Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury
Abstract
:1. Introduction
2. Results
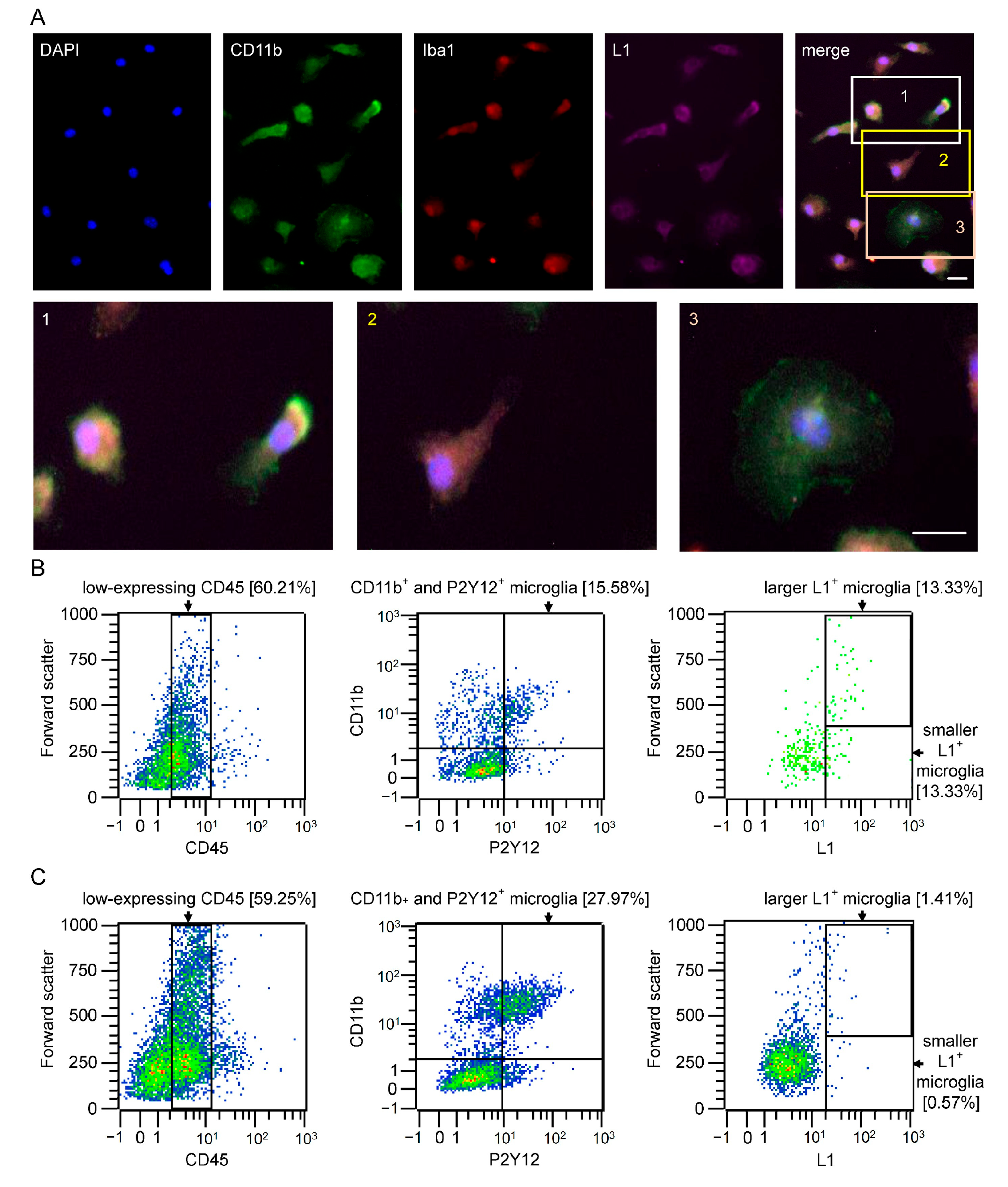
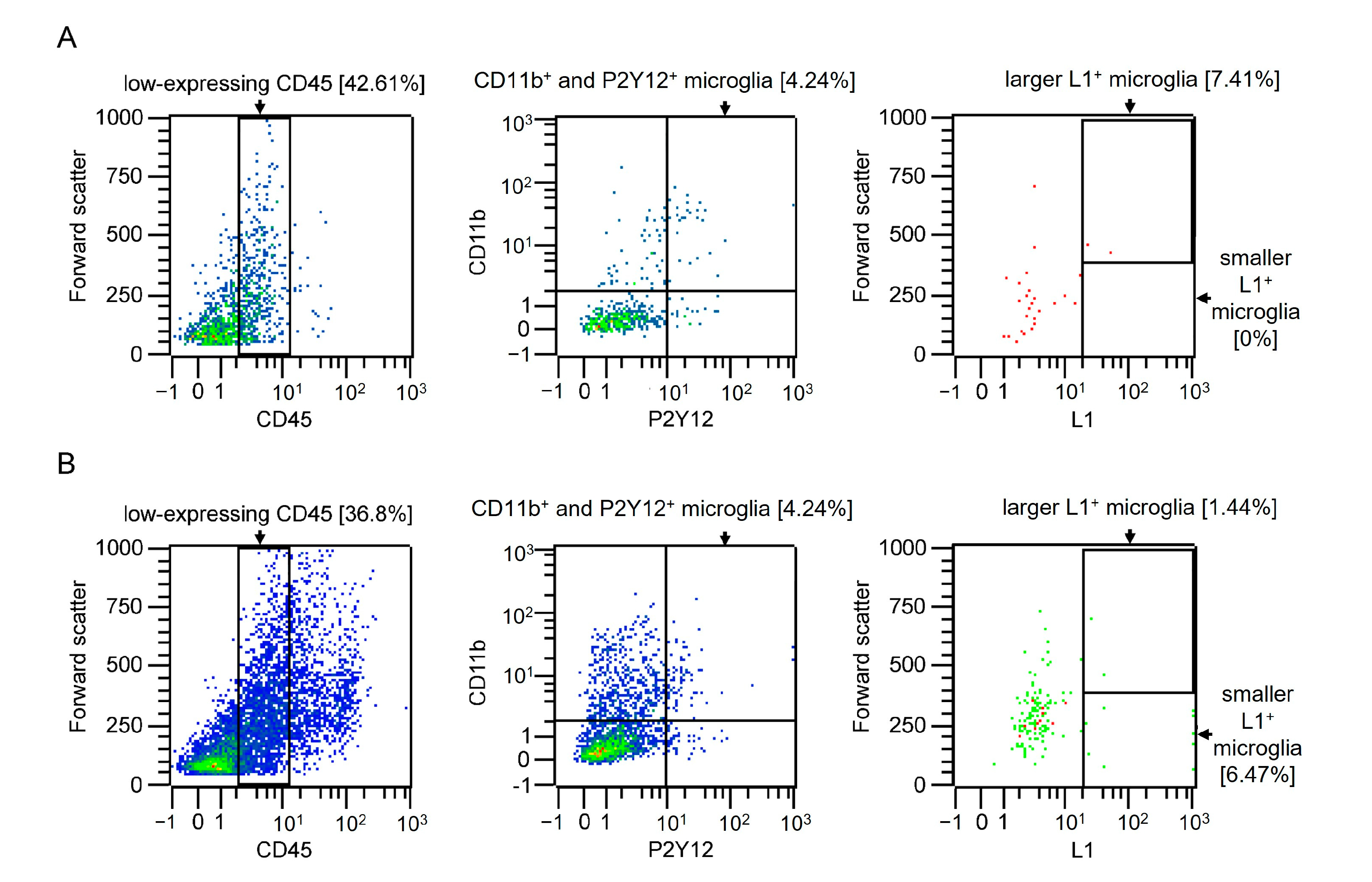
2.1. Subpopulations of Microglial Cells Express L1
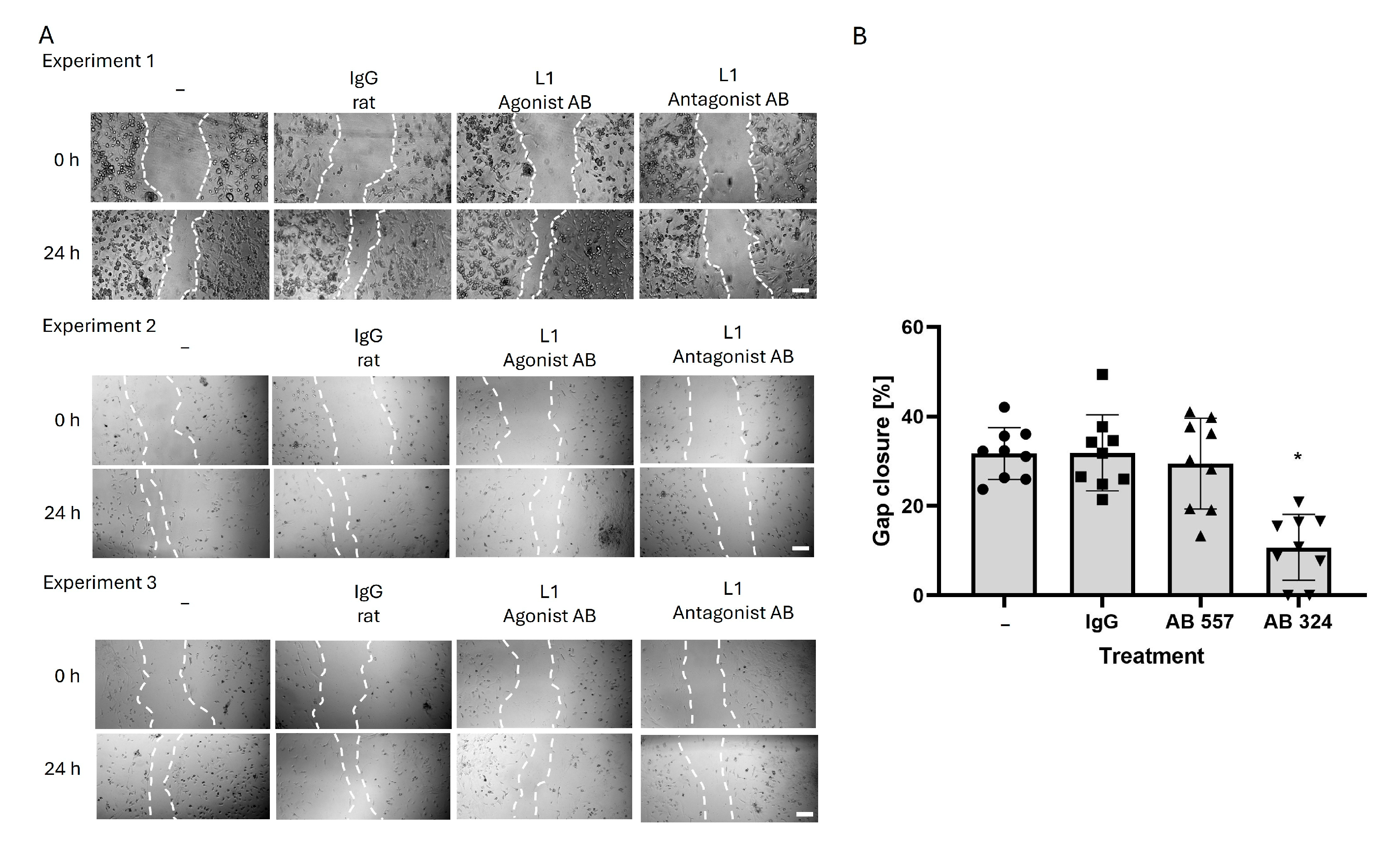
2.2. Reducing L1 Function Inhibits Microglial Cell Migration
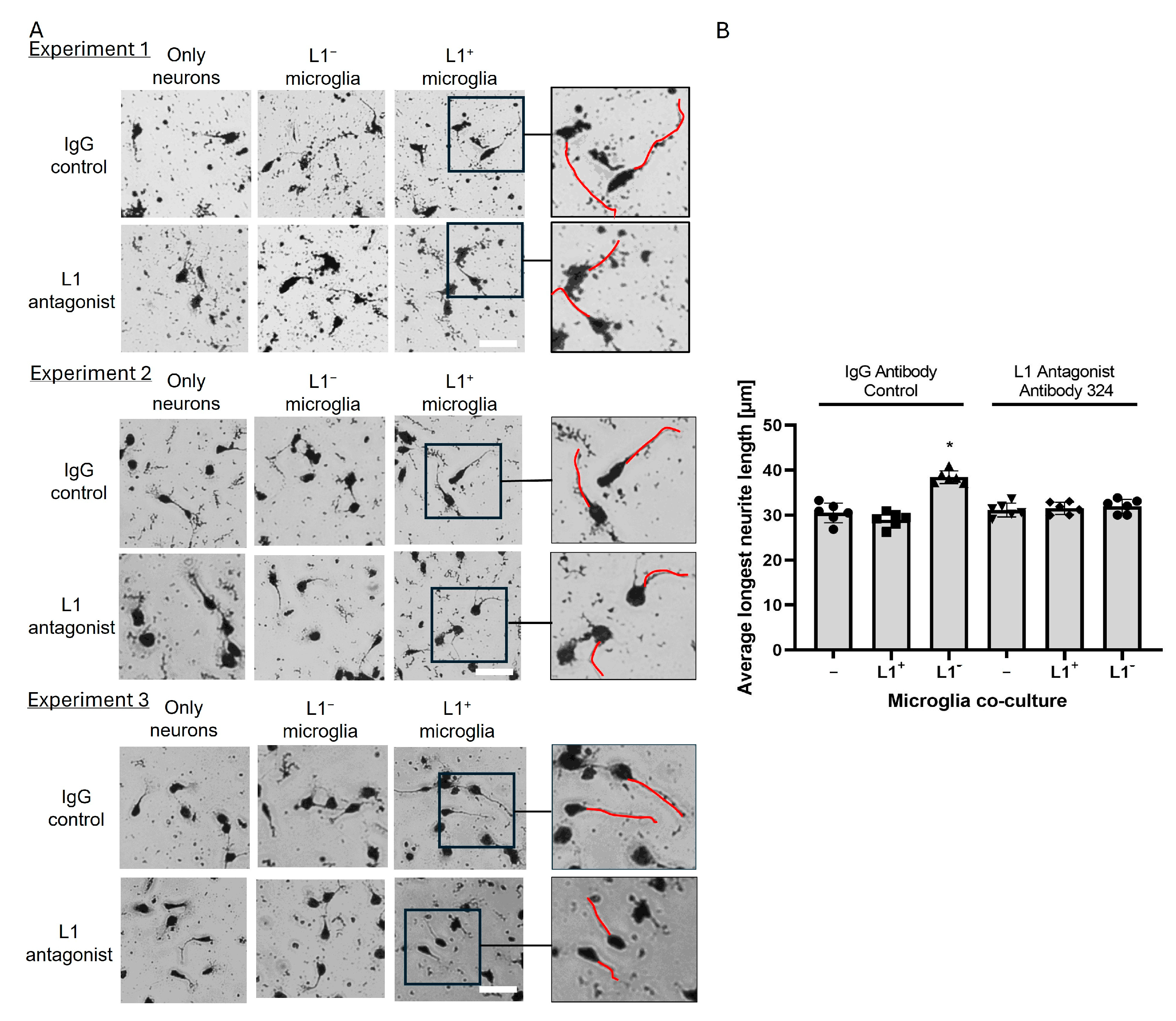
2.3. L1-Expressing Microglial Cells Enhance Neurite Outgrowth of Cerebellar Granule Cells
2.4. L1-Positive Microglial Cells in the Adult Spinal Cord
2.5. Depletion of L1 from Monocytes Reduces Functional Recovery After Spinal Cord Injury
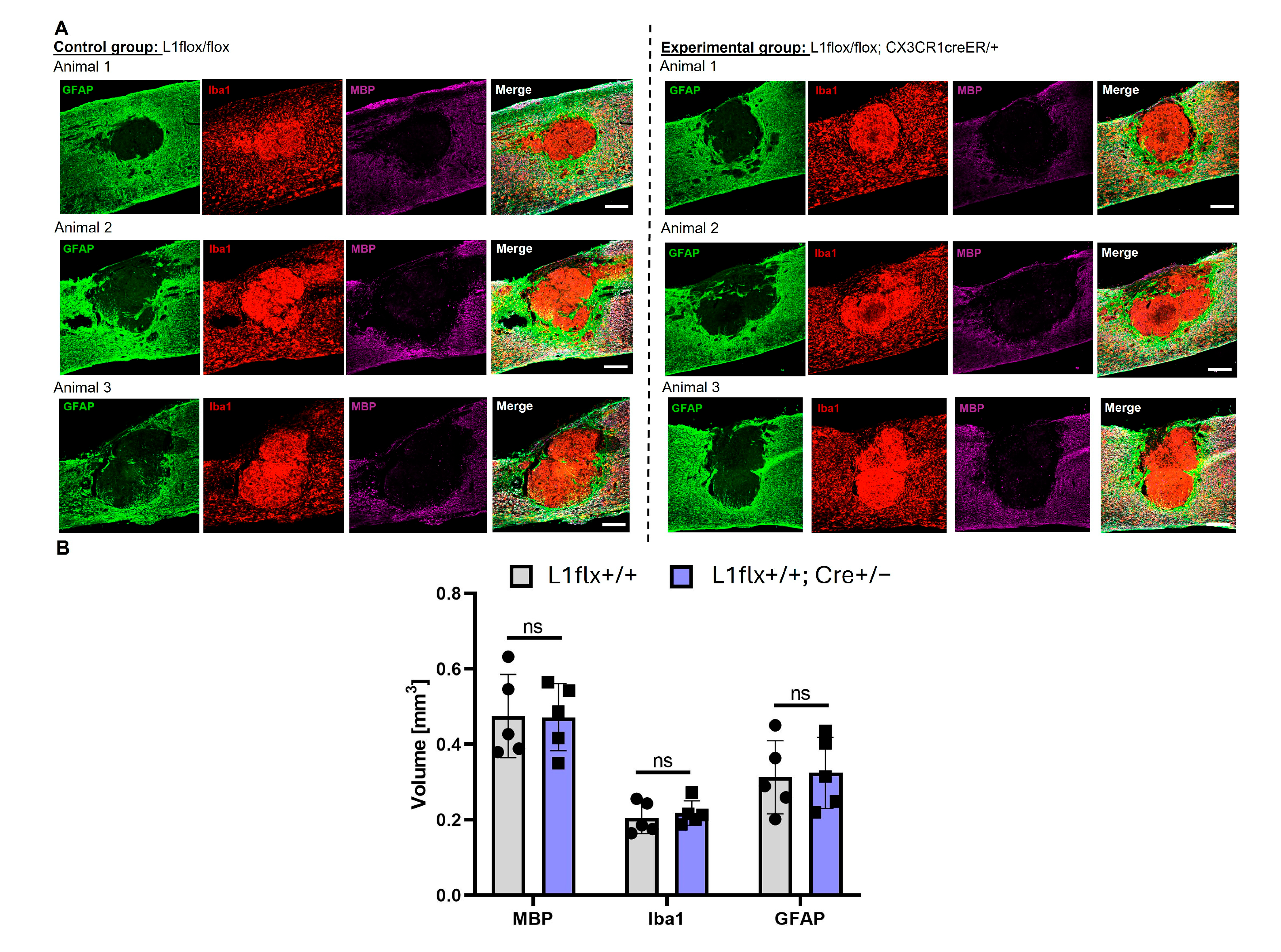
2.6. L1-Expressing Monocytes Do Not Affect Lesion Volume or Myelin Damage
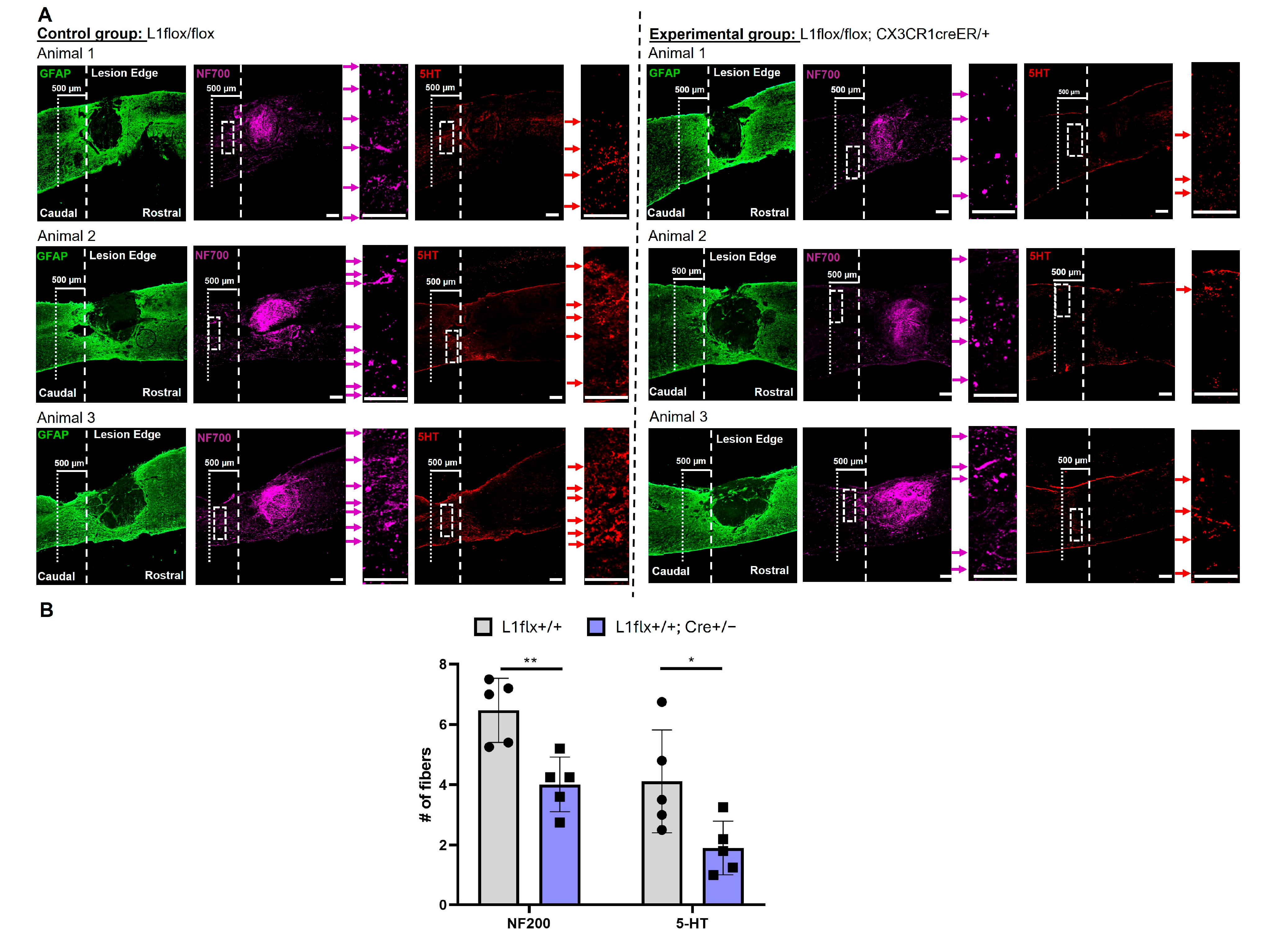
2.7. Depletion of L1 from Monocytes Decreases Fiber Sprouting Caudal to the Lesion Site
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies and Reagents
4.3. Microglia Culture
4.4. Immunocytochemistry
4.5. Flow Cytometry
4.6. Cell Migration Assay
4.7. Cerebellar Granule Cells and Microglia Co-Culture
4.8. Genotyping
4.9. SCI Mouse Model
4.10. Immunohistochemistry
4.11. Quantification of Immunofluorescence
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Tang, Y.; Vogel, L.C.; Devivo, M.J. Causes of Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2013, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, S.; Hammond, E.R.; Haider, A.H.; Abularrage, C.J.; Becker, D.; Dhiman, N.; Hyder, O.; Gupta, D.; Black, J.H.; Schneider, E.B. The Burden of Acute Traumatic Spinal Cord Injury among Adults in the United States: An Update. J. Neurotrauma 2014, 31, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Tang, F.; Tang, J.; Yang, H.; Zhao, Y.; Chen, B.; Han, S.; Wang, N.; Li, X.; Cheng, S.; et al. One-Year Clinical Study of NeuroRegen Scaffold Implantation Following Scar Resection in Complete Chronic Spinal Cord Injury Patients. Sci. China Life Sci. 2016, 59, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poon, W.; Liu, Y.; Leung, G.K.-K.; Wong, Y.; Feng, Y.; Ng, S.C.P.; Tsang, K.S.; Sun, D.T.F.; Yeung, D.K.; et al. Phase I–II Clinical Trial Assessing Safety and Efficacy of Umbilical Cord Blood Mononuclear Cell Transplant Therapy of Chronic Complete Spinal Cord Injury. Cell Transplant. 2016, 25, 1925–1943. [Google Scholar] [CrossRef]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef]
- Lindner, J.; Rathjen, F.G.; Schachner, M. L1 Mono- and Polyclonal Antibodies Modify Cell Migration in Early Postnatal Mouse Cerebellum. Nature 1983, 305, 427–430. [Google Scholar] [CrossRef]
- Wei, H.; Dobkin, C.; Sheikh, A.M.; Malik, M.; Brown, W.T.; Li, X. The Therapeutic Effect of Memantine through the Stimulation of Synapse Formation and Dendritic Spine Maturation in Autism and Fragile X Syndrome. PLoS ONE 2012, 7, e36981. [Google Scholar] [CrossRef]
- Becker, C.G.; Lieberoth, B.C.; Morellini, F.; Feldner, J.; Becker, T.; Schachner, M. L1.1 Is Involved in Spinal Cord Regeneration in Adult Zebrafish. J. Neurosci. 2004, 24, 7837–7842. [Google Scholar] [CrossRef]
- Roonprapunt, C.; Huang, W.; Grill, R.; Friedlander, D.; Grumet, M.; Chen, S.; Schachner, M.; Young, W. Soluble Cell Adhesion Molecule L1-Fc Promotes Locomotor Recovery in Rats after Spinal Cord Injury. J. Neurotrauma 2003, 20, 871–882. [Google Scholar] [CrossRef]
- Kataria, H.; Lutz, D.; Chaudhary, H.; Schachner, M.; Loers, G. Small Molecule Agonists of Cell Adhesion Molecule L1 Mimic L1 Functions In Vivo. Mol. Neurobiol. 2016, 53, 4461–4483. [Google Scholar] [CrossRef]
- Xu, J.; Hu, C.; Jiang, Q.; Pan, H.; Shen, H.; Schachner, M. Trimebutine, a Small Molecule Mimetic Agonist of Adhesion Molecule L1, Contributes to Functional Recovery after Spinal Cord Injury in Mice. Dis. Model. Mech. 2017, 10, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Djogo, N.; Jakovcevski, I.; Müller, C.; Lee, H.J.; Xu, J.-C.; Jakovcevski, M.; Kügler, S.; Loers, G.; Schachner, M. Adhesion Molecule L1 Binds to Amyloid Beta and Reduces Alzheimer’s Disease Pathology in Mice. Neurobiol. Dis. 2013, 56, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.L.; Schachner, M. A Fragment of Cell Adhesion Molecule L1 Reduces Amyloid-β Plaques in a Mouse Model of Alzheimer’s Disease. Cell Death Dis. 2022, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Pancook, J.D.; Reisfeld, R.A.; Varki, N.; Vitiello, A.; Fox, R.I.; Montgomery, A.M. Expression and Regulation of the Neural Cell Adhesion Molecule L1 on Human Cells of Myelomonocytic and Lymphoid Origin. J. Immunol. 1997, 158, 4413–4421. [Google Scholar]
- Schäfer, M.K.E.; Altevogt, P. L1CAM Malfunction in the Nervous System and Human Carcinomas. Cell. Mol. Life Sci. 2010, 67, 2425–2437. [Google Scholar] [CrossRef]
- Raveh, S.; Gavert, N.; Ben-Ze’ev, A. L1 Cell Adhesion Molecule (L1CAM) in Invasive Tumors. Cancer Lett. 2009, 282, 137–145. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Shmuel, A.; Raveh, S.; Ben-Ze’ev, A. L1-CAM in Cancerous Tissues. Expert Opin. Biol. Ther. 2008, 8, 1749–1757. [Google Scholar] [CrossRef]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in Human Cancer. Int. J. Cancer 2016, 138, 1565–1576. [Google Scholar] [CrossRef]
- Grage-Griebenow, E.; Jerg, E.; Gorys, A.; Wicklein, D.; Wesch, D.; Freitag-Wolf, S.; Goebel, L.; Vogel, I.; Becker, T.; Ebsen, M.; et al. L1CAM Promotes Enrichment of Immunosuppressive T Cells in Human Pancreatic Cancer Correlating with Malignant Progression. Mol. Oncol. 2014, 8, 982–997. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Brew, B.J. Microglia, Macrophages, Perivascular Macrophages, and Pericytes: A Review of Function and Identification. J. Leukoc. Biol. 2004, 75, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Haage, V.; Semtner, M.; Vidal, R.O.; Hernandez, D.P.; Pong, W.W.; Chen, Z.; Hambardzumyan, D.; Magrini, V.; Ly, A.; Walker, J.; et al. Comprehensive Gene Expression Meta-Analysis Identifies Signature Genes That Distinguish Microglia from Peripheral Monocytes/Macrophages in Health and Glioma. Acta Neuropathol. Commun. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, V.; Ghosh, H.S. Cx3Cr1-Cre Induction Leads to Microglial Activation and IFN-1 Signaling Caused by DNA Damage in Early Postnatal Brain. Cell Rep. 2022, 38, 110252. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.S.; Lee, A.Y.W.; Sun, M.; Nikonenko, A.G.; Chung, S.K.; Dityatev, A.; Schachner, M.; Morellini, F. Decreased Anxiety, Altered Place Learning, and Increased CA1 Basal Excitatory Synaptic Transmission in Mice with Conditional Ablation of the Neural Cell Adhesion Molecule L1. J. Neurosci. 2003, 23, 10419–10432. [Google Scholar] [CrossRef]
- Cadiz, M.P.; Jensen, T.D.; Sens, J.P.; Zhu, K.; Song, W.-M.; Zhang, B.; Ebbert, M.; Chang, R.; Fryer, J.D. Culture Shock: Microglial Heterogeneity, Activation, and Disrupted Single-Cell Microglial Networks in Vitro. Mol. Neurodegener. 2022, 17, 26. [Google Scholar] [CrossRef]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef]
- Harry, G.J. Microglia during Development and Aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef]
- Xu, P.; Yu, Y.; Wu, P. Role of Microglia in Brain Development after Viral Infection. Front. Cell Dev. Biol. 2024, 12, 1340308. [Google Scholar] [CrossRef]
- Herndon, J.M.; Tome, M.E.; Davis, T.P. Chapter 9-Development and Maintenance of the Blood–Brain Barrier. In Primer on Cerebrovascular Diseases, 2nd ed.; Caplan, L.R., Biller, J., Leary, M.C., Lo, E.H., Thomas, A.J., Yenari, M., Zhang, J.H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 51–56. ISBN 978-0-12-803058-5. [Google Scholar]
- Guedes, J.R.; Ferreira, P.A.; Costa, J.M.; Cardoso, A.L.; Peça, J. Microglia-Dependent Remodeling of Neuronal Circuits. J. Neurochem. 2022, 163, 74–93. [Google Scholar] [CrossRef]
- Dixon, M.A.; Greferath, U.; Fletcher, E.L.; Jobling, A.I. The Contribution of Microglia to the Development and Maturation of the Visual System. Front. Cell. Neurosci. 2021, 15, 659843. [Google Scholar] [CrossRef]
- Watts, R.J.; Schuldiner, O.; Perrino, J.; Larsen, C.; Luo, L. Glia Engulf Degenerating Axons during Developmental Axon Pruning. Curr. Biol. 2004, 14, 678–684. [Google Scholar] [CrossRef]
- Squarzoni, P.; Oller, G.; Hoeffel, G.; Pont-Lezica, L.; Rostaing, P.; Low, D.; Bessis, A.; Ginhoux, F.; Garel, S. Microglia Modulate Wiring of the Embryonic Forebrain. Cell Rep. 2014, 8, 1271–1279. [Google Scholar] [CrossRef]
- Lilienberg, J.; Apáti, Á.; Réthelyi, J.M.; Homolya, L. Microglia Modulate Proliferation, Neurite Generation and Differentiation of Human Neural Progenitor Cells. Front. Cell Dev. Biol. 2022, 10, 997028. [Google Scholar] [CrossRef]
- Cho, K.H.; Cheong, J.S.; Kim, J.H.; Abe, H.; Murakami, G.; Cho, B.H. Site-Specific Distribution of CD68-Positive Microglial Cells in the Brains of Human Midterm Fetuses: A Topographical Relationship with Growing Axons. BioMed Res. Int. 2013, 2013, 762303. [Google Scholar] [CrossRef]
- Hagemeyer, N.; Hanft, K.-M.; Akriditou, M.-A.; Unger, N.; Park, E.S.; Stanley, E.R.; Staszewski, O.; Dimou, L.; Prinz, M. Microglia Contribute to Normal Myelinogenesis and to Oligodendrocyte Progenitor Maintenance during Adulthood. Acta Neuropathol. 2017, 134, 441–458. [Google Scholar] [CrossRef]
- Mehl, L.C.; Manjally, A.V.; Bouadi, O.; Gibson, E.M.; Tay, T.L. Microglia in Brain Development and Regeneration. Dev. Camb. Engl. 2022, 149, dev200425. [Google Scholar] [CrossRef]
- Ung, R.V.; Lapointe, N.P.; Tremblay, C.; Larouche, A.; Guertin, P.A. Spontaneous Recovery of Hindlimb Movement in Completely Spinal Cord Transected Mice: A Comparison of Assessment Methods and Conditions. Spinal Cord 2007, 45, 367–379. [Google Scholar] [CrossRef]
- Boulland, J.-L.; Lambert, F.M.; Züchner, M.; Ström, S.; Glover, J.C. A Neonatal Mouse Spinal Cord Injury Model for Assessing Post-Injury Adaptive Plasticity and Human Stem Cell Integration. PLoS ONE 2013, 8, e71701. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Kawaguchi, R.; Zhang, Y.; Wang, Q.; Monavarfeshani, A.; Yang, Z.; Chen, B.; Shi, Z.; Meng, H.; et al. Microglia-Organized Scar-Free Spinal Cord Repair in Neonatal Mice. Nature 2020, 587, 613–618. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Chen, X.; Zhao, J.; Li, F.; Zhao, Q.; Xie, T.; Huang, L.; Zhang, Z.; Qi, Y.; et al. L1CAM Overexpression Promotes Tumor Progression through Recruitment of Regulatory T Cells in Esophageal Carcinoma. Cancer Biol. Med. 2021, 18, 547–561. [Google Scholar] [CrossRef]
- Loers, G.; Bork, U.; Schachner, M. Functional Relationships between L1CAM, LC3, ATG12, and Aβ. Int. J. Mol. Sci. 2024, 25, 10829. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Floden, A.M.; Combs, C.K. Microglia Demonstrate Age-Dependent Interaction with Amyloid-β Fibrils. J. Alzheimers Dis. 2011, 25, 279–293. [Google Scholar] [CrossRef]
- Lutz, D.; Wolters-Eisfeld, G.; Joshi, G.; Djogo, N.; Jakovcevski, I.; Schachner, M.; Kleene, R. Generation and nuclear translocation of sumoylated transmembrane fragment of cell adhesion molecule L1. J. Biol. Chem. 2012, 287, 17161–17175. [Google Scholar] [CrossRef]
- Lutz, D.; Wolters-Eisfeld, G.; Schachner, M.; Kleene, R. Cathepsin E generates a sumoylated intracellular fragment of the cell adhesion molecule L1 to promote neuronal and Schwann cell migration as well as myelination. J. Neurochem. 2014, 128, 713–724. [Google Scholar] [CrossRef]
- Theis, T.; Ayala, C.; Tschang, M.; Philip, M.; Nagaraj, V.; Shrirao, A.; Voronin, G.; Young, W.; Schachner, M. CHL1-Deficient and Wild-Type Male Mice Do Not Differ in Locomotor Recovery from Spinal Cord Injury. J. Spine Res. Surg. 2022, 4, 96–103. [Google Scholar] [CrossRef]
- Ford, A.L.; Goodsall, A.L.; Hickey, W.F.; Sedgwick, J.D. Normal Adult Ramified Microglia Separated from Other Central Nervous System Macrophages by Flow Cytometric Sorting. Phenotypic Differences Defined and Direct Ex Vivo Antigen Presentation to Myelin Basic Protein-Reactive CD4+ T Cells Compared. J. Immunol. 1995, 154, 4309–4321. [Google Scholar] [CrossRef]
- Costa, A.; Haage, V.; Yang, S.; Wegner, S.; Ersoy, B.; Ugursu, B.; Rex, A.; Kronenberg, G.; Gertz, K.; Endres, M.; et al. Deletion of Muscarinic Acetylcholine Receptor 3 in Microglia Impacts Brain Ischemic Injury. Brain Behav. Immun. 2021, 91, 89–104. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for Locomotion Detects Differences in Recovery after Spinal Cord Injury in Five Common Mouse Strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theis, T.; Kumar, S.; Shah, P.; Patel, M.; Tadmori, I.; Ayala, C.; Tschang, M.; Young, W.; Schachner, M. Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 3285. https://doi.org/10.3390/ijms26073285
Theis T, Kumar S, Shah P, Patel M, Tadmori I, Ayala C, Tschang M, Young W, Schachner M. Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury. International Journal of Molecular Sciences. 2025; 26(7):3285. https://doi.org/10.3390/ijms26073285
Chicago/Turabian StyleTheis, Thomas, Suneel Kumar, Pratiksha Shah, Mukti Patel, Iman Tadmori, Carlos Ayala, Monica Tschang, Wise Young, and Melitta Schachner. 2025. "Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury" International Journal of Molecular Sciences 26, no. 7: 3285. https://doi.org/10.3390/ijms26073285
APA StyleTheis, T., Kumar, S., Shah, P., Patel, M., Tadmori, I., Ayala, C., Tschang, M., Young, W., & Schachner, M. (2025). Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury. International Journal of Molecular Sciences, 26(7), 3285. https://doi.org/10.3390/ijms26073285







