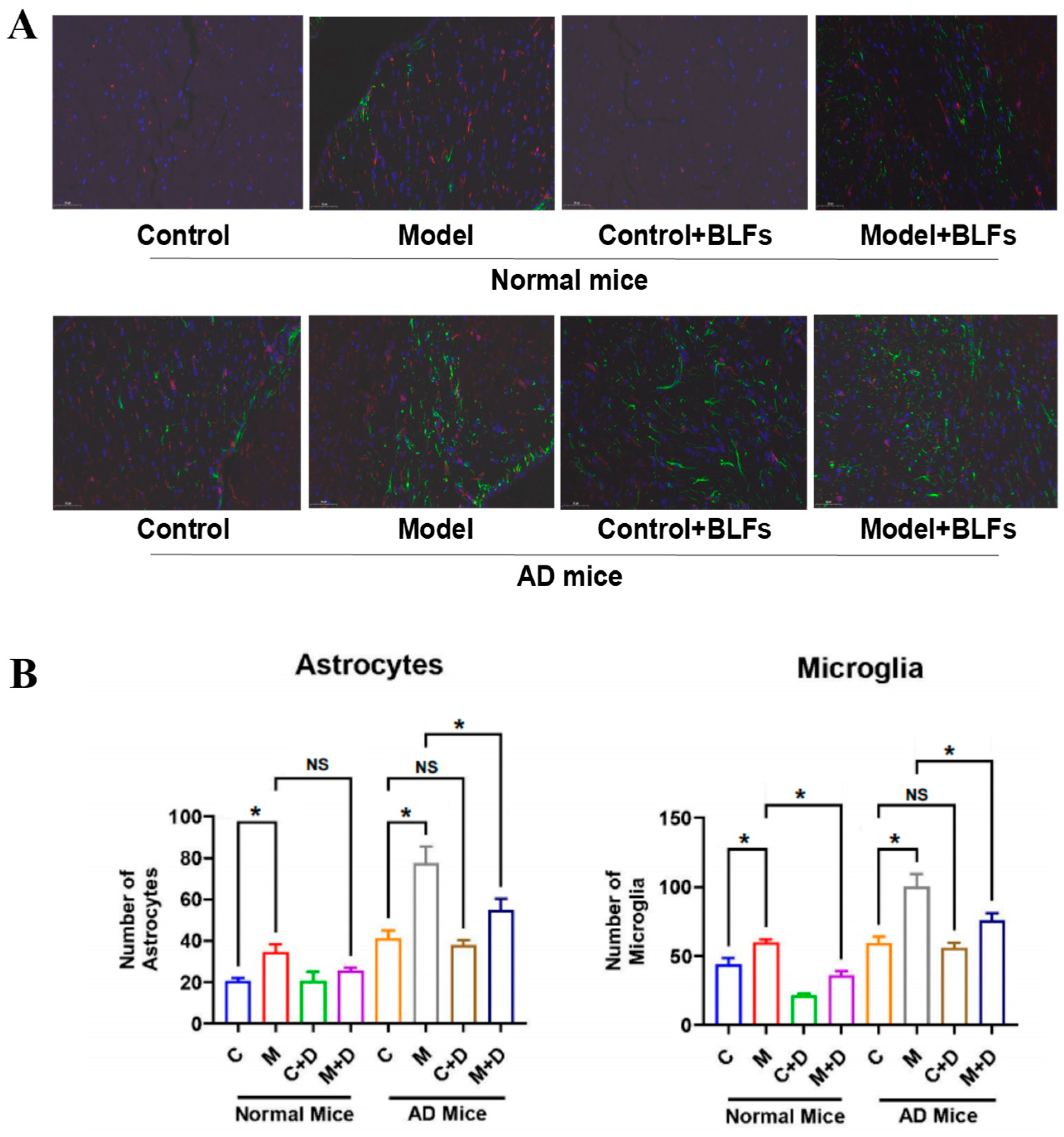
2.4. Astrocyte and Microglia Activation in Brain Tissue: GFAP/Iba-1 Double Staining
2.5. Transcriptional Analysis of Brain Tissue in Response to Circadian Disruption and BLFs Treatment
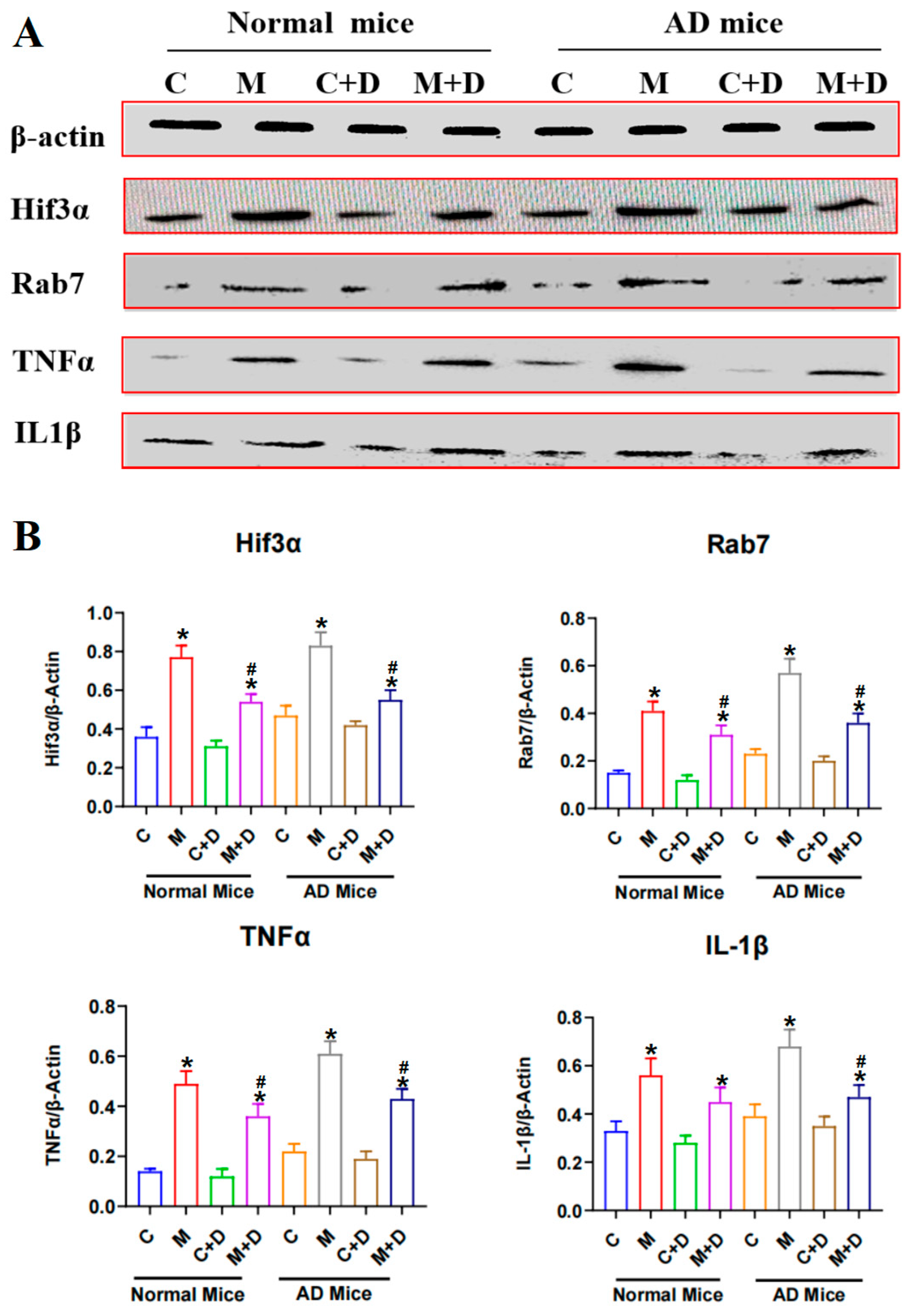
2.6. Expression of the Hif3α/Rab7/TNFα/IL-1β Pathway in Mouse Brain Tissue
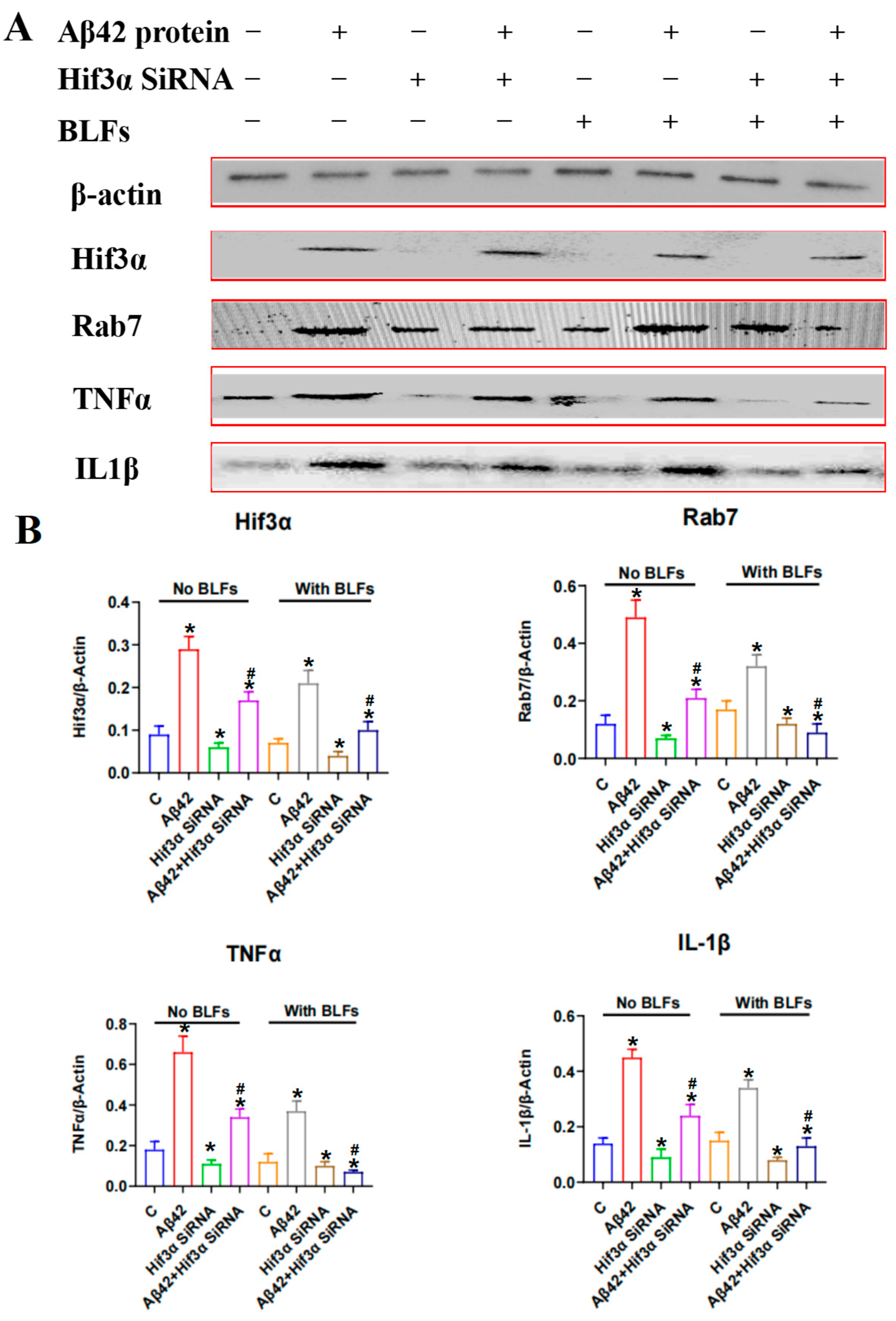
2.7. Expression of the Hif3α/Rab7/TNFα/IL-1β Pathway in PC12 Cells
3. Discussion
4. Methods
4.1. Reagents
4.2. Animal Models
4.3. Detection of Biochemical Indicators
4.4. HE Staining of Liver and Kidney
4.5. Special Factor Staining of Brain Tissues
4.6. Transcriptome Sequencing and Analysis of Brain Tissue
4.7. PC12 Cells Culturing and siRNA Fragment Transfection
4.8. Western Blot Detection of Special Proteins
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid beta |
| AD | Alzheimer’s disease |
| ALT | Alanine Aminotransferase |
| APP/PS1 | Amyloid Precursor Protein/Presenilin 1 (transgenic mice model) |
| AST | Aspartate Aminotransferase |
| BUN | Blood Urea Nitrogen |
| BLFs | Bamboo Leaf Flavonoids |
| CD | Circadian Disruption |
| Cr | Creatinine |
| DAPI | 4′,6-diamidino-2-phenylindole (nuclear stain) |
| DESeq | Differential Expression Sequencing |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECL | Enhanced Chemiluminescence |
| GFAP | Glial fibrillary acidic protein |
| GSH | Glutathione |
| HE | Hematoxylin-Eosin |
| HIF3α | Hypoxia-inducible factor 3 alpha |
| IL-1β | Interleukin 1 beta |
| Iba-1 | Ionized Calcium-binding Adapter Molecule 1 |
| MDA | Malondialdehyde |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| PC12 | Rat adrenal pheochromocytoma cell line |
| PBS | Phosphate-buffered saline |
| PMSF | Phenylmethylsulfonyl fluoride |
| Rab7 | Ras-associated binding protein 7 |
| ROS | Reactive oxygen species |
| siRNA | Small interfering RNA |
| SPF | Specific pathogen-free |
| TBST | Tris-buffered saline with Tween |
References
- Devranis, P.; Vassilopoulou, Ε.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean diet, ketogenic diet or MIND diet for aging populations with cognitive decline: A systematic review. Life 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Albratty, M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm. J. 2022, 30, 1755–1764. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Vergallo, A. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A.; Baig, J.; Islam, M.A.; Reddy, H.; Kshirsagar, S. Amyloid-β and Phosphorylated Tau are the Key Biomarkers and Predictors of Alzheimer’s Disease. Aging Dis. 2025, 16, 2. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Androulakis, I.P. Light entrainment of the SCN circadian clock and implications for personalized alterations of corticosterone rhythms in shift work and jet lag. Sci. Rep. 2021, 11, 17929. [Google Scholar] [CrossRef] [PubMed]
- Nyamugenda, E.; Rosensweig, C.; Allada, R. Circadian Clocks, Daily Stress, and Neurodegenerative Disease. Annu. Rev. Pathol. 2025, 20, 355–374. [Google Scholar] [CrossRef]
- Sharma, A.; Sethi, G.; Tambuwala, M.M.; Aljabali, A.A.; Chellappan, D.K.; Dua, K.; Goyal, R. Circadian Rhythm Disruption and Alzheimer’s Disease: The Dynamics of a Vicious Cycle. Curr. Neuropharmacol. 2020, 19, 248–264. [Google Scholar] [CrossRef]
- Cuomo, F.; Coppola, A.; Botti, C.; Maione, C.; Forte, A.; Scisciola, L.; Cobellis, G. Pro-inflammatory cytokines activate hypoxia-inducible factor 3α via epigenetic changes in mesenchymal stromal/stem cells. Sci. Rep. 2018, 8, 5842. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Xu, Q.; Lu, X.; Yang, X.; Song, Q.; Zou, C. 2-Dodecyl-6-Methoxycyclohexa- 2,5-Diene-1,4-Dione Ameliorates Diabetic Cognitive Impairment Through Inhibiting Hif3α and Apoptosis. Front. Pharmacol. 2021, 12, 708141. [Google Scholar] [CrossRef]
- Maki, T.; Sawahata, M.; Akutsu, I.; Amaike, S.; Hiramatsu, G.; Uta, D.; Kume, T. APP Knock-In Mice Produce E22P-Aβ Exhibiting an Alzheimer’s Disease-like Phenotype with Dysregulation of Hypoxia-Inducible Factor Expression. Int. J. Mol. Sci. 2022, 23, 13259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jia, C.; Ran, L.; Shi, J.; Amarmend, T.; Li, H. The alleviative effects comparison of four flavonoids from bamboo leaves on ulcerative colitis in an Alzheimer mouse model. CNS Neurosci. Ther. 2024, 30, e14620. [Google Scholar] [CrossRef]
- Tao, M.F.; Li, R.; Xu, T.T.; Zhang, Z.; Zheng, D.; Xia, Z.; Xu, X. Vitexin and isovitexin delayed ageing and enhanced stress-resistance through the activation of the SKN-1/Nrf2 signaling pathway. Int. J. Food Sci. Nutr. 2023, 74, 685–694. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Tan, X.; Liu, J.; Zhi, Y.; Yi, L.; Dong, Y. Isoorientin inhibits inflammation in macrophages and endotoxemia mice by regulating glycogen synthase kinase 3β. Mediat. Inflamm. 2020, 2020, 8704146. [Google Scholar] [CrossRef]
- Cui, T.; Lan, Y.; Lu, Y.; Yu, F.; Lin, S.; Fu, Y.; Niu, G. Isoorientin ameliorates H2O2-induced apoptosis and oxidative stress in chondrocytes by regulating MAPK and PI3K/Akt pathways. Aging 2023, 15, 4861. [Google Scholar] [CrossRef]
- Kimura, I.; Kagawa, S.; Tsuneki, H.; Tanaka, K.; Nagashima, F. Multitasking bamboo leaf-derived compounds in prevention of infectious, inflammatory, atherosclerotic, metabolic, and neuropsychiatric diseases. Pharmacol. Ther. 2022, 235, 108159. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sood, A.; Lang, D.K.; Bhatia, S.; Al-Harrasi, A.; Aleya, L.; Behl, T. Potential of flavonoids as anti-Alzheimer’s agents: Bench to bedside. Environ. Sci. Pollut. Res. 2022, 29, 26063–26077. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Jia, X.; Yao, L.; Liu, Y. Research Mechanism and Progress of the Natural Compound Curcumin in Treating Alzheimer’s Disease. Mini Rev. Med. Chem. 2024, 24, 1590–1601. [Google Scholar] [CrossRef]
- Pagotto, G.L.D.O.; Santos, L.M.O.D.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Barbalho, S.M. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Costello, H.M.; Johnston, J.G.; Juffre, A.; Crislip, G.R.; Gumz, M.L. Circadian clocks of the kidney: Function, mechanism, and regulation. Physiol. Rev. 2022, 102, 1669–1701. [Google Scholar] [CrossRef]
- Geng, F.; Zhao, N.; Ren, Q.G. Circadian rhythm, microglia-mediated neuroinflammation, and Alzheimer’s disease. Neurosci. Biobehav. Rev. 2025, 170, 106044. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Poesen, K.; Vandenberghe, R.; De Meyer, S. Alzheimer’s disease neuropathology and its estimation with fluid and imaging biomarkers. Mol. Neurodegener. 2025, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-astrocyte protein markers in the brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Olmedillas, M.; Brawek, B.; Li, K.Z.; Richter, C.; Garaschuk, O. Plaque vicinity as a hotspot of microglial turnover in a mouse model of Alzheimer’s disease. Glia 2023, 71, 2884–2901. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Z.; Cai, Y.; Zeng, S.; Peng, B.; Ren, X.; Gong, Z. Rheostatic balance of circadian rhythm and autophagy in metabolism and disease. Front. Cell Dev. Biol. 2020, 8, 616434. [Google Scholar] [CrossRef]
- Juhász, K.Z.; Hajdú, T.; Kovács, P.; Vágó, J.; Matta, C.; Takács, R. Hypoxic Conditions Modulate Chondrogenesis through the Circadian Clock: The Role of Hypoxia-Inducible Factor-1α. Cells 2024, 13, 512. [Google Scholar] [CrossRef]
- Carús-Cadavieco, M.; de la Fuente, S.G.; Berenguer López, I.; Serrano-Lope, M.A.; Aguado, B.; Guix, F.; Dotti, C.G. Loss of Cldn5-and increase in Irf7-in the hippocampus and cerebral cortex of diabetic mice at the early symptomatic stage. Nutr. Diabetes 2024, 14, 64. [Google Scholar] [CrossRef]
- Gu, X.X.; Tang, Z.Z.; He, Y.L.; Zeng, Z.N.; Shi, W.X.; Qiao, Y.C.; Wei, Y.S. A functional polymorphism in HIF-3α is related to an increased risk of ischemic stroke. J. Mol. Neurosci. 2021, 71, 1061–1069. [Google Scholar] [CrossRef]
- Ting, K.K.Y. Revisiting the role of hypoxia-inducible factors and nuclear factor erythroid 2-related factor 2 in regulating macrophage inflammation and metabolism. Front. Cell. Infect. Microbiol. 2024, 14, 1403915. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Leung, V.I.K.; Xu, Z.; Zhang, T.; Du, J.; Zhang, Y.; Li, H. Bamboo Leaf Flavonoids from Phyllostachys glauca McClure Suppress the Progression of Alzheimer’s Disease Induced by Circadian Rhythm Disruption Through Regulating Hif3α/Rab7/TNFα/IL1β Pathway. Int. J. Mol. Sci. 2025, 26, 3169. https://doi.org/10.3390/ijms26073169
Li J, Leung VIK, Xu Z, Zhang T, Du J, Zhang Y, Li H. Bamboo Leaf Flavonoids from Phyllostachys glauca McClure Suppress the Progression of Alzheimer’s Disease Induced by Circadian Rhythm Disruption Through Regulating Hif3α/Rab7/TNFα/IL1β Pathway. International Journal of Molecular Sciences. 2025; 26(7):3169. https://doi.org/10.3390/ijms26073169
Chicago/Turabian StyleLi, Junru, Victor I. K. Leung, Zixiang Xu, Taiyu Zhang, Jianing Du, Yuqing Zhang, and Huiying Li. 2025. "Bamboo Leaf Flavonoids from Phyllostachys glauca McClure Suppress the Progression of Alzheimer’s Disease Induced by Circadian Rhythm Disruption Through Regulating Hif3α/Rab7/TNFα/IL1β Pathway" International Journal of Molecular Sciences 26, no. 7: 3169. https://doi.org/10.3390/ijms26073169
APA StyleLi, J., Leung, V. I. K., Xu, Z., Zhang, T., Du, J., Zhang, Y., & Li, H. (2025). Bamboo Leaf Flavonoids from Phyllostachys glauca McClure Suppress the Progression of Alzheimer’s Disease Induced by Circadian Rhythm Disruption Through Regulating Hif3α/Rab7/TNFα/IL1β Pathway. International Journal of Molecular Sciences, 26(7), 3169. https://doi.org/10.3390/ijms26073169





