Mechanisms of Exogenous Brassinosteroids and Abscisic Acid in Regulating Maize Cold Stress Tolerance
Abstract
:1. Introduction
2. Results
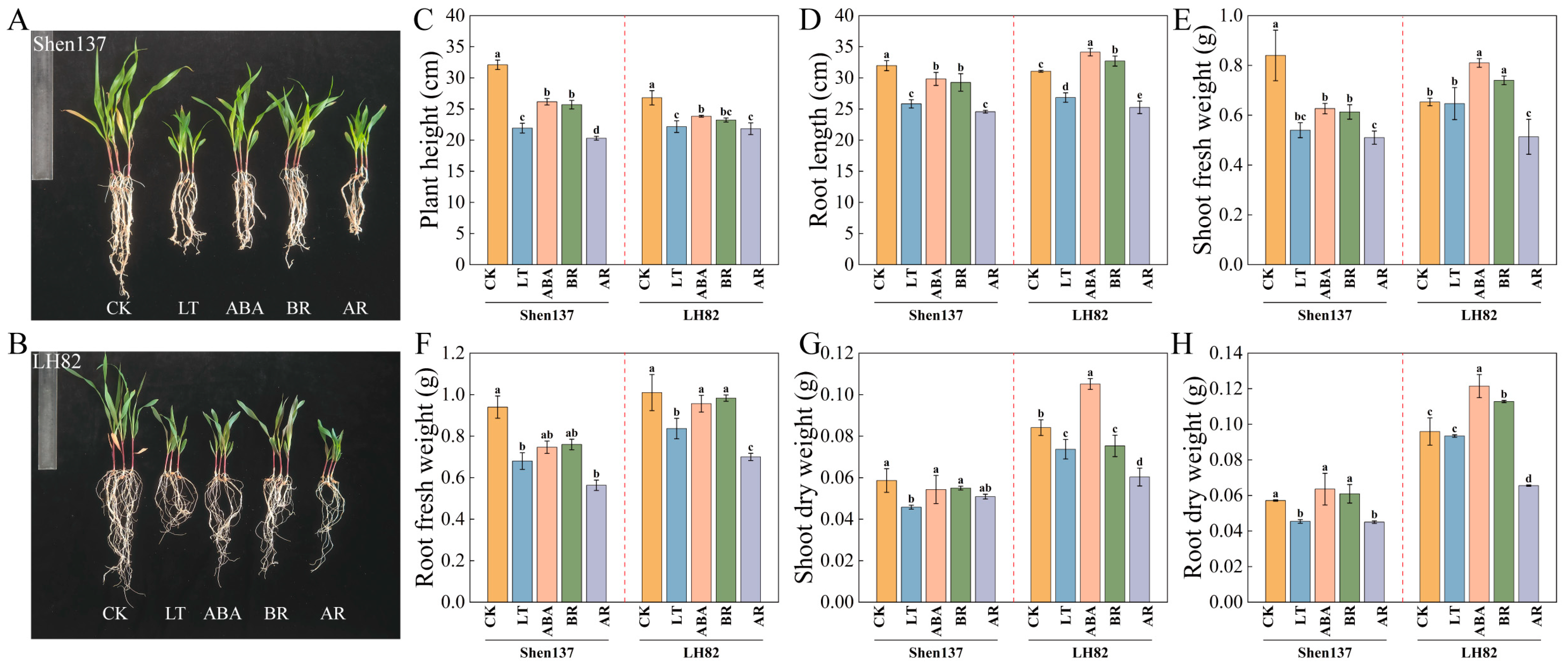
2.1. Effects of Exogenous BR and ABA on Maize Seedling Growth Under Cold Stress
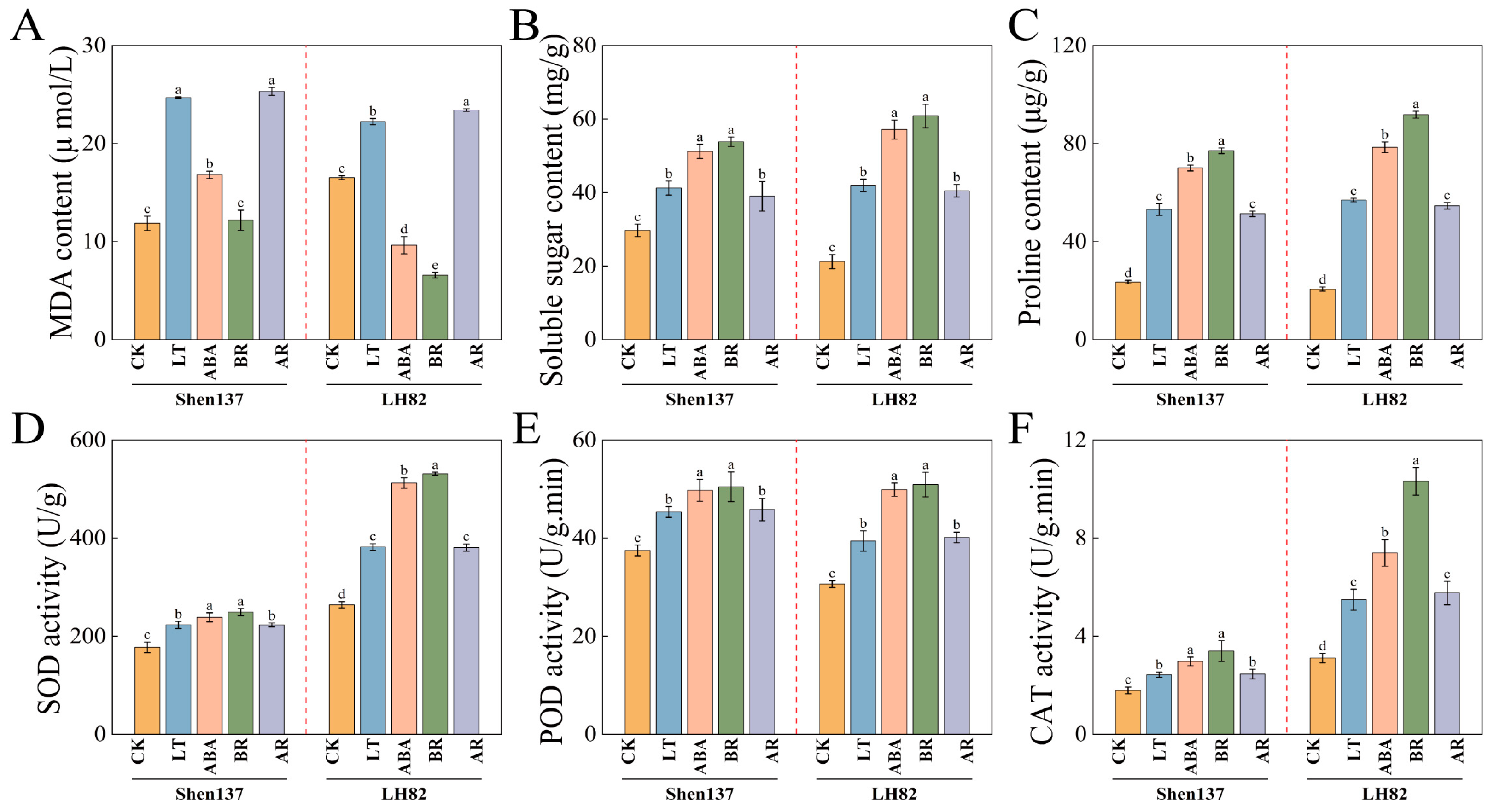
2.2. Effects of Exogenous BR and ABA on the Physicochemical Properties of Maize Seedlings Under Cold Stress
2.3. RNA-seq Data Quality Inspection and Sequencing Results
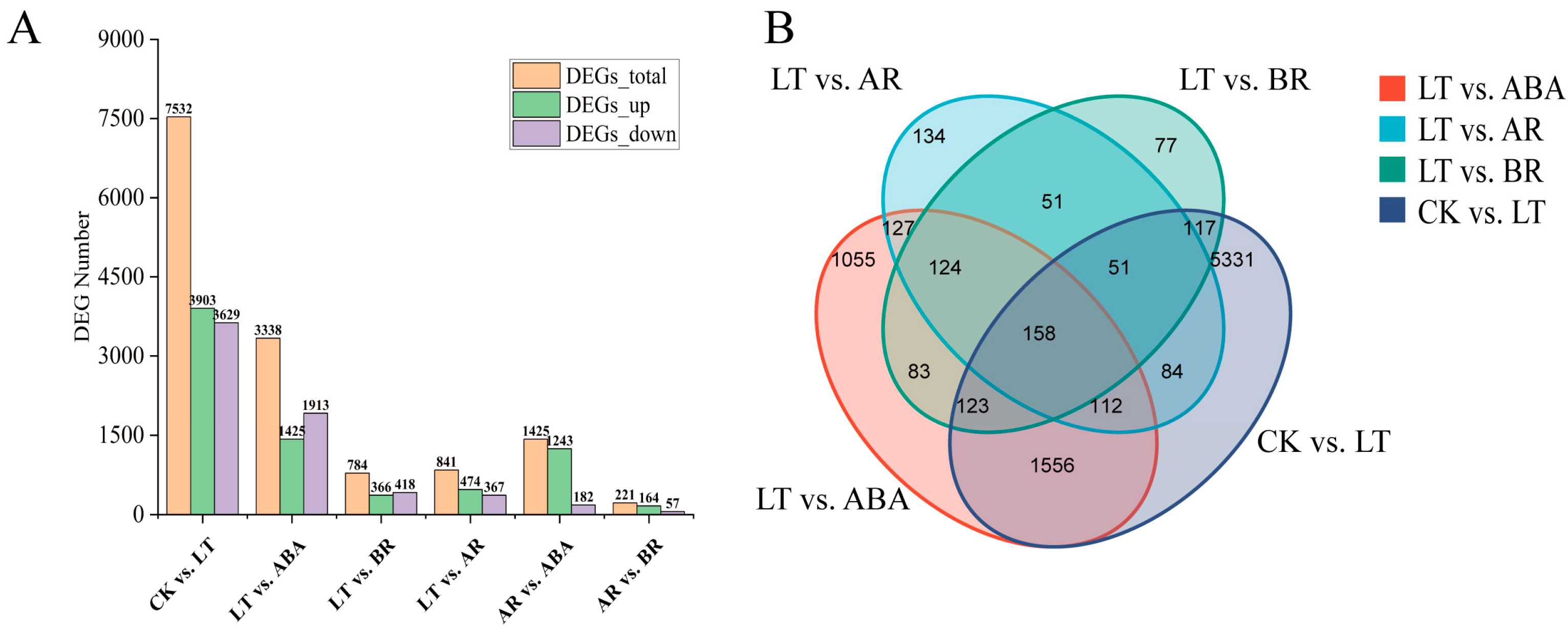
2.4. Screening and Statistical Analysis of DEGs
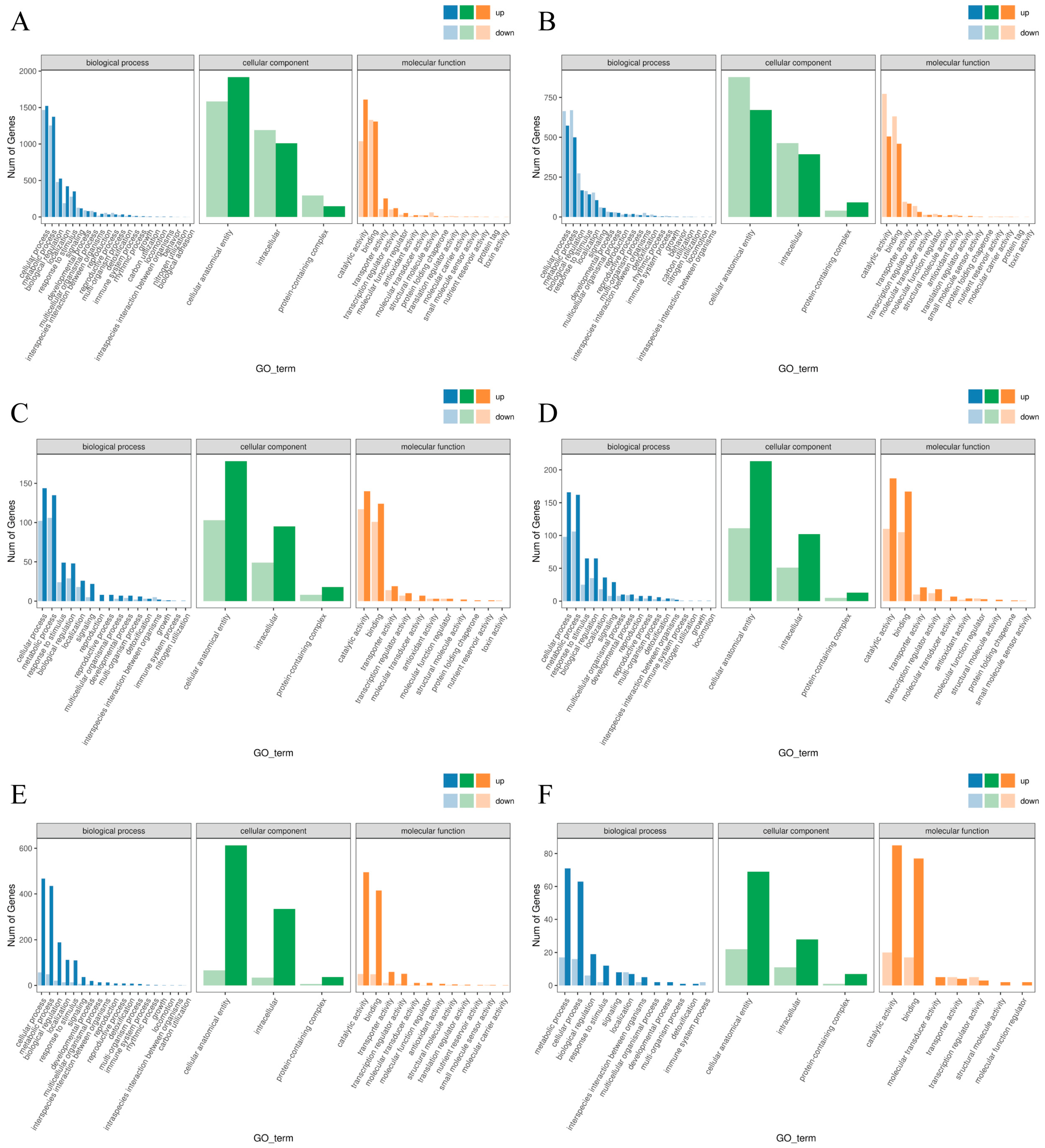
2.5. GO Enrichment Analysis
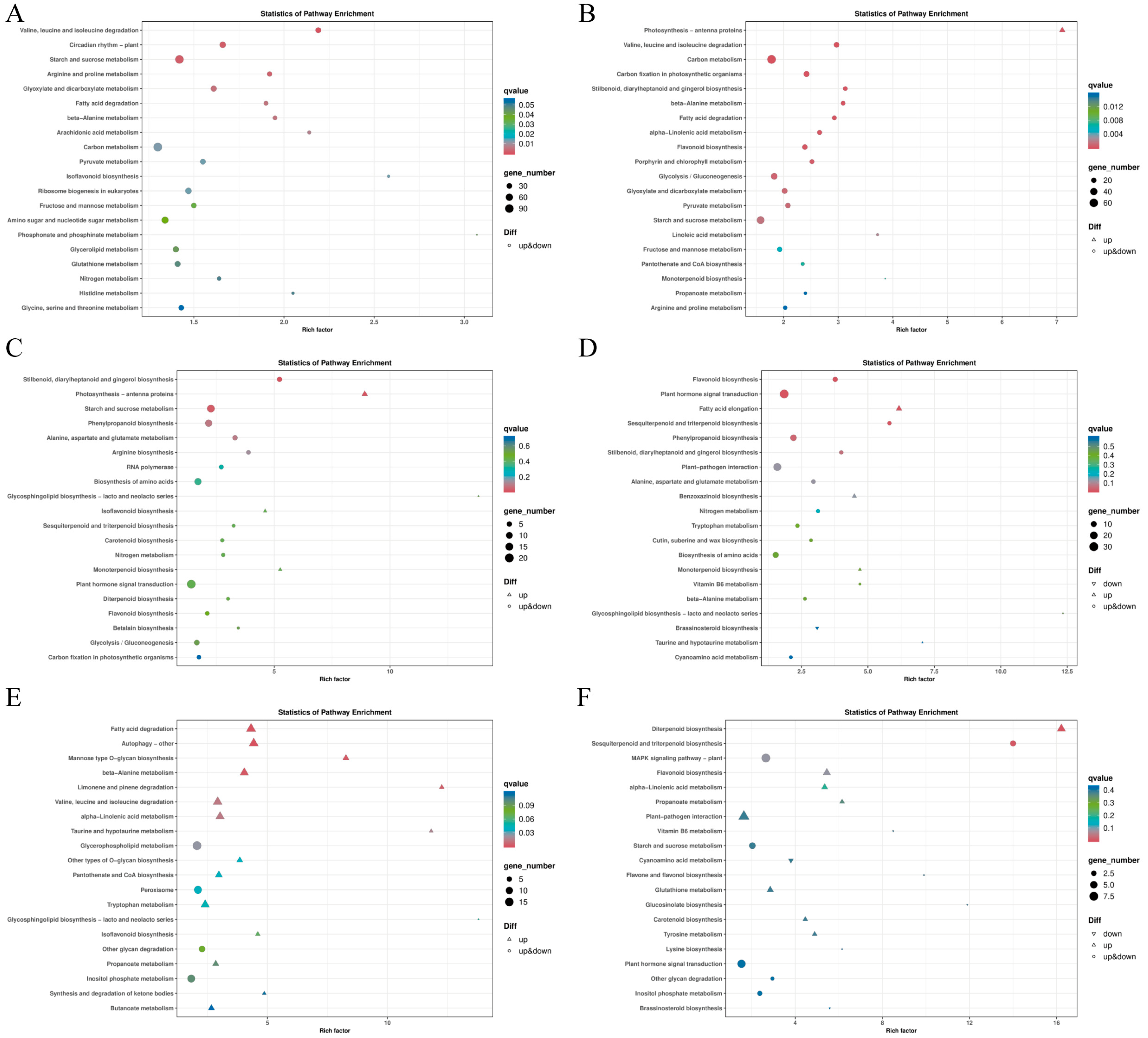
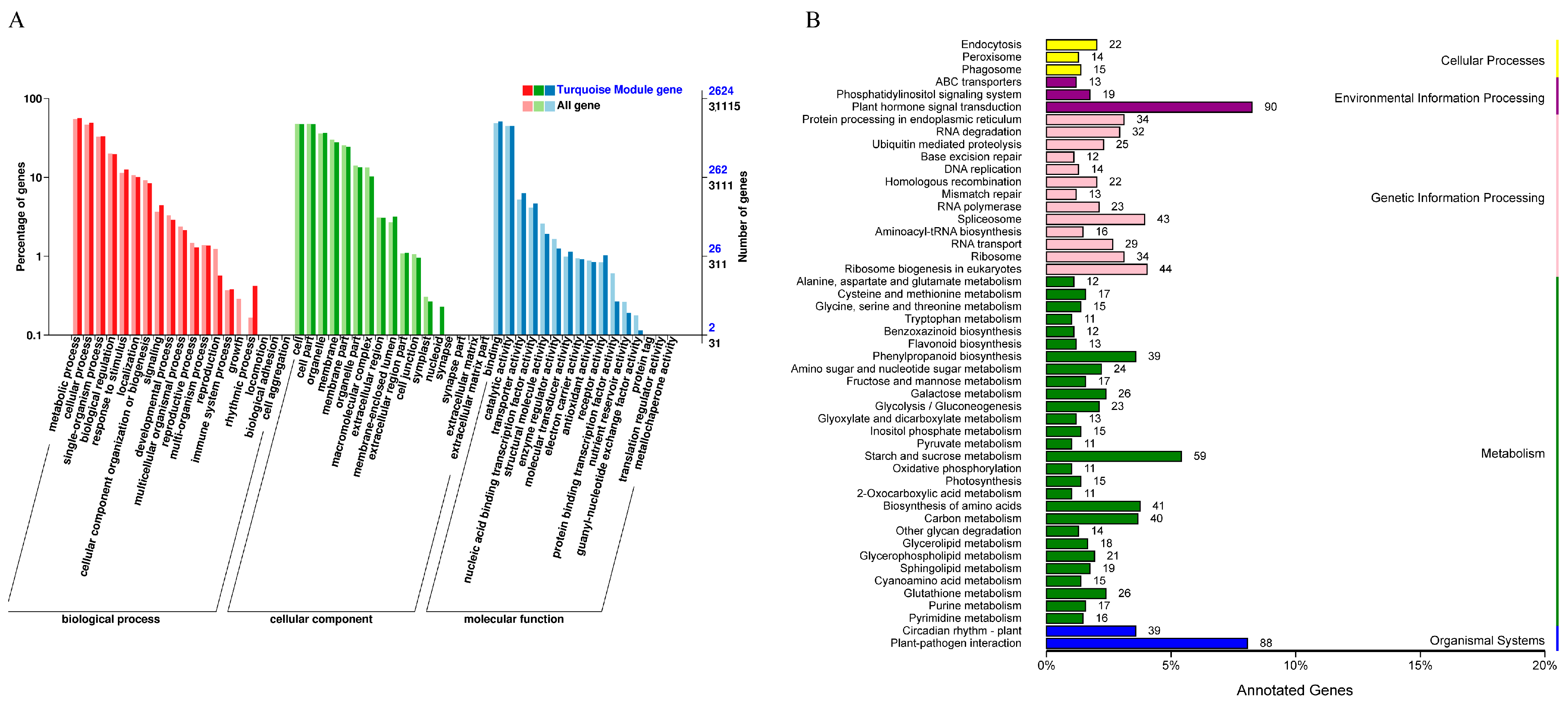
2.6. KEGG Enrichment Analysis
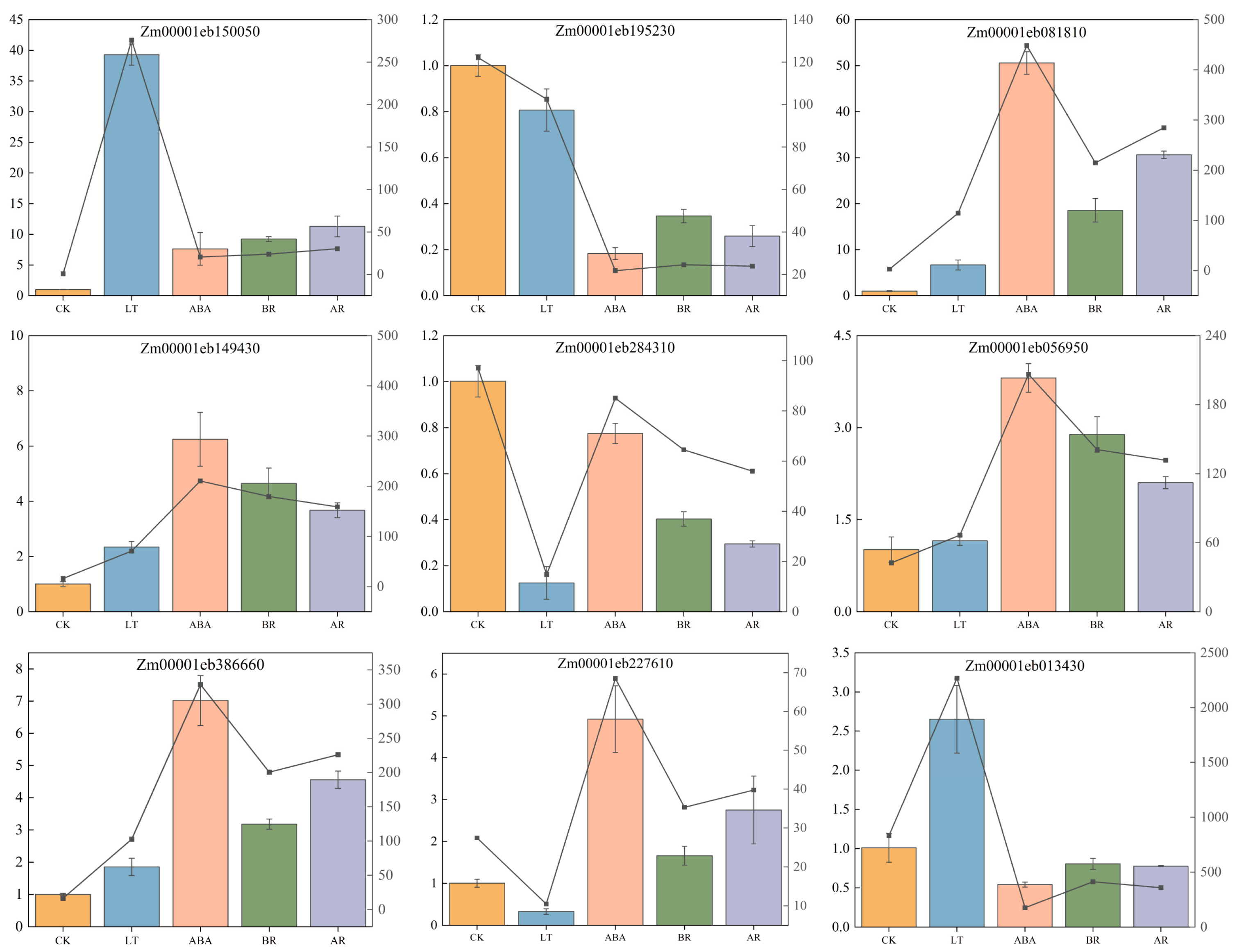
2.7. RT-qPCR Verification
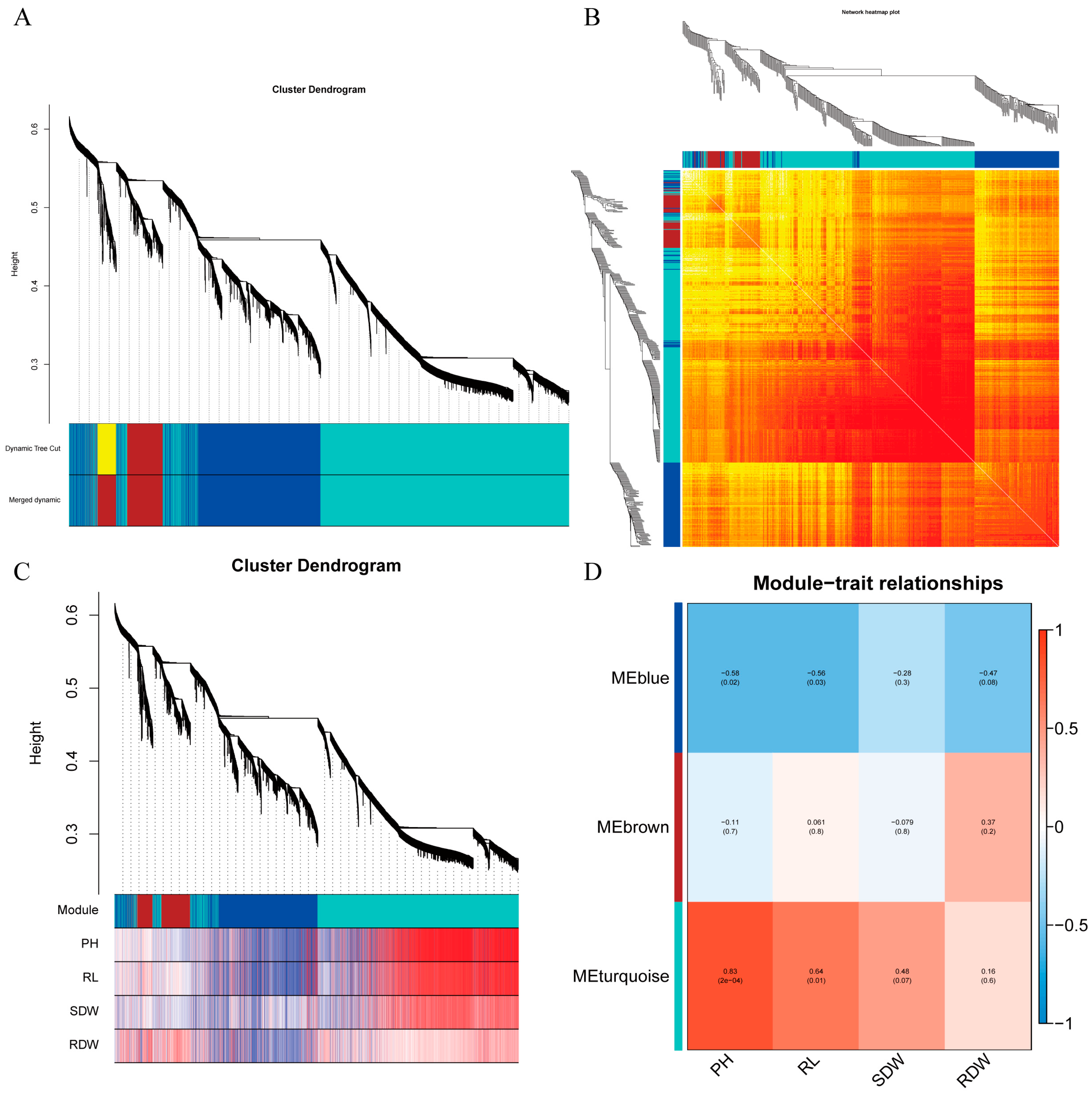
2.8. Weighted Gene Co-Expression Network Analysis (WGCNA) Analysis
2.8.1. Construction of Gene Co-Expression Module
2.8.2. Functional Analysis of Gene Module
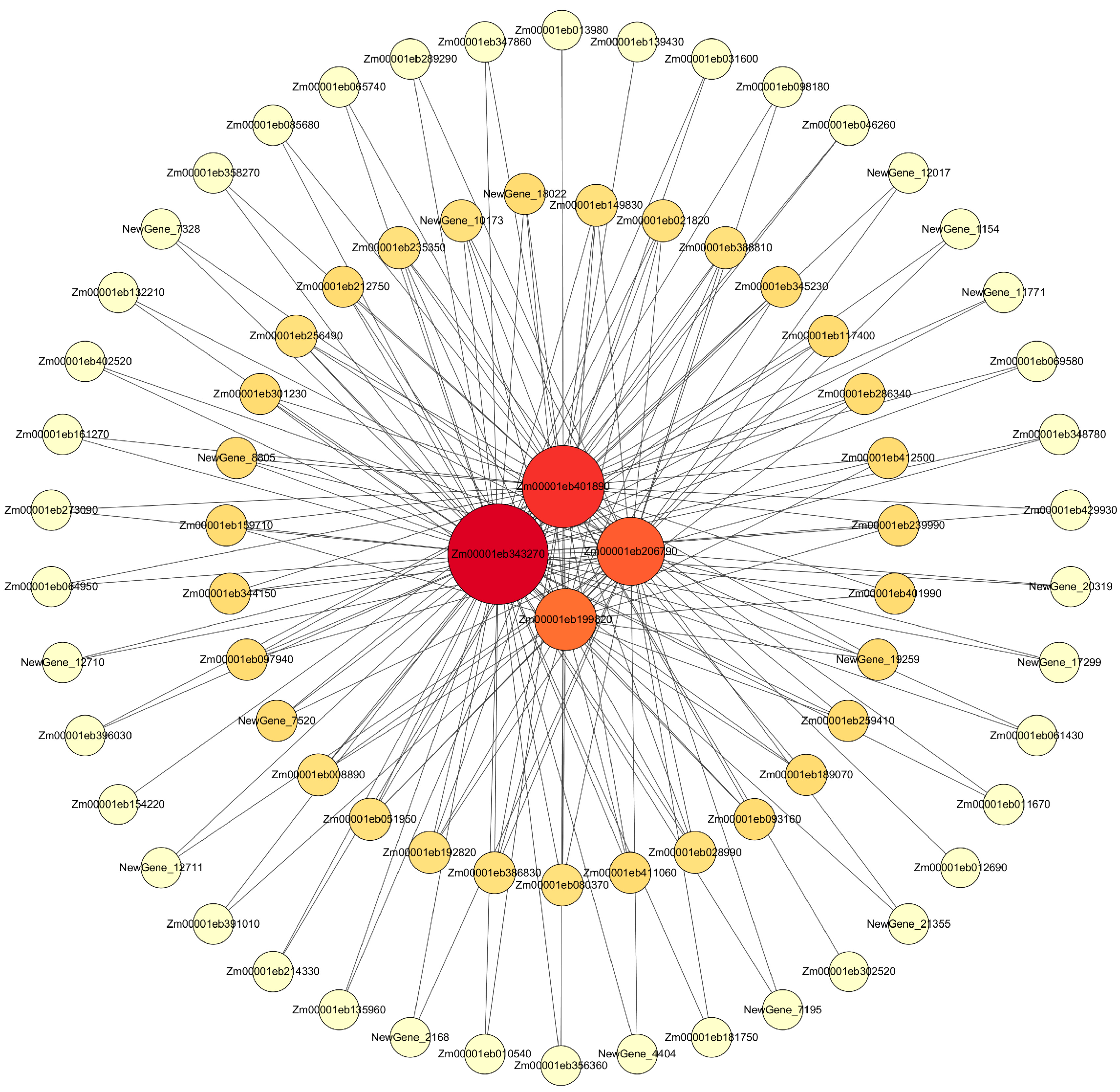
2.8.3. Visualization Analysis of Core Genes of the Gene Module
3. Discussion
3.1. Effects of Low Temperature and Exogenous ABA and BRs on Maize Seedling Growth
3.2. Mechanism of Exogenous ABA in Enhancing Cold Tolerance in Maize Seedlings
3.3. Mechanism of Exogenous BR in Enhancing Cold Tolerance in Maize Seedlings
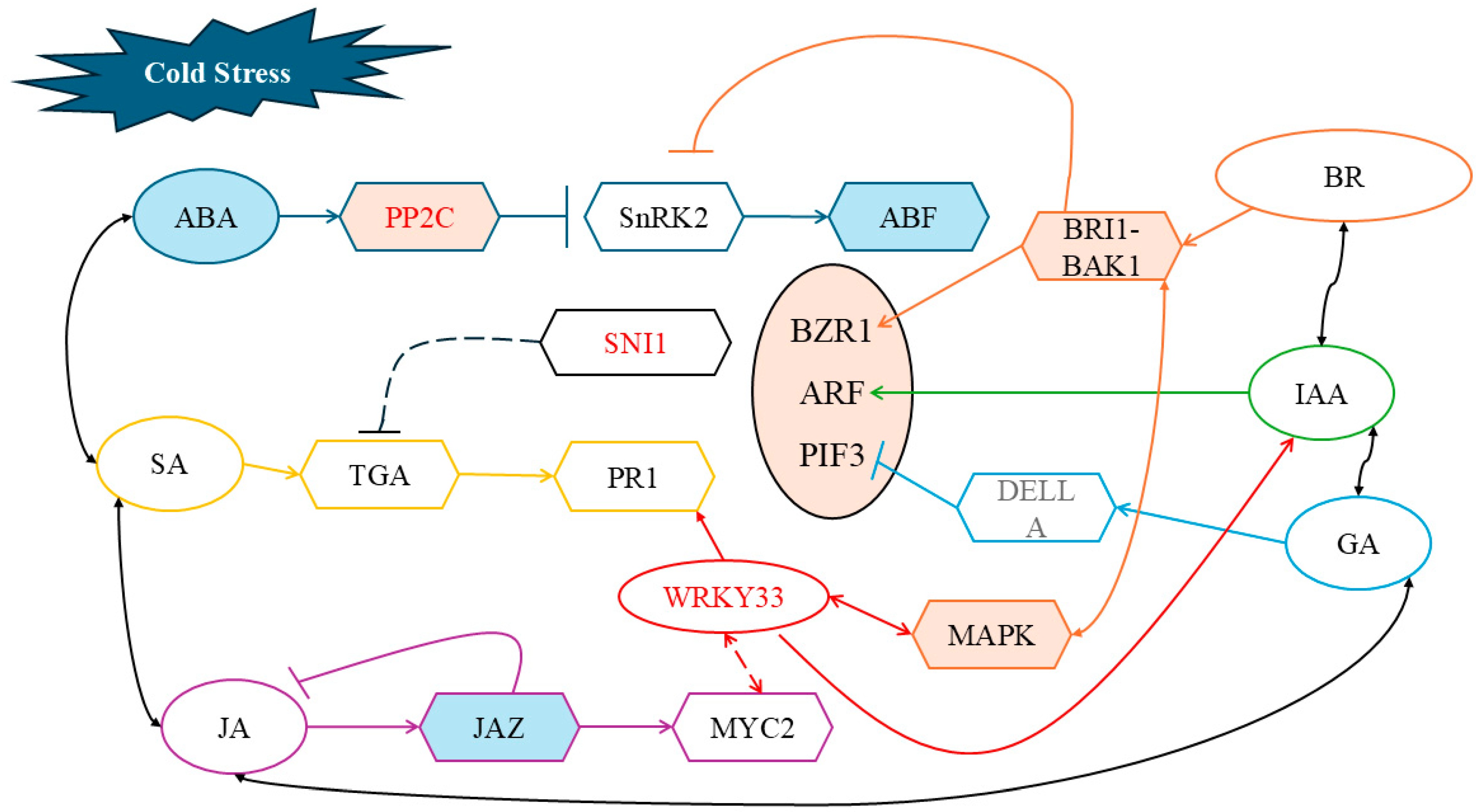
3.4. Regulation of Maize Cold Tolerance by the Interaction Between Exogenous ABA and BR
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design and Treatment
4.3. Index Measurement
4.3.1. Growth Index Measurement
4.3.2. Measurement of Physiological Indexes
4.4. Transcriptomic Analysis
4.4.1. Total RNA Extraction and Illumina Deep Sequencing
4.4.2. Quality Assessment of Sequencing Results
4.4.3. Differential Expression Gene Analysis
4.4.4. Analysis of the Gene Co-Expression Network
4.4.5. Real-Time Fluorescence Quantitative PCR (RT-qPCR) Verification of DEGs
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Waqas, M.A.; Wang, X.; Zafar, S.A.; Noor, M.A.; Hussain, H.A.; Azher Nawaz, M.; Farooq, M. Thermal Stresses in Maize: Effects and Management Strategies. Plants 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Sidhu, A.; Avinash, G.; Sethi, M. Effect of Low-Temperature Stress on Plant Performance and Adaptation to Temperature Change. In Plant Abiotic Stress Responses and Tolerance Mechanisms; IntechOpen: London, UK, 2023. [Google Scholar]
- Qi, X.; Wan, C.; Zhang, X.; Sun, W.; Liu, R.; Wang, Z.; Wang, Z.; Ling, F. Effects of histone methylation modification on low temperature seed germination and growth of maize. Sci. Rep. 2023, 13, 5196. [Google Scholar]
- Jiang, L.; Wang, M.; Chu, Z.; Gao, Y.; Guo, L.; Ji, S.; Jiang, L.; Gong, L. Effects of Temperature on Growth and Grain Maturity of Spring Maize in Northeast China: A Study of Different Sowing Dates. Atmosphere 2023, 14, 1755. [Google Scholar] [CrossRef]
- Miao, R.; Li, C.; Liu, Z.; Zhou, X.; Chen, S.; Zhang, D.; Luo, J.; Tang, W.; Wang, C.; Wu, J.; et al. The Role of Endogenous Brassinosteroids in the Mechanisms Regulating Plant Reactions to Various Abiotic Stresses. Agronomy 2024, 14, 356. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, D.; Li, L.; Sun, X.; He, S.; Li, M.; Chen, J. Physiological Characteristics and Transcriptome Analysis of Exogenous Brassinosteroid-Treated Kiwifruit. Int. J. Mol. Sci. 2023, 24, 19. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, W.; Yu, H. Effects of exogenous epibrassinolide on photosynthetic characteristics in tomato (Lycopersicon esculentum Mill) seedlings under weak light stress. J. Agric. Food Chem. 2010, 58, 3642–3645. [Google Scholar]
- Zhang, H.; Zhao, D.; Tang, Z.; Zhang, Y.; Zhang, K.; Dong, J.; Wang, F. Exogenous brassinosteroids promotes root growth, enhances stress tolerance, and increases yield in maize. Plant Signal. Behav. 2022, 17, 2095139. [Google Scholar]
- Sun, S.; Yao, X.; Liu, X.; Qiao, Z.; Liu, Y.; Li, X.; Jiang, X. Brassinolide can improve drought tolerance of maize seedlings under drought stress: By inducing the photosynthetic performance, antioxidant capacity and ZmMYB gene expression of maize seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 2092–2104. [Google Scholar] [CrossRef]
- Mu, D.; Feng, N.; Zheng, D.; Zhou, H.; Liu, L.; Chen, G. Studies on the Physiological Mechanism of Brassinolide to Improve the Resistance of Rice Seedlings to NaCl Stress. Water Air Soil Pollut. 2022, 233, 238. [Google Scholar]
- Wang, S.-Q.; Zhao, H.-H.; Zhao, L.-M.; Gu, C.-M.; Na, Y.-G.; Xie, B.; Cheng, S.-H.; Pan, G.-J. Application of brassinolide alleviates cold stress at the booting stage of rice. J. Integr. Agric. 2020, 19, 975–987. [Google Scholar]
- Rai, M.K.; Shekhawat, N.S.; Harish; Gupta, A.K.; Phulwaria, M.; Ram, K.; Jaiswal, U. The role of abscisic acid in plant tissue culture: A review of recent progress. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 106, 179–190. [Google Scholar]
- Yao, C.; Zhang, F.; Sun, X.; Shang, D.; He, F.; Li, X.; Zhang, J.; Jiang, X. Effects of S-Abscisic Acid (S-ABA) on Seed Germination, Seedling Growth, and Asr1 Gene Expression Under Drought Stress in Maize. J. Plant Growth Regul. 2019, 38, 1300–1313. [Google Scholar]
- Tian, L.-x.; Li, J. The effects of exogenous ABA applied to maize (Zea mays L.) roots on plant responses to chilling stress. Acta Physiol. Plant. 2018, 40, 77. [Google Scholar]
- Terán, F.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Facing climate change: Plant stress mitigation strategies in agriculture. Physiol. Plant. 2024, 176, e14484. [Google Scholar]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar]
- Yu, J.; Zhang, L.; Cang, J.; Wang, X.; Zhou, Z.S.; Hao, Z.B.; Li, Z.F. Effects of Exogenous ABA on Cold Resistance and Tender Seedlings Growth of Winter Wheat Dongnongdongmai 1 in Cold Area. J. Triticeae Crops 2008, 5, 30. [Google Scholar]
- Saini, L.K.; Singh, N.; Pandey, G.K. Plant Protein Phosphatase 2C: Critical Negative Regulator of ABA Signaling. In Protein Phosphatases and Stress Management in Plants: Functional Genomic Perspective; Pandey, G.K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 83–102. [Google Scholar]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- de Ollas, C.; Dodd, I.C. Physiological impacts of ABA–JA interactions under water-limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant. 2012, 147, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lang, D.; Yong, J.; Zhang, X. Bacillus cereus G2 alleviate salt stress in Glycyrrhiza uralensis Fisch. by balancing the downstream branches of phenylpropanoids and activating flavonoid biosynthesis. Ecotoxicol. Environ. Saf. 2024, 273, 116129. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Yadav, R.K.; Devi, L.L.; Singh, A.P. Chapter 10—Brassinosteroids in plant growth and development. In Plant Hormones in Crop Improvement; Khan, M.I.R., Singh, A., Poór, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 185–203. [Google Scholar]
- Xia, X.-J.; Wang, Y.-J.; Zhou, Y.-H.; Tao, Y.; Mao, W.-H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.-Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef]
- Ding, M.; Wang, L.; Sun, Y.; Zhang, J.; Chen, Y.; Wang, X.; Liu, L. Transcriptome analysis of brassinolide under low temperature stress in winter wheat. AoB PLANTS 2023, 15, plad005. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, Q.; Kong, Y.; Zhu, L.; Tian, W.; Huang, J.; Pan, L.; Jin, Q.; Zhang, J.; Zhu, C. Unearthing the Alleviatory Mechanisms of Brassinolide in Cold Stress in Rice. Life 2022, 12, 833. [Google Scholar] [CrossRef]
- Xie, C.; Yang, L.; Gai, Y. MAPKKKs in Plants: Multidimensional Regulators of Plant Growth and Stress Responses. Int. J. Mol. Sci. 2023, 24, 4117. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Shi, X.Y.; Qian, W.H.; Mehmood, J.; Yin, Y.M.; Jia, H.J. Identification of the Light-Harvesting Chlorophyll a/b Binding Protein Gene Family in Peach (Prunus persica L.) and Their Expression under Drought Stress. Genes 2023, 14, 1475. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, Z.; Tang, Y.; Wu, M.; Yan, F.; Zhang, X.; Zhang, Q.; Yang, F.; Hu, X.; et al. Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hortic. Res. 2019, 6, 1–16. [Google Scholar]
- Zhang, Y.; Raza, A.L.; Huang, H.; Su, W.; Luo, D.; Zeng, L.; Ding, X.Y.; Cheng, Y.; Liu, Z.F.; Li, Q.A.; et al. Analysis of Lhcb gene family in rapeseed (Brassica napus L.) identifies a novel member “BnLhcb3.4” modulating cold tolerance. Environ. Exp. Bot. 2022, 198, 104848. [Google Scholar]
- Chen, Y.E.; Ma, J.; Wu, N.; Su, Y.Q.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Zeng, X.Y.; Yuan, S. The roles of Arabidopsis proteins of Lhcb4, Lhcb5 and Lhcb6 in oxidative stress under natural light conditions. Plant Physiol. Biochem. 2018, 130, 267–276. [Google Scholar] [PubMed]
- Gu, S.; Zhuang, J.; Zhang, Z.; Chen, W.; Xu, H.; Zhao, M.; Ma, D. Multi-omics approach reveals the contribution of OsSEH1 to rice cold tolerance. Front. Plant Sci. 2023, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Krzywinska, E.; Szymanska, K.P.; Dobrowolska, G. Inhibition of SnRK2 Kinases by Type 2C Protein Phosphatases. Methods Mol. Biol. 2022, 2462, 17–30. [Google Scholar]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2013, 147, 15–27. [Google Scholar]
- Zhao, J.; Peng, P.; Schmitz, R.J.; Decker, A.D.; Tax, F.E.; Li, J. Two Putative BIN2 Substrates Are Nuclear Components of Brassinosteroid Signaling. Plant Physiol. 2002, 130, 1221–1229. [Google Scholar]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.-R.; Tang, G.; Barajas-López, J.D.D.; et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Chen, X.; Li, L.; Liu, K.; Zhao, H.; Wang, Y.; Han, S. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in Arabidopsis. J. Exp. Bot. 2018, 69, 3933–3947. [Google Scholar]
- Martínez, C.; Espinosa-Ruíz, A.; de Lucas, M.; Bernardo-García, S.; Franco-Zorrilla, J.M.; Prat, S. PIF4 induced BR synthesis is critical to diurnal and thermomorphogenic growth. EMBO J. 2018, 37, e99552. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Chico, J.M.; Fernández-Calvo, P.; Solano, R. The ZIM domain mediates homo- and heteromeric interactions between Arabidopsis JAZ proteins. Plant J. 2009, 59, 77–87. [Google Scholar]
- Mosher, R.A.; Durrant, W.E.; Wang, D.; Song, J.; Dong, X. A Comprehensive Structure–Function Analysis of Arabidopsis SNI1 Defines Essential Regions and Transcriptional Repressor Activity. Plant Cell 2006, 18, 1750–1765. [Google Scholar] [PubMed]
- Lv, P.; Liu, J.H.; Liu, X.M. The role of ubiquitin-conjugating enzyme in the process of spermatogenesis. Reprod. Biol. Endocrinol. 2024, 22, 110. [Google Scholar]
- Khan, I.U.; Ali, A.; Zareen, S.; Khan, H.A.; Lim, C.J.; Park, J.; Pardo, J.M.; Yun, D.J. Non-Expresser of PR-Genes 1 Positively Regulates Abscisic Acid Signaling in Arabidopsis thaliana. Plants 2022, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.K.; Giri, M.K.; Singh, D.; Chattopadhyay, S.; Nandi, A.K. MYC2 influences salicylic acid biosynthesis and defense against bacterial pathogens in Arabidopsis thaliana. Physiol. Plant. 2021, 173, 2248–2261. [Google Scholar]
- Hussain, R.M.F.; Kim, H.K.; Khurshid, M.; Akhtar, M.T.; Linthorst, H.J.M. Overexpression of AtWRKY50 is correlated with enhanced production of sinapic derivatives in Arabidopsis. Metabolomics 2018, 14, 25. [Google Scholar]
- Zhang, X.; Zhang, Y.; Li, M.; Jia, H.; Wei, F.; Xia, Z.; Zhang, X.; Chang, J.; Wang, Z. Overexpression of the WRKY transcription factor gene NtWRKY65 enhances salt tolerance in tobacco (Nicotiana tabacum). BMC Plant Biol. 2024, 24, 326. [Google Scholar] [CrossRef]
- Guo, M.Y.; Yang, F.J.; Liu, C.X.; Zou, J.P.; Qi, Z.Y.; Fotopoulos, V.; Lu, G.; Yu, J.Q.; Zhou, J. A single-nucleotide polymorphism in WRKY33 promoter is associated with the cold sensitivity in cultivated tomato. New Phytol. 2022, 236, 989–1005. [Google Scholar]
- Wang, Y.; Cao, J.J.; Wang, K.X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Zhou, J. BZR1 Mediates Brassinosteroid-Induced Autophagy and Nitrogen Starvation in Tomato. Plant Physiol. 2019, 179, 671–685. [Google Scholar]
- Zhu, S.S.; Zhou, J.; Sun, X.Y.; Zhou, Z.L.; Zhu, Q.Q. ROS accumulation contributes to abamectin-induced apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway in TM3 Leydig cells. J. Biochem. Mol. Toxicol. 2020, 34, e22505. [Google Scholar]
- Du, Z.Y.; Chen, M.X.; Chen, Q.F.; Xiao, S.; Chye, M.L. Overexpression of Arabidopsis acyl-CoA-binding protein ACBP2 enhances drought tolerance. Plant Cell Environ. 2013, 36, 300–314. [Google Scholar]
- Wang, X.; Miao, J.M.; Kang, W.J.; Shi, S.L. Exogenous application of salicylic acid improves freezing stress tolerance in alfalfa. Front. Plant Sci. 2023, 14, 1091077. [Google Scholar]
- Yang, X.; Zhuang, Z.; Zhou, Y.; Lei, G.; Wan, W.; Peng, Y. Effects of different exogenous hormones on maize germination and seedling physiological characteristics under low temperature stress. J. Gansu Agric. Univ. 2024, 59, 117–128. [Google Scholar]
- Li, X.; Che, W.; Piao, J.; Song, Y.; Wang, X.; Zhang, Y.; Miao, S.; Wang, H.; Xie, L.; Sun, J. Biochar Increases Rice Yield in Soda Saline-Alkali Paddy Fields by Improving Saline-Alkali Stress and Phosphorus Use Efficiency. Agronomy 2024, 14, 2159. [Google Scholar] [CrossRef]
- Wang, X.; Qi, X.; Zhuang, Z.; Bian, J.; Li, J.; Chen, J.; Li, Z.; Peng, Y. Interactions between Brassinosteroids and Strigolactones in Alleviating Salt Stress in Maize. Int. J. Mol. Sci. 2024, 25, 10505. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Zhuang, Z.; Bian, J.; Ren, Z.; Ta, W.; Peng, Y. Mechanisms of Exogenous Brassinosteroids and Abscisic Acid in Regulating Maize Cold Stress Tolerance. Int. J. Mol. Sci. 2025, 26, 3326. https://doi.org/10.3390/ijms26073326
Yang T, Zhuang Z, Bian J, Ren Z, Ta W, Peng Y. Mechanisms of Exogenous Brassinosteroids and Abscisic Acid in Regulating Maize Cold Stress Tolerance. International Journal of Molecular Sciences. 2025; 26(7):3326. https://doi.org/10.3390/ijms26073326
Chicago/Turabian StyleYang, Tao, Zelong Zhuang, Jianwen Bian, Zhenping Ren, Wanling Ta, and Yunling Peng. 2025. "Mechanisms of Exogenous Brassinosteroids and Abscisic Acid in Regulating Maize Cold Stress Tolerance" International Journal of Molecular Sciences 26, no. 7: 3326. https://doi.org/10.3390/ijms26073326
APA StyleYang, T., Zhuang, Z., Bian, J., Ren, Z., Ta, W., & Peng, Y. (2025). Mechanisms of Exogenous Brassinosteroids and Abscisic Acid in Regulating Maize Cold Stress Tolerance. International Journal of Molecular Sciences, 26(7), 3326. https://doi.org/10.3390/ijms26073326




